Abstract
Multidrug resistance-associated protein (Mrp) 2-deficient (TR−) Wistar rats have been used to elucidate the role of Mrp2 in drug disposition. Decreased breast cancer resistance protein (Bcrp) levels were reported in sandwich-cultured hepatocytes (SCH) from TR− rats compared with those from wild-type (WT) rats. This study was designed to characterize hepatic Bcrp expression and function in TR− rats, using nitrofurantoin and pitavastatin as substrates. Bcrp was knocked down by RNA interference in rat SCH. Antibody BXP53, but not BXP21, specifically detected Bcrp knockdown in SCH. Bcrp protein levels were decreased markedly in TR− but not Mrp2-deficient Sprague-Dawley [Eisai hyperbilirubinemic rats (EHBR)] rats. Bcrp mRNA levels were decreased significantly in TR− livers as determined by TaqMan real-time reverse transcriptase-polymerase chain reaction. Biliary excretion of nitrofurantoin, a specific Bcrp substrate, was decreased significantly in SCH and isolated perfused livers from TR− rats compared with those from WT controls, indicating that hepatic Bcrp function is decreased in TR− rats. In Bcrp knockdown SCH, the biliary excretion index and in vitro biliary clearance of pitavastatin were decreased significantly to ∼58 and ∼52% of control, respectively, indicating that Bcrp plays a role in pitavastatin biliary excretion. Pitavastatin biliary excretion was decreased significantly in perfused livers from TR− compared with those from WT rats. In conclusion, expression and function of hepatic Bcrp are decreased significantly in TR− rats. The potential role of both Bcrp and Mrp2 should be considered when data generated in TR− rats are interpreted. TR− and EHBR rats in combination may be useful in differentiating the role of Mrp2 and Bcrp in drug/metabolite disposition.
Introduction
Biliary excretion of xenobiotics and bile acids is mediated primarily by ATP-binding cassette (ABC) transport proteins located on the canalicular membrane of hepatocytes, namely, breast cancer resistance protein (Bcrp; Abcg2), P-glycoprotein, multidrug resistance-associated protein 2 (Mrp2), and the bile salt export pump (Chandra and Brouwer, 2004). Substrates of BCRP include various organic anions, cations, and phase II conjugates including the anticancer drug topotecan, the antibiotic nitrofurantoin, and lipid-lowering statins (e.g., rosuvastatin and pitavastatin) (Hirano et al., 2005; Choudhuri and Klaassen, 2006). A single nucleotide polymorphism in ABCG2 C421A (Q141K) has been associated with altered drug disposition in clinical studies (elevated plasma concentrations of diflomotecan after intravenous administration and of topotecan and rosuvastatin after oral administration) (Sparreboom et al., 2004, 2005; Zhang et al., 2006a). These findings emphasize the important role of BCRP in drug disposition.
MRP2 is one of the most extensively studied hepatic transport proteins. Substrates of MRP2 include numerous antibiotics, anticancer drugs, and various phase II conjugates, including conjugates of endogenous molecules (Choudhuri and Klaassen, 2006). Mutations in human MRP2 are associated with Dubin-Johnson syndrome, an autosomal recessive disorder resulting in chronic conjugated hyperbilirubinemia (Keitel et al., 2000). In Mrp2-deficient (TR−) Wistar rats, a naturally occurring single nucleotide deletion in the Mrp2 gene results in reduced mRNA abundance and absence of the protein (Jansen et al., 1985; Paulusma et al., 1996). A similar mutation in Mrp2 also exists in Sprague-Dawley rats, which are referred to as Eisai hyperbilirubinemic rats (EHBR) (Hirohashi et al., 1998).
Understanding the contribution of individual transport proteins to overall biliary excretion of drugs and metabolites is important to predict the effect of altered transport function on drug/metabolite pharmacokinetics. Canalicular transporter gene knockout mice and Mrp2-deficient TR− and EHBR rats have been used to determine the role of individual transport proteins in the biliary excretion of drugs, metabolites, endogenous compounds, and toxins (Yamazaki et al., 1997; Hirano et al., 2005; Zamek-Gliszczynski et al., 2005, 2006a, 2008; Gavrilova et al., 2007; Lecureux et al., 2009; Maier-Salamon et al., 2009). In addition, adenoviral vector-mediated RNA interference (RNAi) knockdown of transport proteins in rat sandwich-cultured hepatocytes (SCH) is a powerful in vitro tool to determine the contribution of individual transport proteins to drug biliary excretion; using this approach, nitrofurantoin was confirmed as a specific Bcrp probe substrate in rat SCH (Yue et al., 2009), consistent with data published previously (Merino et al., 2005).
Apparent species differences in Bcrp-mediated biliary excretion of drugs/metabolites have been reported on the basis of data obtained from transport-deficient mice and rat models. For example, in mice, Bcrp appeared to be the major transport protein responsible for the biliary excretion of acetaminophen sulfate, 4-methylumbelliferyl sulfate, and harmol sulfate (Zamek-Gliszczynski et al., 2006b), as well as pitavastatin (Hirano et al., 2005) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (van Herwaarden et al., 2003); biliary excretion of these compounds was significantly decreased in Bcrp knockout mice. However, in rats, the biliary excretion of these compounds was impaired significantly in isolated perfused livers (IPLs) (Dietrich et al., 2001; Zamek-Gliszczynski et al., 2005, 2006a, 2008) and SCH (Abe et al., 2008) from TR− rats, implying that Mrp2 primarily was involved in the biliary excretion of these substrates. To date, the underlying mechanism(s) responsible for the apparent species difference in Bcrp-mediated biliary excretion of drugs/metabolites has not been elucidated.
We reported that Bcrp protein levels detected by BXP53 antibody were decreased markedly in TR− rat SCH (Abe et al., 2008), in contrast to previous data generated with BXP21 antibody suggesting that Bcrp protein levels were similar in livers from wild-type (WT) and TR− rats (Johnson et al., 2006). This novel finding supported the hypothesis that hepatic Bcrp protein levels and function are decreased in TR− rats, which may contribute to the apparent species differences regarding the primary role of Bcrp in the biliary excretion of some substrates in rats and mice. The present study was designed to characterize Bcrp expression and function, using nitrofurantoin and pitavastatin as substrates, in TR− rats. These studies suggest that the potential role of both Bcrp and Mrp2 should be considered when impaired biliary excretion of drug/metabolites in livers or SCH from TR− rats is interpreted, and also provide insight regarding the mechanism(s) of apparent species differences in Bcrp-mediated biliary excretion. Furthermore, differences in Bcrp expression in TR− versus EHBR rat livers were investigated to determine whether the roles of Mrp2 and Bcrp could be differentiated using these two transport-deficient rat models.
