Abstract
Inflammation and infiltration of immune cells in white adipose tissue have been implicated in the development of obesity-associated insulin resistance. Likewise, dysregulation of the fuel-sensing enzyme AMP-activated protein kinase (AMPK) has been proposed as a pathogenetic factor for these abnormalities based on both its links to insulin action and its anti-inflammatory effects. In this study, we examined the relationships between AMPK activity, the expression of multiple inflammatory markers in visceral (mesenteric and omental) and abdominal subcutaneous adipose tissue, and whole-body insulin sensitivity in morbidly obese patients (BMI 48 ± 1.9 kg/m2) undergoing gastric bypass surgery. AMPK activity was assessed by western-blots (P-AMPK/T-AMPK) and mRNA levels of various markers of inflammation by qRT-PCR. Patients were stratified as insulin sensitive obese or insulin resistant obese according to their HOMA-IR values. The results indicate that AMPK activity is lower in visceral than in subcutaneous abdominal adipose tissue of these patients and that this is associated with an increased expression of multiple inflammatory genes. They also revealed that AMPK activity is lower in adipose tissue of obese patients who are insulin resistant (HOMA-IR > 2.3) than in BMI-matched insulin sensitive subjects. Furthermore, this difference was evident in all three fat depots. In conclusion, the data suggest that there are close links between reduced AMPK activity and inflammation in white adipose tissue, and whole-body insulin resistance in obese humans. Whether adipose tissue AMPK dysregulation is a causal factor for the development of the inflammation and insulin resistance remains to be determined.
Keywords: AMP-activated protein kinase, adipose tissue, humans, insulin sensitive obese, inflammation, insulin resistance
Introduction
The metabolic syndrome is typically characterized by obesity, systemic insulin resistance and a predisposition to such disorders as type 2 diabetes and atherosclerotic cardiovascular-disease (1). Inflammation, which also accompanies these disorders, has been repeatedly observed in adipose tissue of obese humans and experimental animals (2). The mechanism that triggers this inflammation is still uncertain, but could involve abnormalities in fatty acid metabolism and oxidative stress in the adipocyte (3). Such abnormalities appear to lead to an increase in the expression and secretion of a myriad of pro-inflammatory molecules (3) that in turn lead to the attraction, infiltration, and adhesion of immune cells (2). Thus, several studies have reported an increased presence of macrophages in adipose tissue of obese humans and rodents compared to lean controls (4; 5) and recently, infiltration of immune cells including neutrophils (6) and various subsets of T-lymphocytes has been reported (7; 8). The respective role of each of these immune cells in causing inflammation in adipose tissue is still uncertain, but collectively they have been closely linked to the development of systemic insulin resistance (4; 9).
Dysregulation of the fuel-sensing enzyme AMP-activated protein kinase (AMPK) has been proposed as both a pathogenetic factor for the development of obesity-related diseases and a target for their therapy (10; 11). Activation of AMPK occurs when the cellular AMP:ATP ratio increases and its most well described role is to restore energy state by activating ATP-generating metabolic pathways (e.g. fatty-acid oxidation) and inhibiting pathways that require ATP (energy) and are not acutely essential for cell survival (e.g. lipid and protein synthesis)(12). In addition, AMPK can be activated and downregulated by other mechanisms (12) and it increasingly appears to have other biological roles. For instance AMPK activation has been shown to protect various cell types by reducing inflammation (13; 14) in the basal state and when it is increased by such stimuli as lipopolysaccharide, TNFα and fatty acids (15). Likewise, knocking down AMPKα1 in cultured macrophages increases NFκB signalling and the expression of inflammatory markers (16). Despite this, and the central role of obesity in the pathogenesis of many diseases, very little is known about the role of AMPK in adipose tissue. Furthermore, what is known is based principally on investigations conducted in rodents and cultured cells. In the present study, we examined the relationships between AMPK activity and gene expression of various markers of inflammation in subcutaneous and visceral adipose tissue, and whole-body insulin sensitivity in morbidly obese humans undergoing gastric bypass surgery.
Material and Methods
Study Subjects
Abdominal subcutaneous (SC), omental, and mesenteric adipose tissues were obtained under informed consent from class III obese patients undergoing gastric bypass surgery (n = 8). The patients had a body mass index (BMI) ranging from 42 to 60 kg/m2, were ≥18 years of age, and were receiving care at the Boston Medical Center Nutrition and Weight Management Clinic. Patients with unstable medical conditions such as active coronary syndromes, congestive heart failure, systemic infection, malignancy, or pregnancy were excluded. The study was approved by the Boston University Medical Center Institutional Review Board. Clinical characteristics including medical history, medications, blood pressure, and anthropometric data were recorded for each subject. In addition, biochemical analyses including plasma lipids, glucose, insulin, and homeostasis model assessment of insulin resistance (HOMA-IR) were performed on blood collected in a fasting state (see Table1).
