SUMMARY
Recent efforts to translate basic research to the treatment of clinical disorders have led to a growing interest in exploring mechanisms for diminishing fear. This research has emphasized two approaches: extinction of conditioned fear, examined across species; and cognitive emotion regulation, unique to humans. Here, we sought to examine the similarities and differences in the neural mechanisms underlying these two paradigms for diminishing fear. Using an emotion regulation strategy, we examine the neural mechanisms of regulating conditioned fear using fMRI and compare the resulting activation pattern with that observed during classic extinction. Our results suggest that the lateral PFC regions engaged by cognitive emotion regulation strategies may influence the amygdala, diminishing fear through similar vmPFC connections that are thought to inhibit the amygdala during extinction. These findings further suggest that humans may have developed complex cognition that can aid in regulating emotional responses while utilizing phylogenetically shared mechanisms of extinction.
INTRODUCTION
The ability to eliminate, control, or diminish negative emotional responses is important for adaptive function and critical in the treatment of psychopathology. Recent research examining the neural mechanisms for diminishing fears has focused on two techniques: extinction, which has been explored across species (for review see Phelps and LeDoux, 2005), and cognitive emotion regulation strategies, which are unique to humans (for review see Ochsner and Gross, 2005). In the present study, we examine whether the neural systems involved in regulating conditioned fear using a cognitive strategy take advantage of evolutionarily shared mechanisms of fear extinction to diminish learned fears.
Previous research in rodents has highlighted a role for the amygdala and ventral medial prefrontal cortex (vmPFC) in extinction learning (Garcia et al., 1999; Morgan and LeDoux, 1995; Quirk, 2002). More recently, these results have been corroborated by neuroimaging research in humans (Knight et al., 2004; Milad et al., 2005; Phelps et al., 2004). Using a paradigm that mirrored designs used in rodents, Phelps et al. (2004) presented participants with repeated exposures to two predictive stimuli that were either partially paired or not paired at all with shock. Following an acquisition phase, the stimuli were once again presented repeatedly, but this time with no shock. Behavioral results showed that, during this extinction phase, new inhibitory learning occurred that diminished the expression of conditioned fear. Further corroborating previous animal research, decreased amygdala activation was observed during extinction as compared to acquisition, while a relative increase in activation was observed in vmPFC (see also Milad et al., 2007a). Using a similar behavioral paradigm, Milad et al., (2005) showed that the relative cortical thickness of this vmPFC region, as assessed with anatomical MRI, predicted the rate of extinction, providing additional evidence that the mechanisms of fear extinction are similar across species.
In contrast to extinction, less is known about the neural correlates underlying the use of complex cognitive strategies to regulate emotions such as fear. Given that the use of these strategies may be unique to humans, the neural circuitry of emotion regulation has been primarily investigated using functional magnetic resonance imaging (fMRI). In such experiments, different forms of cognitive control over emotional responses are examined involving modulation of various cortical structures, such as dorsolateral prefrontal cortex (dlPFC). For instance, Ochsner et al. (2002) had participants view emotionally negative pictures (e.g., woman crying on steps of church) and either “attend” (focus on your natural feelings) or “reappraise” (reinterpret the picture in a less negative context; e.g., the woman is crying at a wedding) the stimulus. Behaviorally, the reappraisal technique was successful in decreasing the negative affect as measured through subjective ratings. An examination of changes in the BOLD signal contrasting the reappraisal and attend conditions revealed enhanced BOLD signal in the left dorsolateral prefrontal cortex (dlPFC) and decreased BOLD signal in the amygdala. Several other neuroimaging studies have reported similar modulation of the amygdala response with emotion regulation, along with involvement of regions of the dlPFC (e.g., Beauregard et al., 2001; Kim and Hamann, 2007; Levesque et al., 2003; Ochsner et al., 2004b; Phan et al., 2005), as well as anterior cingulate and other medial prefrontal regions (for review see Ochsner and Gross, 2005).
The dlPFC regions often observed in studies of emotion regulation have also been implicated in other higher cognitive functions, such as executive processing and working memory, or the active maintenance of on-line information (Smith and Jonides, 1999). The finding that the cognitive regulation of responses to emotional scenes alters functional activity in the dlPFC and amygdala suggests that these brain regions are functionally interconnected. However, anatomical connectivity studies have failed to find direct connections between the amygdala and the dlPFC (Barbas, 2000; McDonald et al., 1996; Stefanacci and Amaral, 2002).
We propose that the dlPFC regions linked to the cognitive regulation of emotion may take advantage of the evolutionarily shared mechanisms of extinction to diminish emotional responses. Although these dlPFC regions do not have direct projections to the amygdala, they do project to ventral and medial PFC regions that are more directly connected with the amygdala (Amaral, 2002; Groenewegen et al., 1997; McDonald et al., 1996). It is possible, therefore, that cognitive emotion regulation strategies, which recruit dlPFC regions, may diminish emotional response through connections with vmPFC, which has been shown to inhibit the amygdala in fear extinction (Milad and Quirk, 2002). In other words, humans may have developed complex cognition that can aid in the regulation of emotional responses, but these processes utilize phylogenetically older mechanisms of extinction for diminishing fears that are no longer adaptive.
