Abstract
The heterogeneity of schizophrenia remains an obstacle for understanding its pathophysiology. Studies using a tone discrimination screening test to classify patients have found evidence for two subgroups having either a specific deficit in verbal working memory (WM) or deficits on both verbal and nonverbal memory tests. This study aimed to: (1) replicate in larger samples differences between these subgroups on the word serial position test (WSPT); (2) further evaluate their performance on additional tests of verbal WM, explicit memory, and sustained attention; (3) determine the relation of verbal WM deficits to auditory hallucinations and other symptoms; and (4) examine medication effects. WSPT of verbal WM and tone discrimination performance did not differ between medicated (n=45) and unmedicated (n=38) patients. Patients with schizophrenia who passed the auditory screening test (discriminators, n=60) were compared to those who did not (nondiscriminators, n=23), and healthy controls (n=47). The discriminator subgroup showed poorer verbal WM than controls and a deficit in verbal but not visual memory on Wechsler Memory Scale-Revised, whereas the nondiscriminator subgroup showed overall poorer performance on both verbal and nonverbal tests and a marked deficit in sustained attention. Verbal WM deficits in discriminators on WSPT were correlated with auditory hallucinations but not with negative symptoms. The results are consistent with a verbal memory deficit in a subgroup of schizophrenia having intact auditory perception, which may stem from dysfunction of language-related cortical regions, and a more generalized cognitive deficit in a subgroup having auditory perceptual and attentional dysfunction.
Keywords: schizophrenia, working memory, auditory perception, attention, hallucinations
The clinical and neurocognitive heterogeneity of schizophrenia remains an obstacle to understanding its pathophysiology. Numerous studies have demonstrated deficits in working memory (WM) in patients with schizophrenia using visual (Barch, Csernansky, Conturo, & Snyder, 2002; Callicott et al., 2000; Carter et al., 1998; Park & Holzman, 1992; Perlstein, Carter, Noll, & Cohen, 2001) and auditory tasks (Gold, Carpenter, Randolph, Goldberg, & Weinberger, 1997; Menon, Anagnoson, Mathalon, Glover, & Pfefferbaum, 2001; Wexler, Stevens, Bowers, Sernyak, & Goldman-Rakic, 1998), but few have addressed the issue of individual differences among patients in the nature of their deficits. It has been suggested that subgroups of patients in the general diagnostic category of schizophrenia are marked by differences in their cognitive deficits, and that such “cognitive subtypes” may be more homogeneous in clinical and pathobiological characteristics (Egan et al., 2001).
In a study of auditory WM, Wexler et al. (1998) reasoned that it is important to distinguish between patients who might perform poorly because they could not adequately attend to or perceive the auditory stimuli and those who have intact attention and perception. They divided patients having schizophrenia into two subgroups based on their performance on a tone discrimination test requiring auditory perception and attention. Patients who performed normally on the tone discrimination test, discriminators (D), showed normal performance on a nonverbal WM test, i.e., tone serial position test, but showed a deficit on a parallel verbal WM test, i.e., word serial position test (WSPT), which involves storage and rehearsal of phonological and sequential information over a delay period. In contrast, patients who performed poorly on the tone discrimination test, i.e., non-discriminators (ND), had marked deficits on both word and tone WM tests. Wexler et al. suggested that the global performance deficit in this group may stem from a perceptual or encoding dysfunction early in the auditory processing sequence. Bruder, Wexler, Sage, Gil, & Gorman (2004) confirmed the difference in WSPT performance between the D and ND subgroups and found that the verbal memory deficit in D patients extended to learning and recall of verbal material on the Wechsler Memory Scale-Revised (WMS-R). In contrast to D patients, who showed poorer verbal than visual memory scores on the WMS-R, ND patients showed poor performance on both verbal and visual indexes. Although the D and ND subgroups did not differ in severity of positive symptoms, ND patients had greater negative symptoms than D patients on the Positive and Negative Symptom Scale (PANSS).
Impairments on neuropsychological tests of cognitive function generally have only small to moderate correlations with severity of negative symptoms (Harvey, Koren, Reichenberg, & Bowie, 2006). Deficits in visuospatial WM have been consistently found to be related to negative symptoms of schizophrenia (Carter, Robertson, Nordahl, Chaderjian, & Oshora-Celaya, 1996; Gooding & Tallent, 2002; Park, Puschel, Sauter, Rentsch, & Hell, 1999). There is, however, less agreement on the relationship between auditory verbal WM and symptom features. Thus, performance of patients with schizophrenia on the letter-number sequencing test was negatively correlated with scores on the Scale for the Assessment of Negative Symptoms (SANS) after controlling for the influence of WAIS-R vocabulary or sustained attention (Perry et al., 2001), but in another study, letter-number performance was not associated with negative symptoms on the PANSS (Donohoe, Corvin & Robertson, 2006). Stevens et al. (2000) found that, among patients with schizophrenia who performed normally on the tone discrimination test (i.e., D patients), poorer performance on the WSPT was significantly associated with severity of positive but not negative symptoms on the PANSS. Neuroimaging studies also indicate that severity of positive symptoms of schizophrenia, in particular auditory hallucinations or delusions, is associated with activation of language-related regions during verbal WM tasks (Wible et al., 2009; Hashimoto, Lee, Preus, McCarley & Wible, 2010). These conflicting findings concerning the relationship between verbal WM deficits and symptoms of schizophrenia could arise from a failure to take general cognitive impairment of patients into account (Donohoe et al., 2006) or to a problem with the symptom measures, which may be particularly true for negative symptoms.
The present study aimed to replicate in larger samples the difference in auditory verbal WM between D and ND subgroups on the WSPT (Bruder et al., 2004; Wexler et al., 1998), and to examine medication effects by comparing the tone discrimination and WSPT performance of patients on antipsychotics versus off antipsychotics. To further evaluate the material specificity of memory deficits in D and ND subgroups, we again compared their performance on verbal and non-verbal indexes on WMS-R. Moreover, patients were tested on the Letter-Number Sequencing test (Gold et al., 1997) and the Continuous Performance Test-Identical Pairs (CPT-IP; Cornblatt & Keilp, 1994), so as to further assess the difference between D and ND subgroups in verbal WM and sustained attention. We also aimed to replicate our finding of greater negative symptoms in ND than D patients, and to examine the relation of verbal WM deficits on the WSPT to positive and negative symptoms of patients in these subgroups. Based on the findings of Wible et al. (2009), suggesting that auditory hallucinations may interfere with verbal WM processing, we hypothesized that D patients having auditory hallucinations would show greater deficits on the WSPT when compared to those without hallucinations.
