Abstract
Previous in vitro data suggest that ethanol (EtOH) activates NADPH oxidase (Nox) in osteoblasts leading to accumulation of reactive oxygen species (ROS). This might be a mechanism underlying inhibition of bone formation and increased bone resorption observed in vivo after EtOH exposure. In a rat model in which cycling females were infused intragastrically with EtOH-containing liquid diets, EtOH significantly decreased bone formation and stimulated osteoblast-dependent osteoclast differentiation. These effects were reversed by exogenous 17-β-estradiol coadministration. Moreover, coadministration of N-acetyl cysteine (NAC), an antioxidant, or diphenylene iodonium (DPI), a specific Nox inhibitor, also abolished chronic EtOH-associated bone loss. EtOH treatment up-regulated mRNA levels of Nox1, 2, 4, and the receptor activator of nuclear factor-κB ligand (RANKL), an essential factor for differentiation of osteoclasts in bone. Protein levels of Nox4, a major Nox isoform expressed in nonphagocytic cells, was also up-regulated by EtOH in bone. 17-β-Estradiol, NAC, and DPI were able to normalize EtOH-induced up-regulation of Nox and RANKL. In vitro experiments demonstrated that EtOH directly up-regulated Nox expression in osteoblasts. Pretreatment of osteoblasts with DPI eliminated EtOH-induced RANKL promoter activity. Furthermore, EtOH induced RANKL gene expression, and RANKL promoter activation in osteoblasts was ROS-dependent. These data suggest that inhibition of Nox expression and activity may be critical for prevention of chronic EtOH-induced osteoblast-dependent bone loss.
Introduction
Although positive effects of moderate alcohol consumption on bone health have been suggested (Jonsson et al., 2007), chronic alcohol abuse and binge alcohol exposure are well recognized as major risk factors for development of osteoporotic bone loss (Turner, 2000; Sampson, 2002; Chakkalakal, 2005; Callaci et al., 2006). The molecular mechanisms whereby ethanol (EtOH) induces bone pathophysiology are not yet clearly defined, but we and others have previously demonstrated that EtOH both inhibits osteoblastic bone formation (Dyer et al., 1998; Callaci et al., 2010; Chen et al., 2010; Turner et al., 2010) and stimulates osteoclastic bone resorption (Dai et al., 2000) through direct and indirect actions (Chen et al., 2006; Shankar et al., 2008; Turner et al., 2010). The effect of EtOH on bone resorption appears to be due, at least in part, to its ability to induce receptor activator of nuclear factor-κB ligand (RANKL) expression in bone and bone marrow cells, resulting in stimulation of osteoclastogenesis. EtOH can disrupt vitamin D and growth hormone homeostasis and also induce a variety of cytokines, such as tumor necrosis factor α (TNFα), resulting in indirect actions on bone cells (Turner et al., 1988, 2010; Shankar et al., 2008). The question of how EtOH directly influences osteoblastic cell differentiation remains to be answered (Chen et al., 2010). Evidence from previous studies in our laboratory suggests that EtOH-induced oxidative stress accelerates activation of senescence pathways in osteoblasts (Chen et al., 2009). In addition, EtOH can freely defuse into cells and induce oxidative stress and redox changes as a consequence of metabolism to acetaldehyde and acetate by the enzymes' alcohol and acetaldehyde dehydrogenase (Chen et al., 2006). The toxic effects of EtOH on bone cells may result from intracellular generation of reactive oxygen species (ROS) (Muller et al., 2007). However, oxidative stress in bone has not yet been demonstrated directly following EtOH exposure in vivo, and limited studies have been conducted on the effects of dietary antioxidants on EtOH-induced bone loss (Chen et al., 2008, 2009).
In general, oxidative stress is caused by an imbalance between the production of reactive oxygen and a biological system's ability to readily detoxify ROS. In biological systems, ROS such as superoxide and hydrogen peroxide are ubiquitous signaling molecules, and their role in tissue physiology and pathophysiology has been extensively elucidated (Li and Fukagawa, 2010). Four members of the NADPH oxidase (Nox) enzyme family are important sources of ROS in many tissues: Nox1, Nox2, Nox3, and Nox4. Nox4 is a constitutively active Nox enzyme expressed in nonphagocytic cells, where it appears to serve as a major source of intracellular superoxide involved in redox signaling. We have previously described the expression of Nox1, Nox2, and Nox4 and up-regulation of Nox1 and Nox4 by EtOH in vitro in osteoblasts derived from bone marrow stromal cells (Chen et al., 2008). However, whether overexpression of Nox1, Nox4, or both and increased Nox-derived superoxide production in osteoblasts is associated with bone loss in vivo produced by either chronic EtOH feeding or other conditions, such as sex steroid deficiency or aging, remains unclear. We have hypothesized that Nox4-mediated ROS accumulation in osteoblasts or their precursors is critical for EtOH-induced bone resorption.
Estradiol may have the ability to protect against EtOH-induced bone resorption in cycling female rats (Shankar et al., 2006). The protective effects of estrogens against cellular injury in endothelial cells and neurons are considered to occur through improved defense against oxidative stress (Sack et al., 1994; Arnal et al., 1996; Sudoh et al., 2001). More recently, the effects of estrogens on antioxidant defenses in bone have been recognized and investigated with greater emphasis on pathophysiology of osteoporosis (Manolagas, 2010), particularly on osteoclasts that have been shown to be activated by ROS (Lean et al., 2003). An in vivo experiment demonstrated that decreases in glutathione and thioredoxin and in the enzymes that regenerate their reduced forms in rodent bone marrow could be reversed by estrogens (Lean et al., 2003). Both osteoclasts and osteoblasts express Nox; however, evidence to show whether estrogens are capable of modulating expression or activity Nox in bone cells is lacking.
In this study, we demonstrate that estradiol, the dietary antioxidant N-acetylcysteine (NAC), and a pan Nox inhibitor diphenylene iodonium (DPI) can all protect against EtOH-induced bone loss, most likely by suppressing bone resorption and inhibiting EtOH-induced Nox and RANKL expression. These data may lead to a better strategy for preventing toxic effects of EtOH and other conditions, which result in oxidative stress-induced bone loss such as aging.
Materials and Methods
Animals.
