Abstract
Regulation of glutamate release is an important underlying mechanism in mediating excitotoxic events such as damage to dopamine (DA) and serotonin (5-HT) neurons observed after exposure to methamphetamine (Meth). One way to regulate glutamate release may be through the modulation of α7 nicotinic acetylcholine (nACh) receptors. Meth administration is known to increase acetylcholine release; however, it is unknown whether Meth increases glutamate release and causes long-term damage to both DA and 5-HT terminals through the activation of α7 nACh receptors. To test this hypothesis, the α7 nACh receptor antagonist, methyllycaconitine (MLA), was administered before the administration of repeated doses of Meth while simultaneously monitoring extracellular striatal glutamate with in vivo microdialysis. In addition, the subsequent long-term decreases in markers of dopaminergic and serotonergic terminals, including DA reuptake transporter (DAT), serotonin reuptake transporter (SERT), vesicular monoamine transporter-2, vesicular DA, and vesicular 5-HT content in the rat striatum, were measured. The results show that MLA pretreatment prevented Meth-induced increases in striatal glutamate and protected against the subsequent long-term decreases in striatal DAT and vesicular DA content without affecting the hyperthermia produced by Meth. In contrast, the Meth-induced decreases in striatal SERT immunoreactivity and vesicular 5-HT content were not affected by MLA. This suggests that the α7 nACh receptor differentially mediates glutamate-dependent damage to DA but not 5-HT terminals in a manner that is independent of hyperthermia. Furthermore, antagonism of α7 nACh receptors may be a possible therapeutic strategy for decreasing extracellular glutamate and preventing the excitotoxic damage observed in other DA-related neurodegenerative disorders.
Introduction
Glutamate is commonly accepted as the most abundant excitatory neurotransmitter in the brain, and it plays a major role in normal brain functions, including development, cognition, learning, and memory. However, it is also well known that excess glutamate can be damaging to neurons, and it has been implicated in many diseases of the central nervous system including the addiction and damage produced by drugs of abuse (Choi, 1988; Wolf, 1998). Upon activation of calcium-permeable NMDA and AMPA receptors, glutamate produces an increase in intracellular calcium, which in excess activates calcium-dependent enzymes and generates reactive oxygen and nitrogen species, ultimately leading to excitotoxic neuronal damage (Siman and Noszek, 1988; Staszewski and Yamamoto, 2006). Therefore, the concentrations of extracellular glutamate in the brain must be tightly regulated.
Regulation of glutamate release can be mediated by acetylcholine (ACh). More specifically, acetylcholine can modulate glutamate release through presynaptic nicotinic receptors. Although the presence of α7 nicotinic acetylcholine (nACh) receptors on presynaptic glutamate terminals is controversial, there is substantial evidence for the ability of α7 nACh receptors to modulate glutamate release from isolated striatal synaptosomes (Marchi et al., 2002) and in the prefrontal cortex of freely moving rats (Konradsson-Geuken et al., 2009). Thus, the modulation of extracellular levels of glutamate through α7 nACh receptors may be one way to protect the brain from excitotoxic damage.
Excitotoxicity is known to play a role in damage to dopamine (DA) and serotonin (5-HT) neurons of the striatum (Storey et al., 1992) and is involved in long-term striatal damage after exposure to methamphetamine (Meth) (Staszewski and Yamamoto, 2006). The damage observed in response to Meth is marked by long-term decreases in DA reuptake transporter (DAT) and serotonin reuptake transporter (SERT), vesicular monoamine transporter-2 (VMAT-2), tyrosine hydroxylase, and tryptophan hydroxylase immunoreactivity. In addition, there are long-term decreases in DA and 5-HT tissue content (Hotchkiss and Gibb, 1980; Ricaurte et al., 1980; Frey et al., 1997) and increases in markers of excitotoxicity evidenced by increases in calcium-mediated spectrin proteolysis (Staszewski and Yamamoto, 2006).
One mechanism by which Meth might produce neurotoxicity is through the release of acetylcholine or an increase in α7 nACh receptor binding sites. In fact, there is evidence that systemic Meth administration causes an acute increase in extracellular acetylcholine in the rat striatum (Taguchi et al., 1998), and exposure to Meth increases α7 nACh receptor binding sites in differentiated PC-12 cells (Garcia-Ratés et al., 2007). In addition, activation of the α7 nACh receptor mediates the Meth-induced production of reactive oxygen species in isolated rat striatal synaptosomes (Pubill et al., 2005). Moreover, antagonism of the α7 nACh receptor with methyllycaconitine (MLA) protects against Meth-induced decreases in DAT binding (Escubedo et al., 2005); however, the mechanisms underlying this neuroprotective effect have not been investigated.
The current study tested the hypothesis that the specific α7 nACh receptor antagonist, MLA, will block Meth-induced increases in extracellular glutamate and protect against long-term decreases in DAT, SERT, VMAT-2, and vesicular DA and 5-HT content in the rat striatum. The results indicate that MLA protects against Meth-induced long-term damage to striatal DA terminals, but not to 5-HT neurons, through the attenuation of Meth-induced increases in extracellular glutamate.
Materials and Methods
Animals.
Male Sprague-Dawley rats (180–250 g; Harlan, Indianapolis, IN) were used in all experiments. Rats were group-housed in clear plastic containers (45 × 24 × 20 cm) and allowed 4 to 5 days to acclimate to the animal colony after arrival. Rats were housed in a temperature-controlled (23 ± 1°C), and humidity-controlled (40 ± 5%) environment on a 12-h light/dark cycle (lights on at 7:00 AM and off at 7:00 PM). Rats had ad libitum access to food and water. All treatments were administered during the light cycle. Efforts were made to minimize suffering and reduce the number of animals used in the experiments. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by Boston University and University of Toledo Institutional Animal Care and Use Committees.