Table 1.
Clinical and anthropometric parameters of obese individuals stratified into insulin-sensitive and insulin-resistant according to HOMA-IR.
| Insulin-sensitive (n=4) | Insulin-resistant (n=4) | |
|---|---|---|
| Age (years) | 38.25 ± 7.8 | 37.5 ± 8.5 |
| sex (% female) | 50% | 100% |
| Weight (kg) | 142.5 ± 7.5 | 129.6 ± 10 |
| Height (m) | 1.77 ± 6.4 | 1.60 ± 1.5 * |
| Waist Circumference (cm) | 142.0 ± 3.0 | 143.5 ± 6.9 |
| Hip Circumference (cm) | 141.0 ± 2.3 | 145.0 ± 3.81 |
| Heart Rate (bpm) | 70.5 ± 1.8 | 74.25 ± 9.7 |
| Systolic BP (mmHg) | 125.8 ± 5.0 | 121.3 ± 1.5 |
| Diastolic BP (mmHg) | 74.3 ± 2.6 | 68.8 ± 5.4 |
| Plasma insulin (µIU/ml) | 9.0 ± 0.4 | 23.0 ± 8.8 * |
| Glucose (mM) | 5.2 ± 0.3 | 5.9 ± 0.7 |
| HgAIC (%) | 5.9 ± 0.2 | 6.3 ± 0.5 |
| LDL (mg/dL) | 132.0 ± 19.9 | 103.0 ± 7.1 |
| HDL (mg/dL) | 38.8 ± 2.0 | 44.0 ± 1.9 |
| Total Cholesterol (mg/dL) | 188.3 ± 18.7 | 181.5 ± 15.7 |
| Triacylglycerol (mg/dL) | 87.3 ± 7.7 | 172.8 ± 72.6 |
| hsCRP (mg/L) | 5.2 ± 1.4 | 9.2 ± 2.3 |
| Diabetes (%) | 0% | 50% |
| Hypertension (%) | 50% | 25% |
| CAD (%) | 0% | 0% |
| Family History of CAD (%) | 0% | 25% |
| Hypercholesterolemia (%) | 75% | 50% |
| Metabolic Syndrome (%) | 75% | 100% |
| Thiazolidinedione (%) | 0% | 0% |
| Metformin (%) | 0% | 75% |
| ACEI (%) | 0% | 25% |
| Statin (%) | 25% | 25% |
| NSAID (%) | 25% | 0% |
| Antidepressants (%) | 25% | 75% |
| Beta-adrenergic agonists (%) | 25% | 50% |
| Beta-adrenergic antagonists (%) | 25% | 50% |
| Thyroid hormones (%) | 25% | 0% |
| Phentermine (%) | 25% | 25% |
| Proton-pump inhibitors (%) | 50% | 50% |
| Diuretic/Laxative (%) | 50% | 25% |
| Antihistaminic (%) | 25% | 25% |
| SAID (%) | 0% | 50% |
Results are means ± SEM. Significantly different from insulin sensitive obese individuals:
p<0.05.
CAD: Coronary Artery Disease; ACEI: Angiotensin Converting Enzyme Inhibitor; NSAID: Nonsteroidal Antiinflammatory Drug; SAID: Steroidal Antiinflammatory Drug.