Two-month-old female Sprague-Dawley rats (250 g) were purchased from Charles River Laboratories, Inc. (Wilmington, MA). Animals were housed in an Association for the Assessment and Accreditation of Laboratory Animal Care-approved animal facility. Animal maintenance and experimental treatments were conducted in accordance with the ethical guidelines for animal research established and approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Science (Little Rock, AR). Rats (n = 10/group) were surgically implanted with an intragastric cannula that was used for total enteral nutrition (TEN), as described previously (Badger et al., 1993). Liquid diets were formulated to contain the nutrients recommended for rats by the National Research Council. Diets contained 16% protein, 54% carbohydrate, and 25% fat (corn oil), and EtOH-containing diets were kept isocaloric to the control diets by substituting EtOH for carbohydrate calories. The EtOH dose was 12 g/kg per day). Rats were infused 187 kcal/kg3/4 per day for 14 h from 6:00 PM to 8:00 AM during the dark cycle for 4 weeks. Additional groups of control and EtOH-infused animals were given 17-β-estradiol [(E2) 20 μg/kg per day; Sigma-Aldrich, St. Louis, MO] subcutaneously using Alzet osmotic minipumps (Chen et al., 2006). NAC (1.2 g/kg per day) was given as a part of liquid diets. Diphenylene iodonium (DPI) chloride (1 mg/kg per day) was subcutaneously injected daily according to a previously published method (Kono et al., 2001). Rats were weighed every other day. A small number of rats died during the course of the study as a result of technical problems such as clogged cannulae and loss of head pieces. Final body weights and numbers were as follows: control (n = 10), 278.1 ± 1.8 g; EtOH (n = 7), 263.8 ± 2.1 g; E2 (n = 9), 278.1 ± 2.3 g; E2+EtOH (n = 9), 280.2 ± 3.7 g; NAC (n = 9), 273.1 ± 3.8 g; NAC+EtOH (n = 8), 281.1 ± 1.9 g; DPI (n = 8), 283.3 ± 2.5 g; and DPI+EtOH (n = 7), 277.9 ± 2.5 g.
Peripheral Quantitative Computerized Tomography and Histomorphometry.
At sacrifice, the right rear tibia was removed and frozen in liquid nitrogen. Peripheral quantitative computerized tomography (pQCT) scans were performed on individual tibial bones from each rat using a Stratec XCT 960M unit (XCT Research SA; Norland Medical Systems, Fort Atkins, WI) with software version 5.4. The detail of pQCT scanning was described previously (Chen et al., 2006; Shankar et al., 2006). Several key points are briefly summarized below. The position for pQCT scanning was defined at a distance approximately 3 mm from the proximal tibia growth plate. All analyses were conducted in a blinded fashion. Five consecutive slices separated by 1 mm (1 through 5, 1 being most distal) were scanned for each tibia beginning immediately below the tibial growth plate. Data from slice 2 and 3 were combined and averaged, as presented under Results. A threshold of 470 mg/cm3 was used to distinguish cortical bone, and 107 mg/cm3 was used to distinguish cancellous bone throughout the experiment. Tibial bone mineral density (BMD) was separated into total, trabecular, and cortical compartments, and they were automatically calculated. At sacrifice, left rear tibial bones were removed and fixed, and sequential dehydration was carried out using different concentrations of alcohol. Proximal tibial bone samples were embedded and cut, and von Kossa, tetrachrome, and Masson stained by standard Histology Special Procedures. For histomorphometric analysis, sections were read in a blinded fashion. Parameters of cancellous bones in the proximal tibia were measured with a digitizing morphometry system, which consists of an epifluorescent microscope (model BH-2; Olympus, Tokyo, Japan), a color video camera, and a digitizing pad (Numonics 2206; Numonics, Montomerville, PA) coupled to a computer (Sony) and a morphometry program OsteoMetrics (OsteoMetrics, Inc., Decatur, GA). Total bone area, total bone surface, osteoblast surface, osteoclast surface, and eroded surface were obtained by manual tracing.
Real-Time Reverse Transcription-Polymerase Chain Reaction Analysis.
Rat tibial bone RNA and osteoblastic cell RNA were extracted using TRI Reagent (MRC Inc., Cincinnati, OH) according to the manufacturer's recommendation, followed by DNase digestion and column cleanup using QIAGEN (Valencia, CA) mini columns. The following procedure was used for RNA isolation from bone tissue: at the time of sacrifice, the right femur was taken, and bone marrow cells were flushed with Eagle's minimum essential medium plus Hanks' salts after cleaning the surrounding connective tissue. Femoral bones were then frozen in liquid nitrogen. Femoral bone was placed in 1000 μl of TRI Reagent and homogenized using a Polytron-Aggregate (Kinematica, Littau-Lucerne, Switzerland). One hundred microliters of 1-bromo-3-chloropropane was added, and the mixture was centrifuged at 16,000 rpm at 4°C for 15 min. Four hundred and fifty microliters of supernatant was taken, an equal volume of isopropanol was added, and the mixture was centrifuged for an additional 15 min (16,000 rpm at 4°C). After washing the RNA pellet with 75% ethanol, isolated RNA was resuspended in Rnase-free water. The following procedure was used for RNA isolation from cultured cells: treated cells from 6-well plates were washed twice with phosphate-buffered saline, and 1000 μl of TRI Reagent was added into each well. Cells were scrapped into a 1.5-ml Eppendorf tube. RNA preparation was identical to that of isolation of RNA from bone tissue. Reverse transcription was carried out using an iScript cDNA synthesis kit from Bio-Rad Laboratories (Hercules, CA). Real-time reverse transcription-polymerase chain reaction (PCR) was carried out using SYBR Green and an ABI 7000 sequence detection system (Applied Biosystems, Foster City, CA). Primers for rat Nox1, 2, 3, 4, RANKL, and housekeeping genes GAPDH and 18S were designed using Primer Express software 2.0.0 (Applied Biosystems), and all primer sequences used in this study are listed in Table 1.
TABLE 1.