Methods
Participants
Seventy-four inpatients on a research unit at the New York State Psychiatric Institute and 30 outpatients at the Lieber Center for Schizophrenia Research, an outpatient facility associated with this unit, were recruited for the study. Four patients were excluded because of comorbid medical, neurological or substance abuse problems, and 6 patients were excluded because they had a hearing loss or did not complete the tone discrimination test (see below). An additional 11 patients were excluded because they did not meet the criteria for schizophrenia or schizoaffective disorder. The remaining 83 patients (49 male, 34 female) met DSM-IV (American Psychiatric Association, 2000) criteria for schizophrenia (n = 60) or schizoaffective disorder (bipolar type, n = 14; depressive type, n = 9).1 Most patients (n=70) received a semistructured interview by a trained and reliable rater using the Diagnostic Interview for Genetic Studies (DIGS; Nurnberger et al., 1994), which was developed in the NIMH Genetics Initiative collaboration. It combines items from commonly used research instruments, including clinical rating scales (e.g., SANS and SAPS, Andreasen, 1983 and Andreasen, 1984), the Schedule for Affective Disorders and Schizophrenia (SADS; Endicott & Spitzer, 1978), and the Structured Clinical Interview for DSM-III-R and IV (SCID; Spitzer, Williams, Gibbon & First, 1990 and First, Spitzer, Gibbon & Williams, 2002). The DIGS has undergone extensive reliability testing with good results. DSM-IV research diagnoses for the 70 patients interviewed with the DIGS were made by a consensus of at least two doctoral level research-clinicians (M.D. or Ph.D.) and the clinical research interviewer during regular consensus conferences. The DSM-IV diagnoses of the remaining 13 patients were made by psychiatrists on the research units.2 Symptom ratings were also obtained using the PANSS (Kay, Opler, & Fishbein, 1999). Masters level raters were required to achieve adequately high inter-rater reliability with each other (interclass correlations of greater than 0.85 for symptom ratings). A total Brief Psychiatric Rating Scale (BPRS; Overall, 1974) score was derived from the 18 PANSS items that match those in the BPRS. When tested, 45 patients were receiving risperidone (n = 12), aripriprazole (n = 11), ziprasidone (n = 11), olanzapine (n = 4), quetiapine (n = 4), or clozapine (n = 3). The remaining 38 patients did not receive antipsychotic medications for about two or more weeks before testing.
A control group consisted of 52 healthy adults (23 male, 29 female) who were recruited from the New York metropolitan area and paid $15 per hour for participation. Outpatients were also paid $15 per hour, whereas inpatients received treatment on the research unit but were not paid for their participation.3 Controls were interviewed using the Structured Clinical Interview for DSM-IV Axis I Disorders, non-patient edition (First, Spitzer, Gibbon, & Williams, 1996) to exclude those with current or past psychopathology. Both patients and controls were excluded if they had a history of neurologic insult or illness. Patients were excluded for current substance abuse or past substance dependence sufficient to obscure the diagnosis of schizophrenia, and controls were excluded for past or current substance abuse or dependence. Audiograms were administered to all participants, and they were excluded if the average hearing loss at 500, 1000 and 2000 Hz was greater than 30 dB in either ear or differed by 10 dB or more between ears. After a description of the study to participants, written informed consent was obtained before initiating testing following procedures approved by the Columbia University Institutional Review Board.
Tone discrimination screening test
The tone discrimination test (Wexler, Donegan, Stevens, & Jacob, 2002) was presented over headphones using a laptop running Psyscope 1.2.5 (Cohen, MacWhinney, Flatt, & Provost, 1993). In this test, participants indicated whether two 300 ms pure tones separated by a 100 ms interval were the same or different in pitch by pressing the “s” or “d” key on the laptop. The tone frequencies ranged from 325 to 1994 Hz; when the tones in a pair were different, the frequency ratios were .67, .75, .85, .90 or .95. After 10 practice trials, 60 test trials were presented. The test trials consisted of 30 trials in which the tones in a pair were the same pitch (tone ratio= 1.0) and 30 trials in which the tones were different, with each of the five frequency ratios occurring once per block of 10 trials. Trial types were randomly distributed within each block.
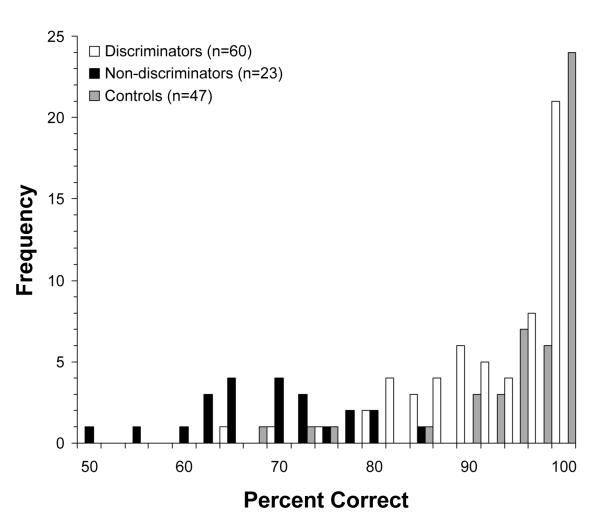
As in our prior studies (Wexler et al., 1998; Bruder et al., 2004), patients were separated into D (n = 60) and ND (n = 23) subgroups based on their performance on this test. Patients were considered to be D if they made at most one error in 12 trials at the two easiest tone discrimination ratios (.67 and .75). The patients who did not pass this screening criterion were considered to be ND. Forty-seven of the 52 controls met this criterion and only these participants were included in the control group. The original rationale given by Wexler et al. (1998) for using this criterion was to identify patients (D) who have perceptual/attentional competence in making simple tone discriminations (i.e., who like most healthy controls perform close to 100% correct at the easiest tone ratios) as opposed to patients (ND) who fail to discriminate tones with clear pitch differences and may therefore have a basic deficit in auditory perception or attention. As evident in Figure 1, the total percent correct scores (including tone ratios of .67, .75, .85, .90, .95, and 1.00) for participants in the current study show a bimodal negatively skewed distribution with two prominent maxima. The distribution of scores for D patients closely resembles that for healthy controls, with most having high accuracy levels at or above 90% correct. In contrast, ND patients showed markedly poorer performance with a mean score (69.5%, SD= 8.4) more than two SDs below the mean for D patients (M= 93.4%, SD=7.9). This indicates that our screening criterion was successful in yielding two distinct subgroups of patients, one that performs as well as controls in tone discrimination and one that shows a marked deficit. Furthermore, a cluster analysis of the tone discrimination scores for patients and controls (including accuracy scores for tone ratios of .67, .75, .85, .90, .95, and 1.00 as variables) yielded two clusters that show a close correspondence to the D and ND subgroups. Thus, 88.3% of the D patients fell in one cluster with high accuracy scores, whereas 91.3% of the ND patients fell in the second cluster with low accuracy scores. This further supports the use of our original screening criterion to define the D and ND subgroups.