Materials and Methods
Chemicals.
Insulin/transferrin/selenium (ITS+) and Matrigel were purchased from BD Biosciences (San Jose, CA). Dexamethasone and nitrofurantoin were purchased from Sigma-Aldrich (St. Louis, MO). Pitavastatin was purchased from Toronto Research Chemicals Inc. (North York, ON, Canada).
Animals.
Male WT Wistar rats (275–330 g) were purchased from Charles River Laboratories, Inc. (Wilmington, MA). Male TR− Wistar rats (275–330 g) were bred in-house [University of North Carolina (UNC) TR−; breeding colony was obtained originally from Dr. Mary Vore (University of Kentucky, Lexington, KY)] unless otherwise specified or purchased from Harlan (Indianapolis, IN). Male Sprague-Dawley (SD) and Mrp2-deficient EHBR rats (200–300 g) were purchased from Japan SLC Inc. (Hamamatsu, Japan).
Isolation and Culture of Rat SCH.
Hepatocytes were isolated from WT or UNC TR− rats using a two-step collagenase perfusion method as described previously (LeCluyse et al., 1994; Liu et al., 1999b). Hepatocytes were seeded in six-well BioCoat plates (BD Biosciences) in Dulbecco's modified Eagle's medium containing 5% (v/v) fetal bovine serum, 1% (v/v) nonessential amino acids, 10 μM insulin, and 1 μM dexamethasone and cultured in a humidified incubator (95% O2 and 5% CO2) at 37°C. Approximately 24 h after seeding, cells were overlaid with Matrigel at a final concentration of 0.25 mg/ml in Dulbecco's modified Eagle's medium containing 0.1 μM dexamethasone and ITS+ premix; culture medium was replaced every 24 h.
RNAi in Rat SCH.
Adenoviral vector-mediated RNAi targeting Bcrp in rat SCH was performed as described previously (Yue et al., 2009). In brief, adenoviral vectors expressing short hairpin RNA targeting rat Bcrp at positions 288 to 306 (Ad-siBcrp) or a nontarget control (Ad-siNT) were packaged and infected into rat hepatocytes 2 h after seeding at a multiplicity of infection of 20; cells were overlaid and cultured in the same manner as noninfected cells described above.
Immunoblot.
Bcrp protein levels in rat SCH and membrane fractions of homogenized rat livers were determined by immunoblot as published previously (Johnson et al., 2006; Yue et al., 2009). In brief, hepatocytes were lysed in lysis buffer [1% SDS and 1 mM EDTA with protease inhibitor. Complete (Roche Diagnostics, Indianapolis, IN)]. Rat livers were homogenized in homogenate buffer [0.1 M Tris/HCl (pH 7.4), containing Complete] followed by centrifugation at 1500g for 10 min at 4°C. The supernatant was ultracentrifuged at 100,000g for 30 min; the pellet (containing membrane fraction) was sonicated, resuspended in homogenate buffer, and then mixed with lysis buffer (final concentration 1% SDS and 1 mM EDTA with Complete TM). Protein concentrations were determined by BCA assay (Pierce Chemical, Rockford, IL). Protein (50 μg) from cell lysates or rat liver membrane fractions was resolved on 4 to 20% NuPAGE gel and subjected to immunoblot using the Bcrp antibody BXP53 or BXP21 (Alexis Biochemicals, San Diego, CA). β-Actin was used as a loading control.
TaqMan Real-Time RT-PCR.
Total RNA from rat livers and SCH was extracted using the ABI RNA isolation system (Applied Biosystems, Foster City, CA). TaqMan real-time RT-PCR was conducted using an ABI Prism 7700 system (Applied Biosystems) to determine Bcrp mRNA levels as described previously (Kim et al., 2002). The TaqMan probe and primer sequences (5′-3′) used for rat Bcrp were CTGCTCGGGAATCCTCAAGCTTCTG (probe), TGGATTGCCAGGCGTTCATT (forward primer), and GTCCCAGTATGACTGTAACAA (reverse primer). The TaqMan probe and primer sequences used for rat β-actin were CACTAATCGGCAATGAGCGGTTCCG (probe), TGCCTGACGGTCAGGTCA (forward primer), and CAGGAAGGAAGGCTGGAAG (reverse primer). Bcrp expression relative to that of control groups was analyzed using the 2−ΔΔCT method with β-actin as an internal control (Livak and Schmittgen, 2001).
Accumulation Studies of Nitrofurantoin and Pitavastatin in Rat SCH.
Accumulation studies were performed in SCH to determine the in vitro biliary excretion of nitrofurantoin and pitavastatin, as described previously (Abe et al., 2008; Yue et al., 2009). In brief, rat SCH were rinsed twice with 2 ml of standard HBSS and preincubated in 2 ml of either Ca2+-free HBSS (to open the tight junctions and disrupt the canalicular networks) or standard HBSS for 10 min at 37°C. Subsequently, cells were incubated for 10 min at 37°C in 1.5 ml of standard HBSS containing nitrofurantoin (5 μM) or pitavastatin (5 μM). After washing 3 times with ice-cold standard HBSS, cells were lysed with 1 ml of methanol-water (70/30, v/v). Drug accumulation in cells plus bile canaliculi (rat SCH preincubated in standard HBSS) and cells (rat SCH preincubated in Ca2+-free HBSS) was determined by LC-MS/MS using the same methods as published previously (Abe et al., 2008; Yue et al., 2009). Accumulation, normalized to protein concentration, was corrected for nonspecific binding by including a blank plate (BioCoat plate overlaid with Matrigel, but without cells). Because of incompatibility of the protein assay with methanol, the average protein concentration for standard HBSS or Ca2+-free HBSS incubations in the same liver preparation was used to normalize the protein content.
LC-MS/MS Analysis of Nitrofurantoin and Pitavastatin.
Concentrations of nitrofurantoin and pitavastatin were determined by LC-MS/MS analysis using the same procedure as described previously (Abe et al., 2008; Yue et al., 2009). In brief, SCH, liver homogenate and perfusate plasma from recirculating IPLs were first deproteinized with methanol-water (70:30, v/v) followed by centrifugation at 4°C (12,000g) for 10 min. The supernatant (20 μl) from the above preparation, outflow perfusate from single-pass IPLs, or diluted bile samples were mixed with 100 μl of methanol and water (3.8:1) containing internal standard (ethyl warfarin for nitrofurantoin, or rosuvastatin for pitavastatin). Nitrofurantoin, pitavastatin, and internal standards were eluted from an Aquasil C18 column (50 × 2.1 mm; particle size 5 μm) (Thermo Fisher Scientific, Waltham, MA) using a mobile phase gradient as detailed previously (Abe et al., 2008; Yue et al., 2009). Analytes were quantified with standard curves (2–1000 nM for nitrofurantoin and 2–2000 nM for pitavastatin) prepared in the appropriate matrix; interday and intraday coefficients of variation were <15%.