Real-time reverse-transcription PCR primer sequences
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Nox1 | CCCTTTGCTTCCTTCTTGAAATC | GCACCCGTCTCTCTACAAATCC |
| Nox2 | TGATCATCACATCCTCCACCAA | GATGGCAAGGCCGATGAA |
| Nox3 | GCAGCATTGGCGTGTTCTT | GAAATGAACGCCCCTAGGATCT |
| Nox4 | CTGCATCTGTCCTGAACCTCAA | TCTCCTGCTAGGGACCTTCTGT |
| RANKL | TGGGCCAAGATCTCTAACATGA | TCATGATGCCTGAAGCAAATG |
| 18S | CCTGTAATTGGAATGAGTCCACTTT | ATACGCTATTGGAGCTGGAATTACC |
| GAPDH | TGAGGTGACCGCATCTTCTTG | TGGTAACCAGGCGTCCGATA |
Western Blotting.
Femoral bone tissue proteins were extracted using a cell lysate buffer as described previously (Chen et al., 2009). Nox4 and RANKL protein expression in bone tissue were assessed by standard Western immunoblotting using goat polyclonal antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) followed by incubation with an anti-goat antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology, Inc.). β-Actin protein in bone tissue was analyzed by immunoblotting, using mouse polyclonal antibody recognizing β-actin (Sigma-Aldrich) followed by incubation with an anti-mouse antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology, Inc.) and SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL). Quantitation of the intensity of the bands in the autoradiograms was performed using a VersaDoc imaging system (Bio-Rad Laboratories).
Cell Culture, DNA Constructs, Transient Transfection, and Luciferase Activity Assays.
The rat osteoblast-like cell line UMR-106 (American Type Culture Collection, Manassas, VA) was cultured in α-minimum essential medium supplemented with 10% fetal bovine serum. When cells were ready to be treated, culture medium was saturated with oxygen and carbon dioxide in an incubator for 2 h, and plates were sealed during EtOH and hydrogen peroxide treatment—these treatment procedures were detailed previously (Chen et al., 2006). P7 RANKL promoter (−6880 to +115 relative to the transcriptional start site) luciferase reporter plasmid was a gift from C.A. O'Brien (University of Arkansas for Medical Science, Little Rock, AR) (Fu et al., 2006). Using 24-well plates, rat osteoblastic UMR-106 cells were transiently transfected with 0.005 μg of p7 RANKL promoter plasmid or 0.025 μg of empty pEGFP-N1 vector. Constitutively active pRL-CMV Renilla luciferase vector (0.005 μg; Promega, Madison, WI) was used as an internal control for transfection efficiency. After transfection, cells were allowed to grow overnight before being treated with EtOH, DPI, and hydrogen peroxide. Twenty-four hours after cell treatment, firefly and Renilla luciferase activity were determined using the Dual-Luciferase assay system according to the manufacturer's instructions (Promega). Luciferase activity was measured on a MLX Microtiter Plate Luminometer (Dynex Technolnogies, Inc., Chantilly, VA).
Statistical Analyses.
Data were expressed as means ± S.E. One-way and two-way analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc analysis was used to compare the treatment groups. Values were considered statistically significant at p < 0.05.
Results
E2 Treatment Elevated Serum E2 and Prevented Chronic EtOH-Induced Bone Loss in Cycling Female Rats.
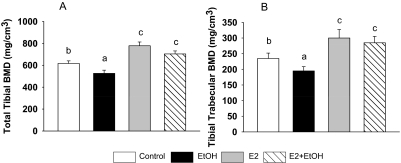
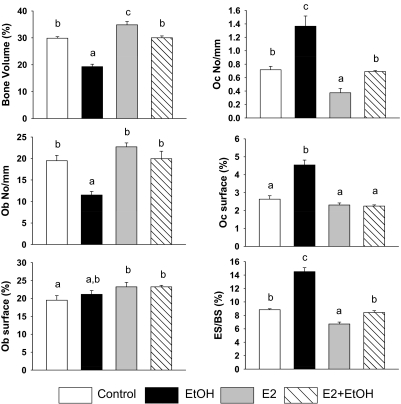
As described previously (Shankar et al., 2006), EtOH treatment reduced serum E2 values in cycling female rats (p < 0.05) (TEN 8.9 ± 1.3 pg/ml versus EtOH 4.5 ± 0.6 pg/ml, n = 7, p < 0.05). E2 treatment significantly increased serum E2 to values commonly found in pregnant rats (Shankar et al., 2006) in both control and EtOH-treated groups (TEN+E2 52.0 ± 8.8 pg/ml; EtOH±E2 51.8 ± 5.9 pg/ml). Using pQCT (Chen et al., 2008), we found that E2 treatment of intact cycling female rats increased both total and trabecular BMD (p < 0.05) accompanied by increases in bone volume and osteoblast surface and decreases in osteoclast number and eroded surface (p < 0.05) (Figs. 1 and 2). The effects of E2 treatment on osteoclast number and eroded surface in intact females were independent of effects on expression of RANKL (Fig. 3). In the EtOH-fed group, both total and trabecular BMD and bone volume were lower compared to the control group (Figs. 1 and 2) (p < 0.05), accompanied by decreased osteoblast number, increased osteoclast number, osteoclast surface, and eroded surface (p < 0.05) (Fig. 2). EtOH also increased expression of RANKL mRNA and protein in bone (p < 0.05) (Fig. 3). E2 cotreatment completely prevented the effects of EtOH on tibial total and trabecular BMD and bone volume (Figs. 1 and 2). E2 cotreatment blocked EtOH-induced decreases in the numbers of osteoblasts on trabecular bone surfaces and increased the extent of bone surface covered by osteoblasts (p < 0.05) (Fig. 2). In addition, E2 cotreatment completely prevented EtOH-induced increases in osteoclast number and surface and increases in eroded surface (p < 0.05) (Fig. 2). These protective effects of E2 on EtOH-induced bone resorption were accompanied by reversal of the induction of RANKL mRNA and protein expression (p < 0.05) (Fig. 3).
Fig. 1.
E2 reversed chronic EtOH-induced bone loss in cycling female rats. pQCT analysis. Five consecutive slices (those closest to the tibial growth plate) from each rat tibia were scanned in a blinded manner. Data were analyzed from slice numbers 2 and 3 (average). A, total tibial BMD. B, tibial trabecular BMD. Bars represent means ± S.E.M.; n = 7 animals/group. Means with different letters are significantly different (p < 0.05, a<b<c), as determined by two-way ANOVA followed by Student-Newman-Keuls post hoc analysis for multiple pairwise comparisons.