Figure 1.
Distribution of percent correct on the tone discrimination test for discriminators, nondiscriminators, and controls. Lower and upper limits for percent correct intervals: 50 ≤ X <52.5, 52.5 ≤ X <55, 55 ≤ X <57.5, 57.5 ≤ X <60, 60 ≤ X <62.5, etc…., X=100.
Word Serial Position Test (WSPT)
The WSPT (Wexler et al., 1998) was presented using the same equipment as the tone discrimination task. Each trial began with four nouns spoken in a male voice, with one second between words. One of these words was then repeated after a delay of 9 seconds. Participants were instructed to remember the 4 words in the order presented and to indicate the position of the repeated word by pressing the “1”, “2”, “3” or “4” key on the laptop. The WSPT consisted of 36 trials, randomly ordered and balanced with regard to the 4 serial positions. No word appeared twice in the test.
Neuropsychological tests
Most patients (n= 78) and controls (n=51) were also tested on the Letter-Number Sequencing Test from the Wechsler Adult Intelligence Scale – 3rd Edition (WAIS-III; Tulsky et al., 1997; Wechsler, 1997) and the CPT-IP test (Cornblatt & Keilp, 1994). The Letter-Number WM test consists of auditory presentation of strings of intermingled letters and numbers and participants are asked to store and reorder the numbers and letters (recite in numeric and alphabetical order). The dependent measure is the total number of correct strings. Sustained attention was assessed with the four-digit fast condition of the CPT-IP test (Cornblatt et al., 1988). Number strings were presented on a Macintosh laptop screen for 50 ms at a constant rate of 1 per second. Subjects responded with a key press if the number string matched the string that had preceded it (same digits in same order). A total of 150 stimuli were presented: 28 target trials, 25 catch trials, and 97 random trials. Performance on the CPT-IP was measured by: (1) correct detections or hits (responses to target trials); (2) false alarms (responses to catch trials); and (3) d’ index of sensitivity computed from hits and false alarms using a signal detection computer program (Cornblatt et al., 1988). Verbal and performance IQ scores on WAIS-III (Wechsler, 1997) were also obtained for 47 patients, and WMS-R indices of verbal and visual memory (Wechsler, 1987) were obtained for 50 patients as part of other ongoing research at the Schizophrenia Research Unit.
Statistical analyses
Comparison of age, education, and handedness (Edinburgh Inventory laterality quotient; Oldfield, 1971) between patient and control groups was performed using a one-way ANOVA followed by Student Newman–Keuls (SNK) pairwise comparisons. Gender was compared between groups with a chi-square test. The influence of medication on the percentage of correct responses in the tone discrimination test was first analyzed using a Group (Unmedicated Patients, Medicated Patients, Controls) by Gender (Male, Female) by Ratio of Tones (.67, .75, .85, .90, .95, 1.00) repeated-measures ANOVA. Similarly, accuracy scores on the WSPT were submitted to a Group (Unmedicated Patients, Medicated Patients, Controls) by Gender (Male, Female) by Serial Position (1,2,3,4) repeated measures ANOVA. Accuracy scores on the WSPT were also submitted to a Group (D, ND, Controls) by Gender (Male, Female) by Serial Position (1,2,3,4) repeated measures ANOVA followed by SNK comparisons. Performance on the Letter-Number Sequencing and CPT-IP tests was analyzed using a Group (D, ND, Controls) by Gender (Male, Female) ANOVA. Main effects of Group were followed by SNK multiple comparisons, and significant interactions were followed by simple effects analyses and pairwise contrasts (BMDP-4V; Dixon, 1992). Greenhouse–Geisser epsilon (ε) correction was used to compensate for violations of sphericity when appropriate (e.g., Keselman, 1998). Eta-squared (η2) and Cohen’s d measures of effect size are also presented. A conventional significance level (p<.05) was applied for all effects.
To examine the impact of auditory hallucinations in patients having a verbal WM deficit, patients in the D subgroup were separated into those who reported experiencing auditory hallucinations in the past week (rating of one or higher on auditory hallucination item of SAPS; n = 18) and those who did not report auditory hallucinations (n = 33). ANOVAs comparing hallucinators, nonhallucinators, and controls were performed using the same statistical analyses as for the D, ND, and control groups.
The relationships between the total accuracy score on the WSPT and age or education were measured with Pearson correlations. Correlations also examined relationships between the total WSPT scores and ratings of auditory hallucinations on the SAPS, which were available for 51 D patients and 19 ND patients. These correlations with WSPT were also performed for positive and negative symptom total scores on the PANSS, available for 53 D patients and 19 ND patients. Correlations also examined relationships between WSPT accuracy and performance on the tone discrimination, Letter-Number Sequencing, CPT-IP, and WMS-R tests.
Results
Unmedicated Patients, Medicated Patients and Healthy Controls
A comparison was made of tone discrimination and WSPT performance for 38 unmedicated patients (24 males), 45 medicated patients (25 males), and 47 healthy controls (23 males). There was no difference between medicated patients and unmedicated patients in age, education or handedness, but both patient groups were somewhat less educated than controls (Table 1). Unmedicated patients were somewhat older than controls, but medicated patients were not significantly different in age from either group. Performance on the WSPT was not significantly correlated with age, r(47)= −0.10, ns, or education, r(47)= 0.24, ns, in controls and only weakly correlated with age, r(83)= −.27, p<.05, and education, r(83)= .22, p<.05 in patients.
Table 1.