IPL Studies.
Rats were maintained on a 12-h light/dark cycle with access to water and chow ad libitum. Rats were anesthetized with ketamine/xylazine (60:12 mg/kg i.p.) before surgical manipulation. The Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill approved all procedures.
For recirculating IPL studies, male WT Wistar rats (>400 g) were used as blood donors, and the experiments were performed according to standard procedures (Hoffmaster et al., 2004). In brief, livers were perfused in situ with oxygenated Krebs-Henseleit bicarbonate buffer after cannulation of the bile duct and portal vein. Livers were removed from the body cavity and placed into a humidified perfusion chamber at 37°C. Livers were perfused with 80 ml of oxygenated buffer containing 20% (v/v) heparinized rat blood at a flow rate of 20 ml/min. Taurocholate (0.5 μmol/min in saline) was infused to maintain bile flow. Livers were equilibrated for 15 min before bolus administration of 8 μmol of nitrofurantoin into the perfusate reservoir. Perfusate pH was maintained at ∼7.4. Livers demonstrating acceptable viability (portal pressure <15 cm of water; initial bile flow >0.8 and >0.2 μl per min/g liver in WT and TR− rat IPLs, respectively, gross morphology) were used in recirculating IPL studies. Perfusate was sampled at 5, 10, 15, 30, 45, 60, 75, and 90 min, and bile samples were collected in toto in 15-min intervals up to 90 min. Noncompartmental pharmacokinetic analysis was performed, and the area under the perfusate concentration-time curve (AUC) was calculated with WinNonlin 5.2 (Pharsight, Mountain View, CA). Biliary clearance (0–90 min) of substrate in IPLs was determined as the cumulative amount excreted in bile in 90 min divided by the perfusate AUC0–90 min.
The single-pass in situ liver perfusion procedure was similar to methods reported previously (Tian et al., 2008). In brief, the common bile duct, portal vein, and inferior vena cava above the liver were cannulated. The liver was perfused at 30 ml/min with continually oxygenated Krebs-Henseleit buffer containing 5 μM taurocholate for an equilibration period of approximately 15 min. Subsequently, the liver was perfused for 70 min with buffer containing 0.5 μM pitavastatin. Bile and outflow perfusate were collected in 10-min intervals in toto, and livers were isolated at the end of perfusion and stored at −80°C until analysis. Bile flow and gross morphology were used to assess liver viability.
Data Analysis.
Substrate accumulation (picomoles per milligram of protein), biliary excretion index (BEI) (percentage), and in vitro biliary clearance (Clbiliary) (milliliters per minute per kilogram) were calculated in SCH using B-CLEAR technology (Qualyst, Inc., Raleigh, NC) on the basis of the following equations (Liu et al., 1999a):
The area under the medium concentration versus time curve (AUCmedium) was determined as the product of the incubation time and the medium concentration of substrate. Medium concentrations at the beginning and end of the incubation did not differ by more than 10%. Thus, the concentration of drug in the medium was defined as the initial substrate concentration in the incubation medium. The in vitro Clbiliary (milliliters per minute per milligram of protein) was scaled to kilograms of body weight assuming the following: 200 mg protein/g of rat liver tissue and 40 g of rat liver tissue/kg b.wt. (Seglen, 1976).
Statistical Analysis.
Data are expressed as the mean ± S.D. Statistical significance was evaluated with a one-way analysis of variance or Student's t test using SigmaStat (SPSS Inc., Chicago, IL). In all cases, p < 0.05 was considered statistically significant.
Results
Antibody BXP53 but Not BXP21 Specifically Detected Rat Bcrp in SCH.
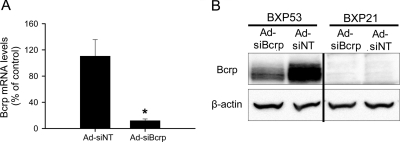
Bcrp can be knocked down specifically by adenoviral vector-mediated RNAi in rat SCH (Yue et al., 2009). Cell lysates from control (Ad-siNT-infected) or Bcrp knockdown (Ad-siBcrp-infected) day 4 rat SCH were used to determine the reactivity and specificity of BXP53 and BXP21 to rat Bcrp. mRNA levels of Bcrp in Ad-siBcrp-infected rat SCH were only ∼8% of control as determined by TaqMan real-time RT-PCR (Fig. 1A), indicating efficient knockdown of Bcrp mRNA. Consistent with the previous report (Yue et al., 2009), protein levels of Bcrp were knocked down markedly in Ad-siBcrp-infected rat SCH compared with those in control Ad-siNT infected cells, as determined by BXP53 antibody (Fig. 1B). However, BXP21 failed to specifically detect rat Bcrp.
Fig. 1.
Antibody BXP53 but not BXP21 specifically recognized rat Bcrp in rat SCH. A, Bcrp mRNA levels in Bcrp knockdown (Ad-siBcrp) and control (Ad-siNT) SCH. Two hours after seeding, rat hepatocytes were infected at multiplicity of infection of 20 with adenoviral vectors that expressed small interfering RNA (siRNA) targeting Bcrp (Ad-siBcrp) or nontarget control siRNA (Ad-siNT). Bcrp mRNA levels were determined by TaqMan real-time RT-PCR. Data are presented as the mean ± S.D.; n = 3 livers. *, p < 0.05, Ad-siBcrp versus Ad-siNT. B, immunoblot of Bcrp in Bcrp knockdown (Ad-siBcrp) and control (Ad-siNT) SCH using BXP53 or BXP21 antibodies. β-Actin was used as a loading control.
Bcrp Expression Was Decreased in Mrp2-Deficient TR− but Not in EHBR Rat Livers.