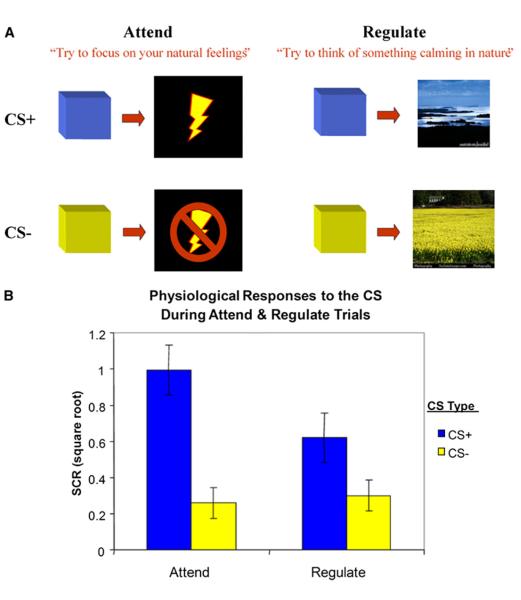
In order to examine whether the mechanisms of emotion regulation overlap with the neural circuitry of extinction, we used a paradigm designed to be as similar as possible to another examining extinction of conditioned fear (e.g., the identity of the conditioned and unconditioned stimuli, timing, probability of reinforcement, measure of conditioned response) and integrated cognitive, emotion regulation instructions. Both paradigms were run simultaneously on different participants to avoid practice effects. The extinction data set had been previously published, and the details will not be presented again here (Phelps et al., 2004). In the emotion regulation paradigm, participants were presented with two conditioned stimuli (CS), one of which was paired with an unconditioned stimulus (US) on a subset of the trials. They were instructed that upon seeing a stimulus, such as a blue square, for example, there was a chance they would receive a shock (CS+), but if they saw a yellow square, for example, they would not receive a shock (CS−). Participants were also asked to pay attention to a cue that preceded the on-set of each colored square, which indicated whether participants should either attend to the predictive qualities of the CS or attempt to regulate their emotional response (Figure 1A). When cued to attend, participants were instructed to think of their feelings and react normally (e.g., “I may get a shock” or “I will not get a shock”). When cued to regulate, participants were instructed to think of something calming in nature that was specific to the color of the square, regardless of whether it predicted a possible shock or not. For instance, upon seeing a blue square, participants might think of the ocean or the sky, while upon seeing the yellow square they might think of sunshine or a field of daffodils. Therefore, participants either attended to their feelings as in a typical fear conditioning paradigm or they attempted to change their emotional reaction by intentionally altering what they were thinking using a specific cognitive strategy. By using similar paradigms, with different means of controlling fear, we can directly compare the differences and similarities in the circuitry mediating extinction and emotion regulation.
Figure 1. Description of Experimental Conditions and Behavioral Data.
(A) Participants were exposed to two different types of trial (Attend, Regulate) that varied with respect to cognitive instruction and two types of stimuli (conditioned stimulus or CS+ and CS−) that were either paired or not paired with a potential aversive consequence (i.e., the unconditioned stimulus, shock).
(B) Physiological responses, measured by skin conductance, to different trial types. Error bars reflect standard error (±SEM).
RESULTS
Physiological Results
Consistent with previous studies of fear conditioning and extinction, the fear response was assessed using a physiological measure of arousal (LaBar et al., 1998; Milad et al., 2007b; Phelps et al., 2004). Skin conductance responses (SCRs) were acquired throughout the experiment for 12 participants (six male, six female; Figure 1B). A repeated-measures ANOVA was performed using type of trial (Attend trials, Regulate trials) and type of stimulus (CS+, CS−) as within-subject factors. A significant main effect of type of trial [F(1, 11) = 18.96, p < 0.001] and a main effect of stimulus [F(1, 11) = 41.55, p < 0.0001] were found. An interaction between type of trial and stimulus was also observed [F(1, 11) = 42.75, p < 0.0001]. The ANOVA results suggested that Attend and Regulate trials modulated the response to the CS in different ways. Responses were higher for CS+ compared to CS− during Attend trials. This differential response was reduced, however, during Regulate trials. A two-tailed paired t test compared the differential SCR to the Attend CS+ and CS− trials (M = 0.74, SD = 0.28) with the differential SCR to the Regulate CS+ and CS− trials (M = 0.32, SD = 0.33). This physiological measure of emotion regulation success indicated that using a cognitive emotion regulation strategy was successful in decreasing the conditioned response to the CS+ [t(11) = 6.54, p < 0.0001].
Neuroimaging Results
Emotion Regulation
The contrast of CS+ and CS− trials yielded regions previously associated with fear conditioning (Buchel and Dolan, 2000; Buchel et al., 1998; Delgado et al., 2006; LaBar et al., 1998; Phelps et al., 2004; Phelps and LeDoux, 2005) during both Attend and Regulate conditions (Table 1). To investigate the effects of emotion regulation on conditioned fear using a contrast analogous to other studies of emotion regulation (for review see Ochsner and Gross, 2005), we conducted a comparison between Attend and Regulate CS+ trials (Table 2). Within the subset of regions defined by this contrast, activation was observed in the three a priori regions of interest (ROIs)–the left dlPFC (middle frontal gyrus), the vmPFC (subgenual anterior cingulate), and the amygdala. To further investigate the response in these regions, a 3 mm3 cube (i.e., ROI specific analysis) was drawn around the peak voxel of each target structure and was applied to each individual participant to acquire mean beta weights for four possible predictors (Attend CS+ and CS−, Regulate CS+ and CS−).
Table 1.