Preparation of Tissue Lysates and Immunoblot Analysis
Adipose tissue excised during gastric bypass surgery was immediately frozen in liquid nitrogen and stored at −80°C until further analysis. On the day of the assay, the frozen adipose tissue was homogenized on ice in buffer A (30 mM Na–Hepes (pH 7.4), 2.5 mM EGTA, 3 mM EDTA, 32% glycerol, 20 mM KCl, 40 mM β-glycerophosphate, 40 mM NaF, 4 mM NaPPi, 1 mM Na3VO4, 0.1% Nonidet P-40, 2 mM diisopropyl fluorophosphates (DFP), 2 mM phenylmethylsulfonyl fluoride (PMSF), 5 µM aprotinin, leupeptin, and pepstatin A, and 1 mM dithiothreitol (DTT)). Homogenates were centrifuged (14,000g × g for 10 min at 4 °C) and supernatants were harvested and supplemented with an equal volume of buffer B (30 mM Na-Hepes, pH 7.4, 2.5 mM EGTA, 3 mM EDTA, 70 mM KCl, 20 mM β-glycerophosphate, 20 mM NaF, 2mM NaPPi, 1 mM Na3VO4, 0.1% Nonidet P-40, 2 mM DFP, 2 mM PMSF, 5 µM aprotinin, leupeptin, and pepstatin A, and 1 mM DTT). The resulting homogenates were centrifuged (14,000g × g for 10 min at 4 °C) and supernatants were harvested. Protein concentrations of cell supernatants were determined by the bicinchoninic acid (BCA) method (Pierce, Rockford, IL) using bovine serum albumin as the standard. Protein lysates (25 µg) were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech, Uppsala, Sweden). Membranes were blocked in Tris-buffered saline (pH 7.5) containing 0.05% Tween 20 (TBST) and 5% milk for 1 h at room temperature and then probed with antibodies to β-actin for 45min at room temperature, or antibodies to P-AMPK Thr172, and total AMPKα (T- AMPKα) overnight at 4°C. Bound antibodies were detected with the appropriate horseradish peroxidase-linked whole secondary antibodies. Protein immunoblots were visualized by enhanced chemiluminescence, and bands were quantified with scanning densitometry. Antibodies for P-AMPK (Thr172), and T-AMPKα, were purchased from Cell Signaling Technology (Beverly, MA), and for β-actin from Sigma-Aldrich (St-Louis, MO). Mouse and rabbit antibodies conjugated to horseradish peroxidase were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Real-time quantitative PCR
Human adipose tissues were analyzed for mRNA levels of various genes involved in inflammation and infiltration of immune cells. Briefly, after collection, biopsy specimens were placed in ice-cold saline and transported to the laboratory within 15 minutes. Samples were cleaned by removing visible blood vessels and clots, immersed in RNA preserving solution (RNAlater, Sigma-Aldrich), and stored at −80°C. Total RNA was isolated from fat tissue via a commercially available kit according to instructions (Ambion Inc, Austin TX) and cDNA was synthesized by reverse transcription. Real-time quantitative PCR was performed for CD3A, CD4, CD68, MPO, CCL2, CCL5, AGT, VCAM, ICAM, and PECAM using Assay-on-Demand gene expression primers and probes and TaqMan Universal Master Mix (Applied Biosystems) in an Applied Biosystems 7500 real-time PCR instrument. Results were analyzed with reference to GAPDH, which was used as an endogenous housekeeping gene. Fold difference in gene expression was calculated as 2−ΔΔCt using GAPDH as the endogenous control gene and subcutaneous fat as the “comparer” (17).