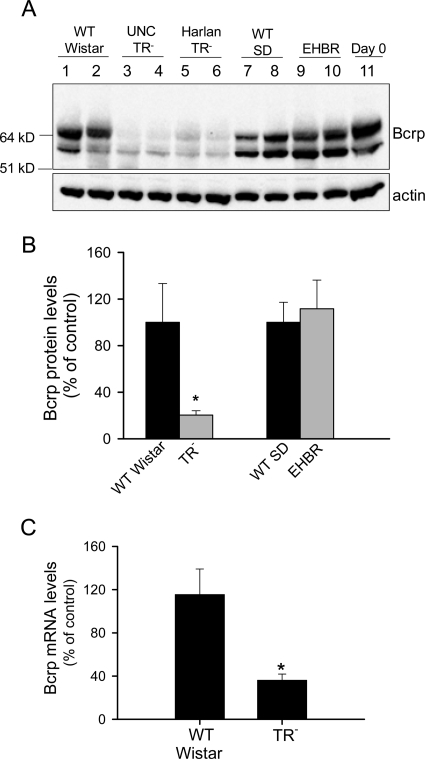
Protein levels of Bcrp in WT Wistar, TR−, WT SD, and EHBR rat livers were determined by immunoblot using BXP53 antibody. As shown in Fig. 2A, two different molecular weights of Bcrp were detected in all of the rat liver samples and day 0 freshly isolated WT rat hepatocytes. Based on a recent publication, the higher molecular weight Bcrp represents N-glycosylated Bcrp (Draheim et al., 2010). Total Bcrp protein levels were markedly decreased in livers of both UNC TR− rats and TR− rats purchased from Harlan, as determined by BXP53 antibody (Fig. 2A). Bcrp protein levels in TR− rat livers were decreased to ∼20% of WT control levels; however, Bcrp protein levels were not significantly different between WT SD and EHBR rat livers (Fig. 2B). BXP21 antibody failed to detect Bcrp expression in any of the liver samples and day 0 freshly isolated hepatocytes (data not shown). Bcrp mRNA levels in WT and TR− rat livers were compared by TaqMan real-time RT-PCR. As shown in Fig. 2C, mRNA levels of Bcrp were decreased significantly in livers from TR− rats compared with those of WT control rats. Overall, these results indicated that Bcrp expression is decreased markedly in Mrp2-deficient TR− rat livers but not in EHBR rat livers.
Fig. 2.
Decreased Bcrp protein levels in UNC TR− and Harlan TR− rat livers but not in EHBR rat livers. A, immunoblot of Bcrp in WT Wistar, UNC TR−, Harlan TR−, WT SD, and EHBR rat livers and freshly isolated WT rat hepatocytes (day 0) using BXP53 antibody (upper panel). β-Actin was used as a loading control (lower panel). Representative results of three to five rats per group are shown. B, relative Bcrp protein levels in TR− and EHBR rat livers compared with those in WT Wistar or WT SD controls, respectively. Protein levels of Bcrp and β-actin were determined by densitometry. Total Bcrp protein levels (sum of the higher and lower molecular weight Bcrp) were normalized to β-actin. Data are presented as mean ± S.D.; n = 3 (WT Wistar), n = 5 (TR−), n = 4 (WT SD), and n = 4 (EHBR) rat livers. *, p < 0.05, TR− or EHBR versus WT control. C, mRNA levels of Bcrp in WT Wistar (control) and TR− rat livers. Bcrp mRNA levels were determined by TaqMan real-time RT-PCR. β-Actin was used as an internal control. Data are presented as mean ± S.D.; n = 8 (WT Wistar) and n = 11 (TR−, including two Harlan TR−) rat livers. *, p < 0.05, TR− versus WT Wistar.
Bcrp Expression and Function Were Decreased in TR− Rat SCH.
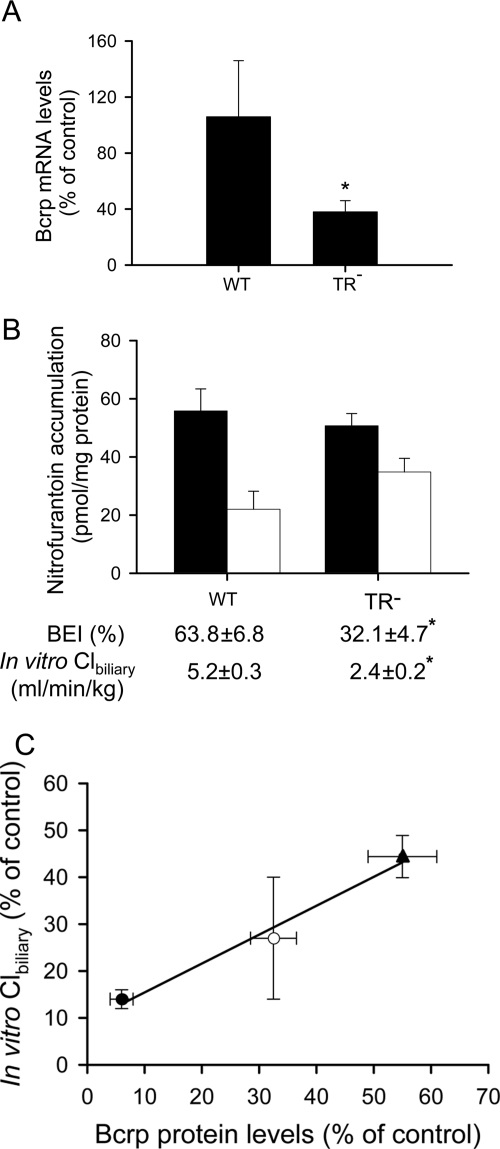
As shown in Fig. 3A, mRNA levels of Bcrp were decreased significantly in TR− SCH compared with those in WT control rats. The function of Bcrp in WT and TR− rat SCH was determined using nitrofurantoin as a probe substrate (Yue et al., 2009). As shown in Fig. 3B, nitrofurantoin accumulation (5 μM, 10 min) in cells plus bile was similar between day 4 WT and TR− rat SCH; however, the BEI and in vitro Clbiliary of nitrofurantoin in TR− rat SCH were decreased significantly to ∼50 and ∼46% of WT control values, respectively, indicating that Bcrp function was decreased in TR− rat SCH.
Fig. 3.
Bcrp expression, nitrofurantoin accumulation, BEI, and in vitro Clbiliary in rat sandwich-cultured hepatocytes. A, mRNA levels of Bcrp in WT (control) and TR− SCH. Bcrp mRNA levels in day 4 SCH were determined by TaqMan real-time RT-PCR. β-Actin was used as an internal control. Data are presented as the mean ± S.D.; n = 3 livers/group. *, p < 0.05, TR− versus WT. B, nitrofurantoin accumulation, BEI, and in vitro Clbiliary in cells plus bile (■) and cells (□) after a 10-min incubation with 5 μM nitrofurantoin in day 4 SCH from WT and UNC TR− rats. Data are presented as the mean ± S.E.; n = 4 livers in triplicate. *, p < 0.05, TR− versus WT. C, relationship between nitrofurantoin in vitro Clbiliary and Bcrp protein levels expressed as a percentage of control values. Nitrofurantoin in vitro Clbiliary in Ad-siBcrp-infected SCH from WT rats exhibiting extensive Bcrp knockdown (day 6; ●) and moderate Bcrp knockdown (day 4; ○), expressed as a percentage of control (Ad-siNT) values in day 6 and day 4 SCH, respectively, and in day 4 noninfected TR− SCH (▴), expressed as a percentage of control (WT) values in day 4 SCH, were plotted on the y-axis. The in vitro Clbiliary values were published previously in Fig. 3, B and C, of Yue et al. (2009) and have been replotted here for comparison. Bcrp protein levels (plotted on x-axis) were determined by immunoblot, followed by densitometry and normalized to β-actin, and drawn on the basis of the findings in Yue et al. (2009) and Abe et al. (2008). Data are presented as the mean ± S.D.