Fig. 2.
Histomorphometry demonstrating preventive effect of E2 on EtOH-induced bone loss. Static histomorphometric parameters, percentage of bone volume to total tissue, osteoclast number (Oc No/mm), osteoblast number (Ob No/mm), osteoclast surface (Oc surface %), osteoblast surface (Ob surface %), and eroded surface per bone surface (ES/BS %) from control, EtOH, E2, and E2+EtOH female rats. Bars represent means ± S.E.M.; n = 4 animals/group. Means with different letters are significantly different (p < 0.05, a<b<c), as determined by two-way ANOVA followed by Student-Newman-Keuls post hoc analysis for multiple pairwise comparisons.
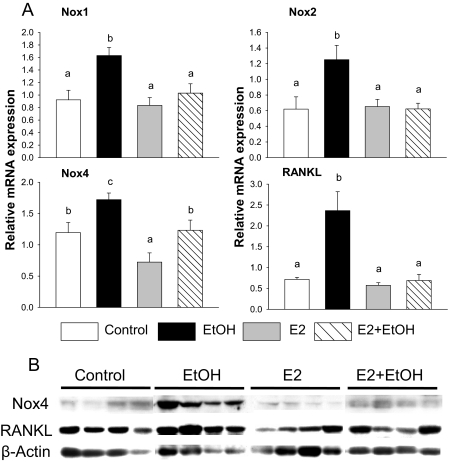
Fig. 3.
EtOH up-regulates Nox mRNA and interacts with E2 in bone. A, total RNA was isolated from femur of each rat with different treatments. Real-time PCR was performed for Nox1, 2, and 4 and RANKL. mRNA gene expression of each gene was normalized by housekeeping gene GAPDH mRNA. B, Western blot analysis of Nox4, RANKL, and β-actin. Proteins were isolated from rat femur after aspiration of bone marrow cells. Bars represent means ± S.E.M.; n = 7 animals/group. Means with different letters are significantly different (p < 0.05, a<b<c), as determined by two-way ANOVA followed by Student-Newman-Keuls post hoc analysis for multiple pairwise comparisons.
E2 Prevention of Chronic EtOH-Induced Bone Loss Is Associated with Normalization of Nox Expression in Bone.
We have previously hypothesized that chronic EtOH-induced bone loss may be due to activation of Nox to generate excessive ROS production in bone. Therefore, we examined the gene expression of Nox in total RNA isolated from bone after aspiration of bone marrow cells. Real-time PCR determined that EtOH-infused animals had higher expression of Nox1, Nox2, and Nox4 mRNAs (p < 0.05) (Fig. 3A), which we have previously shown to be expressed in bone cells in vitro (Chen et al., 2008). Coadministration of E2 with EtOH maintained expression of all three Nox enzymes at control levels (Fig. 3A). In addition, E2 by itself down-regulated Nox4 expression (p < 0.05). EtOH and E2 treatments had similar effects on Nox4 protein expression in Western blots to that seen for its mRNA (Fig. 3B).
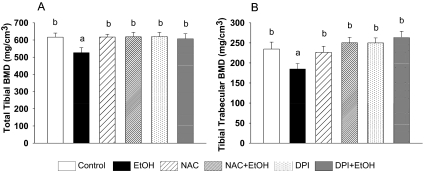
Effects of NAC and DPI on Chronic EtOH-Induced Bone Loss.
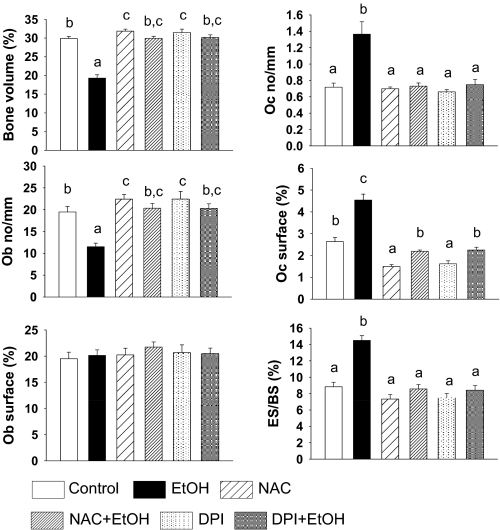
Treatment with the antioxidant NAC or the pan-Nox inhibitor DPI did not significantly alter serum E2 concentrations either alone or in combination with EtOH compared to the control and EtOH-treated groups (EtOH 4.5 ± 0.6 pg/ml, EtOH+NAC 6.76 ± 2.43 pg/ml, EtOH+DPI 3.35 ± 0.53 pg/ml). However, both treatments protected against EtOH-induced loss of both total and trabecular BMD (p < 0.05) (Fig. 4). In contrast, there were no statistically significant changes in either total or trabecular bone densities with either NAC or DPI treatment alone compared to their control animals (Fig. 4). Histomorphometric analysis revealed that coadministration of NAC or DPI with EtOH was able to maintain osteoblast and osteoclast indices in EtOH-treated rats at control levels (p < 0.05) (Fig. 5). There were significant increases in bone volume and the number of osteoblasts covering the trabecular bone surface and a decrease in the extent of bone surface covered by osteoclasts with NAC or DPI treatment alone (p < 0.05). However, we did not find any differences in osteoblast surface among all treatment groups, and there was no difference in the number of osteoclasts in either the NAC- or DPI-treated groups compared to controls (Fig. 5).
Fig. 4.
NAC and DPI prevented chronic EtOH-induced bone loss in cycling female rats. pQCT analysis was performed as described in Fig. 1. Data were analyzed from slice numbers 2 and 3 (average). A, total tibial BMD. B, tibial trabecular BMD is presented. Bars represent means ± S.E.M.; n = 7 animals/group. Means with different letters are significantly different (p < 0.05, a<b<c), as determined by two-way ANOVA followed by Student-Newman-Keuls post hoc analysis for multiple pairwise comparisons.
Fig. 5.