Regions Activated by a Contrast of All CS+ versus All CS− Trials (Irrespective of Condition) and Vice Versa (p < 0.005)
| Brain Region | Brodman’s Area (BA) | Talairach Coordinates (x, y, z) | Number of Voxels |
|---|---|---|---|
| CS+ versus CS− Trials | |||
|
| |||
| Right medial frontal gyrus | BA 8 | 5, 29, 37 | 5764 |
| Right cingulate gyrus | BA 24/32 | 1, 3, 37 | 2112 |
| Right inferior parietal cortex | BA 40 | 50, −45, 36 | 245 |
| Left posterior cingulate gyrus | BA 23/31 | −1, −22, 33 | 391 |
| Right precuneus | BA 7 | 15, −59, 33 | 224 |
| Left inferior parietal cortex | BA 40 | −64, −29, 28 | 113 |
| Right caudate nucleus | 12, 4, 13 | 3199 | |
| Left caudate nucleus | BA 40 | −9, 13, 5 | 1887 |
| Right superior temporal sulcus | BA 42 | 30, −24, 12 | 132 |
| Right posterior cingulate gyrus | BA 23/31 | 8, 39, 12 | 164 |
| Right medial frontal gyrus | BA 10 | 34, 58, 11 | 106 |
| Left putamen | −17, 4, 3 | 365 | |
| Left insula | −32, 20, 2 | 2976 | |
| Right medial frontal gyrus | BA 10/32 | 31, 56, 2 | 184 |
| Left thalamus | −5, −12, 2 | 2856 | |
| Right thalamus | 8, −14, 0 | 3747 | |
| Right middle temporal gyrus | BA 40 | 60, −30, −3 | 439 |
| Right midbrain | 4, −23, −14 | 1405 | |
|
| |||
| CS− versus CS+ Trials | |||
|
| |||
| Left parietal cortex | BA 40/7 | −39, −35, 55 | 312 |
| Left precuneus | BA 7 | −25, −69, 40 | 182 |
| Left cuneus | BA 31/18 | −3, −58, 23 | 446 |
| Right parahippocampus gyrus | BA 35 | 22, −33, −8 | 112 |
| Left parahippocampus gyrus | BA 35 | −25, −26, −13 | 356 |
| Right cerebellum | 25, −29, −17 | 139 | |
Table 2.
Regions Activated by a Contrast of Attend CS+ versus Regulate CS+ and Vice Versa (p < 0.005)
| Brain Region | Brodman’s Area (BA) | Talairach Coordinates (x, y, z) | Number of Voxels |
|---|---|---|---|
| Attend versus Regulate CS+ Trials | |||
|
| |||
| Right medial frontal gyrus | BA 6 | 20, 1, 51 | 61 |
| Right superior frontal gyrus | BA 8 | 5, 40, 51 | 368 |
| Right inferior parietal cortex | BA 7/40 | 35, −47, 46 | 440 |
| Right precuneus | BA 7 | 11, −71, 45 | 293 |
| Left inferior parietal cortex | BA 7/40 | −31, −41, 42 | 216 |
| Right cingulate gyrus | BA 24 | 2, −3, 35 | 5677 |
| Left cerebellum | −26, −68, −30 | 772 | |
| Left cingulate gyrus | BA 32 | −2, 22, 27 | 837 |
| Right superior frontal gyrus | BA 10 | 23, 59, 24 | 49 |
| Right parietal cortex | BA 40 | 53, −28, 21 | 8522 |
| Left parietal cortex | BA 40 | −58, −26, 21 | 6503 |
| Right caudate | 11, −8, 19 | 3153 | |
| Left thalamus | −1, −9, 16 | 821 | |
| Left caudate | −9, −5, 15 | 3395 | |
| Right medial frontal gyrus | BA 10/46 | 29, 46, 15 | 220 |
| Right thalamus | 10, −5, 12 | 831 | |
| Left putamen | −26, −5, 6 | 517 | |
| Right putamen | 29, −5, 6 | 1578 | |
| Right insula | 32, 13, 5 | 595 | |
| Left lingual gyrus | BA 18 | −25, −59, 3 | 29 |
| Right cuneus | BA 17 7, | −89, 2 | 130 |
| Left insula | −35, −20, 0 | 994 | |
| Left amygdala | −20, 0, −20 | 17 | |
|
| |||
| Regulate versus Attend CS+ Trials | |||
|
| |||
| Left dlPFC (middle frontal gyrus) | BA 9/46 | −43, 28, 30 | 167 |
| vmPFC | BA 32 | 0, 35, −8 | 92 |
| Left parahippocampus gyrus | BA 35 | −30, −35, −9 | 22 |
| Right parahippocampus gyrus | BA 35 | 29, −32, −12 | 27 |
| Subgenual cingulate cortex | BA 25 | 0, 14, −11 | 17 |
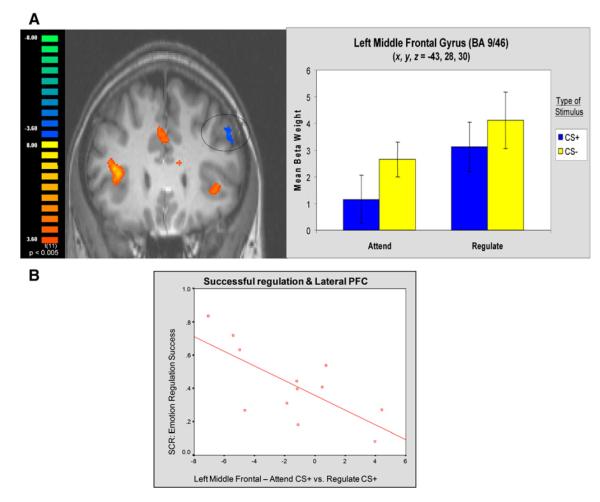
Consistent with previous emotion regulation studies, opposite patterns of activation were observed in PFC ROIs and the amygdala (Ochsner et al., 2002; Schaefer et al., 2002). Activation in the left middle frontal gyrus (BA 9/46: x, y, z = −43, 28, 30) was characterized by an increase in BOLD response to Regulate CS+ trials compared with Attend CS+ trials (Figure 2A). As dictated by the contrast used to define this ROI, a two-tailed paired-samples t test confirmed that the mean beta weights for Regulate CS+ trials were significantly higher than those for Attend CS+ trials [t(11) = 2.83, p < 0.02]. No difference was observed when contrasting the two CS− predictors [t(11) = 1.53, p = n.s.]. Notably, a significant correlation between the differential response of Attend and Regulate CS+ trials and the physiological correlate of emotion regulation success was also observed (r = −0.74, p < 0.006; Figure 2B), consistent with previous studies of emotion regulation (Ochsner et al., 2002). Participants with more activation in this left dlPFC region during Regulate CS+ trials (compared to Attend trials) were also more successful in decreasing their conditioned response, as measured by SCR.
