Abstract
A small library of anilino enaminones was analyzed for potential anticonvulsant agents. We examined the effects of three anilino enaminones on neuronal activity of output neurons, mitral cells (MC), in an olfactory bulb brain slice preparation using whole-cell patch-clamp recording. These compounds are known to be effective in attenuating pentylenetetrazol-induced convulsions. Among the three compounds tested, 5-methyl-3-(4-trifluoromethoxy-phenylamino)-cyclohex-2-enone (KRS-5Me-4-OCF3) showed potent inhibition of MC activity with an EC50 of 24.5 μM. It hyperpolarized the membrane potential of MCs accompanied by suppression of spontaneous firing. Neither ionotropic glutamate receptor blockers nor a GABAB receptor blocker prevented the KRS-5Me-4-OCF3-evoked inhibitory effects. In the presence of GABAA receptor antagonists, KRS-5Me-4-OCF3 completely failed to evoke inhibition of MC spiking activity, suggesting that KRS-5Me-4-OCF3-induced inhibition may be mediated by direct action on GABAA receptors or indirect action through the elevation of tissue GABA levels. Neither vigabatrin (a selective GABA-T inhibitor) nor 1,2,5,6-tetrahydro-1-[2-[[(diphenylmethylene)amino]oxy]ethyl]-3-pyridinecarboxylic acid hydrochloride (NNC-711) (a selective inhibitor of GABA uptake by GABA transporter 1) eliminated the effect of KRS-5ME-4-OCF3 on neuronal excitability, indicating that the inhibitory effect of the enaminone resulted from direct activation of GABAA receptors. The concentration-response curves for GABA are left-shifted by KRS-5Me-4-OCF3, demonstrating that KRS-5Me-4-OCF3 enhanced GABA affinity and acted as a positive allosteric modulator of GABAA receptors. The effect of KRS-5Me-4-OCF3 was blocked by applying a benzodiazepine site antagonist, suggesting that KRS-5Me-4-OCF3 binds at the classic benzodiazepine site to exert its pharmacological action. The results suggest clinical use of enaminones as anticonvulsants in seizures and as a potential anxiolytic in mental disorders.
Introduction
A diverse series of anilino enaminones has been synthesized and investigated as potential anticonvulsant compounds. Structurally, these compounds are uniquely different from currently available antiepileptic drugs (Edafiogho et al., 1992, 2007, 2009; Foster et al., 1999; Abdel-Hamid et al., 2002; Kombian et al., 2005). This class of enaminones has shown good to moderate protection in the traditional preclinical animal models, the subcutaneous pentylenetetrazol test and the maximal electroshock seizure test. Their anticonvulsant activities are comparable with those of some clinically used agents in animal models of seizures with a minimal side effect profile as well as a wider margin of safety than conventional antiepileptic drugs such as carbamazepine, valproate, and phenytoin (Mulzac and Scott, 1993; Eddington et al., 2000). The unique pharmacophoric structure of the anilino enaminones and the variety of bioactivities provide an excellent opportunity for developing new drugs.
We reported previously the anticonvulsant activity of anilino enaminones in vivo and the possible mechanisms of action of these compounds by which they elicit their response (Edafiogho et al., 1992; Mulzac and Scott, 1993; Ananthalakshmi et al., 2007). The anilino enaminone E139 inhibited excitatory postsynaptic currents in the rat nucleus accumbens and hippocampus by enhancing extracellular GABA levels (Kombian et al., 2005; Ananthalakshmi et al., 2007) and inhibiting tetrodotoxin-sensitive sodium currents to modulate excessive firing in individual neurons (Ananthalakshmi et al., 2006). A study aimed at elucidating the essential structural parameters necessary for anticonvulsant activity found that some benzylamino enaminones, which possess a similar chemical structure to anilino enaminones with benzyl-substitution at the NH-moiety, produced anticonvulsant effects in rats and mice neurons by suppressing glutamate-mediated excitation and action potential firing (Edafiogho et al., 2006). The different substitutions at the NH-moiety change the target protein to which enaminones bind. These studies indicate that enaminones with similar chemical structure may possess different modes of action. Here, we hypothesize that the substituted site in enaminones may contribute to the mode of action of these compounds. To study the structure-activity relationships of enaminones, three enaminone compounds with non-ortho-substituted cyclohexenone were synthesized and used to determine the mechanism of their anticonvulsant action.
Epileptic seizures result from poorly controlled neuronal activity at a seizure focus and the subsequent spread of electrical excitation in brain circuits (Rall and Schleifer, 1990). It is not surprising that most effective antiseizure medications have been demonstrated to inhibit neuronal excitability through modulating the function of several types of proteins such as sodium channels, NMDA receptors, and GABA receptors (Rho and Sankar, 1999). The excitability of neurons in the brain is an integral of intrinsic membrane conductances and synaptic inputs. Both excitatory and inhibitory inputs regulate the resting excitability (Traynelis and Dingledine, 1988). Output neurons such as mitral cells (MCs) in the mouse main olfactory bulb (MOB) display their neuronal activity as spontaneous action potential firing, which can be modulated by intrinsic membrane receptors as well as synaptic inputs (Shepherd et al., 2004; Ennis et al., 2007). In the rodent MOB, MCs express high levels of different receptors such as GABA receptors (GABAA, GABAB), ionotropic and metabotropic glutamate receptors (NMDA, AMPA, metabotropic glutamate receptor 1, kainate), and serotonin receptors (5-HT1A, 5-HT2A/C). Most of these receptor proteins are thought to be strongly epilepsy-related (McNamara, 1996; Snell et al., 2000; Wang et al., 2002; Ennis et al., 2007). The functional modulation of these proteins may change synaptic input and neuronal excitability. Thus, in this study, we used acute slices of the mouse MOB and electrophysiological recordings from MCs to determine the effects of enaminones on the activity of MCs and the mechanisms underlying the inhibitory or excitatory actions of these compounds. Our results show that enaminone compounds with non-ortho-substituted cyclohexenone suppress neuronal excitability through activation of GABAA receptors and display the characteristics of a positive allosteric modulator.
Materials and Methods
Synthesis of Anilino Enaminones.
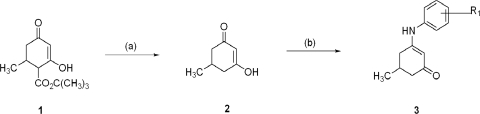
Anilino enaminones 5-methyl-3-(4-trifluoromethoxy-phenylamino)-cyclohex-2-enone (KRS-5Me-4-OCF3), 3-(4-fluoro-phenylamino)-5-methyl-cyclohex-2-enone (KRS-5Me-4-F), and 3-(3-chloro-phenylamino)-5-methyl-cyclohex-2-enone (KRS-5Me-3-Cl) were recently synthesized. The mono methyl anilino enaminones (3) were prepared from the 5-methylcyclohexane-1,3-dione (2) form by the decarboxylation of 4-carbo-tert-butoxy-5-methylcyclohexane-1,3-dione (1) and were refluxed with appropriate substituted aniline derivatives under standard conditions (Fig. 1) (Eddington et al., 2003). The chemical structures of the anilino enaminones are shown in Fig. 2. The β-hydroxy keto tert-butoxy ester was prepared as reported previously (Friary et al., 1973; Edafiogho et al., 1992; Scott et al., 1993). The enaminone structures were confirmed via NMR analyses at 400 MHz.
Fig. 1.
Synthesis of aniline enaminones. Condition a is H2SO4, Δ; condition b is Δ, substituted amine.
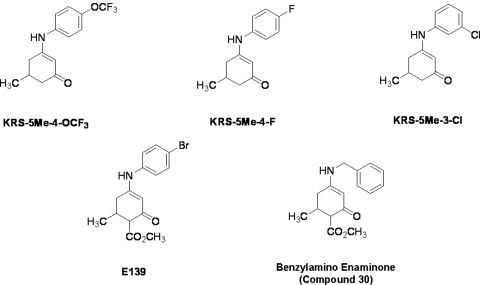
Fig. 2.