Histomorphometry demonstrating preventive effect of NAC and DPI on EtOH-induced bone loss. Static histomorphometric parameters, percentage of bone volume to total tissue, osteoclast number (Oc No/mm), osteoblast number (Ob No/mm), osteoclast surface (Oc surface %), osteoblast surface (Ob surface %), and eroded surface per bone surface (ES/BS %) from control, EtOH, NAC, DPI, NAC+EtOH, and DPI+EtOH female rats. Bars represent means ± S.E.M.; n = 4 animals/group. Means with different letters are significantly different (p < 0.05, a<b<c), as determined by two-way ANOVA followed by Student-Newman-Keuls post hoc analysis for multiple pairwise comparisons.
Effects of NAC and DPI on EtOH-Induced Nox and RANKL Gene Expression in Bone.
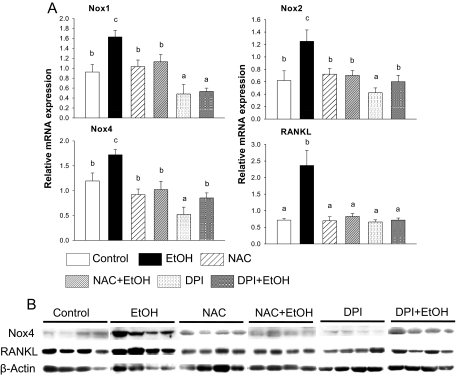
Administration of NAC blocked EtOH-induced increases of all three Nox enzymes, as well as RANKL mRNA expression (p < 0.05), with no obvious effect by NAC treatment alone (Fig. 6A). DPI treatment by itself down-regulated mRNA expression of Nox enzymes in bone (p < 0.05) and inhibited EtOH-induced increases in Nox expression (p < 0.05) (Fig. 6A). Western blot analysis demonstrated similar results for NAC and DPI treatment on Nox4 and RANKL protein expression as described for their mRNA (p < 0.05) (Fig. 6B).
Fig. 6.
EtOH up-regulates Nox mRNA and interacts with NAC and DPI in bone. A, total RNA was isolated from femur of each rat with different treatments. Real-time PCR was performed for Nox1, 2, and 4 and RANKL. mRNA gene expression of each gene was normalized by housekeeping gene GAPDH mRNA. B, Western blot analysis of Nox4, RANKL, and β-actin. Proteins were isolated from rat femur after aspiration of bone marrow cells. Bars represent means ± S.E.M.; n = 7 animals/group. Means with different letters are significantly different (p < 0.05, a<b<c), as determined by two-way ANOVA followed by Student-Newman-Keuls post hoc analysis for multiple pairwise comparisons.
ROS-Dependent Activation of RANKL by EtOH in Osteoblasts.
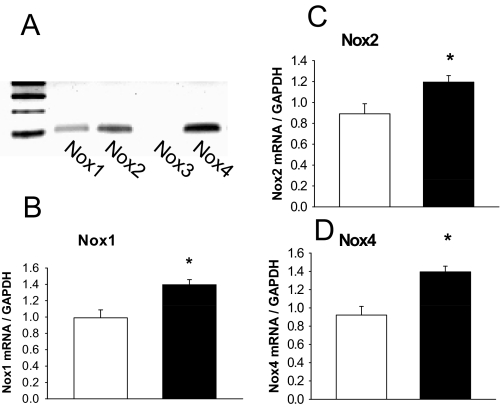
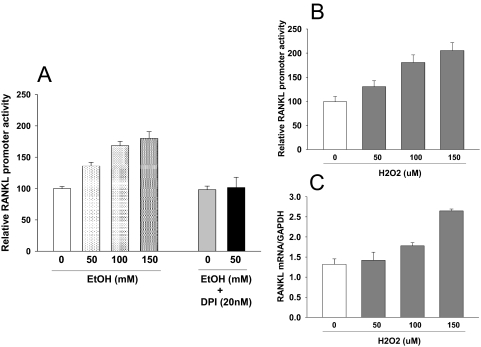
We have previously published in vitro data demonstrating that EtOH induction of Nox mRNA expression in rat osteoblasts derived from bone marrow cells and that EtOH induction of RANKL mRNA expression in this mixed cell population appears to be Nox-dependent (Chen et al., 2008). Here, we confirmed that Nox proteins are expressed and targeted by EtOH in the UMR-106 rat osteoblastic cell line (Fig. 7). To specifically address whether EtOH induces RANKL gene transcription in osteoblasts, a luciferase reporter gene construct containing a 7-kilobase fragment of the mouse RANKL promoter was transfected into UMR-106 cells. We found that EtOH activated RANKL promoter activity in a concentration-dependent manner (p < 0.05) and that this was completely inhibited by pretreatment of the cells with DPI (Fig. 8A). EtOH induction of RANKL mRNA expression and promoter activity were recapitulated in a dose-responsive fashion when these cells were treated directly with hydrogen peroxide (Fig. 8, B and C), suggesting that the effect of EtOH on RANKL in osteoblasts may be ROS-dependent.
Fig. 7.
EtOH activates Nox mRNA in osteoblasts. A, standard PCR was carried out to check the expression of Nox1, 2, 4 in UMR-106 osteoblastic cells. B–D, UMR-106 cells were then treated with 50 mM EtOH for 24 h, RNA was isolated, and real-time PCR was performed for Nox1, 2, and 4. Data are mean ± S.E.M. of triplicate determinations. Control, white bar. EtOH, black bar. *, p < 0.05 versus control by t test.
Fig. 8.
ROS-dependent activation of RANKL by EtOH in osteoblasts. A, a 7-kilobase fragment of the mouse RANKL promoter was transfected into UMR-106 osteoblastic cells. Transfected cells were then treated with three different concentrations of EtOH, and 50 mM EtOH was cotreated with DPI (20 nM) for 24 h. Luciferase activity was measured. B, UMR-106 cells or RANKL promoter transfected UMR-106 cells were treated with three different concentrations of hydrogen peroxide (H2O2); after 24 h, luciferase activity or (C) real-time PCR for RANKL were performed. Data are mean ± S.E.M. of triplicate determinations. *, p < 0.05 versus control by t test.