Mean Percent Correct for Unmedicated Patients, Medicated Patients and Healthy Controls in Tone Discrimination Test and WSPT
| Variable | Unmedicated (n=38, 24 male) |
Medicated (n=45, 25 male) |
Controls (n=47, 23 male) |
Statistic | |||
|---|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | Mean | S.D. | ||
| Age (years) | 31.4a | 10.9 | 29.9a, b | 7.4 | 26.6b | 6.6 | F(2, 127)=3.70* |
| Education (years) |
13.9a | 2.3 | 14.3a | 2.7 | 16.3b | 2.0 | F(2, 127)=12.31*** |
| Handedness (LQ) |
65.2 | 41.5 | 74.9 | 33.4 | 77.2 | 19.25 | F(2, 127)=1.61 |
| Tone Ratio | |||||||
| .67 | 88.16 | 17.72 | 89.26 | 16.73 | 98.23 | 5.19 | |
| .75 | 89.04 | 19.48 | 89.63 | 16.78 | 99.29 | 3.40 | |
| .85 | 84.65 | 23.69 | 81.85 | 26.31 | 94.68 | 13.51 | |
| .90 | 69.30 | 36.25 | 78.89 | 25.48 | 91.13 | 18.99 | |
| .95 | 72.37 | 32.25 | 71.85 | 36.21 | 89.36 | 22.64 | |
| Total Correct | 83.27a | 19.72 | 83.32a | 17.44 | 95.09b | 9.25 | F(2, 124)=7.90 ** |
| WSPT Position | |||||||
| 1 | 81.14 | 19.44 | 81.85 | 22.70 | 95.04 | 9.14 | |
| 2 | 73.25 | 24.67 | 72.22 | 24.62 | 95.04 | 10.94 | |
| 3 | 65.79 | 27.11 | 68.89 | 29.22 | 87.59 | 15.72 | |
| 4 | 89.47 | 19.15 | 83.33 | 18.12 | 98.58 | 5.85 | |
| Total Correct | 77.41a | 16.40 | 76.57a | 18.15 | 94.06b | 6.77 | F(2, 124)=20.47 *** |
Note: WSPT = Word Serial Position Test, LQ = laterality quotient on Edinburgh Inventory. Means with different superscripts differ significantly at p < .05 using Student Newman -Keuls post hoc comparisons.
p<.05
p<.01
p<.001
The ANOVA of the tone discrimination performance of unmedicated patients, medicated patients, and controls revealed significant main effects of Group, F(2,124) = 7.90, p=.001, η2= .11 and Tone Ratio, F(5, 620) = 23.92 p<.001, ε = 0.515, η2= .16. As can be seen in Table 1, the larger the difference in pitch between tone pairs, where a tone ratio of .95 is the smallest and .67 is the largest difference, the greater the accuracy of tone discrimination. Also, healthy controls performed more accurately than the patient groups on the tone discrimination test (p<.05), but there was no difference between unmedicated and medicated patients. The ANOVA of WSPT performance revealed main effects of Group, F(2,124) = 20.47, p<.001, η2= .25 and Serial Position, F(3,372) = 23.78, p<.001, ε=0.89, η2=.16. The controls showed overall greater accuracy than the patient groups on the WSPT (p<.05). Most importantly, there was no difference in WSPT performance between unmedicated and medicated patients, which indicates that there was no evidence of medication effects on this verbal WM test. Neither the gender main effect nor any interaction involving gender was significant for either the WSPT or tone discrimination test.
Discriminators, Nondiscriminators and Healthy Controls
Table 2 gives the demographic variables for the D, ND, and control groups. There was no difference in gender, age, or education between the D and ND groups, but these groups were somewhat older and less educated than controls. There was no difference among groups in handedness. An approximately equal percentage of D patients (46.7%) and ND patients (43.5%) were off antipsychotic medication when tested and the remainder of the patients in each group were receiving atypical antipsychotics. The D and ND patients did not differ in age of onset or illness duration. There was no difference between the patient groups in overall symptom severity as indexed by total BPRS scores.
Table 2.
Demographic, Clinical and Neuropsychological Variables
| Variable | Discriminators (n=60, 35 Male) |
Non discriminators (n = 23, 14 male) |
Controls (n = 47, 23 male) |
Statistic | |||
|---|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | Mean | S.D. | ||
| Age (years) | 30.6 | 8.8 | 30.6 | 10.3 | 26.6 | 6.5 | F(2, 127)=3.33* |
| Education (years) | 14.4a | 2.5 | 13.5a | 2.5 | 16.3b | 2.1 | F(2, 127)=13.47*** |
| Handedness (LQ) | 69.9 | 39.1 | 71.8 | 33.3 | 73.7 | 28.7 | F(2, 127)=0.69 |
| Onset Age (years) | 22.1 | 6.8 | 22.1 | 6.7 | t(81)=0.03 | ||
| Illness Duration (years) |
8.5 | 8.2 | 8.5 | 9.6 | t(81)= 0.01 | ||
| Total BPRS | 35.9 | 13.3 | 35.6 | 10.8 | t(70)=0.08 | ||
| PANSS Positive | 15.0 | 7.1 | 14.2 | 5.7 | t(70)=0.43 | ||
| PANSS Negative | 14.3 | 6.0 | 16.6 | 5.9 | t(70)=1.44 | ||
| Letter Number Sequencing (Number Correct) |
10.6a | 3.0 | 8.3b | 2.9 | 12.5c | 2.8 | F(2, 122)=15.44*** |
| CPT -IP (d’) | 1.75a | .99 | .98b | .66 | 2.59c | .81 | F(2, 122)=25.60*** |
Note: LQ = laterality quotient on Edinburgh Inventory; BPRS = Brief Psychiatric Rating Scale; PANSS = Positive and Negative Syndrome Scale; CPT-IP =Continuous Performance Test-Identical Pairs. BPRS, PANSS: n =53 D group, n = 19 ND group. Letter-Number, CPT-IP: n = 59 D group, n = 22 ND group. Means with different superscripts differ significantly at p < .05 using Student Newman-Keuls post hoc comparisons.
p<.05
p<.01
p<.001
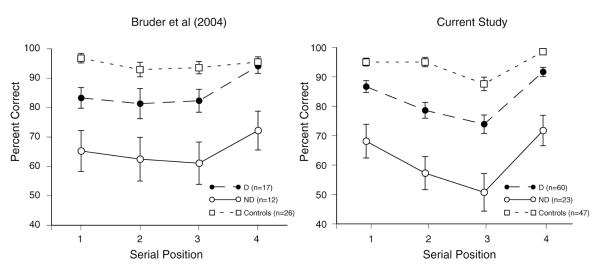
The ANOVA of WSPT performance of D, ND, and controls revealed significant main effects of Group, F(2,124) = 53.20, p<.001, η2= .46, and Serial Position, F(3, 372) = 20.61, p<.001, ε=0.893, η2= .14, but no significant Group by Serial Position interaction.4 Multiple comparisons indicated that ND patients had overall poorer accuracy than D patients (Cohen’s d= 1.44) and controls (d= 2.73) on WSPT (p<.05), and D patients also had significantly poorer accuracy than controls (d= 1.08, p<.05). Neither the Gender main effect nor any interaction involving Gender was significant. Figure 2 shows the mean accuracy for each group at the four serial positions on the WSPT in both the current study and our prior study (Bruder et al., 2004). ND patients performed considerably worse than both D patients and controls at all serial positions. D patients showed smaller deficits compared to controls for words in positions 1 to 3, and their accuracy for the 4th word in the sequence (92% correct) approached that for controls. WSPT performance was not significantly correlated with tone discrimination accuracy in controls, r(47)= .22, ns, D patients, r (60)= −.01, ns, or ND patients, r (23)= −.29, ns.
Figure 2.