Figure 2. BOLD Signals in dlPFC during Regulation.
(A) BOLD responses in dlPFC (in blue) are higher during Regulate compared to Attend CS+ trials.
(B) Correlation between physiological measure of regulation success and BOLD levels in dlPFC reflecting differential response of Attend and Regulate CS+ trials.
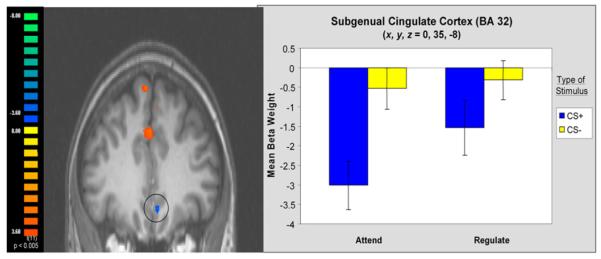
Activation of the subgenual anterior cingulate (BA 32: x, y, z = −3, 36, −8) in the vmPFC was also observed. This region was similar to the vmPFC region previously implicated in studies of fear extinction in humans (BA 32: 0, 35, −8 taken from Phelps et al., 2004; BA 32: 4, 30, −12 taken from Milad et al., 2005), with a pattern of BOLD responses also mirroring that observed during the extinction of conditioned fear (Phelps et al., 2004). There was an overall decrease in activation relative to a resting baseline in response to a CS+ that predicts shock (Figure 3). However, when the fear response was diminished, there was relative increase in BOLD signal as revealed through the comparison of the mean beta weights for Attend CS+ with Regulate CS+ trials [t(11) = −4.07, p < 0.002]. No significant difference between Attend and Regulate CS− trials were observed [t(11) = −0.29, p = n.s.].
Figure 3. Activation of the Subgenual Anterior Cingulate (BA 32) and Mean Beta Weights.
The pattern of BOLD response in this vmPFC ROI resembles observations of similar regions of interest in previous extinction studies (Milad et al., 2005; Phelps et al., 2004). Error bars reflect standard error (±SEM).
The opposite pattern was observed in the left amygdala ROI (x, y, z = −20, 0, 20) where emotion regulation led to a decrease in activation (Figure 4), consistent with studies of emotion regulation (Ochsner and Gross, 2005) and extinction (Phelps et al., 2004). A comparison of mean beta weights in individual participants, as expected, showed a higher overall mean for Attend CS+ as compared to Regulate CS+ trials [t(11) = 4.15, p < 0.002]. No differences were observed when comparing the two CS− predictors [t(11) = −1.20, p = n.s.].
Figure 4. Mean Beta Weights Reflecting BOLD Responses in the Left Amygdala for the Main Conditions.
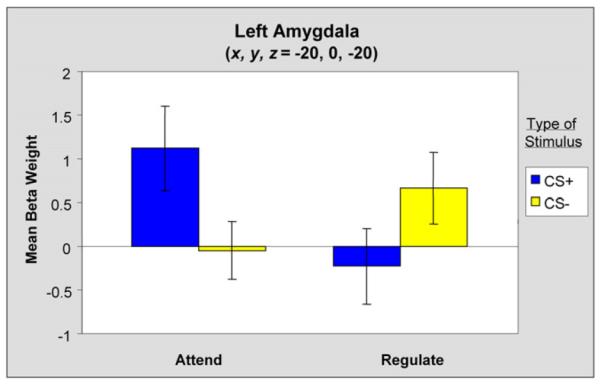
Error bars reflect standard error (±SEM).
Overlap with the Mechanisms of Extinction
The use of an emotion regulation strategy led to a decrease in conditioned fear, along with opposite patterns of activation in the prefrontal cortex and the amygdala. As mentioned above, the pattern of BOLD response observed in the current emotion regulation study is strikingly similar to data on extinction of conditioned fear (Phelps et al., 2004) in both the amygdala and vmPFC, suggesting similar neural mechanisms may be recruited.
To further investigate the overlap in the neurocircuitry of emotion regulation and extinction of conditioned fear, additional analyses were conducted. Using the vmPFC and amygdala ROIs identified in the extinction data set (Phelps et al., 2004), we explored the similarity and differences in the pattern of BOLD response when conditioned fear was diminished through emotion regulation. An examination of the subgenual anterior cingulate ROI identified in extinction (BA 32: x, y, z = 0, 35, −8), revealed a similar pattern of results when extracting mean beta weights from the data of participants in the emotion regulation paradigm. Specifically, differences were observed between Attend and Regulate CS+ trials [t(11) = −2.40, p < 0.04], but not between Attend and Regulate CS− trials [t(11) = −0.28, p = n.s.]. As can be seen in Figure 5, the presentation of a CS+ that predicted possible shock led to a decrease in BOLD signal in this vmPFC region (relative to rest) in both extinction and emotion regulation. However, as the conditioned fear response was diminished through either extinction training or a cognitive regulation strategy, responses in this region increased, and the observed decrease in the BOLD response to the CS+ was attenuated.
Figure 5. Similarities between BOLD Responses in the vmPFC in Two Separate Fear Conditioning Experiments.