Chemical structure of aniline enaminone analogs. The difference among the three mono methyl compounds is the anilino substitution represented by meta-chloro; para-fluoro, and para-trifluoro. E139 is a para-bromo anilino enaminone derivative with ortho-methyl ester substituted moiety at cyclohexenone, and compound 30 is the benzylamino analog.
Slice Preparation.
Wild-type mice (C57BL/6J; The Jackson Laboratory, Bar Harbor, ME) were used in agreement with Institutional Animal Care and Use Committee and National Institutes of Health guidelines. Juvenile (16–25 days old) mice were decapitated, and the MOBs were dissected out and immersed in artificial cerebrospinal fluid (ACSF) at 4°C as described previously (Heinbockel et al., 2004). Horizontal slices (400 μm thick) were cut parallel to the long axis using a vibratome (Vibratome Series 1000; Ted Pella, Redding, CA). After 30 min at 30°C, slices were incubated in a holding bath at room temperature (22°C) until use. For recording, a brain slice was placed in a recording chamber mounted on a microscope stage and maintained at 30 ± 0.5°C by superfusion with oxygenated ACSF flowing at 2.5 to 3 ml/min.
Electrophysiological Recording and Data Acquisition.
Visually guided recordings were obtained from cells in the mitral cell layer with near-infrared differential interference contrast optics and a BX51WI microscope (Olympus Optical, Tokyo, Japan) equipped with a camera (C2400-07; Hamamatsu Photonics, Hamamatsu, Japan). Images were displayed on a Sony Trinitron Color Video monitor (PVM-1353MD; Sony Corp., Tokyo, Japan). Recording pipettes (5–8 MΩ) were pulled on a Flaming-Brown P-97 puller (Sutter Instrument Co., Novato, CA) from 1.5-mm o.d. borosilicate glass with filament. Seal resistance was routinely >1 GΩ, and liquid junction potential was 9 to 10 mV; reported measurements were not corrected for this potential. Data were obtained using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Signals were low-pass Bessel-filtered at 2 kHz and digitized on computer disc (Clampex 10.1; Molecular Devices). Data were also collected through a Digidata 1440A Interface (Molecular Devices) and digitized at 10 kHz. Holding currents were generated under control of the Multiclamp 700B Commander.
The ACSF consisted of 124 mM NaCl, 3 mM KCl, 2 mM CaCl2, 1.3 mM MgSO4, 10 mM glucose, 26 mM sucrose NaHCO3, 1.25 mM NaH2PO4 (pH 7.4, 300 mOsm), saturated with 95 O2/5% CO2 (modified from Heyward et al., 2001). The standard pipette-filling solution consisted of 125 mM K gluconate, 2 mM MgCl2, 10 mM HEPES, 2 mM Mg2ATP, 0.2 mM Na3GTP, 1 mM NaCl, and 0.2 mM EGTA.
Chemicals and Solutions.
Drugs were bath-perfused at the final concentration as indicated by dissolving aliquots of stock in ACSF. The three enaminone compounds that we tested, KRS-5Me-4-OCF3, KRS-5Me-4-F, and KRS-5Me-3-Cl, were recently synthesized. All enaminones were dissolved in DMSO to make 20 mM stock solution (final concentration of DMSO in bath <0.1%). For all experiments, the drugs were applied by bath perfusion. Control recordings showed that 0.1% DMSO had no detectable effects on the firing rate and membrane potential. The following drugs were also bath applied: l-2-amino-5-phosphonopentanoic acid (d-AP5), CNQX, gabazine, (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385), (2S)-3[[(1S0–1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl) phosphinic acid (CGP55845), (R)-baclofen, 1,2,5,6-tetrahydro-1-[2-[[(diphenylmethylene)amino]oxy]ethyl]-3-pyridinecarboxylic acid hydrochloride (NNC 711), vigabatrin, flumazenil, and 1-[2-[tris(4-methoxyphenyl)methoxy]ethyl]-(S)-3piperidinecarboxylic acid [(S)-SNAP 5114]. Chemicals and drugs were supplied by Tocris Bioscience (Ellisville, MO) and Sigma-Aldrich (St. Louis, MO).
Numerical data are expressed as the mean ± S.E.M. Tests for statistical significance were performed using paired Student's t tests and one-way ANOVA followed by the Bonferroni test for multiple comparisons.
Results
Recordings were obtained from 166 MCs with whole-cell recordings in mouse MOB slices from 94 animals. All recorded cells showed measurable responses to enaminone KRS-5Me-4-OCF3. MCs were identified visually by their soma location and relatively large soma size and their input resistance (297 ± 19.2 MΩ, n = 46). The membrane potential of MCs in this study was −52.2 ± 0.7 mV (n = 46).
Enaminones KRS-5Me-3-Cl and KRS-5Me-4-F Slightly Depressed Activity of Mitral Cells.
MCs are principal neurons and play a crucial role in processing sensory information in MOB. They receive direct synaptic inputs from the axons of olfactory receptor neurons, send excitatory projections to olfactory cortical areas, and receive strong feedback inhibition primarily through reciprocal dendrodendritic synapses with local interneurons (Shepherd et al., 2004; Ennis et al., 2007). MCs generate spontaneous action potentials (1–6 Hz) in slices. Here, we made use of the intrinsic properties of MCs such as spontaneous firing, membrane potential, and membrane conductance to test the effect of enaminones on MC activity and determine the possible binding target of enaminones.
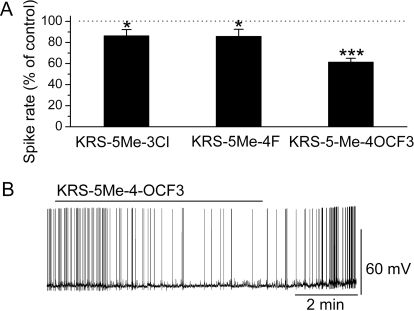
Bath application of either KRS-5Me-3-Cl or KRS-5Me-4-F modulated the spike rate of MCs (Fig. 3). Compared with control conditions, 20 μM KRS-5Me-3-Cl slightly reduced the MC firing rate (in control: 3.1 ± 0.5 Hz; in drug: 2.6 ± 0.5 Hz; n = 5; p < 0.05; paired t test). The other compound, KRS-5Me-4-F (20 μM), decreased MC firing from 4.8 ± 0.8 to 4.0 ± 0.7 Hz (n = 5; p < 0.05; paired t test) and slightly hyperpolarized the membrane potential by −0.4 ± 0.1 mV (n = 5; p < 0.05; paired t test).
Fig. 3.
Anilino enaminones depressed the spiking activity of MCs. A, normalized bar graph shows inhibition of spiking of MCs in response to bath application of KRS-5Me-4-OCF3, KRS-5Me-3-Cl, and KRS-5Me-4-F. Responses to enaminones were normalized with respect to control conditions. *, p < 0.05; ***, p < 0.001. B, original recording from a representative MC illustrated the inhibition in firing rate and hyperpolarization after application of KRS-5Me-4-OCF3.
KRS-5Me-4-OCF3 Inhibited Spontaneous Spiking of Mitral Cells and Hyperpolarized the Membrane Potential.
For the concentration tested, KRS-5Me-4-OCF3 showed a difference in potency of inhibition of MC activity compared with the above two compounds (Fig. 3A). KRS-5Me-4-OCF3 (20 μM) reversibly decreased MC firing from 4.4 ± 0.4 to 2.9 ± 0.3 Hz (n = 50; p < 0.001; paired t test). The reduction of the firing rate was accompanied by hyperpolarization of the MC membrane potential by −0.9 ± 0.2 mV (n = 50; p < 0.001; paired t test). Figure 3B illustrates the inhibitory effect in an original recording from a MC.