Mean percentage of correct responses for discriminators, nondiscriminators, and controls as a function of serial position of words on the WSPT (error bars= standard errors of mean) for Bruder et al. (2004) and current study.
Ratings of symptom severity were available for 53 D patients and 19 ND patients on the PANSS. There was a trend for ND patients to have the expected higher negative symptoms when compared to D patients (Table 2), but there was no significant difference between these groups in either the PANSS positive or negative symptom total scores..
Auditory Hallucinators, Nonhallucinators and controls
To examine whether the verbal WM deficit in D patients is greater for those who are prone to auditory hallucinations, we compared the WSPT performance for 18 D patients (10 males) who reported experiencing auditory hallucinations in past week (rating of one or higher on auditory hallucination item of SAPS), 33 Dsz patients (18 males) without auditory hallucinations, and the 47 healthy controls (23 males). There was no significant difference between hallucinators and nonhallucinators in gender, age, education or handedness, but hallucinators (M= 14.4 years, SD= 2.4) and nonhallucinators (M= 14.5 years, SD= 2.5) were somewhat less educated than controls (M=16.3 years, SD= 2.0), F(2,97)= 7.70, p=.001. As expected, there was also no difference in tone discrimination performance among D patients having hallucinations, D patients without hallucinations, and controls.
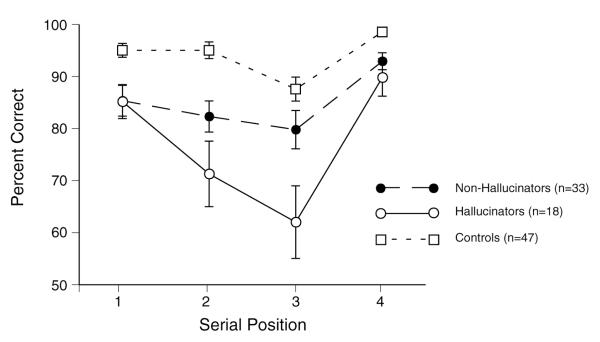
An ANOVA revealed significant main effects of Group, F(2,92) = 19.01, p<.001, η2= .29 and Serial Position, F(3,276) = 24.36, p<.001, ε=0.89, η2= .21, and a Group by Serial Position interaction, F(6,276) = 3.18, p<.01, ε=0.89, η2= .06. Although analysis of simple effects yielded significant group differences at each serial position, group differences at the 2nd and 3rd serial positions, F(2,92) ≥ 9.84, p<.001, were more marked than those at the 1st and 4th serial positions, F(2,92) ≥ 6.59, p<.01 (see Figure 3). Pairwise comparisons indicated that hallucinators had significantly poorer accuracy than controls at all serial positions, F(1,92) ≥ 7.33, p<.01; d= .94 to 1.44. Nonhallucinators showed significantly poorer accuracy than controls at serial positions 1, 2 and 4, F(1,92) ≥7.08, p<.01; d= .76 to .93, but not at the 3rd serial position (d= .43). Most importantly, hallucinators performed more poorly than nonhallucinators only at the middle serial positions 2 and 3, F(1,92) ≥ 5.04, p<.05, d= .54 & .74. There was no significant effect of or interactions involving Gender.
Figure 3.
Mean percentage of correct responses for hallucinators, nonhallucinators, and controls as a function of serial position of words on the WSPT (error bars= standard errors of mean).
Correlations of WSPT scores and Symptom Ratings
To examine whether the verbal WM deficit in D patients was correlated with auditory hallucinations, their overall accuracy scores on the WSPT were correlated with ratings on the SAPS. Poorer performance significantly correlated with greater Auditory Hallucination ratings, r(51)= −.35, p=.01. Hallucinatory Behavior ratings in D patients on the PANSS were also significantly correlated with poorer WSPT performance, r(53) = −.27, p<.05. There were, however, no significant correlations between WSPT scores and either PANSS total positive or negative symptom ratings for D patients, r(53)≤ .18, ns. WSPT performance in ND patients was not correlated with hallucination ratings on the SAPS, r(19) = .02, ns, or PANSS, r(19) = .04, ns. Nor were there significant correlations of performance on the Letter-Number Sequencing or CPT-IP tests and symptom ratings in either D or ND patients (all ps >.14).
Neuropsychological Tests
There was a significant difference among the D, ND and control groups in their performance on the Letter-Number Sequencing test, F(2,122)= 15.44, p<.001, η2= .20 . As shown in Table 2, ND patients performed more poorly than both D patients and controls (p<.05). Although D patients also differed significantly from controls (d = 0.60), their deficit was smaller than that for ND patients (d= 1.41). There was no significant gender effect. The findings for this auditory WM test confirm the group differences seen on the WSPT. The Letter-Number test was significantly correlated with performance on the WSPT in D patients (r [59]=.47, p<.001), ND patients (r [22]= .52, p= .01) and controls (r [47]= .44, p<.01).
ND patients also performed significantly worse than D patients and controls on the CPT-IP, F(2,122)= 25.60, p<.001, η2= .30 (Table 2). Their deficit was on the average more than two SDs below the mean for controls (d = 2.18), which is consistent with a marked deficit in sustained attention. D patients showed a more moderate deficit in CPT-IP performance compared to controls (d= 0.93). There was no significant gender effect. The CPT-IP test was not significantly correlated with performance on the WSPT in D patients (r[59]= .22, ns), ND patients (r[22]=.31, ns), or controls (r[52]= .24, ns).
ND patients had lower verbal and performance WAIS-IQ scores than D patients in keeping with a generalized cognitive deficit, while D patients had IQs in the normal range (see Table 3). On the WMS-R, however, D patients performed as poorly as ND patients on the verbal memory index, but tended to show better visual memory. D patients performed significantly poorer on the verbal than visual memory index, t(38)= 4.36, p<.001, whereas ND patients did not show a difference between their verbal and visual memory, t(10)= 0.83, ns. The WMS-R indexes were standardized to have a mean of 100 and an SD of 15. The WMS-R scores for D and ND patients with an education level of 14.4 and 13.5 years respectively were compared to norms of the standardization sample with an education level greater than 12 years (Wechsler, 1987). The verbal memory index for D patients was between one and two SDs below the mean for the standardization sample (M= 107.6, SD=14.7), whereas their visual memory index was within a half a SD of the norms (M= 105.5, SD= 13.4). The ND patients showed deficits in both verbal and visual memory, which were between one and two SDs below the norms. D and ND patients also showed a different pattern of correlations between performance on the WMS-R and the WSPT. Among D patients, performance on the WSPT was associated with the verbal memory index on the WMS-R, r(39)=.32, p=.05, with a significant correlation only for words in the 3rd serial position, r(39)=.41, p<.01, but was not correlated with the visual memory index, r(40)= .18, ns. In ND patients, performance on the WSPT was strongly correlated with the visual memory index on the WMS-R, r(11)= .75, p<.01, with a significant correlation only for words in the 2nd serial position, r(11)= .72, p=.01, but was not correlated with the verbal memory index on the WMS-R, r(11)= .16, ns.