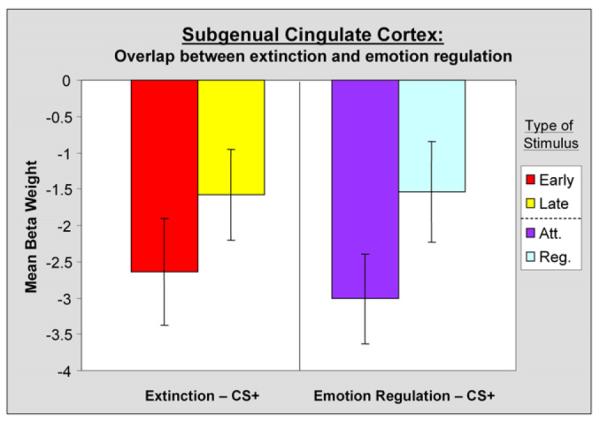
The left panel displays mean beta weights in the vmPFC for CS+ trials during both early (red) and late (yellow) extinction of conditioned fear (data from Milad et al., 2005; Phelps et al., 2004). The right panel displays mean beta weights in the vmPFC ROI from the current experiment for both Attend (purple) and Regulate (light blue) CS+ trials. Error bars reflect standard error (±SEM).
Although the left amygdala showed the greatest changes with emotion regulation as previously mentioned, investigating the right amygdala ROI (x, y, z = 15, −3, −13) identified in the extinction data set revealed similar differences between the Attend CS+ trials and Regulate CS+ trials [t(11) = 2.54, p < 0.03], but not between Attend and Regulate CS− trials [t(11) = 0.82, p = n.s.]. These results support a potential overlap and similarities between the neural circuitry of diminishing fears through either passive extinction or cognitive emotion regulation strategies.
Perhaps not surprisingly, the dlPFC region observed in the emotion regulation data set did not show any significant differential BOLD responses during extinction (Phelps et al., 2004). It is proposed that this region may be specifically linked to the online manipulation and maintenance of the cognitive regulation strategy, which was not a component of the passive extinction paradigm. Interestingly, the left amygdala ROI defined in the emotion regulation data set also did not show any significant BOLD responses during extinction, while the right amygdala response was similar across both data sets.
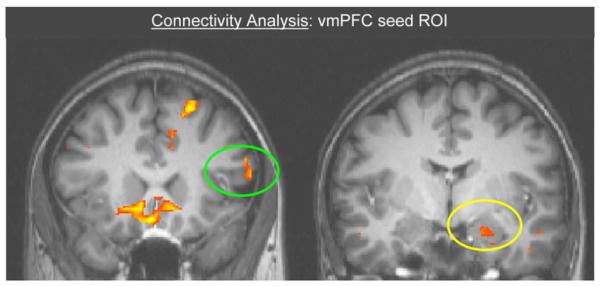
To further support the assertion that the vmPFC may be mediating the impact of the cognitive strategy on the amygdala response, we conducted an exploratory connectivity analysis. If the vmPFC is mediating the influence of the dlPFC on the amygdala, we would expect that the pattern of activation in this vmPFC ROI during the regulation of conditioned fear should correlate with both the amygdala and dlPFC. Using the vmPFC (subgenual anterior cingulate) ROI identified in the emotion regulation data set, a time course of activation was extracted for the entire experiment and used as a covariate in the data. The time course for the ROI was extracted for each run for each subject, then z transformed and used as a single predictor in a general linear model. Resulting activation maps thresholded at p < 0.00001 displayed areas where the activation pattern was correlated with this seed ROI. As expected, both the left amygdala (see Figure 6: x, y, z = −20, −8, −16) and dlPFC (middle frontal gyrus, x, y, z = −50, 23, 19; see Figure 6) emerged as correlated with the vmPFC ROI in this connectivity analysis. The results of this exploratory connectivity analysis are consistent with the interpretation that the vmPFC, and the mechanisms of fear extinction, may be mediating the impact that cognitive emotion regulation strategies and the dlPFC have on the amygdala and the expression of conditioned fear.
Figure 6. Connectivity Analysis Using vmPFC Seed ROI Time Course as Predictor.
Regions in the dlPFC (green circle) and amygdala (yellow circle) are found to correlate with the seed ROI.
DISCUSSION
The present study explored the overlap in the neural circuitry of diminishing learned fears through emotion regulation and extinction. In order to examine the similarities and differences of diminishing fear through these two techniques, we chose a paradigm where the procedure (fear conditioning) and dependent measure (physiological arousal) were identical to an extinction protocol (Phelps et al., 2004) and typical in studies of extinction (Knight et al., 2004; Milad et al., 2005, 2007a), while integrating a cognitive emotion regulation strategy. Our results support a model in which the lateral PFC regions engaged by the online manipulation of information characteristic of cognitive emotion regulation strategies (for review see Ochsner and Gross, 2005) influences amygdala function through connections to vmPFC regions that are also thought to inhibit the amygdala during extinction (Milad and Quirk, 2002). These results are consistent with the suggestion that vmPFC may play a general regulatory role in diminishing fear across a range of paradigms (e.g., Kim et al., 2003; Urry et al., 2006).
Previous studies of emotion regulation have generally invoked emotional reactions by using more diverse and complex stimuli, primarily graphic scenes, and have assessed reactions and the success of the regulation strategy most frequently by subjective report. In addition, many studies of emotion regulation have used distinct visual images and asked participants to apply a “reappraisal” strategy: reinterpret the context of the current image along with the actions of the actors in it. In contrast, the present study used repeated stimuli (the CSs), a physiological measure of emotion and regulation success, and a practiced imagerybased emotion regulation task. A particular advantage of the imagery strategy is its similarities with traditional cognitive behavioral therapies (CBT) as compared to reappraisal. In some forms of CBT, patients are taught, and practice, specific strategies, including imagery, to deal with specific situations, much like the current paradigm that enforces a specific strategy to use in response to a specific cue (Beck et al., 2005). A potential disadvantage of this strategy is that even though participants are instructed to specifically think of something soothing, it is difficult to determine whether any regulation effect is due to the effortful regulation of emotion, or simple distraction, which can also effectively alter emotional responses (Ochsner and Gross, 2005). Nevertheless, even with these fundamental differences, our physiological and brain imaging results are quite consistent with previous studies of emotion regulation. Our subjects were able to use a simple imagery strategy to diminish their physiological response to a conditioned fear stimulus. Similar to other fMRI studies of emotion regulation, the use of this strategy resulted in decreased activation of the amygdala and increased activation of the left dlPFC, the magnitude of which was correlated with regulation success.