Discussion
Toxic effects of alcohol abuse on the skeleton have involved both indirect effects associated with endocrine disruption and direct effects on bone cells. Alcohol abuse decreases bone formation and impairs osteoblast function to unbalance bone remodeling, leading to a net bone loss (Zhang et al., 2002; Chakkalakal, 2005; Chen et al., 2006; Shankar et al., 2008). This toxic effect of alcohol on osteoblastic bone formation may be due to suppression of Wnt signaling (Himes et al., 2008; Yeh et al., 2008; Callaci et al., 2010; Chen et al., 2010). To further examine mechanisms underlying EtOH effects on bone, we have studied cycling female rats in which an EtOH-containing diet is infused intragastrically during the dark cycle when animals are normally awake and eating. In our current study, the mean blood EtOH concentrations were at levels (200 ± 30 mg/dl) that are commonly observed in alcoholics (Wadstein and Skude, 1979). By using this animal model, we have found that bone loss occurred after chronic EtOH infusion. This was associated with increased expression of NADPH oxidase (Nox) in osteoblastic cells. Consistent with our previous studies (Chen et al., 2006), chronic EtOH-induced bone loss could be prevented by coadministration of a dose of E2 sufficient to mimic plasma concentrations found in pregnancy. It should be noted that the dose of E2 used in this study might cause deleterious side effects on other tissues, such as increased risk of cardiovascular disease and breast cancer, as has been observed in women on hormone replacement therapy. Protection against bone resorption was associated with prevention of EtOH induction of Nox expression. Likewise, dietary supplementation with the antioxidant NAC at levels previously observed to block EtOH-induced oxidative stress in vivo (Shankar et al., 2008) and by subcutaneous injection of a pan Nox inhibitor DPI also prevented EtOH-induced bone loss and elevation of Nox expression in bone. Our data are consistent with the hypothesis that EtOH induction of Nox expression and activity leads to production of ROS in osteoblasts, which in turn, increases expression of the essential osteoclastogenic factor RANKL (Simonet et al., 1997). It is accepted that EtOH itself can freely diffuse into a cell. However, whether there is a specific alcohol receptor functioning within bone cells to mediate the effect of alcohol on bone has been questioned (Gimble et al., 2006). We have previously shown that EtOH inhibits estrogen receptor (ER) signaling in bone cells (Chen et al., 2009), and, therefore, the effect of EtOH on RANKL expression, reported here and elsewhere, may be mediated via inhibitory cross-talk between EtOH-generated ROS and ER signaling pathways (Chen et al., 2008, 2009).
Our results suggest that the protective effects of E2 on chronic EtOH-induced bone resorption occurs, at least in part, via its ability to attenuate Nox-mediated oxidative stress in bone and in osteoblasts. We previously published in vitro data that, in the presence of the ER antagonist ICI 182,780 (Wakeling et al., 1991), the effect of E2 on inhibiting EtOH-induced RANKL expression in osteoblasts was completely blunted. Based on these findings, we believe that the action of E2 to prevent EtOH-induced bone loss is mediated through ERs. Our study was primarily focused on the protective effects of E2, dietary antioxidants, and Nox inhibitors on alcohol-induced bone loss in female rats. It remains to be seen whether selective estrogen receptor modulators such as raloxifene or phytoestrogens such as genistein or equol have similar effects to E2 in this model. In addition, the effectiveness of E2, NAC, and DPI treatments against alcohol-induced bone loss in males remains to be determined. Other studies have shown that removal of E2 can lower oxidant defenses and that exogenous estrogens have the ability to maintain thiol antioxidants in rodent bone (Lean et al., 2003; Almeida et al., 2007). Our results suggest that estrogens exert their antioxidant effects via regulation of several different enzymes that control the sequential generation of ROS in osteoblasts. Future studies in our laboratory will focus on how estrogens affect the expression and activities of specific Nox subtypes in osteoblasts and their precursors. By itself, E2 treatment in intact cycling females also significantly increased osteoblast numbers and surface and decreased osteoclast numbers without affecting RANKL mRNA expression, and E2 also appears to have additional actions on bone turnover independent of ROS, which may contribute to its protective actions.
In recent years, knowledge of the signaling role of ROS in vascular and pulmonary physiology and pathophysiology has increased tremendously; however, less is known about ROS in the skeletal system. Almeida et al. (2007) recently reported an age-related decrease in bone strength, BMD, and formation rates. They demonstrated that all changes in bone phenotype were temporally associated with increased ROS levels and decreased glutathione reductase activity in aging and gonadectomized female and male mice. Likewise, we have found that chronic alcohol-induced bone loss and decreased bone formation rate were also associated with increased ROS generation and oxidative stress. We believe that Nox enzymes are critical targets for alcohol in bone tissue and play essential roles in the pathophysiology of the skeletal system. Nox proteins have emerged as major sources of ROS in cells and tissues, multiple Nox subtypes have been cloned and analyzed structurally and functionally, and the relationship of Nox enzymes to signaling pathways, cellular function, and vascular disease has begun to be investigated. It has been demonstrated that primary osteoblasts derived from bone marrow stromal cells express three Nox subtypes: Nox1, 2, and 4 (Chen et al., 2008). The osteoblastic rat UMR-106 cell line used in this study was found to also express Nox1, 2, and 4, with Nox4 being a major subtype in this particular cell line. All three Nox were significantly up-regulated by EtOH in vitro. Nox1 and 2 have been suggested to be important in maintaining activity and possibly differentiation potential of phagocytic cells such as osteoclasts in bone. On the other hand, Nox4 is considered as a regulator of nonphagocytic cell proliferation and differentiation, and maybe apoptosis (Schröder et al., 2009). Activation mechanisms for Nox1 and 2 are similar and involve complex formation with regulatory cytosolic subunits. The regulation of Nox4 remains poorly understood. It may be primarily regulated at the level of transcriptional expression (Ago et al., 2010), although a Nox4 regulatory protein was recently identified (Janiszewski et al., 2005).