Statistical analysis
Results were analyzed using Statview version 5.0.1 and are presented as means ± S.E.M. Statistical significance of continuous variables was determined by non-parametric Mann–Whitney U tests, Wilcoxon Signed Rank Tests, or 2-way ANOVA as appropriate and correlations were examined with non-parametric Spearman Rank Tests. Categorical group differences were examined using the Fisher’s exact test. For all analyses, a probability value <0.05 was considered statistically significant.
Results
AMPK activity is lower and mRNA levels of inflammatory markers are higher in visceral than in abdominal subcutaneous adipose tissue of morbidly obese humans
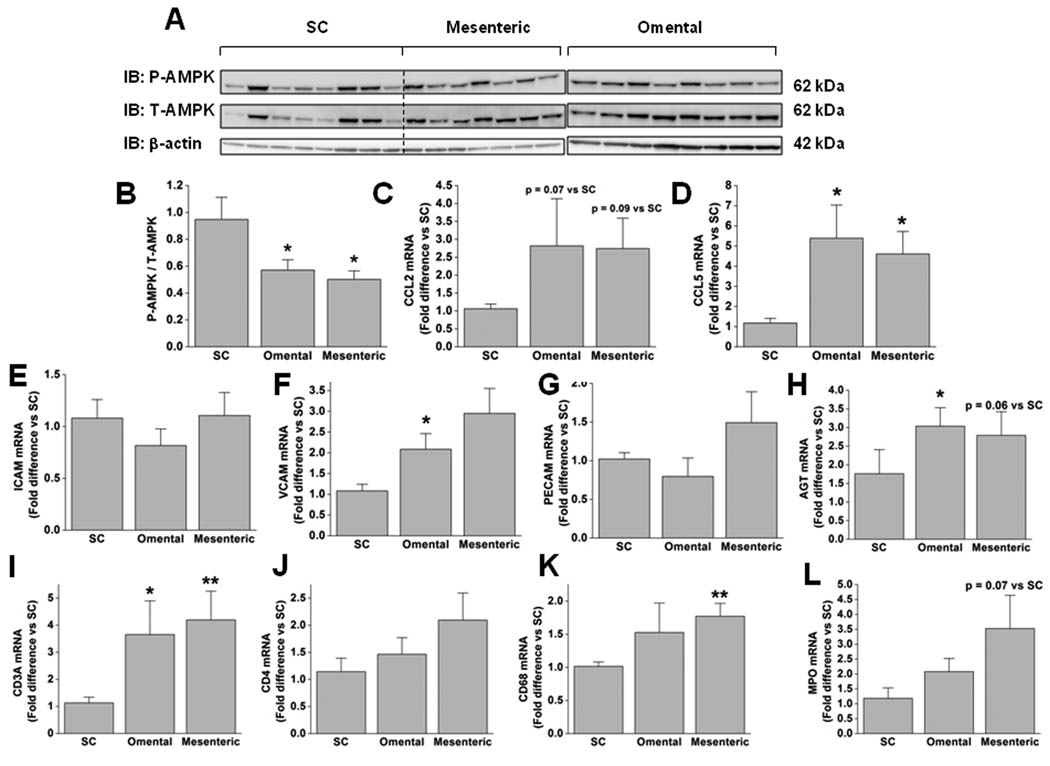
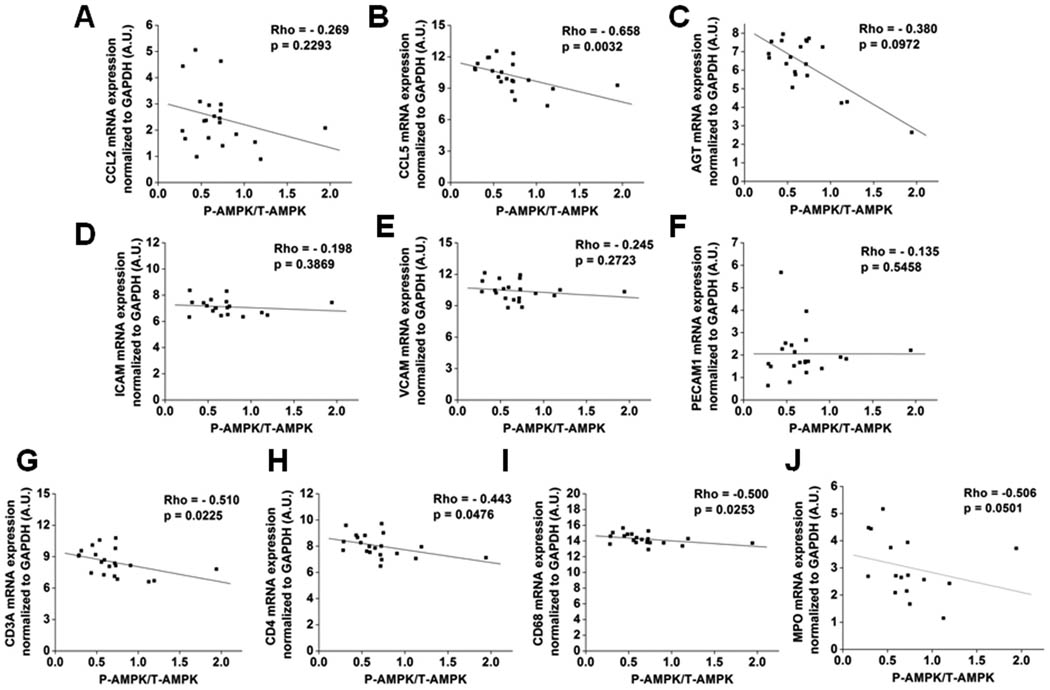
AMPK activity and inflammation were examined in abdominal subcutaneous and visceral (omental and mesenteric) adipose tissues that were excised from eight obese patients (Class III obesity, mean BMI 48.0 ± 1.9 kg/m2) undergoing gastric bypass surgery. AMPK activity, assessed by its phosphorylation state (P-AMPK/T-AMPK), was significantly lower (~2-fold) in omental and mesenteric, than in subcutaneous abdominal fat (Fig 1B). As shown in Fig. 1C–G, the visceral adipose tissue depots also exhibited higher mRNA levels of the pro-inflammatory chemokines CCL5 (also known as RANTES) and CCL2 (also known as MCP-1), and of vascular cell adhesion molecule 1 (VCAM1), but showed no differences in inter-cellular adhesion molecule 1 (ICAM) or platelet endothelial cell adhesion molecule (PECAM) expression. In addition, mRNA levels of the cell surface receptors of immune cells CD3A (T-cell) and CD68 (monocytes/macrophages), and myeloperoxidase (MPO), an enzyme most abundant in neutrophils, were higher in visceral fat, suggesting that it is more infiltrated with immune cells than is subcutaneous adipose tissue (Fig 1I–L). Visceral adipose tissue also exhibited higher mRNA levels of angiotensinogen (AGT) than the abdominal subcutaneous depot (Fig. 1H). As shown in Fig. 2, when values for the three depots were pooled, a significant negative correlation was found between adipose tissue AMPK activity and mRNA levels of CCL5, CD3A, CD4, CD68, and MPO but not of CCL2, ICAM, VCAM, PECAM, or AGT. Altogether, these results indicate that AMPK activity is lower in visceral than in subcutaneous fat and that this is closely associated with increased inflammation and the infiltration of immune cells.