Most previous research on emotion regulation has emphasized the importance of the lateral PFC, although there is considerable variability in the precise region within the lateral PFC engaged across studies and emotion regulation paradigms (for review see Ochsner and Gross, 2005). Although less frequently emphasized, several of these studies also report activation in regions of the vmPFC (i.e., the subgenual ACC or immediately adjacent vmPFC region) similar to that observed in the present study (e.g., Harenski and Hamann, 2006; Kalisch et al., 2005b; Kim and Hamann, 2007; Ochsner et al., 2004a; Urry et al., 2006), supporting a general role for this region in mediating inhibition of the amygdala response. Other studies of emotion regulation have reported involvement of other medial PFC regions, including the orbitofrontal cortex, more dorsal regions of the mPFC, and anterior cingulate (Beauregard et al., 2001; Eippert et al., 2007; Kalisch et al., 2005a; Levesque et al., 2003; Ochsner et al., 2002; Phan et al., 2005), while suggesting a similar role. The reasons for the differences between studies in the precise regions of the mPFC observed may be due to a number of factors, including differences in stimuli, emotional state, or regulation strategy employed (e.g., Ochsner et al., 2004b). Since a null differential BOLD response does not rule out involvement of a specific brain region in a task, it is also possible that a similar vmPFC region is mediating the influence of the lateral PFC on the amygdala across different types of emotional stimuli, assessments of emotion, and regulation strategies. Future studies with additional techniques will be needed to determine the functional role of different mPFC regions in the cognitive control of emotion. The present results demonstrate that when using a paradigm and dependent measure typically used in studies of extinction of conditioned fear, the use of a cognitive regulation strategy results in involvement of an overlapping region of the vmPFC across tasks.
In addition to the amygdala, other regions that showed decreased responses in the emotion regulation condition included posterior insular cortex, cingulate regions, and dorsal medial PFC. These regions have previously been involved in anticipation of aversive, at times painful stimuli (Jensen et al., 2003; Mohr et al., 2005) as well as other regulation studies that involve attentional resources (Ochsner and Gross, 2005). Commonly reported in studies of fear conditioning (LaBar et al., 1998; Phelps et al., 2004), it is unclear how such regions interact with other prefrontal regions that are involved in regulatory control. A recent study, however, suggested a critical role for ventral lateral PFC regions in the regulation of pain while influencing regions such as posterior insula and anterior cingulate (Salomons et al., 2007).
Besides the involvement of left dlPFC during emotion regulation, another difference in the pattern of activation observed between emotion regulation and extinction (Phelps et al., 2004) was the laterality of the most robust amygdala activation. During extinction, the right amygdala showed the greatest differential BOLD response between the acquisition and extinction stages, whereas in emotion regulation the left amygdala showed the greatest differential BOLD response between attend and regulate conditions. Although an exploratory analysis revealed a similar pattern of activation in the right amygdala with emotion regulation, this difference in the laterality of peak differential amygdala response may reflect the different paradigms. A similar pattern has been observed in studies of the acquisition of fear. Although activation of the right and left amygdala has been reported in studies of fear conditioning and damage to either disrupts fear conditioning (LaBar et al., 1995), it is more often the right amygdala that correlates with expression of learning (LaBar et al., 1998; Phelps et al., 2004) or shows the peak response (Buchel et al., 1998), especially when the stimuli are processed automatically (Morris et al., 1999). In contrast, previous studies of instructed fear, in which the potentially threatening quality of a stimulus is symbolically conveyed, found only the left amygdala was involved in the expression of the learning (Funayama et al., 2001; Phelps et al., 2001). The reason for the left hemisphere bias is not clear, but it may be due to the cognitive, linguistic nature of the task instructions, or the left hemisphere involvement in tasks that require the on-line cognitive interpretation of stimuli (Gazzaniga, 2000). It should be noted, however, that other studies of emotion regulation have reported involvement of both the right and left dlPFC, although activation of left dlPFC and regulation of the left amygdala is observed more frequently (e.g., Kim and Hamann, 2007; for review see Ochsner and Gross, 2005). It is possible that the left hemisphere bias in the present study may reflect the specific technique for diminishing fear, but strong claims of laterality should be interpreted with caution with fMRI due to the possibility of subthreshold differential BOLD responses in regions that may play an important role in the task.
The present results suggest that, even though humans may have developed unique capabilities for using complex cognitive strategies to control emotion, these strategies may influence the amygdala through phylogenetically shared mechanisms of extinction. A similar pattern has been observed in studies of fear acquisition in which cognitive and social means of fear learning depend on the amygdala for expression (Olsson and Phelps, 2007). By identifying an overlap in the neural circuitry across these different techniques for diminishing fear, it is possible to gain insight from detailed animal models of fear extinction to help understand a subset of the processes that may underlie emotion regulation. There is growing interest in taking advantage of animal models of extinction to explore novel pharmacological and therapeutic treatments for anxiety disorders (e.g., Davis et al., 2006; LeDoux and Gorman, 2001; Rauch et al., 2006). How these potential treatments might interact with more standard therapeutic tools, such as cognitive-behavioral training for diminishing fears, is largely unknown. The present result suggests that these techniques may be, in part, complementary in that they rely on an overlapping neural circuitry and, perhaps, similar neurophysiological and neurochemical mechanisms.