Nitrofurantoin in vitro Clbiliary, expressed as a percentage of control values in day 4 and day 6 Bcrp knockdown SCH, was calculated on the basis of data published previously (Fig. 3, B and C, in Yue et al., 2009). The Bcrp protein levels, expressed as a percentage of control, in TR− versus WT controls and in day 4 and day 6 Ad-siBcrp-infected versus Ad-siNT-infected SCH controls, were determined by immunoblot, followed by densitometry and normalized to β-actin. Representative Western blot results were published previously in Fig. 6 of Abe et al. (2008) and Fig. 2A of Yue et al. (2009). Of note, nitrofurantoin in vitro Clbiliary was positively correlated with Bcrp protein levels (R2 = 0.98) (Fig. 3C) in TR− rat SCH (46 ± 4 and 55 ± 6% of WT control, respectively; ▴), WT SCH with moderate Bcrp knockdown at day 4 in culture (27 ± 12 and 32 ± 4% of the Ad-siNT control, respectively; ○), and WT SCH with extensive Bcrp knockdown at day 6 in culture (14 ± 2 and 6.0 ± 2.1% of the Ad-siNT control, respectively; ●) (Yue et al., 2009).
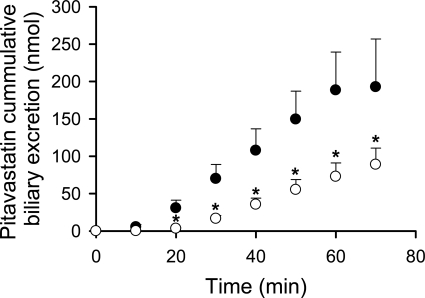
Fig. 6.
Decreased biliary excretion of pitavastatin in TR− rat in situ IPLs. Cumulative biliary excretion of pitavastatin in WT (●) and UNC TR− (○) rat in situ single-pass IPLs. Data are presented as mean ± S.D.; n = 3 livers/group; *p < 0.05, TR− versus WT.
Decreased Biliary Excretion of Nitrofurantoin in TR− Rat Livers.
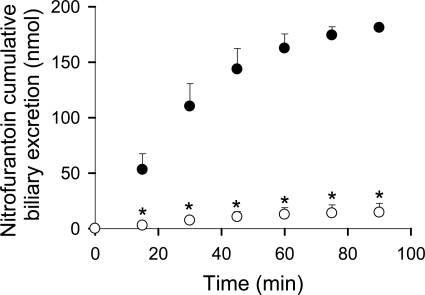
The cumulative biliary excretion (0–90 min) of nitrofurantoin in recirculating IPLs from UNC TR− rats was significantly lower than that in WT rats (15 ± 8 versus 180 ± 4 nmol) (Fig. 4). Nitrofurantoin Clbiliary was ∼8-fold lower in UNC TR− than in WT rat IPLs (0.36 ± 0.11 versus 3.2 ± 0.4 ml/h), although the perfusate AUC0–90 min of nitrofurantoin was not significantly different (data not shown).
Fig. 4.
Decreased nitrofurantoin biliary excretion in TR− rat IPLs. Cumulative biliary excretion of nitrofurantoin in recirculating WT (●) and UNC TR− (○) rat IPLs after bolus administration of 8 μmol of nitrofurantoin to the perfusate reservoir. Data are presented as the mean ± S.D.; n = 3 to 5 livers/group. *, p < 0.05, TR− versus WT.
Role of Bcrp in Pitavastatin Biliary Excretion in Rat SCH.
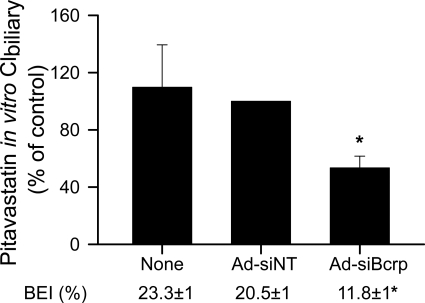
The BEI and in vitro Clbiliary of pitavastatin (5 μM) in day 6 WT Wistar rat SCH after a 10-min incubation were compared between noninfected (None), Ad-siNT-infected, and Ad-siBcrp-infected rat SCH. As shown in Fig. 5, in Ad-siBcrp-infected SCH, pitavastatin BEI (Fig. 5) and in vitro Clbiliary were significantly decreased to 58 ± 6 and 52 ± 4% of control (Ad-siNT), respectively, suggesting that Bcrp is involved in the biliary excretion of pitavastatin in rat SCH.
Fig. 5.
Role of Bcrp in pitavastatin biliary excretion in rat SCH. Pitavastatin BEI and in vitro Clbiliary (percentage of control) after a 10-min incubation with 5 μM pitavastatin were compared in noninfected, Ad-siNT-infected (control), and Ad-siBcrp-infected day 6 rat SCH. Data represent the mean ± S.D.; n = 3 livers. *, p < 0.05, Ad-siBcrp-infected versus Ad-siNT-infected. See Materials and Methods and the legend to Fig. 1A for details regarding adenoviral vector-mediated RNAi.
Decreased Biliary Excretion of Pitavastatin in TR− Rat IPLs.
Pitavastatin cumulative biliary excretion in single-pass in situ IPLs from UNC TR− rats was significantly lower than that in WT rat IPLs (Fig. 6); outflow perfusate concentrations were not statistically different between these two groups (data not shown). Of interest, the initial biliary excretion of pitavastatin was noticeably delayed in TR− compared with WT rat IPLs.