Figure 1. AMPK activity is lower and mRNA levels of inflammatory markers higher in visceral than in subcutaneous adipose tissue of obese humans.
(A) Immunoblots with specific antibodies for AMPKα phosphorylated at Thr172, T-AMPKα, and β-actin. (B) Densitometric analysis of P-AMPK normalized to T-AMPK. (C–L) Fold difference in the expression of various genes involved in inflammation and immunity in visceral compared to subcutaneous fat are shown. Results are means ± SEM (n=7–8). Significantly different from subcutaneous fat: * p < 0.05, ** p < 0.01. Significantly different from omental fat: + p < 0.05. IB: immunoblot; SC: subcutaneous.
Figure 2. AMPK activity inversely correlates with the expression of various genes involved in inflammation and immunity in adipose tissue of obese humans.
AMPK activity in the three depots was assessed by immunoblots of P-AMPK, normalized to T-AMPKα abundance, and correlated to the mRNA levels of the indicated genes. Individual values were obtained in the experiments presented in Fig. 1.
AMPK activity is higher in adipose tissue of BMI-matched obese humans who are not insulin resistant
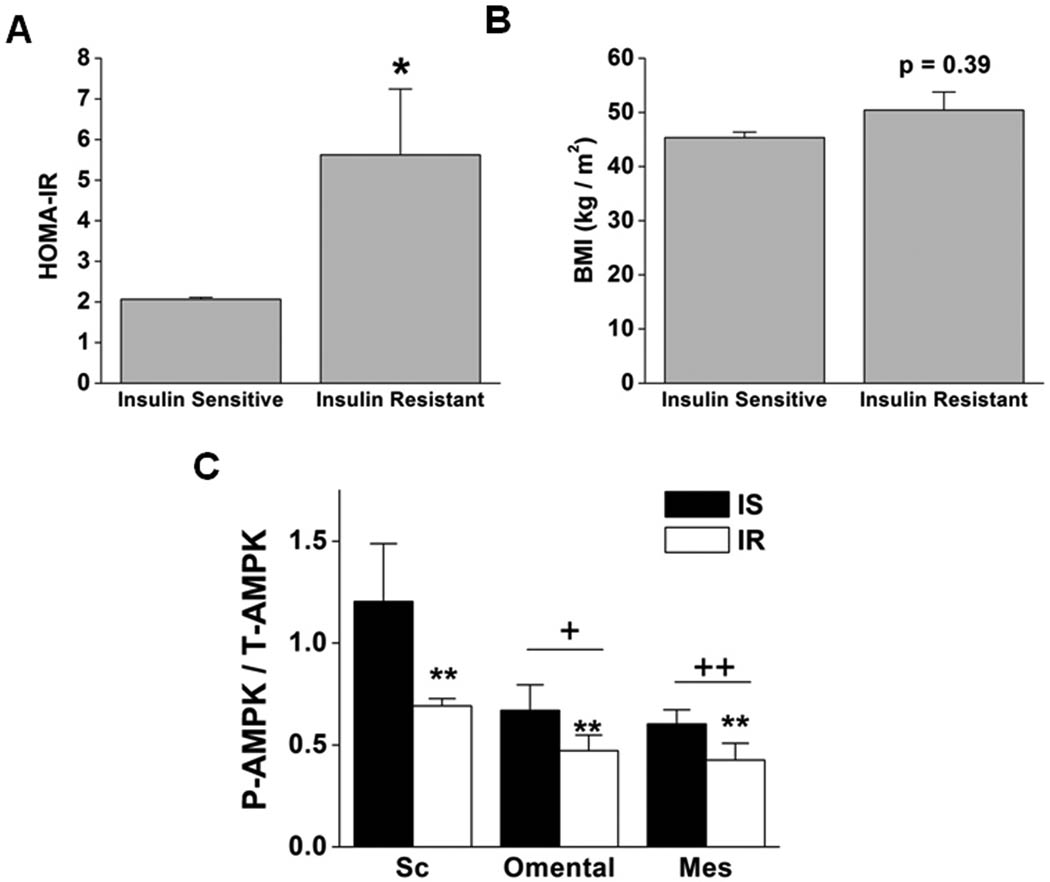
Obesity is a risk factor for the development of insulin resistance and diabetes; however, not all obese subjects are insulin resistant (18–20). Indeed a relatively normal degree of insulin sensitivity can be found in 30–40% of obese U.S. adults (BMI ≥30 kg/m2)(21) and even in a significant percentage of class III obese individuals (BMI ≥40 kg/m2)(22). To examine the relationship between AMPK activity and insulin resistance, we stratified the obese patients into BMI-matched groups in which insulin sensitivity was evaluated by HOMA-IR (Fig 3 A,B). A HOMA-IR value of 2.3 was used as a cut-off point to categorize obese subjects as insulin-sensitive (HOMA-IR < 2.3) or insulin-resistant (HOMA-IR > 2.3) as previously described (23). Of the patients studied, four were insulin sensitive and four insulin resistant. As shown in Fig. 3C, insulin-resistant individuals exhibited lower AMPK activity in all three adipose tissue depots. Anthropometric and clinical characteristics and medications taken by the patients in the two subgroups are presented in Table 1. As expected, insulin-resistant patients had higher plasma insulin levels than did comparably fasted insulin-sensitive patients. Two of the patients stratified as insulin-resistant, but none of the insulin-sensitive subgroup, had type 2 diabetes, three were medicated with metformin and two with anti-inflammatory drugs. We cannot rule out that these differences influenced some of our results. On the other hand, it is known that metformin activates AMPK; yet we still found increased AMPK activity in the insulin-sensitive subgroup in which none of the patients were taking this medication.
Figure 3. AMPK activity is higher in adipose tissues of obese, insulin-sensitive humans than in the same tissues of comparably obese individuals who are insulin resistant.