Table 3.
Neuropsychological Measures for Verbal and Nonverbal Tests
| Discriminators | Nondiscriminators | Statistics | |||
|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | ||
| WAIS-II | |||||
| Verbal IQ | 104.3 | 14.6 | 88.1 | 15.8 | t(45) = 3.23** |
| Performance IQ | 95.8 | 15.1 | 79.8 | 21.2 | t(45) = 2.84** |
| Wechsler Memory | |||||
| Verbal Memory | 85.6 | 18.1 | 82.9 | 12.4 | t(48) = 0.46 |
| Visual Memory | 99.3 | 19.8 | 88.5 | 21.0 | t(48) = 1.57 |
Note: WAIS-II: n = 35 D group, n = 12 ND group; Wechsler Memory: n = 39 D group, n = 11 ND group.
p<.05
p<.01
p<.001
Discussion
Patients with schizophrenia having normal performance on a tone discrimination test of auditory perception and attention (D patients) had deficits in verbal WM on the WSPT, which replicates our prior findings (Bruder et al., 2004; Wexler et al., 1998; Wexler, Donegan, Stevens, & Jacob, 2002). These patients also showed deficits on another test of auditory verbal WM, i.e., the Letter-Number Sequencing test, and on the WMS-R index of verbal explicit memory, while having relatively preserved nonverbal performance. As in our prior study (Bruder et al., 2004), their verbal memory scores on the WMS-R were one to two SD below published norms, a large effect size (Cohen, 1988), but they showed little or no deficit on the visual memory index. The present study included inpatients and outpatients, generating a large sample with a range of symptoms and symptom severity. We were therefore able to demonstrate for the first time associations between the verbal WM deficit in D patients and symptoms of auditory hallucinations. In addition, 46% of patients were evaluated while off antipsychotic medications, making it possible to show that there were no differences between medicated and unmedicated patients on the tone discrimination test that defined the subgroups or on the WSPT that revealed the verbal WM deficit in D patients.
Patients who failed the tone discrimination test (i.e., ND) performed more poorly than D patients and controls on the WSPT and the Letter-Number Sequencing test of auditory WM. They also showed poor verbal and visual memory on the WMS-R, which agrees with prior findings of both verbal and nonverbal memory deficits in this subgroup (Bruder et al., 2004; Wexler et al., 1998). One interpretation of the ND patients’ poor performance on the tone discrimination test and WSPT is that they have a basic deficit in auditory processing. This possibility receives some support from the findings of Javitt, Strous, Grochowski, Ritter & Cowan (1997), who compared the tone matching performance of patients with schizophrenia and healthy controls. Patients showed deficits in the ability to match two tones in both easy and difficult pitch discriminations, even when the interval between tones was brief (≤ 1 sec). They suggest that this deficit in auditory sensory or echoic processing reflects impaired precision with which schizophrenic patients encode the physical properties of auditory stimuli. We found that both unmedicated and medicated patients with schizophrenia showed a deficit in tone discrimination, but this deficit was present in only a subgroup of patients, who we refer to as ND patients. Although their poor performance on the WM tests may stem from an auditory processing deficit, it might also reflect generalized cognitive dysfunction that cuts across modality. Their marked deficit on the CPT-IP and visual memory index of the WMS-R is suggestive of a more global problem, which may in part involve reduced sustained attention to both auditory and visual stimuli.
The normal tone discrimination and nonverbal memory in D patients indicates that they do not suffer from a generalized cognitive deficit and their poor verbal WM cannot be explained by nonspecific factors, such as lack of attention or perceptual dysfunction. Neuroimaging studies suggest that a neural network involving prefrontal and parietal regions underlies WM performance (Goldman-Rakic, 1991; Smith & Jonides, 1999). Healthy adults were found to activate the left inferior frontal cortex, temporal cortex and left inferior and superior parietal lobe during performance of the WSPT (Stevens, Goldman-Rakic, Gore, Fulbright & Wexler, 1998). Activation of the left inferior frontal cortex was reduced in patients with schizophrenia who performed at least 90% correct on the tone discrimination test (i.e., D patients), and they also failed to show greater activation of this region during a word than a tone serial position test, as was seen in healthy adults (Stevens et al., 1998). Similarly, Barch et al. (2002) measured fMRI during both WM (n-back) and recognition memory tasks with words and unfamiliar faces and found that patients with schizophrenia failed to show greater activation for words than faces in regions that normally show enhanced activation to verbal stimuli, including left inferior prefrontal, left parietal and left temporal cortex. Recently, Kayser et al. (2010) recorded event-related brain potentials (ERPs) of patients with schizophrenia and controls during recognition memory tasks with words and unfamiliar faces. Old-new ERP effects were markedly reduced in patients over the left lateral parietotemporal region, and this deficit was more pronounced for words than faces despite the greater difficulty in recalling faces, which indicates that it was not due to a generalized deficit. Similarly, in a study recording ERPs of patients with schizophrenia during the WSPT, Kayser et al. (2006) found evidence of disturbed processing in a frontal-parietotemporal network during encoding and early storage of the words. These findings suggest that both verbal WM and explicit memory deficits in schizophrenia may reflect a common disturbance of frontal and left parietotemporal regions. This is consistent with our findings for D patients who showed poorer verbal memory not only on WM tests but also the WMS-R.
Overall severity of clinical symptoms could not account for the marked difference in performance of the D and ND subgroups on the verbal WM tests. ND patients in our prior study did show somewhat higher negative symptoms than D patients on the PANSS (Bruder et al., 2004), but this difference was smaller and not statistically significant in the current study. Although it could be argued that higher negative symptoms and possible reduced motivation or effort might be related to the generally poorer performance in ND patients, severity of negative symptoms was not correlated with performance on the WSPT.4
Poorer verbal WM on the WSPT in D patients, but not in ND patients, was significantly correlated with auditory hallucination ratings on the SAPS and hallucination ratings on the PANSS, but not with negative symptoms. As predicted based on fMRI findings of reduced activity in language-related cortical regions in patients with auditory hallucinations (Wible et al., 2009), D patients having auditory hallucinations showed poorer WSPT performance than those without hallucinations and healthy adults. Although this could be due to hallucinations interfering with auditory processing of words, it is important to note that hallucinators performed more poorly than nonhallucinators only at the middle serial positions on the WSPT. An alternative interpretation is that patients with auditory hallucinations may be more prone to “cognitive” sources of interference, e.g., proactive and retroactive interference (Stevens et al., 2000). The relation between poorer WSPT performance and auditory hallucinations is consistent with the hypothesis that verbal WM deficits in schizophrenia stem from dysfunction of language-related regions in left inferior prefrontal and parietotemporal cortex (Stevens et al., 1998; Wible et al., 2009).