Discussion
The current studies demonstrate, for the first time, that expression and function of Bcrp is decreased significantly in the livers of Mrp2-deficient TR− rats. In previous studies, Bcrp protein levels were reported to be slightly, but not significantly, decreased in livers from UNC TR− compared with those from WT rats (Johnson et al., 2006) by immunoblot using BXP21 antibody. BXP21 antibody has been used to detect human BCRP expression by immunoblot (Merino et al., 2005) but has not been validated to detect rat Bcrp. Results of the present study clearly indicate that BXP53, but not BXP21, is a specific antibody for immunoblot of rat Bcrp (Fig. 1B). Immunoblot of Bcrp using BXP53 antibody demonstrated markedly decreased Bcrp protein levels in TR− rat livers compared with those in WT controls. Decreased hepatic Bcrp protein levels were not restricted to the UNC TR− colony; livers from commercially available TR− rats (Harlan) also exhibited lower Bcrp protein levels (Fig. 2A, top panel). BXP21 antibody could not specifically detect Bcrp in either SCH (Fig. 1B) or rat livers (data not shown); thus, Bcrp protein levels may not have been determined accurately in the previous study (Johnson et al., 2006). The decreased Bcrp protein levels in TR− rat livers may not be caused by Mrp2 deficiency because Bcrp protein levels are similar to WT control values in livers from EHBR, another Mrp2-deficient rat strain (Fig. 2, A and B). Likewise, Bcrp protein levels were similar in Abcc2(−/−) and wild-type mice (Chu et al., 2006; Nezasa et al., 2006).
To explore the mechanism(s) underlying the decreased Bcrp protein levels in TR− rat hepatocytes, the mRNA levels of Bcrp were compared in SCH and livers from WT and TR− rats. Bcrp mRNA levels were decreased significantly in livers (Fig. 2C) and SCH (Fig. 3A) from TR− rats compared with those from WT controls, suggesting that Bcrp expression in TR− rats was down-regulated at the mRNA level. To date, the promoter sequence of rat Bcrp has not been identified, and the transcriptional regulation of rat Bcrp expression has not been well characterized. Although it has been reported that Bcrp mRNA levels are higher in male rat kidney compared with those in female rat kidney (Lu and Klaassen, 2008), the mechanisms underlying such differences are not known. It has been reported that human BCRP mRNA levels can be up-regulated by some nuclear factors and 17β-estradiol (Ebert et al., 2005; Zhang et al., 2006b) and down-regulated by microRNA (Pan et al., 2009). A single nucleotide polymorphism in the BCRP promoter region was identified, although this single nucleotide polymorphism did not affect BCRP promoter activity (Lee et al., 2007). The different mRNA levels in TR− and WT rats may be due to different levels of Bcrp modulators in WT and TR− rat livers or to variation in the Abcg2 gene in TR− rats.
In addition to Bcrp expression, the function of hepatic Bcrp was compared in WT and TR− rats in vitro in SCH and ex vivo in IPLs, using nitrofurantoin as a probe substrate (Yue et al., 2009). Compared with WT controls, nitrofurantoin BEI and in vitro Clbiliary in SCH (Fig. 3B), as well as the cumulative biliary excretion in recirculating IPLs (Fig. 4), from TR− rats were decreased significantly, confirming decreased Bcrp function in TR− rats. Notably, there was a positive relationship (R2 = 0.99) between nitrofurantoin in vitro Clbiliary and Bcrp protein levels expressed as a percentage of control values (Fig. 3C). The fraction of nitrofurantoin biliary excretion mediated by transporter(s) other than Bcrp, as estimated from the y-axis intercept of Fig. 3C, was only ∼6%. These findings are consistent with a previous report that nitrofurantoin was not transported by P-glycoprotein and MRP2 (Merino et al., 2005) and further validate the utility of nitrofurantoin as a specific Bcrp substrate in rat SCH.
Our laboratory previously reported that in TR− SCH, which exhibited Mrp2-deficiency and decreased Bcrp protein levels, there was a ∼70% decrease in pitavastatin in vitro Clbiliary compared with that in WT Wistar controls (Abe et al., 2008). In Mrp2-deficient EHBR rats, which have Bcrp protein levels similar to those in WT SD controls (Fig. 2, A and B), the in vivo biliary excretion of pitavastatin was similar to that in WT SD control rats, implying that Mrp2 does not play a major role in pitavastatin biliary excretion (Hirano et al., 2005). To elucidate the role of Bcrp in pitavastatin biliary excretion, pitavastatin BEI and in vitro Clbiliary were compared in noninfected, Ad-siNT- and Ad-siBcrp-infected day 6 SCH, which exhibited efficient and specific Bcrp knockdown as published previously (Yue et al., 2009) and confirmed in the present studies. Infection with Ad-siNT and Ad-siBcrp did not affect pitavastatin accumulation in SCH (data not shown). However, in SCH exhibiting Bcrp knockdown (Ad-siBcrp), pitavastatin BEI and in vitro Clbiliary were decreased to ∼58 and ∼52% of control (Ad-siNT) values, respectively (Fig. 5), indicating that Bcrp does play a role, although moderate, in pitavastatin biliary excretion in rat SCH. Moreover, pitavastatin biliary excretion was assessed in situ in TR− and WT rat IPLs. As shown in Fig. 6, the cumulative biliary excretion of pitavastatin was significantly lower by more than 2-fold in perfused livers from TR− rats, consistent with decreased Bcrp function in TR− rat livers. In Abcg2(−/−) mice, pitavastatin biliary excretion was ∼10-fold lower than in controls (Hirano et al., 2005). Such species differences between mice and rats in the contribution of Bcrp to the biliary excretion of some compounds may be explained by the significantly higher amount of Bcrp protein in mice (∼1.21 fmol/mg protein) (Y. Lai, unpublished results) than in rats (∼0.28 fmol/mg protein) (Li et al., 2009), as determined by absolute quantification using LC-MS/MS.
In summary, the results of this study indicate that Bcrp expression and function are significantly decreased in Mrp2-deficient TR− rat livers. TR− rats have been used frequently to elucidate the role of Mrp2 in the biliary excretion of drugs, metabolites, endogenous compounds, and toxins. Decreased Bcrp protein levels were detected in both the UNC TR− colony and in commercially available Harlan TR− rats and may be generalized to TR− Wistar rats from other sources. Therefore, data obtained from TR− rats should be interpreted with caution; decreased biliary excretion in TR− rats may be attributed to the absence of Mrp2, decreased Bcrp, or both. The important role of Bcrp in the biliary excretion of many drugs/metabolites may have been underestimated previously because of the unrecognized fact that Bcrp protein levels and function in TR− rats are decreased. Specific Bcrp or Mrp2 knockdown in SCH is a useful in vitro tool to determine the contribution of individual transport proteins to the biliary excretion of drugs/metabolites (Tian et al., 2004; Yue et al., 2009), and may be used to distinguish the role of Bcrp and Mrp2 in the biliary excretion of compounds reported to undergo decreased biliary excretion in TR− rats. A novel finding of these studies is that unlike TR− rats, Mrp2-deficient EHBR rats exhibit normal Bcrp expression. Therefore, TR− and EHBR rats, in combination, could serve as a unique tool to differentiate the contribution of Mrp2 and Bcrp to the biliary excretion of drugs/metabolites in vivo.