EXPERIMENTAL PROCEDURES
Participants
Eighteen right-handed volunteers participated in this study (nine male, nine female). Three participants were removed due to failure to acquire conditioned fear during Attend trials, as assessed with SCR, since they provided no physiological evidence of conditioning or regulation, thus making it difficult to interpret their neuroimaging data. Three others were removed due to failure to comply with task requirements (assessed by postexperimental questions and self-reports), which included misattribution of Attend and Regulate instructions and switching instructions midway through the experiment. Final analysis was therefore conducted on 12 participants (six male, six female). Participants responded to posted advertisement (average age: M = 23.29, SD = 3.31), and all participants gave informed consent.
Procedure
A partial reinforcement, fear conditioning paradigm with instruction was used. The paradigm involved a series of 66 interleaved trials, divided into three runs of 22 trials each. Each trial began with the presentation of a word cue, presented for 2 s, which instructed the participant on the type of trial. It was followed by either a blue or yellow square that served as a conditioned stimulus (CS) and was presented for 4 s. A mild shock to the wrist served as the unconditioned stimulus (US) and was administered during the last 200 ms for six of the CS trials. During one experimental session, a specific colored square (e.g., blue) was paired with the US, thus serving as the CS+, while the other square (e.g., yellow) served as the CS−. This contingency was counterbalanced across participants. The trial concluded with a 12 s intertrial interval.
Prior to scanning, participants were trained on the emotion regulation technique. They were told that in the scanner they would see a series of blue and yellow squares, one of which would be paired with a mild shock to the wrist for a subset of trials. Prior to the presentation of each colored square, they were told they would be presented with an instruction cue. Participants viewed two types of cues during the experiment: “ATTEND” or “REAPPRAISE.” When instructed to “attend,” participants were asked to view the stimulus and attend to their natural feelings regarding which CS was presented. In these Attend trials, for example, participants might focus on the fact that they may receive a shock (if the cue was followed by a CS+) or would never receive a shock (if the cue was followed by a CS−). When instructed to “reappraise,” participants were asked to view the CS and try to imagine something in nature that was calming, prompted by the color of the CS. During these Regulation trials, for example, participants could think of an image of the ocean or a blue sky when viewing the blue square, or they could think of the sunshine or a field of daffodils when viewing the yellow square. Participants were trained on repeated trials of both instructions and all four types of trials (“attend” CS+ and CS−, and “reappraise” CS+ and CS−). They were asked to keep the same mental picture they selected during training throughout the experiment. After training, the subjects were placed in the scanner and were reminded of the instructions and imagery task prior to the first functional run. After the experiment, participants filled out questionnaires regarding their subjective judgment of success at engaging in the regulation task and following the instructions.
The experiment included four types of trials (Attend and Regulate CS+ and CS−), each of which was presented 15 times throughout the experimental session. Six additional CS+ trials were paired with a US and were discarded from further physiological or neuroimaging analysis. Of these six extra CS+ trials, three were Attend CS+ trials paired with shock and three were Regulate CS+ trials paired with shock, with one presentation of each per fMRI run.
Physiological Set-Up and Assessment
A Grass Instruments stimulator was used to administer mild shocks to participants. The stimulator was shielded from magnetic interference and grounded through an RF filter. A bar electrode attached to the right wrist delivered the shocks. Prior to scanning, participants received a mild shock (200 ms duration, 50 pulses/s), which was gradually increased according to the participant’s self report of intensity and pain. They were instructed to determine a level where the shock felt “uncomfortable, but not painful” (maximum = 50 V). Skin conductance responses (SCR) were acquired from the participant’s middle phalanges of the second and third fingers in the left hand using BIOPAC systems skin conductance module and shielded Ag-AgCl electrodes grounded through an RF filter panel. AcqKnowledge software was used to analyze SCR wave-forms. Trials where a shock was administered (six total) were not included in the analysis. The level of SCR response was assessed as the base to peak difference in the 0.5 to 4.5 s window following the onset of a CS, the blue or yellow square (see LaBar et al., 1995). SCRs for each participant were converted to standardized T scores and averaged per participant, per condition (Funayama et al., 2001). A repeated-measures ANOVA was conducted with type of trial (Attend, Regulate) and type of stimulus (CS+, CS−) as within-subjects factor to investigate the effects of emotion regulation during conditioned fear. A physiological measure of emotion regulation success was calculated by subtracting the mean SCR for Regulate CS+ trials from Attend CS+ trials for each individual participant.
fMRI Acquisition and Analysis
A 3T Siemens Allegra head-only scanner and a Siemens standard head coil were used for data acquisition at NYU’s Center for Brain Imaging. Anatomical images were acquired using a T1-weighted protocol (256 × 256 matrix, 176 1 mm sagittal slices). Functional images were acquired using a single-shot gradient echo EPI sequence (TR = 2000 ms, TE = 20 ms, FOV = 192 cm, flip angle =75°, bandwith = 4340 Hz/px, echo spacing = 0.29 ms). Thirty-five contiguous oblique-axial slices (3 × 3 × 3 mm voxels) parallel to the AC-PC line were obtained. Analysis of imaging data was conducted using Brain Voyageur software (Brain Innovation, Maastricht, The Netherlands). The data were initially corrected for motion (using a threshold of 2 mm or less), and slice scan time using sinc interpolation was applied. Further, spatial smoothing was performed using a three-dimensional Gaussian filter (4 mm FWHM), along with voxel-wise linear detrending and high-pass filtering of frequencies (three cycles per time course). Structural and functional data of each participant were then transformed to standard Talairach stereotaxic space (Talairach and Tournoux, 1988).
A random-effects analysis was performed on the functional data using a general linear model (GLM), which estimated beta weights for each of four different CS presentations (Attend CS+, Attend CS−, Regulate CS+, Regulate CS−). Statistical maps of interest were created using a threshold of p < 0.005. The primary contrast of interest was the differential response between Attend CS+ and Regulate CS+, which directly investigated the effect of using emotion regulation to diminish conditioned fear.