Obese subjects were stratified according to their HOMA-IR values into insulin-sensitive (IS) (HOMA-IR < 2.3) and insulin-resistant (IR) (HOMA-IR > 2.3). (A) HOMA-IR and (B) body mass index (BMI). (C) Densitometric analyses of P-AMPK normalized to T-AMPKα in subcutaneous, omental and mesenteric adipose tissue of IS and IR obese subjects. Results are means ± SEM (n=4). Significantly different from IS individuals: * p < 0.05, ** p < 0.01. Significantly different from subcutaneous fat: + p < 0.05, ++ p < 0.01. IS: insulin sensitive; IR: insulin resistant; Sc: subcutaneous; Mes: mesenteric.
Discussion
We investigated the interrelationship between AMPK activity, inflammation, and whole-body insulin sensitivity in white adipose tissue of massively obese humans (BMI 42.3 – 59.7 kg/m2) undergoing gastric bypass surgery. The major findings were as follows: 1) AMPK activity was lower and the expression of genes involved in inflammation higher in mesenteric and omental than in subcutaneous adipose depots of the same individual; 2) an inverse relation between AMPK activity and inflammatory gene expression was observed in all of these fat depots; 3) AMPK activity was lower in all three adipose tissue depots in insulin resistant subjects than in their BMI-matched counterparts who were insulin sensitive.
To our knowledge, this is the first study to examine concurrently AMPK activity and markers of inflammation and adhesion in abdominal subcutaneous and visceral adipose tissue of obese humans. AMPK activity, as assessed by its phosphorylation state, was ~45% lower in visceral fat (omental and mesenteric) than in abdominal subcutaneous fat of the same individual; no difference was found in the abundance of total AMPKα protein between the depots. While this manuscript was in preparation, Martinez-Agustin et al. (24) reported a higher abundance of total AMPKα in subcutaneous than in visceral adipose tissue of morbidly obese subjects; however, AMPK phosphorylation and activity were not assessed. Studies in both humans and rodents have established that the accumulation of predominantly visceral adipose tissue is associated with the metabolic syndrome and the diseases that accompany it (19; 25; 26). An increasing body of evidence has linked this to the fact that visceral adipose tissue is more lipolytic and exhibits more inflammatory changes including a greater infiltration of immune cells (27). In keeping with this, we found that the mRNA levels of various markers of inflammation (CCL2, CCL5, VCAM) and of immune cells (CD3A, CD68, MPO) were greater in visceral than in subcutaneous fat. A novel finding was that this pro-inflammatory profile in visceral fat was associated with decreased AMPK activity. It remains to be determined in which cell types AMPK activity is diminished as adipose tissue is not only comprised of adipocytes but also of immune cells, fibroblasts, endothelial cells, and preadipocytes (28).