Drugs and Drug Treatment.
(+)Methamphetamine-hydrochloride (Meth) was purchased from Sigma-Aldrich (St. Louis, MO). Meth was dissolved in 0.9% NaCl (saline) and administered intraperitoneally, at a dose of 10 mg/kg, for four injections, every 2 h. This dosing paradigm was used because it has been shown that it produces neurotoxicity in rats. Controls for Meth treatment were rats treated with four intraperitoneal injections of saline (1 ml/kg) every 2 h.
MLA was purchased from Tocris Bioscience (Ellisville, MO). MLA was dissolved in saline, and MLA pretreatments consisted of 5.5 mg/kg i.p. injections 15 min before each Meth or saline injection. MLA is a potent α7 nACh receptor antagonist (Ki = 1.4 nM); however, at concentrations greater than 40 nM, it can also interact with α4β2 and α6β2 nACh receptors. A dose of 5.5 mg/kg was chosen for our experiments based on previous studies indicating that similar doses produce brain concentrations, which would selectively antagonize the nACh receptors containing α7 subunits (Turek et al., 1995; Escubedo et al., 2005). To control for MLA pretreatment, rats were treated with 1 ml/kg saline 15 min before each Meth or saline injection. These treatments resulted in a total of four experimental groups (saline + saline, MLA + saline, saline + Meth, and MLA + Meth) for each experiment. Separate groups of animals were used for in vivo microdialysis experiments and experiments investigating the neurotoxic effects of Meth. Those animals that were used in the in vivo microdialysis experiments were killed 24 h after treatment, whereas those used for the neurotoxicity studies were killed 7 days after treatment.
During all treatments, rectal temperatures were measured via a digital thermometer with probe (Thermalert TH-8; Physitemp Instruments Inc., Clifton, NJ) before the first injection and 1 h after each injection. To minimize animal death, rats that reached temperatures higher than 41°C were cooled briefly by placing ice packs on top of their cages while their temperatures were closely monitored.
In Vivo Microdialysis.
For in vivo microdialysis experiments, rats underwent intracranial surgery for probe implantation into the striatum, and the next day rats were treated and brain dialysate was collected.
Rats were anesthetized with a solution of xylazine (5 mg/kg) and ketamine (75 mg/kg) via intramuscular injection and placed into a stereotaxic frame (David Kopf Instruments, Tujunga, CA). The skull was exposed, and a small hole was drilled through the skull above the striatum. A microdialysis probe, constructed as described previously (Yamamoto and Pehek, 1990) with an active membrane (13,000 molecular weight cutoff; 216-μm outer diameter) (Spectrum Laboratories, Rancho Dominguez, CA) length of 4 mm, was slowly lowered into the striatum (anteroposterior + 1.2; mediolateral ± 3.0) using the stereotaxic frame, so that the tip of the probe was 6.25 mm ventral to the surface of the brain. This resulted in the actively dialyzed region of the striatum to be 2.25 to 6.25 mm from the brain surface. Probes were secured in the appropriate position with cranioplastic cement (CO-ORAL-ITE Dental MFG. CO., Diamond Springs, CA).
After surgery, all rats were housed individually in round buckets and attached to a spring tether and swivel, which allowed the rats to move freely. The probes were attached to polyethylene 50 tubing, connected to a Harvard Apparatus Inc. (Holliston, MA) 22 syringe perfusion pump. Dulbecco's phosphate-buffered saline (138 mM NaCl, 2.1 mM KCl, 0.5 mM MgCl2, 1.5 mM KH2PO4, 8.1 mm NaHPO4, 1.2 mm CaCl2, and 0.5 mM d-glucose, pH 7.4) (Sigma-Aldrich) was perfused at a flow rate of 0.25 μl/min overnight. Approximately 15 h after surgery, the pump flow rate was increased to 1.5 μl/min. After a 2-h equilibration period, baseline dialysate samples were collected every hour for 2 h, before the first Meth or saline injection, and all subsequent samples were collected every hour during treatment through 2 h after the last Meth or saline injection. Rats were killed by rapid decapitation 24 h after treatment, and their brains were removed and frozen on dry ice. A cryostat (Microm HM 550; Thermo Fisher Scientific, Waltham, MA) was used to cut coronal slices to verify probe placement.
High-Performance Liquid Chromatography for Measurement of Extracellular Glutamate.
Extracellular concentrations of striatal glutamate were determined by high-performance liquid chromatography (HPLC) with electrochemical detection. Each dialysate sample was derivatized with an o-pthaldialdehyde (OPA) (Sigma-Aldrich) solution, comprised of OPA, methanol, β-mercaptoethanol, and sodium tetraborate, pH 9.3 before injection onto the column. An ESA model 542 autosampler (ESA Inc., Chelmsford, MA) was used to add 10 μl of the OPA solution to 20 μl of the dialysate sample. The autosampler mixed the solution and allowed it to incubate for 90 s, before the mixture was injected onto a Hyperclone 3 μm C18 column, 150 × 2.00 mm (Phenomenex, Torrance, CA). The mobile phase used for detection of glutamate consisted of 0.1 M Na2HPO4 and 0.1 mM EDTA in 12% methanol, at a pH of 6.6. Electrochemical detection of glutamate was performed with an LC-4C amperometric detector (BAS Bioanalytical Systems, Inc., Lafayette, IN) using a 6-mm glassy working electrode maintained at a potential of 0.7 V relative to an Ag-AgCl reference electrode. Data were collected and analyzed using EZ Chrome software (Scientific Software, Pleasanton, CA).
Striatal extracellular glutamate levels were represented as a percentage of baseline. To obtain these values, the concentration of glutamate at each time point for an animal was calculated as a percentage of that animal's baseline concentration. For each group, the mean and S.E.M. was calculated and plotted.