Even though we did not perform a detailed comparison, the findings indicated that, at 20 μM, KRS-5Me-4-OCF3 was the most potent compound compared with the other two enaminones, KRS-5Me-3-Cl and KRS-5Me-4-F, in terms of depressing spiking activity of MCs. Therefore, the enaminone KRS-5Me-4-OCF3 was selected for the remainder of the study to characterize the cellular actions of an enaminone and the mechanism underlying its inhibitory effect on neuronal activity.
Ionotropic Glutamate Receptors Were Not Involved in the KRS-5Me-4-OCF3-Induced Inhibition of Neuronal Activity.
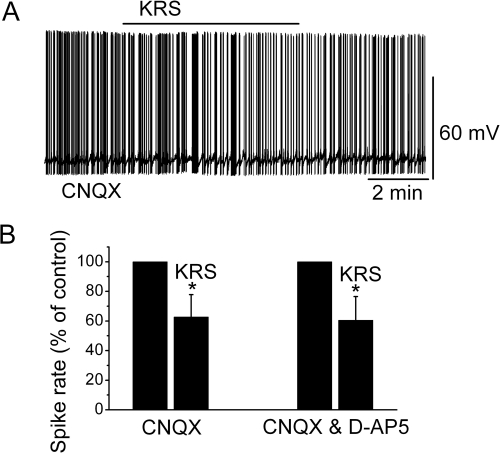
Ionotropic glutamate receptors play a critical role in the regulation of neuronal excitability in the MOB (Ennis et al., 2007). Blockade of ionotropic glutamate receptors may result in neuronal inhibition. To determine whether the inhibitory effect of the anticonvulsant agent KRS-5Me-4-OCF3 was mediated through interaction with ionotropic glutamate receptors, we examined the effects of neuronal inhibition evoked by KRS-5Me-4-OCF3 in the presence of AMPA/kainate and NMDA receptor inhibitors. In the presence of CNQX (10 μM), a potent AMPA/kainate receptor antagonist, the inhibitory effects of KRS-5Me-4-OCF3 persisted as seen by a reduction of the firing rate (in CNQX: 3.2 ± 0.56 Hz; in CNQX plus KRS-5Me-4-OCF3: 2.3 ± 0.50 Hz; n = 4; p < 0.05; paired t test) and hyperpolarization of MCs by −0.8 ± 0.2 mV (n = 4; p < 0.05; paired t test) (Fig. 4). In comparison with the results shown in Fig. 3A, these values indicated that CNQX had no any additional effect on KRS-5Me-4-OCF3-induced suppression of MC activity (p > 0.05; ANOVA and Bonferroni post hoc analysis).
Fig. 4.
Neither AMPA/kainate nor NMDA receptor antagonists blocked the KRS-5Me-4-OCF3-induced inhibition of MCs. A, KRS-5Me-4-OCF3-induced suppression of neuronal firing recorded in a representative MC in the presence of CNQX. B, the normalized and averaged results showed the persistence of KRS-5Me-4-OCF3-induced inhibitory effects on spontaneous spiking of MCs in the presence of either CNQX or CNQX plus d-AP5. *, p < 0.05.
d-AP5 is a potent NMDA receptor antagonist. Blockade of both AMPA/kainate and NMDA receptors antagonizes the excitatory activities of ionotropic glutamate receptors. In the presence of both CNQX and d-AP5, KRS-5Me-4-OCF3 reduced the firing rate (in CNQX + d-AP5: 3.4 ± 0.4 Hz; in CNQX+d-AP5 plus KRS-5Me-4-OCF3: 2.2 ± 0.40 Hz; n = 4; p < 0.05; paired t test) and hyperpolarized MCs by −0.9 ± 0.2 mV (n = 4; p < 0.05; paired t test) (Fig. 4). These values were not significantly different from the values of KRS-5Me-4-OCF3-induced suppression recorded in ACSF control condition (see Fig. 3A) (p > 0.05; determined by ANOVA and Bonferroni post hoc analysis). These results showed that ionotropic glutamate receptors were not involved in KRS-5Me-4-OCF3-induced MC inhibition.
The KRS-Induced Inhibition of MC Excitability Was Not Influenced by GABAB Receptors.
GABAB receptors (GABABRs) are restricted mostly to the glomerular layer in the MOB (Bowery et al., 1987; Chu et al., 1990). GABABRs are metabotropic transmembrane receptors for GABA and linked via G proteins to potassium channels (Chen et al., 2005). Activation of GABABRs can stimulate the opening of K+ channels that will hyperpolarize the neuron, quiet down excitable cells, and hence stop neurotransmitter release. In the MOB, activation of GABABRs has been observed to reduce MC excitability (Palouzier-Paulignan et al., 2002; Isaacson and Vitten, 2003).
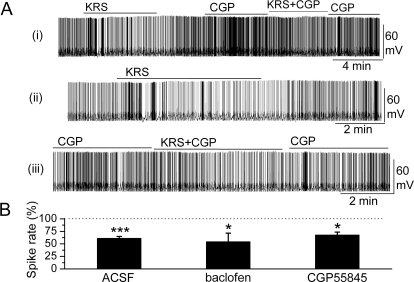
In the presence of the GABABR antagonist CGP55845 (10 μM), the KRS-5Me-4-OCF3-evoked modulation of MC firing persisted (in CGP55845: 3.7 ± 0.6 Hz; in CGP55845 plus KRS-5Me-4-OCF3: 2.8 ± 0.5 Hz; n = 5; p < 0.05; paired t test), and MCs were hyperpolarized −1.0 ± 0.2 mV (n = 5; p < 0.05; paired t test). Figure 5A shows a representative recording from one MC. No significant difference was observed for KRS-5Me-4-OCF3-evoked inhibition in the control condition (see Fig. 3A) and in the presence of CGP55845 (p > 0.05; determined by ANOVA and Bonferroni post hoc analysis).
Fig. 5.
GABAB receptors were not involved in KRS-5Me-4-OCF3-evoked inhibition. A, original recording from an MC during KRS-5Me-4-OCF3 application in the ACSF condition and in the presence of GABABR antagonist CGP55845. The upper trace (i) illustrates the effect of KRS-5Me-4-OCF3 on spiking of an MC and the effect of adding CGP55845. The upper trace is shown at an extended time scale in the middle (ii) and lower trace (iii). B, the normalized results showed the persistence of KRS-5Me-4-OCF3-evoked inhibitory effects on spiking of MCs in the presence of the GABABR antagonist CGP55845 (10 μM) and the GABABR agonist (R)-baclofen (50 μM). *, p < 0.05; ***, p < 0.001. The data for the effect of KRS-5Me-4-OCF3 on spiking of MCs were normalized with respect to ACSF, CGP88545, or baclofen alone.
Compared with control conditions, the GABABR agonist (R)-baclofen (50 μM) evoked a strong decrease in the firing rate of MCs (in control: 2.6 ± 0.4 Hz; in baclofen: 1.3 ± 0.3 Hz; n = 4; p < 0.01; paired t test) and hyperpolarization of MCs by −1.6 ± 0.7 mV (n = 4; p < 0.05; paired t test). In the presence of the GABABR agonist baclofen, KRS-5Me-4-OCF3 further reduced the MC firing rate to 0.7 ± 0.1 Hz (n = 4; p < 0.05, paired t test) and hyperpolarized MCs by −0.9 ± 0.3 mV (n = 4; p < 0.05, paired t test). In comparison with the inhibitory effects of KRS-5Me-4-OCF3 in control conditions (percentage of control firing; see Fig. 3A), the effects of KRS-5Me-4-OCF3 recorded in the presence of (R)-baclofen [percentage of values recorded in (R)-baclofen] did not significantly change (p > 0.05 determined by ANOVA and Bonferroni post hoc analysis). These results indicated that the inhibitory effect of KRS-5Me-4-OCF3 persisted irrespective of GABABR activation or blockade, suggesting that neither a GABABR antagonist nor an agonist influenced the enaminone-induced MC inhibition.