Acknowledgments
We gratefully acknowledge Dr. Arlene S. Bridges for analytical support, Dr. Joseph Polli and Lindsey Webster for assistance with Bcrp real-time RT-PCR pilot experiments, Dr. Brandon Swift for expertise with in situ single-pass perfused livers, and Dr. Hiroyuki Kusuhara and Naoki Kotani for assistance with the EHBR rat livers.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM41935] (to K.L.R.B.); and a scholarship from Daiichi-Sankyo (to K.A.).
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.035188.
ABBREVIATIONS:
- ABC
- ATP-binding cassette
- BCRP/Bcrp
- breast cancer resistance protein
- Mrp/MRP
- multidrug resistance-associated protein
- EHBR
- Eisai hyperbilirubinemic rats
- RNAi
- RNA interference
- SCH
- sandwich-cultured hepatocytes
- IPL
- isolated perfused liver
- WT
- wild-type
- UNC
- University of North Carolina
- SD
- Sprague-Dawley
- RT
- reverse transcriptase
- PCR
- polymerase chain reaction
- HBSS
- Hanks' balanced salt solution
- LC
- liquid chromatography
- MS/MS
- tandem mass spectrometry
- AUC
- area under the perfusate concentration-time curve
- BEI
- biliary excretion index.
Authorship Contributions
Participated in research design: Yue, Lee, and Brouwer.
Conducted experiments: Yue, Lee, and Abe.
Contributed new reagents or analytic tools: Sugiyama.
Performed data analysis: Yue and Abe.
Wrote or contributed to the writing of the manuscript: Yue and Brouwer.
References
- Abe K, Bridges AS, Yue W, Brouwer KLR. (2008) In vitro biliary clearance of angiotensin II receptor blockers and 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors in sandwich-cultured rat hepatocytes: comparison with in vivo biliary clearance. J Pharmacol Exp Ther 326:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra P, Brouwer KLR. (2004) The complexities of hepatic drug transport: current knowledge and emerging concepts. Pharm Res 21:719–735 [DOI] [PubMed] [Google Scholar]
- Choudhuri S, Klaassen CD. (2006) Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int J Toxicol 25:231–259 [DOI] [PubMed] [Google Scholar]
- Chu XY, Strauss JR, Mariano MA, Li J, Newton DJ, Cai X, Wang RW, Yabut J, Hartley DP, Evans DC, et al. (2006) Characterization of mice lacking the multidrug resistance protein MRP2 (ABCC2). J Pharmacol Exp Ther 317:579–589 [DOI] [PubMed] [Google Scholar]
- Dietrich CG, de Waart DR, Ottenhoff R, Bootsma AH, van Gennip AH, Elferink RP. (2001) Mrp2-deficiency in the rat impairs biliary and intestinal excretion and influences metabolism and disposition of the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Carcinogenesis 22:805–811 [DOI] [PubMed] [Google Scholar]
- Draheim V, Reichel A, Weitschies W, Moenning U. (2010) N-Glycosylation of ABC transporters is associated with functional activity in sandwich-cultured rat hepatocytes. Eur J Pharm Sci 41:201–209 [DOI] [PubMed] [Google Scholar]
- Ebert B, Seidel A, Lampen A. (2005) Identification of BCRP as transporter of benzo[a]pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. Carcinogenesis 26:1754–1763 [DOI] [PubMed] [Google Scholar]
- Gavrilova O, Geyer J, Petzinger E. (2007) In vivo relevance of Mrp2-mediated biliary excretion of the Amanita mushroom toxin demethylphalloin. Biochim Biophys Acta 1768:2070–2077 [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Matsushima S, Nozaki Y, Kusuhara H, Sugiyama Y. (2005) Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol Pharmacol 68:800–807 [DOI] [PubMed] [Google Scholar]
- Hirohashi T, Suzuki H, Ito K, Ogawa K, Kume K, Shimizu T, Sugiyama Y. (1998) Hepatic expression of multidrug resistance-associated protein-like proteins maintained in Eisai hyperbilirubinemic rats. Mol Pharmacol 53:1068–1075 [PubMed] [Google Scholar]
- Hoffmaster KA, Zamek-Gliszczynski MJ, Pollack GM, Brouwer KLR. (2004) Hepatobiliary disposition of the metabolically stable opioid peptide [d-Pen2,d-Pen5]-enkephalin (DPDPE): pharmacokinetic consequences of the interplay between multiple transport systems. J Pharmacol Exp Ther 311:1203–1210 [DOI] [PubMed] [Google Scholar]
- Jansen PL, Peters WH, Lamers WH. (1985) Hereditary chronic conjugated hyperbilirubinemia in mutant rats caused by defective hepatic anion transport. Hepatology 5:573–579 [DOI] [PubMed] [Google Scholar]
- Johnson BM, Zhang P, Schuetz JD, Brouwer KLR. (2006) Characterization of transport protein expression in multidrug resistance-associated protein (Mrp) 2-deficient rats. Drug Metab Dispos 34:556–562 [DOI] [PubMed] [Google Scholar]
- Keitel V, Kartenbeck J, Nies AT, Spring H, Brom M, Keppler D. (2000) Impaired protein maturation of the conjugate export pump multidrug resistance protein 2 as a consequence of a deletion mutation in Dubin-Johnson syndrome. Hepatology 32:1317–1328 [DOI] [PubMed] [Google Scholar]
- Kim HS, Lee G, John SW, Maeda N, Smithies O. (2002) Molecular phenotyping for analyzing subtle genetic effects in mice: application to an angiotensinogen gene titration. Proc Natl Acad Sci USA 99:4602–4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCluyse EL, Audus KL, Hochman JH. (1994) Formation of extensive canalicular networks by rat hepatocytes cultured in collagen-sandwich configuration. Am J Physiol 266:C1764–C1774 [DOI] [PubMed] [Google Scholar]
- Lecureux L, Dieter MZ, Nelson DM, Watson L, Wong H, Gemzik B, Klaassen CD, Lehman-McKeeman LD. (2009) Hepatobiliary disposition of thyroid hormone in Mrp2-deficient TR− rats: reduced biliary excretion of thyroxine glucuronide does not prevent xenobiotic-induced hypothyroidism. Toxicol Sci 108:482–491 [DOI] [PubMed] [Google Scholar]