Whether the decrease in AMPK activity is a cause or a consequence of the inflammation is unclear. One factor that contributes to this conundrum is that AMPK and inflammation have both been shown to impact on the other. Thus, AMPK activation inhibits the ability of high glucose, TNFα and palmitate to cause inflammation, apoptosis (13; 14; 16; 29) and where studied, insulin resistance (29) in various cell types, whereas decreased AMPK activity enhances their ability to do so (16). On the other hand, inflammation induced by TNFα in skeletal muscle (30) and palmitate in aortic endothelium (31) has been shown to diminish AMPK activity in both instances, apparently due to their action on phosphatases. Recent studies in 3T3-L1 adipocytes have also demonstrated that knocking out the histone/protein deacetylase SIRT1 enhances the inflammation induced by incubation with recombinant TNFα (32). Since SIRT1 has been shown to activate AMPK in various cells (33), it will be of interest to determine if these effects are the result of decreased AMPK activity. In particular, studies comparing adipose tissue of patients or experimental animals as they become obese and/or develop the metabolic syndrome may help to determine the order of events.
Another striking finding was that AMPK activity was lower in all three adipose tissue depots of obese patients who were insulin-resistant than in BMI-matched individuals who were insulin-sensitive. Such a decrease in adipose tissue AMPK activity has also been found in mice fed a high-fat diet (34), another model of obesity-associated insulin resistance. In addition, Kola et al. (35) have recently reported that AMPK activity is decreased in visceral adipose tissue of patients with Cushing’s syndrome. Individuals with this disorder exhibit many features of the metabolic syndrome, including insulin resistance and central obesity, which as suggested by the authors, may be due to a glucocorticoid-induced decrease in adipose tissue AMPK activity. Presumably such patients would not have inflammation in their adipose tissue because of high levels of circulating glucocorticoids; however, this does not appear to have been directly examined. The exact mechanism responsible for decreased adipose tissue AMPK activity in obese insulin resistant patients is not known. Among the factors that could hypothetically mediate it are decreased circulating levels of adiponectin (36) and/or altered lipolysis (37) since increases in both have been shown to activate AMPK (38; 39). Likewise, as already noted the possibility that inflammation is the primary event and leads to a decrease in AMPK has not been ruled out.
In conclusion, AMPK activity is suppressed in visceral compared to abdominal subcutaneous adipose tissue of massively obese patients undergoing bariatric surgery and this is associated with increased expression of markers of inflammation and infiltration of immune cells. In addition we found that, AMPK activity is lower in adipose tissue of insulin-resistant obese than in BMI-matched insulin sensitive individuals. In freshly isolated adipocytes and adipose tissue of rats in vivo, stimulation of lipolysis by exercise and epinephrine (40) leads to an increase in AMPK activity associated with a decrease in energy state. Similar findings are observed in cultured adipocytes when lipolysis is stimulated (39). When AMPK activation is inhibited pharmacologically or genetically downregulated in such cells, it is accompanied by a further decrease in energy state, oxidative stress and monocyte adhesion (19; 41). Collectively, these results suggest a close link between dysregulation of AMPK activity and the development of adipose tissue dysfunction associated with insulin-resistance. Whether this relationship is causal warrants further investigation.
Acknowledgements
The authors thank Kathleen Tumelty for technical assistance, and Sharon Mosher and Anupriya Mundra for their assistance in preparing the manuscript. This study was supported in part by USPHS grants, RO1 DK19514 and DK067509, PO1-HL68758 and a Mentor-based Fellowship Award from the American Diabetes Association (NR). It was also supported by USDA Contract 58-1950-7-707 and a grant from the Robert C. and Veronica Atkins Foundation (C.M.A). Marie-Soleil Gauthier was supported by a postdoctoral research fellowship from Fonds de la Recherche en Santé du Québec and is a Canadian Diabetes Association fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Parts of this work were presented at the Obesity Society Meeting in Washington DC in October 2009 and published as an abtract (42).