Blockade of GABAA Receptor Reversed KRS-Induced Inhibition of MC Excitability.
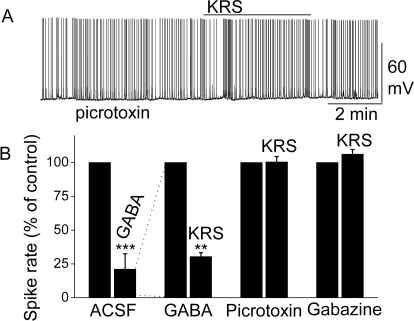
GABAARs play an important role in regulating MC excitability (Laurie et al., 1992; Panzanelli et al., 2005) by suppressing neuronal activity. Bath application of GABA (50 μM) dramatically decreased the firing rate of MCs (in control: 5.0 ± 0.8 Hz; in GABA: 1.7 ± 0.3 Hz; n = 6; p < 0.001; paired t test) and hyperpolarized MCs by −1.1 ± 0.3 mV (n = 6; p < 0.05; paired t test), indicating that GABA induced a large direct inhibition of MC activity via GABA receptors (Fig. 6B). The potent inhibition by GABA is consistent with previous reports showing that GABA receptors are abundant in MCs (Laurie et al., 1992; Persohn et al., 1992). In the presence of GABA, KRS-5Me-4-OCF3 reduced the MC firing rate to 0.7 ± 0.2 Hz (n = 4; p < 0.01; paired t test). The enhancement of the KRS-5Me-4-OCF3-evoked inhibition in the presence of increased extracellular GABA levels (50 μM) suggested that KRS-5Me-4-OCF3-evoked inhibition may be mediated by direct activation of GABA receptors rather than by increased GABA levels. Potentially, increased endogenous GABA levels could result from increasing GABA release or reducing GABA reabsorption and GABA degradation in the slice.
Fig. 6.
KRS-5Me-4-OCF3-evoked inhibition of MC spiking activity was blocked by GABAA receptor antagonists. A, original recording obtained from a MC showed that picrotoxin (20 μM) blocked 20 μM KRS-5Me-4-OCF3-evoked suppression of neuronal firing. B, the normalized and averaged results showed KRS-5Me-4-OCF3-evoked inhibitory effects on spiking of MCs in the presence of the GABAA receptor agonist GABA (50 μM) and antagonist picrotoxin (20 μM) and gabazine (5 μM). **, p < 0.01; ***, p < 0.001. The data for the effect of GABA or KRS-5Me-4-OCF3 on spiking of MCs were normalized with respect to control condition.
Blockade of GABAA receptors can result in overexcitation of neurons. In the presence of the GABAA receptor antagonist picrotoxin, the inhibition of MC activity by KRS-5Me-4-OCF3 was completely blocked (in picrotoxin: 6.1 ± 1.0 Hz; in picrotoxin plus KRS-5Me-4-OCF3: 6.2 ± 1.0 Hz; n = 9, p > 0.05; paired t test) (Fig. 6). Gabazine is another selective GABAA receptor antagonist. Likewise, in the presence of gabazine, the KRS-5Me-4-OCF3-induced inhibition of MC activity was completely abolished (in gabazine: 5.4 ± 0.7 Hz; in gabazine plus KRS-5Me-4-OCF3: 5.7 ± 0.7 Hz; n = 6, p > 0.05; paired t test) (Fig. 6B). These results indicated that blockade of GABAA receptors prevented KRS-5Me-4-OCF3-evoked inhibition of MC activity and suggested that KRS-5Me-4-OCF3 acted through enhanced GABA levels or direct action on GABAA receptors.
Further evidence for the involvement of GABAA receptors came from measurements of MC ionic currents induced by KRS-5Me-4-OCF3 with or without blockade of GABAA receptors using the antagonist gabazine. In voltage-clamp mode at a holding potential of −60 mV, KRS-5Me-4-OCF3 produced an outward current in MCs of 14.7 ± 3.5 pA (n = 5; the steady-state current at −60 mV in KRS-5Me-4-OCF3 was measured and subtracted from that in ACSF). In the presence of gabazine, KRS-5Me-4-OCF3-induced outward currents were blocked (−0.2 ± 1.6 pA; n = 4; the current at −60 mV in KRS-5Me-4-OCF3 plus gabazine was subtracted from that in gabazine).
Enhancement of Extracellular GABA Levels Did Not Block the KRS-5ME-4-OCF3-Induced Inhibition of Neuronal Excitability.
The above results (Fig. 6) showed that inhibition of MC firing evoked by KRS-5Me-4-OCF3 could be blocked by GABAA receptor antagonists. This result suggested that the enaminone either binds to GABAA receptors to produce the inhibitory effects or acts to enhance extracellular GABA levels. Extracellular GABA levels are in part controlled by GABA reuptake and degradation (Errante et al., 2002). GABA released from synaptic terminals may be removed from the extracellular space by GABA uptake back into synaptic terminals and/or into glial cells by plasma membrane transporters (GATs). Subsequently, the captured GABA is degraded by the enzyme GABA transaminase (GABA-T). To test the possible involvement of endogenous GABA in the KRS-5Me-4-OCF3-evoked neuronal inhibition, we examined the role of the enzyme GABA-T and membrane transporters, GATs, in enaminone-mediated inhibition of MC activity.
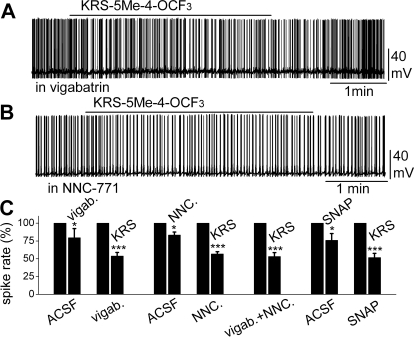
Bath application of vigabatrin (200 μM), an irreversible and selective GABA-T inhibitor that results in extracellular accumulation of GABA in the synaptic cleft, induced a reduction in MC firing rate (in control: 4.3 ± 0.5 Hz; in vigabatrin: 3.4 ± 0.4 Hz; n = 4; p < 0.05; paired t test). In the presence of vigabatrin, bath application of KRS-5Me-4-OCF3 (20 μM) resulted in further reduction of the firing rate (in vigabatrin: 3.5 ± 0.5 Hz; in vigabatrin plus KRS: 2.0 ± 0.2 Hz; n = 7; p < 0.001; paired t test) accompanied by hyperpolarization of the membrane potential (ΔVm = −0.8 ± 0.3 mV; n = 7; p < 0.05; paired t test) (Fig. 7, A and C). The persistence of the KRS-5Me-4-OCF3 effect in the presence of vigabatrin indicated that enaminone-evoked neuronal inhibition was not mediated by GABA-T.
Fig. 7.
Neither an inhibitor of GABA reuptake nor an inhibitor of the GABA degradation enzyme GABA-T blocked KRS-5Me-4-OCF3-evoked MC inhibition. A, original recording from a MC showing that spiking inhibition evoked by KRS-5Me-4-OCF3 persisted in the presence of vigabatrin (200 μM). B, original recording from a MC showing that spiking inhibition evoked by KRS-5Me-4-OCF3 persisted in the presence of NNC-711 (10 μM). C, summary of results from normalized and averaged data. *, p < 0.05; ***, p < 0.001.