Western Blot for VMAT-2, DAT, and SERT.
Detection of VMAT-2, DAT, and SERT protein was measured in the striatum 7 days after treatment using Western blot. Rats were rapidly decapitated 7 days after treatment, brains were dissected, and tissue was rapidly frozen on dry ice and stored at −80°C until use.
DAT and SERT were measured in the synaptosomal fraction of the striatal tissue, whereas VMAT-2 was measured in the vesicular fraction. The following procedure was used to obtain subcellular fractions. Striatal tissue was homogenized in ice-cold 0.32 M sucrose (20 μl per 1 mg of tissue) and centrifuged for 24 min at 800g at 4°C to pellet insoluble material. The supernatant (S1) was collected and centrifuged for 17 min at 22,000g at 4°C. The supernatant from the second spin (S2) was discarded, and the pellet (P2) containing the synaptosomes was resuspended in ice-cold Millipore-purified water (Millipore Bioscience Research Reagents, Temecula, CA). A portion of this synaptosomal preparation was retained for later use and the remaining sample was centrifuged at 22,000g for 17 min, at 4°C. The resulting supernatant (S3) contained the vesicular fraction and the pellet (P3) contained the membrane fraction.
The Bradford assay (Bio-Rad Laboratories, Hercules, CA) was used to determine total protein content in the synaptosomal and vesicular fractions. Samples were then diluted with 2× SDS loading buffer (Invitrogen, Carlsbad, CA), boiled at 85°C for 5 min, and stored at −80°C until use.
Equal amounts of protein were loaded per well for each sample (10 μg of synaptosomal protein for DAT and SERT, 5 μg of vesicular protein for VMAT-2) onto a 10% Tris-glycine SDS-polyacrylamide gel electrophoresis gel (Invitrogen). After electrophoresis, proteins were transferred onto polyvinylidene fluoride membranes. Membranes were blocked with Tris-buffered saline (TBS) (10 mM Tris, 150 mM NaCl), containing 0.5% Tween 20 and 5% nonfat powdered milk, for 1 h at room temperature. Membranes were incubated with primary antibodies [anti-DAT, 1:2500 (Santa Cruz Biotechnology Inc., Santa Cruz, CA); anti-SERT, 1:1000 (Santa Cruz Biotechnology Inc.); anti-VMAT-2, 1:5000 (Millipore Bioscience Research Reagents); anti-α-tubulin, 1:2000 (Sigma-Aldrich)] in blocking buffer for approximately 17 h at 4°C. After 3- and 5-min washes with TBS containing 0.5% Tween 20, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies [rabbit anti-goat, 1:2500 (Santa Cruz Biotechnology Inc.); goat anti-rabbit, 1:2500 (Millipore Bioscience Research Reagents); goat anti-mouse IgG, 1:2500 (Santa Cruz Biotechnology Inc.)] in blocking buffer for 1 h at room temperature. Membranes were washed again, three times for 5 min, with TBS containing 0.5% Tween 20.
HyGLO-enhanced chemiluminescence (Denville Scientific Inc., Metuchen, NJ) was used for antibody detection. Chemiluminescence was imaged using the Fuji LAS-4000 mini system (FujiFilm Corp. Life Science Division, Tokyo, Japan) and analyzed using Multi Gauge software (FujiFilm Corp. Life Science Division). Optical densities were measured for bands of all proteins. DAT and SERT, in the synaptosomal fractions, were normalized to an internal α-tubulin control. The VMAT-2 was not normalized to α-tubulin, because there was little to no α-tubulin in the isolated vesicular fraction. Each sample was calculated as a percentage of the mean of all saline + saline samples on the same gel to compare across gels. VMAT-2, DAT, and SERT immunoreactivity were presented as percentage of saline + saline-treated animals.
Measurement of Vesicular DA and 5-HT Content via HPLC.
Decreases in vesicular DA and 5-HT content are indicative of Meth-induced striatal damage (Sandoval et al., 2003); therefore, vesicular DA and 5-HT content was measured in a pure vesicular fraction. The vesicular fraction was prepared as described by Sandoval et al. (2003). In brief, the crude vesicular preparation from the subcellular fractionation above was centrifuged at 100,000g for 45 min at 4°C to pellet the vesicles from the cytosolic fraction. The supernatant was discarded and the pellet was resuspended and sonicated, using a Thermo Fisher Scientific model 150E ultrasonic dismembrator at 30% amplitude in 0.1 N perchloric acid. Samples were then centrifuged at 14,000g for 20 min to pellet proteins. Supernatants containing DA and 5-HT were injected onto the HPLC column, and pellets containing protein were resuspended in 1 N NaOH for protein quantification using the Bradford Assay (Bio-Rad Laboratories).
An ESA model 542 autosampler (ESA, Chelmsford, MA) was used to inject 20 μl of each sample onto a Varian Microsorb-MV 100, 5-μm C-18 column, 250 × 4.6 mm (Varian Inc., Palo Alto, CA). The mobile phase used for detection of monoamines consisted of 11 mM citric acid, 0.81 mM 1-octanesulfonic acid sodium salt, 75 mM sodium phosphate dibasic, and 11% methanol, at pH 4.4. Electrochemical detection of DA and 5-HT was performed with an LC-4C amperometric detector (BAS Bioanalytical Systems, Inc.) using a 6-mm glassy working electrode maintained at a potential of 0.7 V relative to an Ag-AgCl reference electrode. Data were collected and analyzed using EZ Chrome software (Scientific Software, Pleasanton, CA). DA and 5-HT HPLC values were normalized to protein levels and presented as percentage of saline + saline controls.
Statistical Analyses.