The importance of our findings stems from the need to parse the heterogeneous clinically-diagnosed disorder of schizophrenia into subgroups having more homogeneous pathophysiology. The tone discrimination test introduced by Wexler et al. (1998) provides a quick and inexpensive way of identifying patients who have marked deficits in auditory perception or attention and display wide-spread cognitive dysfunction on both verbal and nonverbal tests. These ND patients differ from those who have normal tone discrimination (i.e., D patients) in showing poorer performance on auditory verbal WM tests (WSPT and Letter-Number Sequencing), poorer sustained attention (CPT-IP), and lower verbal and performance IQ. This subgroup therefore has global cognitive deficits as might result from perceptual or attentional dysfunction, and differs from D patients who have a more focal deficit in verbal memory. Deficits in early perceptual processing could have downstream impact on higher-order cognitive or social functions (Javitt et al., 1997; Wynn, Sugar, Horan, Kern, & Green, 2010). A cognitive rehabilitation strategy using auditory training was found to improve verbal WM in schizophrenia (Fisher, Holland, Merzenich, & Vinogradov, 2009) and may prove particularly beneficial for ND patients having an auditory processing deficit.
This study has several limitations. First, the tests included in this study were not sufficiently broad for determining the specificity of the verbal WM deficit in D patients. Wexler et al. (1998) and Stevens et al. (2000) did, however, show that D patients had deficits on the WSPT, but not on a tone serial position test, which supports the specificity of their verbal WM deficit. Also, Wexler et al (2002) found that D patients had marked deficits on an serial position test with easily named environmental sounds (e.g., telephone ringing) but performed nearly as well as healthy controls on same test with birdsongs that could not be verbally labeled and were much more difficult for controls. Second, it is not clear whether the poor performance in ND patients on the verbal WM tests was due to an auditory processing deficit or more global attentional dysfunction. This could be addressed by measuring early auditory ERPs (N1, P2) during the WSPT and also mismatch negativity (MMN) to assess preattentive auditory processing. Third, while D patients have been found to show reduced verbal WM on both auditory and visual versions of the WSPT (Stevens et al., 2000), the extent to which the deficits in ND patients are specific to the auditory modality needs further study. Lastly, the D subgroup included 60-70 % of patients with schizophrenia in our studies and was defined by their normal performance in one cognitive task. This raises a question as to whether they represent a homogenous subtype of schizophrenia or could benefit from further subdivision on the basis of their clinical features (e.g., those with or without auditory hallucinations) or distinctive cognitive or neurophysiologic deficits.
Acknowledgments
This research was supported by MH066597 from the National Institute of Mental Health. We thank John Keilp for his help with the neuropsychological tests.
Footnotes
Both patients meeting criteria for schizophrenia and schizoaffective disorder were included because we did not find a significant difference in their tone discrimination or WSPT performance. Nor did they differ in gender, age, education or handedness. The percentage of correct responses on the tone discrimination test was analyzed using a Group (Schizophrenia, Schizoaffective, Controls) by Gender (Male, Female) by Ratio of Tones (.67, .75, .85, .90, .95, 1.00) repeated measures ANOVA. There was a significant difference in tone discrimination among groups, F (2,124)= 8.40, p< .001. Both patients with schizophrenia (M= 82.8, SD= 18.6) and schizoaffective disorder (M=84.6, SD= 18.2) showed poorer tone discrimination than controls (M= 95.1, SD= 9.3, both p<.05), but there was no significant difference between the schizophrenia and schizoaffective groups. The same was true for the WSPT. There was a significant difference in WSPT accuracy among groups, F (2, 124)= 22.58, p<.001, with both patients with schizophrenia (M=75.4, SD= 19.0) and schizoaffective (M=81.0, SD= 11.0) performing more poorly than controls (M=94.1, SD= 6.8, both p<.05), but there was no significant difference between the schizophrenia and schizoaffective group.
Group differences reported below on the tone discrimination, WSPT, Letter-Number test, CPT, and WMS-R remained the same when we excluded the 13 patients who did not have a DIGS interview.
To check whether non-payment of inpatients may have impacted their incentive to perform the tests, we compared their performance with that of outpatients (who were paid) on the tone discrimination test and WSPT. There was no significant difference between inpatients (n=56) and outpatients (n=27) in their tone discrimination, F (1, 81)= 0.04, p= .85, or WSPT performance, F (1, 81)= 0.34, p= .56. It is therefore unlikely that non-payment of outpatients differentially affected their performance.
Differences in WSPT performance among the D, ND and control groups remained the same when the 23 patients having a diagnosis of schizoaffective disorder were excluded from the analyses. The ANOVA revealed the same main effect of Group, F(2, 101)= 43.61, p<.001, but no Group by Serial Position interaction. Multiple comparisons indicated that ND patients (n= 18) had overall poorer accuracy than D patients (n= 42) and controls (p<.05), and D patients had poorer accuracy than controls (p<.05).