To determine whether enaminones interacted with GATs to regulate GABA reuptake and whether GATs modulate KRS-5Me-4-OCF3-evoked neuronal inhibition, we applied NNC-711, a potent and selective inhibitor of GABA uptake by GAT-1, and (S)-SNAP 5114, a selective inhibitor by GAT-3 and GAT-2. Bath application of NNC-711 (10 μM) reduced MC spiking (in control: 4.4 ± 0.5 Hz; in NNC-711: 3.8 ± 0.3 Hz; n = 5; p < 0.05; paired t test) but did not significantly modulate the membrane potential (ΔVm = −0.38 ± 0.09 mV; n = 5; p > 0.05). In the presence of NNC-711, KRS-5Me-4-OCF3 further reduced the firing rate (in NNC-711: 3.9 ± 0.3 Hz; in NNC-711 plus KRS: 2.3 ± 0.2 Hz; n = 22; p < 0.001; paired t test) and hyperpolarized MCs by −0.8 ± 0.1 mV (n = 22; p < 0.01). Likewise, bath application of (S)-SNAP 5114 (20 μM) reduced MC spiking (in control: 3.5 ± 0.6 Hz; in SNAP: 2.7 ± 0.4 Hz; n = 4; p < 0.05; paired t test) and hyperpolarized membrane potential (ΔVm = −0.6 ± 0.1 mV; n = 4; p < 0.05). In the presence of (S)-SNAP 5114, KRS-5Me-4-OCF3 further reduced the firing rate (in SNAP: 3.0 ± 0.5 Hz; in SNAP 5114 plus KRS: 1.8 ± 0.3 Hz; n = 7; p < 0.001; paired t test) and hyperpolarized MCs by −0.9 ± 0.1 mV (n = 7; p < 0.05). The results indicate that the inhibitory effects of KRS-5Me-4-OCF3 persisted in the presence of GABA reuptake inhibitors (Fig. 7, B and C).
The effects of KRS-5Me-4-OCF3 on MC activity were also tested in the presence of both the GABA reuptake inhibitor NNC-711 (10 μM) and the GABA-T inhibitor vigabatrin (100 μM). Under this condition, application of 20 μM KRS-5Me-4-OCF3 produced firing inhibition (in NNC711 + vigabatrin: 3.8 ± 0.5 Hz; in NNC-711 + vigabatrin plus KRS: 2.1 ± 0.4 Hz; n = 9; p < 0.001; paired t test) and hyperpolarization by −0.7 ± 0.2 mV (n = 9; p < 0.05; paired t test) (Fig. 7C). These results indicated that GABA reuptake transporters, GATs, or GABA transaminase (GABA-T) did not block KRS-5Me-4-OCF3-evoked neuronal inhibition, suggesting that extracellular enhancement of GABA levels did not contribute to the pharmacological effects of KRS-5ME-4-OCF3.
Substituted Anilino Enaminone Exhibited Characteristics of a Positive Allosteric Modulator of GABAA Receptor.
We showed that KRS-5Me-4-OCF3 significantly enhanced the inhibitory effect of GABA on MC activity (Fig. 6B), i.e., the enaminone inhibited the firing rate to 2.9 ± 0.3 Hz in ACSF (Fig. 3) and it suppressed the firing rate to 0.7 ± 0.2 Hz in the presence of 50 μM GABA (Fig. 6B) (p < 0.05; determined by ANOVA and Bonferroni post hoc analysis). The enhancement of the inhibitory effect of bath-applied GABA suggested that KRS-5Me-4-OCF3 acted as a positive allosteric modulator of GABAA receptors.
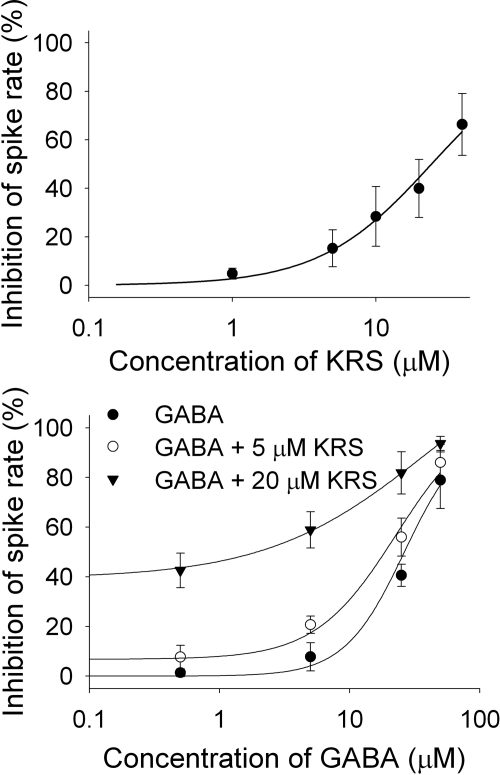
It has been established that the GABAA receptor is the main target for positive allosteric modulators such as benzodiazepines (Möhler et al., 2002; Munro et al., 2008; Fisher, 2009). By binding at a site distinct from the GABA binding site and by increasing GABA affinity for the GABAA receptor, positive allosteric modulators facilitate an augmentation of GABAA receptor function. To examine the concentration dependence of the inhibitory effect of KRS-5Me-4-OCF3 on neuronal activity and to test whether KRS-5Me-4-OCF3 behaved like a positive allosteric modulator the concentration-response relationships of KRS-5Me-4-OCF3 and GABA in the absence and presence of KRS-5Me-4-OCF3 (0, 5, and 20 μM) were measured (Fig. 8). The averaged inhibitory effects evoked by varying concentrations of the enaminone (Fig. 8A) and GABA (Fig. 8B) were well fit by the Hill equation and allowed us to estimate an EC50. Based on a fitted Hill coefficient (n) value of 1.11 in Fig. 8A, it seemed that the stoichiometry of drug and receptor interaction was 1:1. The inhibitory effect of KRS-5Me-4-OCF3 on neuronal activity was concentration-dependent with the estimated value of 24.5 μM EC50. Figure 8B shows that GABA evoked concentration-dependent inhibition, and the concentration-response curves were left-shifted by KRS-5Me-4-OCF3. The shift of the concentration-response relationships suggested that KRS-5Me-4-OCF3 mostly likely bound at non-GABA binding sites on the GABA receptor. The EC50 of GABA fitted by the Hill equation was 28.8 μM for GABA only, 19.9 μM for GABA plus 5 μM KRS-5Me-4-OCF3, and 10.5 μM for GABA plus 20 μM KRS-5Me-4-OCF3. The affinity of GABA for GABA binding sites was enhanced by KRS-5Me-4-OCF3, which suggested an action of KRS-5Me-4-OCF3 as a positive allosteric modulator of the GABAA receptor. The property provides a cellular mechanism that accounts for the anticonvulsant effects of KRS-5Me-4-OCF3 in vivo.
Fig. 8.
The concentration-response curves of KRS-5Me-4-OCF3-evoked inhibition and GABA in the presence of KRS-5Me-4-OCF3. A, the KRS-5Me-4-OCF3-evoked change in spiking rate was normalized to the control condition, and then averaged. Each point was the mean value ± S.E.M. of four to seven cells. The line is fit for the data to the Hill equation: y = Y0 + Axn/(Kan + xn), where y is the inhibition of spiking rate, x is concentration of drugs, Y0 is minimal inhibition, A is maximal inhibition, Kd is the apparent dissociation constant for agents, and n is the Hill coefficient. Kd and n were estimated using a Marquadt nonlinear least-squares routine. B, shift of the concentration-response curves of GABA in the presence of KRS-5Me-4-OCF3 at different concentrations (0, 5, and 20 μM). The lines are fits for the data to the Hill equation.
The Enhancement of GABA by KRS-5Me-4-OCF3 Is through Binding at the Classic Benzodiazepine Site.
Previous in vivo studies reported that enaminones show potent anticonvulsion effects in chemical-induced epilepsy animal models but fewer side effects such as sedation, drowsiness, and dizziness (Mulzac and Scott, 1993; Eddington et al., 2003). Based on these in vivo results and our in vitro results, we hypothesized that KRS-5Me-4-OCF3 might bind to benzodiazepine sites to exert its pharmaceutical actions. Therefore, an antagonist of the benzodiazepine site was used to ascertain whether the enhancement of GABA by KRS-5Me-4-OCF3 was mediated at the classic benzodiazepine site.