Body temperatures and striatal extracellular glutamate concentrations were analyzed with a two-way analysis of variance with repeated measures, followed by Tukey's post hoc analyses. A two-way analysis of variance was used to compare the effects of Meth or saline treatment and MLA or saline pretreatments on VMAT-2, DAT, and SERT immunoreactivity and vesicular DA and 5-HT content. To compare the effects of MLA pretreatment in Meth-treated rats, a post hoc t test was used. In all cases where a two-way interaction was revealed, Tukey's post hoc analyses were used to identify significant differences between treatment groups. For all experiments, statistical significance was set at p < 0.05.
Results
Effects of MLA Pretreatment on Meth-Induced Hyperthermia.
The effects of drug treatment on body temperatures are illustrated in Fig. 1. Meth administration (10 mg/kg i.p. four times every 2 h) significantly increased body temperature by approximately 3°C during the treatment period compared with those rats receiving saline treatment (F12,239 = 14.493; p < 0.001). Furthermore, MLA pretreatment (5.5 mg/kg), administered 15 min before each Meth or saline injection, had no effect on Meth- or saline-induced body temperatures. It is important to note anecdotally that MLA pretreatment did not seem to have any behavioral effects on its own and did not alter Meth-induced hyperlocomotion.
Fig. 1.
Rectal temperatures after Meth or saline exposure. Rectal temperatures were significantly increased over the period of Meth treatment. ***, p < 0.001. MLA pretreatment, administered 15 min before each Meth or saline injection had no effect on saline or Meth-induced body temperatures (n = 10–14 for each group). Large arrows indicate Meth or saline injections, and small arrows indicate MLA or saline pretreatment injections.
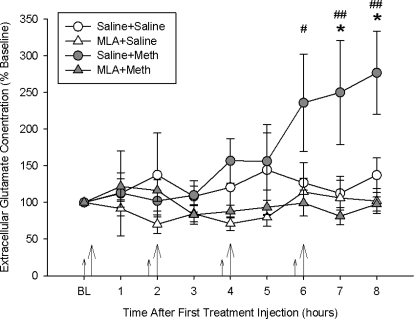
Effects of MLA Pretreatment on Meth-Induced Acute Increases in Extracellular Glutamate.
Extracellular glutamate levels were measured before, during, and after treatments and are illustrated as a percentage of baseline glutamate (Fig. 2). The average baseline concentration of glutamate in the dialysate was 1359.89 ± 201.80 pg/20 μl. Meth administration significantly increased striatal extracellular concentrations of glutamate over all other treatment groups, as revealed by a significant two-way interaction between drug treatment and time (F24,236 = 2.149; p < 0.001). Tukey's post hoc analyses indicated that the saline + Meth treatment group produced a significant 2.5-fold increase, compared with all of the other treatment groups at hours 7 and 8 (p < 0.05). MLA pretreatment significantly blocked Meth-induced increases in extracellular glutamate, because the MLA + Meth group was significantly different from the saline + Meth group at hours 6, 7, and 8 (p < 0.05). The MLA + Meth group was not different from saline-treated controls at any time.
Fig. 2.
Striatal extracellular glutamate concentrations during Meth or saline treatment. Meth significantly increased glutamate release over time, and MLA completely blocked the Meth-induced increase in glutamate. *, p < 0.05, saline + Meth compared with saline + saline; #, p < 0.05; ##, p < 0.01, MLA + Meth compared with saline + Meth (n = 6–9 for each group). Large arrows indicate Meth or saline injections, and small arrows indicate MLA or saline pretreatment injections.
Effects of MLA Pretreatment on Long-Term Meth-Induced Decreases in Striatal Monoaminergic Markers.
Rats were treated with Meth (10 mg/kg i.p. every 2 h four times a day) or saline (1 ml/kg i.p. every 2 h four times a day) and pretreated with MLA (5.5 mg/kg) or saline 15 min before each Meth or saline injection. Seven days after treatment, markers of dopaminergic and serotonergic terminals were assessed in the striatum.
Figure 3 illustrates striatal vesicular VMAT-2 immunoreactivity 7 days after treatment. Meth treatment significantly decreased VMAT-2 immunoreactivity, as revealed by a significant main effect of Meth treatment (F1,47 = 27.358; p < 0.001). MLA pretreatment had no effect in saline-treated rats. MLA + Meth-treated rats showed a partial protection in VMAT-2 compared with saline + Meth rats (27 ± 7% depletion in the MLA + Meth rats versus a 65 ± 7% depletion in the saline + Meth rats), as illustrated by a significant two-way interaction between Meth treatment and MLA pretreatment (F1,47 = 4.046; p < 0.05). Tukey's post hoc analyses revealed that saline + Meth treatment produced a significant 65 ± 7% depletion of VMAT-2 immunoreactivity compared with saline + saline-treated rats (p < 0.05). MLA + Meth treatment produced a slight, but significant, 27 ± 7% depletion compared with the MLA + saline-treated group (p < 0.05).
Fig. 3.
Striatal vesicular VMAT-2 immunoreactivity 7 days after Meth or saline treatment. A, Meth administration significantly decreased VMAT-2 immunoreactivity, and MLA pretreatment significantly attenuated the Meth-induced decrease. ***, p < 0.001, saline + Meth compared with saline + saline; ##, p < 0.01, MLA + Meth compared with saline + Meth; &, p < 0.05, MLA + Meth compared with MLA + saline (n = 8–14 for each group). B, representative VMAT-2 Western blot image illustrating VMAT-2 at 68 kDa.