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/ABN
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, D.C.: 2000. text revision. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) The University of Iowa; Iowa City, IA: 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) The University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- Barch DM, Csernansky JG, Conturo T, Snyder AZ. Working and long-term memory deficits in schizophrenia: Is there a common prefrontal mechanism? Journal of Abnormal Psychology. 2002;111(3):478–494. doi: 10.1037//0021-843x.111.3.478. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Wexler BE, Sage MM, Gil RB, Gorman JM. Verbal memory in schizophrenia: Additional evidence of subtypes having different cognitive deficits. Schizophrenia Research. 2004;68:137–147. doi: 10.1016/S0920-9964(03)00156-7. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cerebral Cortex. 2000;10(11):1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, Robertson LC, Nordahl TE, Chaderjian M, Oshora-Celaya L. Perceptual and attentional asymmetries in schizophrenia: Further evidence for a left hemisphere deficit. Psychiatry Research. 1996;62(2):111–119. doi: 10.1016/0165-1781(96)02849-1. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for behavioral sciences. 2nd ed Erlbaum; Hillside, NJ: 1988. [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments & Computers. 1993;25(2):257–271. [Google Scholar]
- Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophrenia Bulletin. 1994;20(1):31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Research. 1988;26(2):223–338. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Dixon WJ, editor. BMDP statistical software manual: To accompany the 7.0 software release. University of California Press; Berkeley, CA: 1992. [Google Scholar]
- Donohoe G, Corvin A, Robertson IH. Evidence that specific executive functions predict symptom variance among schizophrenia patients with a predominantly negative symptom profile. Cognitive Neuropsychiatry. 2006;11(1):13–32. doi: 10.1080/13546800444000155. [DOI] [PubMed] [Google Scholar]
- Egan MF, Duncan CC, Suddath RL, Kirch DG, Mirsky AF, Wyatt RJ. Event-related potential abnormalities correlate with structural brain alterations and clinical features in patients with chronic schizophrenia. Schizophrenia Research. 1994;11(3):259–271. doi: 10.1016/0920-9964(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Hyde TM, Weinberger DR. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biological Psychiatry. 2001;50(2):98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: The schedule for affective disorders and schizophrenia. Archives of General Psychiatry. 1978;35(7):837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Non-patient ed. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1996. SCID-NP. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Patient ed. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 2002. Research Version. SCID-I/P. [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. American Journal of Psychiatry. 2009;166:805–811. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, White PM, Csernansky JG, Faustman WO, Roth WT, Pfefferbaum A. ERPs in schizophrenia: Effects of antipsychotic medication. Biological Psychiatry. 1994;36(3):153–170. doi: 10.1016/0006-3223(94)91221-1. [DOI] [PubMed] [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Archives of General Psychiatry. 1997;54(2):159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Prefrontal cortical dysfunction in schizophrenia: The relevance of working memory. In: Carroll BJ, Barrett JE, editors. Psychopathology and the brain. Raven Press; New York, NY: 1991. pp. 1–23. [Google Scholar]
- Gooding DC, Tallent KA. Spatial working memory performance in patients with schizoaffective psychosis versus schizophrenia: A tale of two disorders? Schizophrenia Research. 2002;53(3):209–218. doi: 10.1016/s0920-9964(01)00258-4. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Koren D, Reichenberg A, Bowie CR. Negative symptoms and cognitive deficits: What is the nature of their relationship? Schizophrenia Bulletin. 2006;32(2):250–258. doi: 10.1093/schbul/sbj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Lee K, Preus A, McCarley RW, Wible CG. An fMRI study of functional abnormalities in the verbal working memory system and the relationship to clinical symptoms in chronic schizophrenia. Cerebral Cortex. 2010;20(1):46–60. doi: 10.1093/cercor/bhp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. Journal of Abnormal Psychology. 1997;106(2):315–324. doi: 10.1037//0021-843x.106.2.315. [DOI] [PubMed] [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: Patients, paradigms, and practical implications. Psychophysiology. 2003;40(5):684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Fishbein A. Positive and Negative Syndrome Scale (PANSS) rating manual. MultiHealth Systems; Toronto, Canada: 1999. [Google Scholar]
- Kayser J, Tenke CE, Gates NA, Kroppmann CJ, Gil RB, Bruder GE. ERP/CSD indices of impaired verbal working memory subprocesses in schizophrenia. Psychophysiology. 2006;43:237–252. doi: 10.1111/j.1469-8986.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Kroppmann CJ, Fekri S, Alschuler DM, Gates NA, Bruder GE. Current source density (CSD) old/new effects during recognition memory for words and faces in schizophrenia and in healthy adults. International Journal of Psychophysiology. 2010;75:194–210. doi: 10.1016/j.ijpsycho.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keselman HJ. Testing treatment effects in repeated measures designs: An update for psychophysiological researchers. Psychophysiology. 1998;35(4):470–478. [PubMed] [Google Scholar]
- Menon V, Anagnoson RT, Mathalon DH, Glover GH, Pfefferbaum A. Functional neuroanatomy of auditory working memory in schizophrenia: Relation to positive and negative symptoms. Neuroimage. 2001;13(3):433–446. doi: 10.1006/nimg.2000.0699. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Reich T. Diagnostic Interview for Genetic Studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Overall JE. The Brief Psychiatric Rating Scale in psychopharmacology research. Modern Problems of Pharmacopsychiatry. 1974;7:67–78. [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Archives of General Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Park S, Puschel J, Sauter BH, Rentsch M, Hell D. Spatial working memory deficits and clinical symptoms in schizophrenia: A 4-month follow-up study. Biological Psychiatry. 1999;46(3):392–400. doi: 10.1016/s0006-3223(98)00370-9. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. The American Journal of Psychiatry. 2001;158(7):1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Perry W, Heaton RK, Potterat E, Roebuck T, Minassian A, Braff DL. Working memory in schizophrenia: Transient “online” storage versus executive functioning. Schizophrenia_Bulletin. 2001;27(1):157–176. doi: 10.1093/oxfordjournals.schbul.a006854. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Stafiniak P. Neuropsychological function in schizophrenia: Selective impairment in memory and learning. Archives of General Psychiatry. 1991;48(7):618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Archives of General Psychiatry. 1994;51(2):124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R. Patient edition American Psychiatric Press; Washington, DC: 1990. [Google Scholar]
- Stevens AA, Donegan NH, Anderson M, Goldman-Rakic PS, Wexler BE. Verbal processing deficits in schizophrenia. Journal of Abnormal Psychology. 2000;109(3):461–471. [PubMed] [Google Scholar]
- Stevens AA, Goldman-Rakic PS, Gore JC, Fulbright RK, Wexler BE. Cortical dysfunction in schizophrenia during auditory word and tone working memory demonstrated by functional magnetic resonance imaging. Archives of General Psychiatry. 1998;55(12):1097–1103. doi: 10.1001/archpsyc.55.12.1097. [DOI] [PubMed] [Google Scholar]
- Tulsky D, Zhu J, Ledbetter MF. WAIS-III WMS-III Technical Manual. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler Memory Scale – Revised manual. Psychological Corporation; New York: 1987. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. Harcourt Assessment; San Antonio, TX: 1997. WAIS-3®. [Google Scholar]
- Wexler BE, Donegan N, Stevens AA, Jacob SA. Deficits in language-mediated mental operations in patients with schizophrenia. Schizophrenia Research. 2002;53:171–179. doi: 10.1016/s0920-9964(01)00194-3. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Stevens AA, Bowers AA, Sernyak MJ, Goldman-Rakic PS. Word and tone working memory deficits in schizophrenia. Archives of General Psychiatry. 1998;55:1093–1096. doi: 10.1001/archpsyc.55.12.1093. [DOI] [PubMed] [Google Scholar]
- Wible CG, Lee K, Molina I, Hashimoto R, Preus AP, Roach BJ, Lauriello J. fMRI activity correlated with auditory hallucinations during performance of a working memory task: data from the FBIRN consortium study. Schizophrenia Bulletin. 2009;35(1):47–57. doi: 10.1093/schbul/sbn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn JK, Sugar C, Horan WP, Kern R, Green MF. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biological Psychaitry. 2010;67:940–947. doi: 10.1016/j.biopsych.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]