Bath application of flumazenil (10 μM), a benzodiazepine site antagonist, slightly increased MC spiking from 3.3 ± 0.6 Hz (in ACSF) to 3.8 ± 0.8 Hz (n = 6; p < 0.05; paired t test), but did not significantly modulate the membrane potential (ΔVm = −0.02 ± 0.08 mV; n = 6; p > 0.05). In the presence of flumazenil, KRS-5Me-4-OCF3 failed to evoke the inhibitory effect on the firing rate (in flumazenil: 3.7 ± 0.7 Hz; in flumazenil plus KRS: 3.7 ± 0.8 Hz; n = 19; p > 0.05; paired t test) and on the membrane potential (Vm = 0.01 ± 0.1 mV; n = 19; p > 0.05). The blocked inhibitory effects of KRS-5Me-4-OCF3 by flumazenil suggested that KRS-5Me-4-OCF3 binds at the benzodiazepine site to exert its pharmacological actions.
Discussion
Here, we provide the first report that the enaminone KRS-5Me-4-OCF3 acted as a novel positive allosteric modulator to decrease neuronal activity via direct regulation of GABAA receptors. Our study showed that the anilino enaminone KRS-5Me-4-OCF3 and its analogs displayed inhibitory effects on neuronal activity with different potencies. The inhibitory potency depended on the chemical structure and concentration of the enaminone. Among the three compounds, at the concentration tested, KRS-5Me-4-OCF3 showed the most potent inhibition of spiking of MCs and evoked hyperpolarization of the membrane potential. These results are consistent with previous in vivo results that KRS-5Me-4-OCF3 is the most potent anticonvulsant agent (Eddington et al., 2003). Neither excitatory ionotropic glutamate receptors (NMDA and non-NMDA receptors) nor inhibitory GABAB receptors were involved in KRS-5Me-4-OCF3-evoked inhibition of neuronal activity. The KRS-5Me-4-OCF3-induced inhibition of activity was abolished by GABAA receptor antagonists, suggesting that the inhibition may act directly through activation of GABAA receptors or indirectly through an increase of extracellular GABA levels. Our results showed that neither blockade of GABA reuptake nor blockade of GABA-T influenced KRS-5Me-4-OCF3-evoked neuronal inhibition, indicating that the inhibition by KRS-5Me-4-OCF3 was mediated through direct activation of GABAA receptors. The left-shift of the concentration-response relationship of enaminone KRS-5Me-4-OCF3 in the presence of GABA implies that KRS-5Me-4-OCF3 binds to a site distinct from the GABA binding site to enhance GABA activity. This property indicates that KRS-5Me-4-OCF3 acted as a positive allosteric modulator of the GABAA receptor. Thus, our results suggest that KRS-5Me-4-OCF3 could be a potential medication as anxiolytic, anticonvulsant, anesthetic, and sedative-hypnotic.
Previously, nucleus accumbens and coronal hippocampal slices in the rat brain have been used to study anticonvulsant enaminone suppression of excitatory synaptic transmission and epileptiform activity (Kombian et al., 2005; Ananthalakshmi et al., 2007). The MOB is anatomically different from nucleus accumbens and hippocampus and provides three advantages to test cellular mechanisms of anilino enaminone action. First, epilepsy-related proteins such as GABAA receptors, sodium channels, ionotropic glutamate receptors, and metabotropic glutamate are expressed in MCs. Second, MCs show the property of spontaneous spiking, and epilepsy-related proteins participate in the regulation of neuronal spiking. Third, the excitability of MCs can be regulated by synaptic input. Thus, MCs in MOB slices serve as a good model for testing the bioactivity of enaminone compounds and exploring the mechanisms underlying their activity. The strategy we used was to 1) test the effects of synthesized enaminones on neuronal activity and 2) determine the cellular basis of their pharmacological actions.
Mechanism Underlying the Suppression of Neuronal Activity.
The subcutaneous pentylenetetrazol seizure model identifies compounds that inhibit the GABA antagonistic effects of pentylenetetrazol or raise the seizure threshold (Stables and Kupferberg, 1997). Studies have shown that a number of enaminone compounds display inhibition against glutamate-mediated excitatory synaptic transmission by modulation of GABAergic transmission (Kombian et al., 2005; Ananthalakshmi et al., 2006). Based on the above findings and the results we present, we hypothesize the existence of an essential pharmacophore within the enaminone structure that possibly interacts with the GABA receptor, which is significant for achieving anticonvulsant activity. Even though the exact site and structural requirements for optimal binding are unknown, we believe, because of the molecular similarities between the enaminone analogs, the compounds we tested share a common binding pocket on the GABA receptor, which explains the probability of eliciting similar biological responses.
The three compounds that we tested in the present study have been reported previously for their different potency of anticonvulsion in vivo (Eddington et al., 2003). The compound KRS-5Me-4-OCF3, which is the most potent anticonvulsant in vivo (Eddington et al., 2003), possessed the most potent inhibitory effect on neuronal activity in vitro. The consistency of the neuronal inhibition in vitro and the anticonvulsant activity in vivo suggests that the anticonvulsant activity of KRS-5Me-4-OCF3 results from preventing overexcitability in epilepsy. The consistent in vitro and in vivo results also suggest that recording in MCs is an appropriate means for elucidating the bioactivity of enaminones.
Both GABAA and GABAB receptors are present in the MOB. They participate in the regulation of MC excitability in distinct ways. GABAA receptors directly regulate the excitability of MCs, whereas GABAB receptors mediate the regulation of MC excitability via presynaptic inhibition in the MOB (Shepherd et al., 2004; Ennis et al., 2007). Antiepileptic drugs such as benzodiazepines are known to interact with GABAA receptors (Rall and Schleifer, 1990). This supports our results that the enaminone KRS-5Me-4-OCF3 does not interact with GABAB receptors. Rather it interacts directly with GABAA receptors to decrease neuronal activity of MCs.
Enaminones with different chemical structure have been reported to display distinct mechanisms underlying their neuronal inhibition and anticonvulsant effects. Another anticonvulsant enaminone, E139 (Fig. 2), was reported to suppress excitatory synaptic transmission by enhancing extracellular GABA levels (Kombian et al., 2005) and blocking tetrodotoxin-sensitive sodium channels and, thereby, directly inhibiting postsynaptic neuronal excitability (Ananthalakshmi et al., 2006). Meanwhile, several enaminones with chemical moieties different from KRS-5Me-4-OCF3 were described to interact with GABAA receptors (Reitz et al., 1999; Yohannes et al., 2003; Hogenkamp et al., 2007). Other enaminone derivatives that probably target GABA receptors were provided by using the comparative molecular field analysis and comparative molecular similarity techniques, which can generate models to define the specific structural and electrostatic features essential for enhanced binding of enaminones to the putative GABA receptor (Jackson et al., 2009).
The GABAA receptor is a ligand-gated ion channel responsible for mediating the effects of GABA, the major inhibitory neurotransmitter in the brain. The GABAA receptor complex has been reported to have distinct binding sites for GABA, benzodiazepines, barbiturates, ethanol (Santhakumar et al., 2007), inhaled anesthetics, and neuroactive steroids. Positive allosteric modulators enhance the affinity of GABA for the binding site. Allosteric modulators of GABAA receptors such as benzodiazepines, neuroactive steroids, and barbiturates have been identified that are useful as anxiolytics, anticonvulsants, anesthetics, and sedative-hypnotics. Previous in vivo studies reported that enaminones show potent anticonvulsion effects in chemical-induced epilepsy animal models but fewer side effects such as sedation, drowsiness, and dizziness (Mulzac and Scott, 1993; Eddington et al., 2003). Based on these in vivo results and our results, we hypothesized and confirmed that KRS-5Me-4-OCF3 binds to benzodiazepine sites to exert its pharmaceutical actions. Therefore, in addition to the reported pharmacological action of KRS-5Me-4-OCF3 as anticonvulsant, it is reasonable to speculate that KRS-5Me-4-OCF3 might display an anxiolytic effect in a clinical setting.