Vesicular DA content was measured in the striatum 7 days after treatment (Fig. 4). Meth significantly depleted vesicular DA content, and MLA pretreatment completely blocked Meth-induced DA depletions (13 ± 10% depletion in the MLA + Meth versus a 54 ± 6% depletion in the saline + Meth groups), as revealed by a two-way interaction between drug treatment and pretreatment (F1,62 = 5.699; p < 0.05). Tukey's post hoc analyses indicated that whereas saline + Meth-treated rats had a significant 54 ± 6% vesicular DA depletion compared with saline + saline and MLA + saline treatments (p < 0.05), MLA + Meth treatment resulted in a nonsignificant 13 ± 10% depletion from saline + saline-treated rats and no depletion compared with MLA + saline-treated rats.
Fig. 4.
Striatal vesicular DA content 7 days after Meth or saline treatment. Meth administration significantly depleted vesicular DA content, and MLA pretreatment significantly blocked the Meth-induced depletion. ***, p < 0.001, saline + Meth compared with saline + saline; ##, p < 0.01, MLA + Meth compared with saline + Meth (n = 8–22 for each group).
Figure 5 illustrates DAT immunoreactivity in striatal synaptosomes 7 days after treatment. Meth produced a significant depletion of striatal DAT immunoreactivity, as revealed by a main effect of Meth treatment (F1,39 = 14.785; p < 0.001). Post hoc t tests revealed that saline + Meth treatment produced a 58 ± 9% depletion of DAT compared with saline + saline controls (p < 0.05), and MLA + Meth treatment was significantly (p < 0.05) different from saline + Meth treatment (a 27 ± 10% depletion in the MLA + Meth group versus a 58 ± 9% depletion in the saline + Meth group), but did not differ from saline + saline or MLA + saline treatment. Furthermore, MLA pretreatment had no effect on saline-treated rats.
Fig. 5.
Striatal synaptosomal DAT immunoreactivity 7 days after Meth or saline treatment. A, Meth administration significantly decreased DAT immunoreactivity, and MLA pretreatment significantly blocked the Meth-induced decrease. ***, p < 0.001, saline + Meth compared with saline + saline; #, p < 0.05, MLA + Meth compared with saline + Meth (n = 8–12 for each group). B, representative Western blot image illustrating an immunoreactive band for DAT at 70 kDa and α-tubuin at 50 kDa.
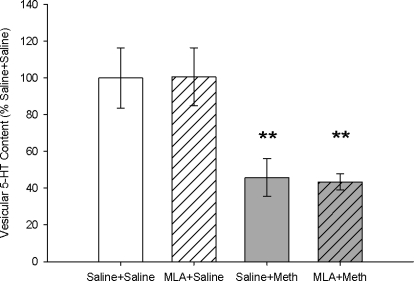
Vesicular serotonin content was measured in the striatum 7 days after treatment (Fig. 6). Meth treatment significantly depleted vesicular serotonin content, as indicated by a significant main effect of Meth treatment (F1,17 = 9.348; p < 0.05). Saline + Meth-treated rats had a significant 54 ± 10% depletion of vesicular serotonin content compared with saline + saline-treated rats (p < 0.05), as indicated by a Tukey's post hoc analysis. MLA pretreatment had no effect on saline- or Meth-induced depletions of vesicular serotonin, indicated by the 56 ± 5% depletion in the MLA + Meth-treated group, compared with the MLA + saline-treated group.
Fig. 6.
Striatal vesicular 5-HT content 7 days after Meth or saline treatment. Meth treatment significantly depleted vesicular 5-HT content in both saline- and MLA-pretreated rats. **, p < 0.01 (n = 4–8 for each group).
Figure 7 illustrates SERT immunoreactivity in striatal synaptosomes 7 days after treatment. Meth treatment significantly decreased SERT immunoreactivity in striatal synaptosomes, as revealed by a significant main effect of drug treatment (F1,15 = 6.01; p < 0.05). Tukey's post hoc analyses indicated a significant 51 ± 20% depletion of SERT in the saline + Meth group compared with the saline + saline group (p < 0.05), and the MLA + Meth treatment resulted in a significant 45 ± 21% decrease in SERT compared with MLA + saline treatment (p < 0.05). Thus, MLA had no effect on SERT in saline- or Meth-treated rats.
Fig. 7.
Striatal synaptosomal SERT immunoreactivity 7 days after Meth or saline treatment. A, Meth treatment significantly depleted synaptosomal SERT immunoreactivity in both saline- and MLA-pretreated rats. **, p < 0.01 (n = 4–8 for each group). B, representative Western blot image illustrating an immunoreactive band for SERT at 68 kDa and α-tubuin at 50 kDa.
Discussion
The role of the α7 nACh receptor in Meth-induced increases in extracellular glutamate and long-term damage to DA and 5-HT terminals in the rat striatum was investigated. Antagonism of the α7 nACh receptor with MLA blocked Meth-induced increases in striatal extracellular glutamate. In addition, MLA blocked Meth-induced decreases in markers of striatal DA terminals including DAT and vesicular dopamine content without affecting markers of 5-HT terminals or Meth-induced hyperthermia.
Meth significantly increased body temperature, but MLA pretreatment did not affect Meth-induced hyperthermia (Fig. 1). Hyperthermia is known to play an important role in the toxic effects of Meth (Bowyer et al., 1992). Meth may cause hyperthermia through an increase in ACh release (Taguchi et al., 1998), because there is evidence that ACh is involved in thermoregulation (Crawshaw, 1973). Because MLA did not alter Meth-induced hyperthermia, the effects of MLA on the acute increases in extracellular glutamate and the long-term decreases in DA and 5-HT terminal markers after Meth probably are caused by its pharmacological properties that are distinct from the role of ACh in thermoregulation. Therefore, the α7 nACh receptor does not seem to be involved in Meth-induced hyperthermia.