- Lee SS, Jeong HE, Yi JM, Jung HJ, Jang JE, Kim EY, Lee SJ, Shin JG. (2007) Identification and functional assessment of BCRP polymorphisms in a Korean population. Drug Metab Dispos 35:623–632 [DOI] [PubMed] [Google Scholar]
- Li N, Palandra J, Nemirovskiy OV, Lai Y. (2009) LC-MS/MS mediated absolute quantification and comparison of bile salt export pump and breast cancer resistance protein in livers and hepatocytes across species. Anal Chem 81:2251–2259 [DOI] [PubMed] [Google Scholar]
- Liu X, Chism JP, LeCluyse EL, Brouwer KR, Brouwer KLR. (1999a) Correlation of biliary excretion in sandwich-cultured rat hepatocytes and in vivo in rats. Drug Metab Dispos 27:637–644 [PubMed] [Google Scholar]
- Liu X, LeCluyse EL, Brouwer KR, Gan LS, Lemasters JJ, Stieger B, Meier PJ, Brouwer KLR. (1999b) Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. Am J Physiol 277:G12–G21 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Lu H, Klaassen C. (2008) Gender differences in mRNA expression of ATP-binding cassette efflux and bile acid transporters in kidney, liver, and intestine of 5/6 nephrectomized rats. Drug Metab Dispos 36:16–23 [DOI] [PubMed] [Google Scholar]
- Maier-Salamon A, Trauner G, Hiltscher R, Reznicek G, Kopp B, Thalhammer T, Jäger W. (2009) Hepatic metabolism and biliary excretion of valerenic acid in isolated perfused rat livers: role of Mrp2 (Abcc2). J Pharm Sci 98:3839–3849 [DOI] [PubMed] [Google Scholar]
- Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. (2005) The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol Pharmacol 67:1758–1764 [DOI] [PubMed] [Google Scholar]
- Nezasa K, Tian X, Zamek-Gliszczynski MJ, Patel NJ, Raub TJ, Brouwer KLR. (2006) Altered hepatobiliary disposition of 5 (and 6)-carboxy-2′, 7′-dichlorofluorescein in Abcg2 (Bcrp1) and Abcc2 (Mrp2) knockout mice. Drug Metab Dispos 34:718–723 [DOI] [PubMed] [Google Scholar]
- Pan YZ, Morris ME, Yu AM. (2009) MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol 75:1374–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RP. (1996) Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science 271:1126–1128 [DOI] [PubMed] [Google Scholar]
- Seglen PO. (1976) Preparation of isolated rat liver cells. Methods Cell Biol 13:29–83 [DOI] [PubMed] [Google Scholar]
- Sparreboom A, Gelderblom H, Marsh S, Ahluwalia R, Obach R, Principe P, Twelves C, Verweij J, McLeod HL. (2004) Diflomotecan pharmacokinetics in relation to ABCG2 421C>A genotype. Clin Pharmacol Ther 76:38–44 [DOI] [PubMed] [Google Scholar]
- Sparreboom A, Loos WJ, Burger H, Sissung TM, Verweij J, Figg WD, Nooter K, Gelderblom H. (2005) Effect of ABCG2 genotype on the oral bioavailability of topotecan. Cancer Biol Ther 4:650–658 [DOI] [PubMed] [Google Scholar]
- Tian X, Swift B, Zamek-Gliszczynski MJ, Belinsky MG, Kruh GD, Brouwer KLR. (2008) Impact of basolateral multidrug resistance-associated protein (Mrp) 3 and Mrp4 on the hepatobiliary disposition of fexofenadine in perfused mouse livers. Drug Metab Dispos 36:911–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Zamek-Gliszczynski MJ, Zhang P, Brouwer KLR. (2004) Modulation of multidrug resistance-associated protein 2 (Mrp2) and Mrp3 expression and function with small interfering RNA in sandwich-cultured rat hepatocytes. Mol Pharmacol 66:1004–1010 [DOI] [PubMed] [Google Scholar]
- van Herwaarden AE, Jonker JW, Wagenaar E, Brinkhuis RF, Schellens JH, Beijnen JH, Schinkel AH. (2003) The breast cancer resistance protein (Bcrp1/Abcg2) restricts exposure to the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res 63:6447–6452 [PubMed] [Google Scholar]
- Yamazaki M, Akiyama S, Ni'inuma K, Nishigaki R, Sugiyama Y. (1997) Biliary excretion of pravastatin in rats: contribution of the excretion pathway mediated by canalicular multispecific organic anion transporter. Drug Metab Dispos 25:1123–1129 [PubMed] [Google Scholar]
- Yue W, Abe K, Brouwer KLR. (2009) Knocking down breast cancer resistance protein (Bcrp) by adenoviral vector-mediated RNA interference (RNAi) in sandwich-cultured rat hepatocytes: a novel tool to assess the contribution of Bcrp to drug biliary excretion. Mol Pharm 6:134–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Hoffmaster KA, Humphreys JE, Tian X, Nezasa K, Brouwer KLR. (2006a) Differential involvement of Mrp2 (Abcc2) and Bcrp (Abcg2) in biliary excretion of 4-methylumbelliferyl glucuronide and sulfate in the rat. J Pharmacol Exp Ther 319:459–467 [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Hoffmaster KA, Nezasa K, Brouwer KLR. (2008) Apparent differences in mechanisms of harmol sulfate biliary excretion in mice and rats. Drug Metab Dispos 36:2156–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Hoffmaster KA, Tian X, Zhao R, Polli JW, Humphreys JE, Webster LO, Bridges AS, Kalvass JC, Brouwer KLR. (2005) Multiple mechanisms are involved in the biliary excretion of acetaminophen sulfate in the rat: role of Mrp2 and Bcrp1. Drug Metab Dispos 33:1158–1165 [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Nezasa K, Tian X, Kalvass JC, Patel NJ, Raub TJ, Brouwer KLR. (2006b) The important role of Bcrp (Abcg2) in the biliary excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in mice. Mol Pharmacol 70:2127–2133 [DOI] [PubMed] [Google Scholar]
- Zhang W, Yu BN, He YJ, Fan L, Li Q, Liu ZQ, Wang A, Liu YL, Tan ZR, Fen-Jiang, et al. (2006a) Role of BCRP 421C>A polymorphism on rosuvastatin pharmacokinetics in healthy Chinese males. Clin Chim Acta 373:99–103 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhou G, Wang H, Zhang X, Wei F, Cai Y, Yin D. (2006b) Transcriptional upregulation of breast cancer resistance protein by 17β-estradiol in ERα-positive MCF-7 breast cancer cells. Oncology 71:446–455 [DOI] [PubMed] [Google Scholar]