A Specific Substituted Site in the Chemical Structure of Enaminones May Be Required for Targeting GABAA Receptors and Conferring Anticonvulsant Activity.
The three enaminones studied in the present article, KRS-5Me-4-OCF3, KRS-5Me-4-F, and KRS-5Me-3-Cl (Fig. 2), differed in their ability to suppress neuronal activity at the concentration tested. Our results indicated that a para-substitution of the phenyl group with –OCF3 evoked the most potent suppression of neuronal excitability, whereas a para- or meta-substitution of the phenyl group with fluoro, chloro decreased the potency of the inhibitory activity. This result suggests that a substitution in the phenyl group most strongly influences the potency of the inhibitory action of enaminones. The importance of the substitution group was also demonstrated in benzylamino enaminones in which unsubstituted benzylamine analog compound 30 (Fig. 2) showed the most potent activities in anticonvulsion and excitatory synaptic depression (Edafiogho et al., 2006).
The mechanism underlying the neuronal inhibition by the anilino enaminone KRS-5Me-4-OCF3 is different from recent evidence obtained for another anilino enaminone, E139 (Fig. 2). The suppression of excitatory synaptic transmission evoked by E139 may be indirectly mediated through enhancement of GABA levels (Kombian et al., 2005). The most striking difference in the chemical structure of E139 and KRS-5Me-4-OCF3 is in the ortho-substitution of the cyclohexenone moiety. E139 has an ortho-substitution of the cyclohexenone moiety, whereas the three compounds we tested in this study were not ortho-substituted in cyclohexenone. Our results indicate that nonsubstituted enaminones in ortho-cyclohexenone such as KRS-5Me-4-OCF3 act as a positive allosteric modulator of GABAA receptors. Based on the chemical structure and our bioactivity analysis, we postulate that the ortho-site of cyclohexenone plays an important role in determining the interaction with a target protein. A study on benzylamino enaminones, which possess a similar structure to anilino enaminones, demonstrates a completely different cellular mechanism of action on excitatory synaptic depression and anticonvulsion (Edafiogho et al., 2006). Benzylamino enaminone compound 30 was found to depress glutamate-mediated excitatory synaptic transmission. In addition, enaminones without ortho-substitution of cyclohexenone that target GABAA receptors were described in several patented enaminones (Reitz et al., 1999; Yohannes et al., 2003). Therefore, we presume that substituted enaminones with different site substitutions may form different pharmacophores targeting specialized proteins. The chemical and pharmacological analysis of the structure-response relationship may provide a new means for guiding rational drug design for potential anticonvulsant and anxiolytic compounds.
This work was supported in part by the National Institute of Health National Institute of General Medical Sciences [Grant S06-GM08016]; the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant U54-NS039407]; and the Howard University New Faculty Research Program, the Howard University Health Sciences Faculty Seed Grant Program, the Whitehall Foundation.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.173740.
Abbreviations:
- NMDA
- N-methyl-d-aspartate
- ACSF
- artificial cerebrospinal fluid
- MOB
- main olfactory bulb
- MC
- mitral cell
- KRS-5Me-4-OCF3
- 5-methyl-3-(4-trifluoromethoxy-phenylamino)-cyclohex-2-enone
- KRS-5Me-4-F
- 3-(4-fluoro-phenylamino)-5-methyl-cyclohex-2-enone
- KRS-5Me-3-Cl
- 3-(3-chloro-phenylamino)-5-methyl-cyclohex-2-enone
- DMSO
- dimethyl sulfoxide
- d-AP5
- l-2-amino-5-phosphonopentanoic acid
- CNQX
- 6-cyano-7-nitroquinoxaline-2-3-dione
- gabazine (SR-95531)
- 2-(3-carboxypropyl)-3-amino-6-(4 methoxyphenyl)-pyridazinium bromide
- LY367385
- (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid
- CGP55845
- (2S)-3[[(1S0–1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl) phosphinic acid
- (R)-baclofen
- (R)-4-amino-3-(4-chlorophenyl) butanoic acid
- NNC 711
- 1,2,5,6-tetrahydro-1-[2-[[(diphenylmethylene)amino]oxy]ethyl]-3-pyridinecarboxylic acid hydrochloride
- vigabatrin
- (±)-γ-vinyl GABA
- flumazenil
- 8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylic acid, ethyl ester
- (S)-SNAP 5114
- 1-[2-[tris(4-methoxyphenyl)methoxy]ethyl]-(S)-3piperidinecarboxylic acid
- ANOVA
- analysis of variance
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- GABABR
- GABAB receptor
- GAT
- GABA transporter
- GABA-T
- GABA transaminase.
Authorship Contributions
Participated in research design: Wang and Heinbockel.
Conducted experiments: Wang and Sun.
Contributed new reagents or analytic tools: Jackson and Scott.
Performed data analysis: Wang and Heinbockel.
Wrote or contributed to the writing of the manuscript: Wang, Jackson, Scott, and Heinbockel.
Other: Heinbockel acquired funding for the research.
References
- Abdel-Hamid ME, Edafiogho IO, Scott KR. (2002) LC/MS determination of the enaminones E139, DM5 and DM27 in rat serum. J Pharm Biomed Anal 30:1001–1011 [DOI] [PubMed] [Google Scholar]
- Ananthalakshmi KV, Edafiogho IO, Kombian SB. (2006) Concentration-dependent effects of anticonvulsant enaminone methyl 4-(4′-bromophenyl)aminocyclohex-3-en-6-methyl-2-oxo-1-oate on neuronal excitability in vitro. Neuroscience 141:345–356 [DOI] [PubMed] [Google Scholar]
- Ananthalakshmi KV, Edafiogho IO, Kombian SB. (2007) Anticonvulsant enaminone E139 suppresses epileptiform activity in rat hippocampal slices. Epilepsy Res 76:85–92 [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. (1987) GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience 20:365–383 [DOI] [PubMed] [Google Scholar]
- Chen K, Li HZ, Ye N, Zhang J, Wang JJ. (2005) Role of GABAB receptors in GABA and baclofen-induced inhibition of adult rat cerebellar interpositus nucleus neurons in vitro. Brain Res Bull 67:310–318 [DOI] [PubMed] [Google Scholar]
- Chu DC, Albin RL, Young AB, Penney JB. (1990) Distribution and kinetics of GABAB binding sites in rat central nervous system: a quantitative autoradiographic study. Neuroscience 34:341–357 [DOI] [PubMed] [Google Scholar]
- Edafiogho IO, Ananthalakshmi KV, Kombian SB. (2006) Anticonvulsant evaluation and mechanism of action of benzylamino enaminones. Bioorg Med Chem 14:5266–5272 [DOI] [PubMed] [Google Scholar]
- Edafiogho IO, Hinko CN, Chang H, Moore JA, Mulzac D, Nicholson JM, Scott KR. (1992) Synthesis and anticonvulsant activity of enaminones. J Med Chem 35:2798–2805 [DOI] [PubMed] [Google Scholar]
- Edafiogho IO, Kombian SB, Ananthalakshmi KV, Salama NN, Eddington ND, Wilson TL, Alexander MS, Jackson PL, Hanson CD, Scott KR. (2007) Enaminones: exploring additional therapeutic activities. J Pharm Sci 96:2509–2531 [DOI] [PubMed] [Google Scholar]
- Edafiogho IO, Phillips OA, Udo EE, Samuel S, Rethish B. (2009) Synthesis, antibacterial and anticonvulsant evaluations of some cyclic enaminones. Eur J Med Chem 44:967–975 [DOI] [PubMed] [Google Scholar]
- Eddington ND, Cox DS, Khurana M, Salama NN, Stables JP, Harrison SJ, Negussie A, Taylor RS, Tran UQ, Moore JA, et al. (2003) Synthesis and anticonvulsant activity of enaminones. Part 7. Synthesis and anticonvulsant evaluation of ethyl 4-[(substituted phenyl)amino]-6-methyl-2-oxocyclohex-3-ene-1-carboxylates and their corresponding 5-methylcyclohex-2-enone derivatives. Eur J Med Chem 38:49–64 [DOI] [PubMed] [Google Scholar]