Although there are a few reports investigating the association between Nox1/2 and osteoclastic bone resorption (Lee et al., 2005; Sasaki et al., 2009), there is a lack of information on how Nox enzymes regulate osteoclastogenesis. Similar to interferon-γ, which up-regulates Nox1 (Kamizato et al., 2009), EtOH up-regulation of Nox1 and 2 may stimulate osteoclast activity and protect osteoclasts from apoptosis. Consistent with our previous studies (Chen et al., 2008), the current data demonstrate that ROS stimulates RANKL expression in osteoblasts and increases RANKL promoter activity. Increased expression of RANKL will stimulate osteoclast differentiation (Chen et al., 2006). On the other hand, a recent study has suggested that RANKL can also stimulate osteoclast differentiation through Nox-induced ROS production in osteoclast precursors downstream of RANKL-RANK binding (Lee et al., 2005). The signaling pathways connecting EtOH, Nox1/2, ROS, and RANKL to determine the final fate of osteoclasts or their precursors are complex and have not been clearly defined. Based on previous data from our laboratory and others, it is likely that signaling through mitogen-activated protein kinases and STAT3 is involved (Thakur et al., 2006; Chen et al., 2008). Our data demonstrate that E2, NAC, and DPI blocked increased expression of Nox proteins by EtOH in vivo, which resulted in protection from bone loss. It is not well documented whether deficiency of Nox1 or Nox2, either experimentally with Nox1/Nox2 knockout mice or clinically in chronic granulomatous disease, is associated with an altered skeletal phenotype. Moreover, the roles of other Nox enzymes, especially Nox4, in osteoblast differentiation, proliferation, and survival have also not been investigated. Most recently, Nox4-dependent generation of hydrogen peroxide was found to be required for transforming growth factor-β1-induced myofibroblast differentiation (Hecker et al., 2009). Schröder et al. (2009) showed that Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Since our data demonstrate that E2, NAC, and DPI blocked effects of EtOH on bone formation as well as resorption, this suggests that Nox enzymes play an important role in the overall regulation of bone turnover. However, whether overexpression of Nox4 in bone by EtOH is able to alter the process of osteoblast or osteoclast differentiation requires further investigation.
In conclusion, EtOH-induced bone loss is, at least in part, due to overexpression of Nox in osteoblasts. Increased production of ROS in response to Nox enzymes triggers expression of the essential osteoclastogenic factor RANKL. Suppression of Nox expression in osteoblasts to reduce ROS production may be critical for prevention of EtOH-induced bone loss and perhaps other oxidative stress-associated conditions in which bone resorption is stimulated, such as aging.
Acknowledgments
We thank Matt Ferguson, Tammy Dallari, Trae Pittman, and Jamie Badeaux for technical assistance.
This work was supported in part by the National Institutes of Health National Institute of Alcohol and Alcoholism [Grant R01-AA18282] (to M.J.R.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.175091.
Abbreviations:
- EtOH
- ethanol
- RANKL
- receptor activator of nuclear factor-κB ligand
- TNFα
- tumor necrosis factor α
- ROS
- reactive oxygen species
- Nox
- NADPH oxidase
- NAC
- N-acetyl cysteine
- DPI
- diphenylene iodonium
- TEN
- total enteral nutrition
- E2
- 17-β-estradiol
- pQCT
- peripheral quantitative computerized tomography
- BMD
- bone mineral density
- PCR
- polymerase chain reaction
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- ANOVA
- analysis of variance
- ER
- estrogen receptor
- ICI 182,780
- 7α-[9-[(4,4,5,5,5,-pentafluoropentyl)-sulfinyl]nonyl]-estra-1,3,5(10)-triene-3,17β-diol.
Authorship Contributions
Participated in research design: Chen and Ronis.
Conducted experiments: Chen, Lazarenko, and Blackburn.
Performed data analysis: Chen, Lazarenko, and Shankar.
Wrote or contributed to the writing of the manuscript: Chen, Blackburn, Lumpkin, Badger, and Ronis.
Other: Ronis and Chen acquired funding for the research.
References
- Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. (2010) Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106:1253–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, et al. (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal JF, Clamens S, Pechet C, Negre-Salvayre A, Allera C, Girolami JP, Salvayre R, Bayard F. (1996) Ethinylestradiol does not enhance the expression of nitric oxide synthase in bovine endothelial cells but increases the release of bioactive nitric oxide by inhibiting superoxide anion production. Proc Natl Acad Sci USA 93:4108–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger TM, Ronis MJ, Lumpkin CK, Valentine CR, Shahare M, Irby D, Huang J, Mercado C, Thomas P, Ingelman-Sundberg M, et al. (1993) Effects of chronic ethanol on growth hormone secretion and hepatic cytochrome P450 isozymes of the rat. J Pharamcol Exp Ther 264:438–447 [PubMed] [Google Scholar]
- Callaci JJ, Himes R, Lauing K, Roper P. (2010) Long-term modulations in the vertebral transcriptome of adolescent-stage rats exposed to binge alcohol. Alcohol Alcohol 45:332–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaci JJ, Juknelis D, Patwardhan A, Wezeman FH. (2006) Binge alcohol treatment increases vertebral bone loss following ovariectomy: compensation by intermittent parathyroid hormone. Alcohol Clin Exp Res 30:665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal DA. (2005) Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res 29:2077–2090 [DOI] [PubMed] [Google Scholar]
- Chen JR, Haley RL, Hidestrand M, Shankar K, Liu X, Lumpkin CK, Simpson PM, Badger TM, Ronis MJ. (2006) Estradiol protects against ethanol-induced bone loss by inhibiting up-regulation of receptor activator of nuclear factor-kappaB ligand in osteoblasts. J Pharmacol Exp Ther 319:1182–1190 [DOI] [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Haley RL, Blackburn ML, Badger TM, Ronis MJ. (2009) Ethanol impairs estrogen receptor signaling resulting in accelerated activation of senescence pathways, whereas estradiol attenuates the effects of ethanol in osteoblasts. J Bone Miner Res 24:221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Badger TM, Ronis MJ. (2010) A role for ethanol-induced oxidative stress in controlling lineage commitment of mesenchymal stromal cells through inhibition of Wnt/beta-catenin signaling. J Bone Miner Res 25:1117–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Shankar K, Nagarajan S, Badger TM, Ronis MJ. (2008) Protective effects of estradiol on ethanol-induced bone loss involve inhibition of reactive oxygen species generation in osteoblasts and downstream activation of the extracellular signal-regulated kinase/signal transducer and activator of transcription 3/receptor activator of nuclear factor-kappaB ligand signaling cascade. J Pharmacol Exp Ther 324:50–59 [DOI] [PubMed] [Google Scholar]