To further investigate the effects of cognitive regulation strategy on conditioned fear, we examined BOLD responses in three a priori regions of interest based on previous studies of emotion regulation (Ochsner et al., 2002) and extinction (Phelps et al., 2004). The three ROIs were the dlPFC (previously implicated in emotion regulation), vmPFC (previously implicated in extinction), and amygdala (linked to both emotion regulation and extinction). For these ROIs, the peak differential BOLD response was identified in the group analysis (Attend versus Regulate CS+), and a cube was drawn around it (3 mm3). For the a priori ROIs, a multistudy GLM was performed on each individual participant, allowing for acquisition of mean beta weights in each participant for each ROI. Correlational analyses between functional and SCR data were performed using the averaged beta weights and physiological measure of emotion regulation success.
Additional Analyses
The emotion regulation of fear data set was also compared to a previously published fear extinction data set (Phelps et al., 2004). Both data sets were collected at the same time with the intent of comparison across paradigms. While a within-subject comparison might have been more useful, several limitations of such a design led to two separate experiments being carried out. Such limitations include potential carry over effects from subsequent fear conditioning sessions in an experiment where participant naivety is important and potential strategy contaminations (e.g., participants who undergo emotion regulation first may apply those strategies during extinction). Thus, we restricted the examination of the overlap in the neurocircuitry of emotion regulation and extinction of conditioned fear to two exploratory analyses done between experiments.
First, using the vmPFC and amygdala ROIs identified in the previous data set investigating extinction (Phelps et al., 2004), we explored the similarity and differences in the pattern of BOLD response when conditioned fear was diminished through emotion regulation. Specifically, we applied the ROIs from the extinction study in the emotion regulation data set and extracted mean beta weights from the set of ROIs of the previous study. A similar analysis was used to investigate the role of the dlPFC ROI identified in the emotion regulation study on the extinction data set.
Second, an exploratory connectivity analyses was performed. The goal of this analysis was to provide additional evidence regarding a potential role of the vmPFC in mediating top-down influence from dlPFC to the amygdala, with the expectation that patterns of activation in both regions correlate with observed time courses in the vmPFC ROI during the regulation of conditioned fear. The analysis was performed by extracting the time course of activation across the entire experiment from the vmPFC (subgenual anterior cingulate) ROI, defined from the emotion regulation study and used as a covariate in the data. Specifically, the time course for the ROI was extracted for each run for each subject, then z transformed and used as a single predictor in a general linear model. The resulting activation map, thresholded at p < 0.00001, displayed brain regions whose activation patterns correlated with patterns from the seed ROI (vmPFC).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institute of Mental Health grants to E.A.P (MH072279) and to J.E.L (MH058911) and the Beatrice and Samuel A. Seaver Foundation. The authors wish to acknowledge Kevin Ochsner and Jennifer Trujillo for discussion and assistance.
Footnotes
SUPPLEMENTAL DATA The Supplemental Data include Supplemental Results and a table and can be found with this article online at http://www.neuron.org/cgi/content/full/59/5/829/DC1/.
REFERENCES
- Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol. Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res. Bull. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J. Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Emery G, Greenberg RL. Anxiety Disorders and Phobias: A Cognitive Perspective. 15th Anniversary Edition Basic Books; Cambridge, MA: 2005. [Google Scholar]
- Buchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Curr. Opin. Neurobiol. 2000;10:219–223. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol. Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol. Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Hum. Brain Mapp. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama ES, Grillon C, Davis M, Phelps EA. A double dissociation in the affective modulation of startle in humans: effects of unilateral temporal lobectomy. J. Cogn. Neurosci. 2001;13:721–729. doi: 10.1162/08989290152541395. [DOI] [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Uylings HB. The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J. Psychopharmacol. 1997;11:99–106. doi: 10.1177/026988119701100202. [DOI] [PubMed] [Google Scholar]
- Harenski CL, Hamann S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage. 2006;30:313–324. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Delfino M, Murer MG, Auer DP. The phenylephrine blood pressure clamp in pharmacologic magnetic resonance imaging: reduction of systemic confounds and improved detectability of drug-induced BOLD signal changes. Psychopharmacology (Berl.) 2005a;180:774–780. doi: 10.1007/s00213-005-2252-0. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Seymour B, O’Doherty JP, Oakley DA, Allen P, Dolan RJ. Anxiety reduction through detachment: subjective, physiological, and neural effects. J. Cogn. Neurosci. 2005b;17:874–883. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J. Cogn. Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn. Affect. Behav. Neurosci. 2004;4:317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. J. Neurosci. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. Am. J. Psychiatry. 2001;158:1953–1955. doi: 10.1176/appi.ajp.158.12.1953. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biol. Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoag-glutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc. Natl. Acad. Sci. USA. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry. 2007a;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatr. 2007b;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Mohr C, Binkofski F, Erdmann C, Buchel C, Helmchen C. The anterior cingulate cortex contains distinct areas dissociating external from self-administered painful stimulation: a parametric fMRI study. Pain. 2005;114:347–357. doi: 10.1016/j.pain.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav. Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc. Natl. Acad. Sci. USA. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J. Cogn. Neurosci. 2004a;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004b;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Social learning of fear. Nat. Neurosci. 2007;10:1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat. Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn. Mem. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biol. Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Shackman AJ, Davidson RJ. Individual differences in the effects of perceived controllability on pain perception: critical role of the prefrontal cortex. J. Cogn. Neurosci. 2007;19:993–1003. doi: 10.1162/jocn.2007.19.6.993. [DOI] [PubMed] [Google Scholar]
- Schaefer SM, Jackson DC, Davidson RJ, Aguirre GK, Kimberg DY, Thompson-Schill SL. Modulation of amygdalar activity by the conscious regulation of negative emotion. J. Cogn. Neurosci. 2002;14:913–921. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J. Comp. Neurol. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: An Approach to Medical Cerebral Imaging. Thieme Medical Publishers; Stuttgart, New York: 1988. [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.