The current results confirm that Meth administration increases extracellular glutamate in the rat striatum (Nash and Yamamoto, 1992; Abekawa et al., 1994) and support previous in vitro studies that demonstrated that the α7 nACh receptor modulates glutamate release. Nicotinic receptor agonists did not affect basal release of [3H]-d-aspartate from isolated rat striatal synaptosomes; however, the nicotinic receptor agonist, anatoxin-a, enhanced [3H]-d-aspartate overflow when synaptosomes were depolarized with potassium chloride (Marchi et al., 2002). This effect was inhibited by the α7 nACh receptor antagonists, α-bungarotoxin and MLA, suggesting that α7 nACh receptors regulate glutamate release from striatal presynaptic glutamatergic terminals. More recently, nicotine or choline was found to stimulate glutamate release in the prefrontal cortex in a manner that was attenuated or blocked, respectively, by α-bungarotoxin (Konradsson-Geuken et al., 2009). Regardless, the present study is the first report that antagonism of the α7 nACh receptor can block Meth-induced increases in striatal extracellular glutamate.
Because high extracellular concentrations of glutamate have been implicated in excitotoxic damage to striatal DA and 5-HT neurons (Storey et al., 1992; Staszewski and Yamamoto, 2006), and MLA blocks Meth-induced increases in extracellular striatal glutamate, we hypothesized that MLA would also block Meth-induced long-term damage to striatal dopaminergic and serotonergic terminals. Pretreatment with MLA, 15 min before each Meth injection, blocked Meth-induced decreases in vesicular DA content (Fig. 4) and DAT (Fig. 5) immunoreactivity, while having no effect on Meth-induced decreases in markers of 5-HT terminals (Figs. 6 and 7). MLA only partially protected against Meth-induced decreases in VMAT-2 immunoreactivity (Fig. 3), which most likely reflects the selective protection of DA terminals despite the localization of VMAT-2 to both DA and 5-HT terminals and the long-term damage to both DA and 5-HT terminals produced by Meth.
These data seem to be in opposition to the protective effects of nicotine against Meth-induced striatal dopaminergic damage. Nicotine binds nonselectively to all nicotinic receptors and its protective effects probably are caused by activation of nACh receptors other than α7 nACh receptors. In fact, while acute nicotine pretreatment protected against Meth-induced decreases in [3H]mazindol binding sites in wild-type mice, nicotine had no effect in α4 nACh receptor subunit knockout mice (Ryan et al., 2001). In addition, there is evidence that nicotine has a greater affinity for nACh receptors containing the α4 subunits (Xiao et al., 2009), suggesting that nicotine affords protection through the activation of nACh receptors containing α4 subunits, whereas ACh affects glutamate release and excitotoxicity through the α7 receptor.
Escubedo et al. (2005) suggested that MLA protects against Meth-induced striatal dopaminergic damage in mice through the attenuation of Meth-induced oxidative stress; however the initiation of a pro-oxidant process was not identified. The ability of MLA to attenuate oxidative stress may be related to the blockade of Meth-induced increases in extracellular glutamate (Fig. 2). It is well known that glutamate, via activation of its ionotropic receptors, activates nitric-oxide synthase and produces nitric oxide (NO) and peroxynitrite (ONOO−) (Bhardwaj et al., 1997). Furthermore, NO and ONOO− can damage proteins of monoaminergic terminals. For example, tyrosine hydroxylase and tryptophan hydroxylase are inactivated after nitration by NO and ONOO− in rat tissue (Kuhn and Geddes, 1999; Sadidi et al., 2005), and DAT function in humans is inhibited by ONOO− (Park et al., 2002). In addition, neuronal nitric-oxide synthase inhibition prevents Meth-induced nitrosylation of VMAT-2 1 h after treatment and prevents long-term decreases in VMAT-2 and DAT immunoreactivity and vesicular DA content in the rat striatum (Eyerman and Yamamoto, 2007). Serotonin terminals are also vulnerable to oxidative stress as evidenced by the attenuation of Meth-induced SERT depletions in copper-zinc superoxide dismutase transgenic mice (Hirata et al., 1995).
Despite the similarities in the oxidative mechanisms that mediate damage to both DA and 5-HT terminals, the reasons for the selective protection to DA terminals afforded by MLA are unclear. Key differences may reside in the specificity of interactions between neurotransmitter systems in the striatum. One possibility is that DA and 5-HT terminals differentially express calcium-permeable α7 nACh receptors. Therefore, the effects of MLA would be relatively selective for the cholinergic modulation of calcium influx (Séguéla et al., 1993) into dopaminergic terminals via α7 nACh receptors. However, there is no direct evidence for the localization of α7 nACh receptors to DA terminals, and the effect of α7 nACh ligands on DA release in the striatum is most likely mediated through the modulation of glutamate release (Wonnacott et al., 2000; Marchi et al., 2002). Another possibility that could explain the relatively selective effect of MLA on the protection against the long-term decreases in markers of DA compared with 5-HT terminals is the differential expression of calcium-permeable NMDA and AMPA receptors on dopaminergic and serotonergic terminals. There is evidence of calcium-permeable NMDA and AMPA receptors on striatal DA terminals (Segovia et al., 1997; Hernández et al., 2003) but less evidence of their expression on 5-HT terminals. However, there are studies indicating that both DA and 5-HT neurons are susceptible to excitotoxic damage in response to excess glutamate (Storey et al., 1992; Staszewski and Yamamoto, 2006). A third possibility is that glutamatergic terminals that synapse onto DA terminals express α7 nACh receptors, whereas those that synapse onto 5-HT terminals do not express α7 nACh receptors. Therefore, glutamatergic projections that synapse onto 5-HT terminals would be refractory to the antagonist effects of MLA, continue to release glutamate in response to Meth despite the presence of MLA, and eventually damage 5-HT terminals. This, too, is unlikely because MLA completely blocked Meth-induced increases in striatal extracellular glutamate (Fig. 2).