- Dai J, Lin D, Zhang J, Habib P, Smith P, Murtha J, Fu Z, Yao Z, Qi Y, Keller ET. (2000) Chronic alcohol ingestion induces osteoclastogenesis and bone loss through IL-6 in mice. J Clin Invest 106:887–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer SA, Buckendahl P, Sampson HW. (1998) Alcohol consumption inhibits osteoblastic cell proliferation and activity in vivo. Alcohol 16:337–341 [DOI] [PubMed] [Google Scholar]
- Fu Q, Manolagas SC, O'Brien CA. (2006) Parathyroid hormone controls receptor activator of NF-kappaB ligand gene expression via a distant transcriptional enhancer. Mol Cell Biol 26:6453–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. (2006) Playing with bone and fat. J Cell Biochem 98:251–266 [DOI] [PubMed] [Google Scholar]
- Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. (2009) NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15:1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes R, Wezeman FH, Callaci JJ. (2008) Identification of novel bone-specific molecular targets of binge alcohol and ibandronate by transcriptome analysis. Alcohol Clin Exp Res 32:1167–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiszewski M, Lopes LR, Carmo AO, Pedro MA, Brandes RP, Santos CX, Laurindo FR. (2005) Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J Biol Chem 280:40813–40819 [DOI] [PubMed] [Google Scholar]
- Jonsson IM, Verdrengh M, Brisslert M, Lindblad S, Bokarewa M, Islander U, Carlsten H, Ohlsson C, Nandakumar KS, Holmdahl R, et al. (2007) Ethanol prevents development of destructive arthritis. Proc Natl Acad Sci USA 104:258–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamizato M, Nishida K, Masuda K, Takeo K, Yamamoto Y, Kawai T, Teshima-Kondo S, Tanahashi T, Rokutan K. (2009) Interleukin 10 inhibits interferon gamma- and tumor necrosis factor alpha-stimulated activation of NADPH oxidase 1 in human colonic epithelial cells and the mouse colon. J Gastroenterol 44:1172–1184 [DOI] [PubMed] [Google Scholar]
- Kono H, Rusyn I, Uesugi T, Yamashina S, Connor HD, Dikalova A, Mason RP, Thurman RG. (2001) Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am J Physiol Gastrointest Liver Physiol 280:G1005–G1012 [DOI] [PubMed] [Google Scholar]
- Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ. (2003) A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest 112:915–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY. (2005) A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106:852–859 [DOI] [PubMed] [Google Scholar]
- Li M, Fukagawa NK. (2010) Age-related changes in redox signaling and VSMC function. Antioxid Redox Signal 12:641–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC. (2010) From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev 31:266–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. (2007) Trends in oxidative aging theories. Free Radic. Biol. Med 43:477–503 [DOI] [PubMed] [Google Scholar]
- Sack MN, Rader DJ, Cannon RO., 3rd (1994) Oestrogen and inhibition of oxidation of low-density lipoproteins in postmenopausal women. Lancet 343:269–270 [DOI] [PubMed] [Google Scholar]
- Sampson HW. (2002) Alcohol and other factors affecting osteoporosis risk in women. Alcohol Res Health 26:292–298 [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Yamamoto H, Tominaga K, Masuda K, Kawai T, Teshima-Kondo S, Rokutan K. (2009) NADPH oxidase-derived reactive oxygen species are essential for differentiation of a mouse macrophage cell line (RAW264.7) into osteoclasts. J Med Invest 56:33–41 [DOI] [PubMed] [Google Scholar]
- Schröder K, Wandzioch K, Helmcke I, Brandes RP. (2009) Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol 29:239–245 [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et al. (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319 [DOI] [PubMed] [Google Scholar]
- Shankar K, Hidestrand M, Haley R, Skinner RA, Hogue W, Jo CH, Simpson P, Lumpkin CK, Jr, Aronson J, Badger TM, et al. (2006) Different molecular mechanisms underlie ethanol-induced bone loss in cycling and pregnant rats. Endocrinology 147:166–178 [DOI] [PubMed] [Google Scholar]
- Shankar K, Liu X, Singhal R, Chen JR, Nagarajan S, Badger TM, Ronis MJ. (2008) Chronic ethanol consumption leads to disruption of vitamin D3 homeostasis associated with induction of renal 1,25 dihydroxyvitamin D3–24-hydroxylase (CYP24A1). Endocrinology 149:1748–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudoh N, Toba K, Akishita M, Ako J, Hashimoto M, Iijima K, Kim S, Liang YQ, Ohike Y, Watanabe T, et al. (2001) Estrogen prevents oxidative stress-induced endothelial cell apoptosis in rats. Circulation 103:724–729 [DOI] [PubMed] [Google Scholar]
- Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. (2006) Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol 79:1348–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RT. (2000) Skeletal response to alcohol. Alcohol Clin Exp Res 24:1693–1701 [PubMed] [Google Scholar]
- Turner RT, Aloia RC, Segel LD, Hannon KS, Bell NH. (1988) Chronic alcohol treatment results in disturbed vitamin D metabolism and skeletal abnormalities in rats. Alcohol Clin Exp Res 12:159–162 [DOI] [PubMed] [Google Scholar]
- Turner RT, Rosen CJ, Iwaniec UT. (2010) Effects of alcohol on skeletal response to growth hormone in hypophysectomized rats. Bone 46:806–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadstein J, Skude G. (1979) Serum ethanol, hepatic enzymes and length of debauch in chronic alcoholics. Acta Med Scand 205:317–318 [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Dukes M, Bowler J. (1991) A potent specific pure antiestrogen with clinical potential. Cancer Res 51:3867–3873 [PubMed] [Google Scholar]
- Yeh CH, Chang JK, Wang YH, Ho ML, Wang GJ. (2008) Ethanol may suppress Wnt/beta-catenin signaling on human bone marrow stroma cells: a preliminary study. Clin Orthop Relat Res 466:1047–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Dai J, Lin DL, Habib P, Smith P, Murtha J, Fu Z, Yao Z, Qi Y, Keller ET. (2002) Osteoprotegerin abrogates chronic alcohol ingestion-induced bone loss in mice. J Bone Miner Res 17:1256–1263 [DOI] [PubMed] [Google Scholar]