- Eddington ND, Cox DS, Roberts RR, Stables JP, Powell CB, Scott KR. (2000) Enaminones-versatile therapeutic pharmacophores. Further advances. Curr Med Chem 7:417–436 [DOI] [PubMed] [Google Scholar]
- Ennis M, Hamilton KA, Hayar A. (2007) Neurochemistry of the main olfactory system, in Handbook of Neurochemistry and Molecular Neurobiology (Lajtha A. ed), Sensory Neurochemistry (Johnson DA, volume ed), 3rd ed, vol 20, pp 137–204, Springer, Heidelberg, Germany [Google Scholar]
- Errante LD, Williamson A, Spencer DD, Petroff OA. (2002) Gabapentin and vigabatrin increase GABA in the human neocortical slice. Epilepsy Res 49:203–210 [DOI] [PubMed] [Google Scholar]
- Fisher JL. (2009) The anti-convulsant stiripentol acts directly on the GABA(A) receptor as a positive allosteric modulator. Neuropharmacology 56:190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JE, Nicholson JM, Butcher R, Stables JP, Edafiogho IO, Goodwin AM, Henson MC, Smith CA, Scott KR. (1999) Synthesis, characterization and anticonvulsant activity of enaminones. Part 6: Synthesis of substituted vinylic benzamides as potential anticonvulsants. Bioorg Med Chem 7:2415–2425 [DOI] [PubMed] [Google Scholar]
- Friary RJ, Gilligan JM, Szajewski RP, Falci KJ, Franck RW. (1973) Heterocyclic synthesis via the intramolecular acylation of enamines derived from amino acids. J Org Chem 38:3487–3491 [Google Scholar]
- Heinbockel T, Heyward P, Conquet F, Ennis M. (2004) Regulation of main olfactory bulb mitral cell excitability by metabotropic glutamate receptor mGluR1. J Neurophysiol 92:3085–3096 [DOI] [PubMed] [Google Scholar]
- Heyward P, Ennis M, Keller A, Shipley MT. (2001) Membrane bistability in olfactory bulb mitral cells. J Neurosci 21:5311–5320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenkamp DJ, Johnstone TB, Huang JC, Li WY, Tran M, Whittemore ER, Bagnera RE, Gee KW. (2007) Enaminone amides as novel orally active GABAA receptor modulators. J Med Chem 50:3369–3379 [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Vitten H. (2003) GABA(B) receptors inhibit dendrodendritic transmission in the rat olfactory bulb. J Neurosci 23:2032–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Scott KR, Southerland WM, Fang YY. (2009) Enaminones 8: CoMFA and CoMSIA studies on some anticonvulsant enaminones. Bioorg Med Chem 17:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombian SB, Edafiogho IO, Ananthalakshmi KV. (2005) Anticonvulsant enaminones depress excitatory synaptic transmission in the rat brain by enhancing extracellular GABA levels. Br J Pharmacol 145:945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W. (1992) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci 12:1063–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JO. (1996) Drugs effective in the therapy of the epilepsies, in The Pharmacological Basis of Therapeutics (Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG. eds), pp 461–486, McGraw–Hill, New York [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. (2002) A new benzodiazepine pharmacology. J Pharmacol Exp Ther 300:2–8 [DOI] [PubMed] [Google Scholar]
- Mulzac D, Scott KR. (1993) Profile of anticonvulsant activity and minimal toxicity of methyl 4-[(p-chlorophenyl)amino]-6-methyl-2-oxo-cyclohex-3-en-1-oate and some prototype antiepileptic drugs in mice and rats. Epilepsia 34:1141–1146 [DOI] [PubMed] [Google Scholar]
- Munro G, Lopez-Garcia JA, Rivera-Arconada I, Erichsen HK, Nielsen EØ, Larsen JS, Ahring PK, Mirza NR. (2008) Comparison of the novel subtype-selective GABAA receptor-positive allosteric modulator NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile] with diazepam, zolpidem, bretazenil, and gaboxadol in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther 327:969–981 [DOI] [PubMed] [Google Scholar]
- Palouzier-Paulignan B, Duchamp-Viret P, Hardy AB, Duchamp A. (2002) GABA(B) receptor-mediated inhibition of mitral/tufted cell activity in the rat olfactory bulb: a whole-cell patch-clamp study in vitro. Neuroscience 111:241–250 [DOI] [PubMed] [Google Scholar]
- Panzanelli P, Perazzini AZ, Fritschy JM, Sassoè-Pognetto M. (2005) Heterogeneity of γ-aminobutyric acid type A receptors in mitral and tufted cells of the rat main olfactory bulb. J Comp Neurol 484:121–131 [DOI] [PubMed] [Google Scholar]
- Persohn E, Malherbe P, Richards JG. (1992) Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp Neurol 326:193–216 [DOI] [PubMed] [Google Scholar]
- Rall TW, Schleifer LS. (1990) Drugs effective in the therapy of the epilepsies, in The Pharmacological Basis of Therapeutics (Goodman AG, Rall TW, Nies AS, Taylor P. eds) pp 436–462, Pergamon Press, New York [Google Scholar]
- Reitz AB, Fitzpatrick LJ, Jordan AD, Sanfilippo PJ. (1999) inventors; Ortho Pharmaceutical Corporation, assignee. 5-[(Heteroaryl)alkyl]-3-oxo-pyrido[1,2-a]benzimidazole-4-carboxamide derivatives useful in treating central nervous system disorders. US patent: 5,922,731 1999 July 13
- Rho JM, Sankar R. (1999) The pharmacologic basis of antiepileptic drug action. Epilepsia 40:1471–1483 [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Wallner M, Otis TS. (2007) Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol 41:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KR, Edafiogho IO, Richardson EL, Farrar VA, Moore JA, Tietz EI, Hinko CN, Chang H, el-Assadi A, Nicholson JM. (1993) Synthesis and anticonvulsant activity of enaminones. 2. Further structure-activity correlations. J Med Chem 36:1947–1955 [DOI] [PubMed] [Google Scholar]
- Shepherd GW, Chen WR, Greer CA. (2004) Olfactory bulb, in The Synaptic Organization of the Brain (Shepherd GM. ed) pp 165–216, Oxford, New York [Google Scholar]
- Snell LD, Claffey DJ, Ruth JA, Valenzuela CF, Cardoso R, Wang Z, Levinson SR, Sather WA, Williamson AV, Ingersoll NC, et al. (2000) Novel structure having antagonist actions at both the glycine site of the N-methyl-d-aspartate receptor and neuronal voltage-sensitive sodium channels: biochemical, electrophysiological, and behavioral characterization. J Pharmacol Exp Ther 292:215–227 [PubMed] [Google Scholar]
- Stables JP, Kupferberg HJ. (1997) The NIH Anticonvulsant Drug Development (ADD) Program: preclinical anticonvulsant screening project, in Molecular and Cellular Targets for Anti-epileptic Drugs (Avanzini G, Regesta G, Tanganelli P, Avoli M. eds), pp 191–198, John Libbey & Company Ltd, London [Google Scholar]
- Traynelis SF, Dingledine R. (1988) Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J Neurophysiol 59:259–276 [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Snell LD, Tabakoff B, Levinson SR. (2002) Inhibition of neuronal Na+ channels by the novel antiepileptic compound DCUKA: identification of the diphenylureido moiety as an inactivation modifier. Exp Neurol 178:129–138 [DOI] [PubMed] [Google Scholar]
- Yohannes D, Maynard G, Yuan J, Xie L, Lee K, Ghosh M, Luke G, Liu X, Nagal A, Vincent L, et al. (2003) inventors; Neurogen Corporation, assignee. Heterocyclic compounds as ligands of the GABAA receptor. U.S. patent 6,653,471 2003 Nov 25