A more likely explanation for why MLA protects against Meth-induced DA terminal damage, and not 5-HT terminal damage, is that the damage to 5-HT terminals produced by Meth is not mediated by a glutamate-dependent mechanism but occurs either through inflammation, mitochondrial dysfunction or hyperthermia. Proinflammatory mechanisms, such as microglial activation, are observed after Meth and MDMA administration (Thomas et al., 2004). Furthermore, MDMA-induced decreases in SERT binding are prevented by minocycline pretreatment, which also prevents MDMA-induced nuclear factor κB activation, interleukin-1β release, and microglial activation (Orio et al., 2010). MLA, however, has been shown to prevent Meth-induced microglial activation (Escubedo et al., 2005), which may depend on the ability of MLA to prevent increases in glutamate and the subsequent activation of AMPA and kainate receptors on microglia (Noda et al., 2000).
Previous studies have shown that mitochondrial dysfunction or metabolic compromise is involved in serotonergic damage produced by Meth as evidenced by the ability of malonate, a mitochondrial complex II inhibitor, to synergize with Meth to cause serotonergic damage (Burrows et al., 2000). In addition, α7 nACh receptors have been implicated in ethanol-induced mitochondrial dysfunction (Li et al., 2002). However, there is little evidence for a differential role of mitochondrial dysfunction in mediating damage to 5-HT versus DA terminals that would explain the selective protection afforded by MLA. Therefore, the most likely explanation for differential effects of MLA on DA and 5-HT terminals is the dependence on glutamate and the α7 nACh receptor for Meth-induced damage to DA terminals relative to the importance of hyperthermia in mediating damage to 5-HT terminals (LaVoie and Hastings, 1999; Haughey et al., 2000).
In conclusion, although both DA and 5-HT terminals are damaged by Meth, the α7 nACh receptor mediates damage to DA but not 5-HT terminals in a manner that is independent of hyperthermia and depends on the release of glutamate. Therefore, antagonism of α7 nACh receptors may be a plausible way of decreasing extracellular glutamate and preventing excitotoxic damage in other neurodegenerative disorders, such as Parkinson's disease.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA07606].
This work was presented previously: Fazo NA, Smith LP, Yamamoto BK, and Eyerman DJ (2010) Methyllycaconitine protects against methamphetamine-induced damage to dopamine terminals in the rat striatum, at the Society for Neurosciences Conference, 2010 Nov 13–17, San Diego, CA. Program 477.11. Society for Neuroscience, Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.177287.
Abbreviations:
- NMDA
- N-methyl-d-aspartate
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ACh
- acetylcholine
- nAChR
- nicotinic acetylcholine
- DA
- dopamine
- DAT
- DA reuptake transporter
- 5-HT
- serotonin
- Meth
- methamphetamine
- MLA
- methyllycaconitine
- SERT
- serotonin reuptake transporter
- VMAT-2
- vesicular monoamine transporter-2
- HPLC
- high-performance liquid chromatography
- NO
- nitric oxide
- ONOO−
- peroxynitrite
- MDMA
- 3,4-methylenedioxymethamphetamine
- OPA
- o-pthaldialdehyde
- TBS
- Tris-buffered saline.
Authorship Contributions
Participated in research design: Northrop, Smith, Yamamoto, and Eyerman.
Conducted experiments: Northrop, Smith, and Eyerman.
Contributed new reagents or analytic tools: Yamamoto.
Performed data analysis: Northrop, Smith, Yamamoto, and Eyerman.
Wrote or contributed to the writing of the manuscript: Northrop, Yamamoto, and Eyerman.
References
- Abekawa T, Ohmori T, Koyama T. (1994) Effects of repeated administration of a high dose of methamphetamine on dopamine and glutamate release in rat striatum and nucleus accumbens. Brain Res 643:276–281 [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Northington FJ, Ichord RN, Hanley DF, Traystman RJ, Koehler RC. (1997) Characterization of ionotropic glutamate receptor-mediated nitric oxide production in vivo in rats. Stroke 28:850–856; discussion 856–857 [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. (1992) The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther 260:817–824 [PubMed] [Google Scholar]
- Burrows KB, Nixdorf WL, Yamamoto BK. (2000) Central administration of methamphetamine synergizes with metabolic inhibition to deplete striatal monoamines. J Pharmacol Exp Ther 292:853–860 [PubMed] [Google Scholar]
- Choi DW. (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1:623–634 [DOI] [PubMed] [Google Scholar]
- Crawshaw LI. (1973) Effect of intracranial acetylcholine injection on thermoregulatory responses in the rat. J Comp Physiol Psychol 83:32–35 [DOI] [PubMed] [Google Scholar]
- Escubedo E, Chipana C, Pérez-Sánchez M, Camarasa J, Pubill D. (2005) Methyllycaconitine prevents methamphetamine-induced effects in mouse striatum: involvement of α7 nicotinic receptors. J Pharmacol Exp Ther 315:658–667 [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. (2007) A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J Neurochem 103:1219–1227 [DOI] [PubMed] [Google Scholar]
- Frey K, Kilbourn M, Robinson T. (1997) Reduced striatal vesicular monoamine transporters after neurotoxic but not after behaviorally-sensitizing doses of methamphetamine. Eur J Pharmacol 334:273–279 [DOI] [PubMed] [Google Scholar]
- Garcia-Ratés S, Camarasa J, Escubedo E, Pubill D. (2007) Methamphetamine and 3,4-methylenedioxymethamphetamine interact with central nicotinic receptors and induce their up-regulation. Toxicol Appl Pharmacol 223:195–205 [DOI] [PubMed] [Google Scholar]
- Haughey HM, Fleckenstein AE, Metzger RR, Hanson GR. (2000) The effects of methamphetamine on serotonin transporter activity: role of dopamine and hyperthermia. J Neurochem 75:1608–1617 [DOI] [PubMed] [Google Scholar]
- Hernández LF, Segovia G, Mora F. (2003) Effects of activation of NMDA and AMPA glutamate receptors on the extracellular concentrations of dopamine, acetylcholine, and GABA in striatum of the awake rat: a microdialysis study. Neurochem Res 28:1819–1827 [DOI] [PubMed] [Google Scholar]
- Hirata H, Ladenheim B, Rothman RB, Epstein C, Cadet JL. (1995) Methamphetamine-induced serotonin neurotoxicity is mediated by superoxide radicals. Brain Res 677:345–347 [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. (1980) Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther 214:257–262 [PubMed] [Google Scholar]
- Konradsson-Geuken A, Gash CR, Alexander K, Pomerleau F, Huettl P, Gerhardt GA, Bruno JP. (2009) Second-by-second analysis of α7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse 63:1069–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DM, Geddes TJ. (1999) Peroxynitrite inactivates tryptophan hydroxylase via sulfhydryl oxidation. Coincident nitration of enzyme tyrosyl residues has minimal impact on catalytic activity. J Biol Chem 274:29726–29732 [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. (1999) Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci 19:1484–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Meyer EM, Walker DW, Millard WJ, He YJ, King MA. (2002) Alpha7 nicotinic receptor activation inhibits ethanol-induced mitochondrial dysfunction, cytochrome c release and neurotoxicity in primary rat hippocampal neuronal cultures. J Neurochem 81:853–858 [DOI] [PubMed] [Google Scholar]
- Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. (2002) Direct evidence that release-stimulating α7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J Neurochem 80:1071–1078 [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. (1992) Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain Res 581:237–243 [DOI] [PubMed] [Google Scholar]
- Noda M, Nakanishi H, Nabekura J, Akaike N. (2000) AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci 20:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio L, Llopis N, Torres E, Izco M, O'Shea E, Colado MI. (2010) A study on the mechanisms by which minocycline protects against MDMA (‘ecstasy’)-induced neurotoxicity of 5-HT cortical neurons. Neurotox Res 18:187–199 [DOI] [PubMed] [Google Scholar]
- Park SU, Ferrer JV, Javitch JA, Kuhn DM. (2002) Peroxynitrite inactivates the human dopamine transporter by modification of cysteine 342: potential mechanism of neurotoxicity in dopamine neurons. J Neurosci 22:4399–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pubill D, Chipana C, Camins A, Pallàs M, Camarasa J, Escubedo E. (2005) Free radical production induced by methamphetamine in rat striatal synaptosomes. Toxicol Appl Pharmacol 204:57–68 [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. (1980) Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res 193:153–163 [DOI] [PubMed] [Google Scholar]
- Ryan RE, Ross SA, Drago J, Loiacono RE. (2001) Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in α4 nicotinic receptor subunit knockout mice. Br J Pharmacol 132:1650–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadidi M, Geddes TJ, Kuhn DM. (2005) S-thiolation of tyrosine hydroxylase by reactive nitrogen species in the presence of cysteine or glutathione. Antioxid Redox Signal 7:863–869 [DOI] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. (2003) Methylphenidate alters vesicular monoamine transport and prevents methamphetamine-induced dopaminergic deficits. J Pharmacol Exp Ther 304:1181–1187 [DOI] [PubMed] [Google Scholar]
- Segovia G, Del Arco A, Mora F. (1997) Endogenous glutamate increases extracellular concentrations of dopamine, GABA, and taurine through NMDA and AMPA/kainate receptors in striatum of the freely moving rat: a microdialysis study. J Neurochem 69:1476–1483 [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. (1993) Molecular cloning, functional properties, and distribution of rat brain α7: a nicotinic cation channel highly permeable to calcium. J Neurosci 13:596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R, Noszek JC. (1988) Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron 1:279–287 [DOI] [PubMed] [Google Scholar]
- Staszewski RD, Yamamoto BK. (2006) Methamphetamine-induced spectrin proteolysis in the rat striatum. J Neurochem 96:1267–1276 [DOI] [PubMed] [Google Scholar]
- Storey E, Hyman BT, Jenkins B, Brouillet E, Miller JM, Rosen BR, Beal MF. (1992) 1-Methyl-4-phenylpyridinium produces excitotoxic lesions in rat striatum as a result of impairment of oxidative metabolism. J Neurochem 58:1975–1978 [DOI] [PubMed] [Google Scholar]
- Taguchi K, Atobe J, Kato M, Chuma T, Chikuma T, Shigenaga T, Miyatake T. (1998) The effect of methamphetamine on the release of acetylcholine in the rat striatum. Eur J Pharmacol 360:131–137 [DOI] [PubMed] [Google Scholar]
- Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. (2004) Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci Lett 367:349–354 [DOI] [PubMed] [Google Scholar]
- Turek JW, Kang CH, Campbell JE, Arneric SP, Sullivan JP. (1995) A sensitive technique for the detection of the α7 neuronal nicotinic acetylcholine receptor antagonist, methyllycaconitine, in rat plasma and brain. J Neurosci Methods 61:113–118 [DOI] [PubMed] [Google Scholar]
- Wolf ME. (1998) The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol 54:679–720 [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones IW. (2000) Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur J Pharmacol 393:51–58 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Abdrakhmanova GR, Baydyuk M, Hernandez S, Kellar KJ. (2009) Rat neuronal nicotinic acetylcholine receptors containing α7 subunit: pharmacological properties of ligand binding and function. Acta Pharmacol Sin 30:842–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Pehek EA. (1990) A neurochemical heterogeneity of the rat striatum as measured by in vivo electrochemistry and microdialysis. Brain Res 506:236–242 [DOI] [PubMed] [Google Scholar]