References
- 1.Ruderman NB, Shulman GI. The metabolic Syndrom. In: Jamieson JL, DeGroot LJ, editors. Textbook of Endocrinology. 6th Edition. Philadelphia, PA: Elsevier; 2010. pp. 822–839. [Google Scholar]
- 2.Clement K, Langin D. Regulation of inflammation-related genes in human adipose tissue. J Intern Med. 2007;262:422–430. doi: 10.1111/j.1365-2796.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 3.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elgazar-Carmon V, Rudich A, Hadad N, et al. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high fat feeding. J. Lipid Res. 2008 doi: 10.1194/jlr.M800132-JLR200. M800132-JLR800200. [DOI] [PubMed] [Google Scholar]
- 7.Wu H, Ghosh S, Perrard XD, et al. T-Cell Accumulation and Regulated on Activation, Normal T Cell Expressed and Secreted Upregulation in Adipose Tissue in Obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 8.Duffaut C, Zakaroff-Girard A, Bourlier V, et al. Interplay Between Human Adipocytes and T Lymphocytes in Obesity: CCL20 as an Adipochemokine and T Lymphocytes as Lipogenic Modulators. Arterioscler Thromb Vasc Biol. 2009;29:1608–1614. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 9.Apovian CM, Bigornia S, Mott M, et al. Adipose Macrophage Infiltration Is Associated With Insulin Resistance and Vascular Endothelial Dysfunction in Obese Subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruderman N, Prentki M. AMP Kinase and Malonyl-CoA: Targets for Therapy of the Metabolic Syndrome. Nature Reviews Drug Discovery. 2004;3:340–351. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 11.Viollet B, Mounier R, Leclerc J, et al. Targeting AMP-activated protein kinase as a novel therapeutic approach for the treatment of metabolic disorders. Diabetes Metab. 2007;33:395–402. doi: 10.1016/j.diabet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 13.Cacicedo JM, Yagihashi N, Keaney J, John F, et al. AMPK inhibits fatty acid-induced increases in NF-[kappa]B transactivation in cultured human umbilical vein endothelial cells. Biochemical and Biophysical Research Communications. 2004;324:1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 14.Jeong HW, Hsu KC, Lee J-W, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–E964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- 15.Ruderman NB, Cacicedo JM, Itani S, et al. Malonyl-CoA and AMP-activated protein kinase (AMPK): possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochem. Soc. Trans. 2003;31:202–206. doi: 10.1042/bst0310202. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Kahn BB, Shi H, et al. Macrophage alpha1 AMP-activated Protein Kinase (alpha1AMPK) Antagonizes Fatty Acid-induced Inflammation through SIRT1. Journal of Biological Chemistry. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. 34.31 - 34.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sims EAH. Characterization of the syndromes of obesity. In: Brodoff BN, Bleicher SJ, editors. Diabtes Mellitus and Obesity. Baltimore: Williams & Wilkins; 1982. pp. 219–226. [Google Scholar]
- 19.Gauthier MS, Ruderman NB. Adipose tissue inflammation and insulin resistance: all obese humans are not created equal. Biochemical Journal. 2010;430:e1–e4. doi: 10.1042/BJ20101062. [DOI] [PubMed] [Google Scholar]
- 20.Primeau V, Coderre L, Karelis AD, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) doi: 10.1038/ijo.2010.216. (In Press) [DOI] [PubMed] [Google Scholar]
- 21.Wildman RP, Muntner P, Reynolds K, et al. The Obese Without Cardiometabolic Risk Factor Clustering and the Normal Weight With Cardiometabolic Risk Factor Clustering: Prevalence and Correlates of 2 Phenotypes Among the US Population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 22.Barbarroja N, Lopez-Pedrera R, Mayas MD, et al. The obese healthy paradox: is inflammation the answer? Biochemical Journal. 2010;430:141–149. doi: 10.1042/BJ20100285. [DOI] [PubMed] [Google Scholar]
- 23.Puri V, Ranjit S, Konda S, et al. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proceedings of the National Academy of Sciences. 2008;105:7833–7838. doi: 10.1073/pnas.0802063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martínez-Agustin O, Hernández-Morante JJ, Martínez-Plata E, et al. Differences in AMPK expression between subcutaneous and visceral adipose tissue in morbid obesity. Regulatory Peptides. 2010;163:31–36. doi: 10.1016/j.regpep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Bjorntorp P. Visceral obesity: a "civilization syndrome". Obes Res. 1993;1:206–222. doi: 10.1002/j.1550-8528.1993.tb00614.x. [DOI] [PubMed] [Google Scholar]
- 26.Peiris AN, Sothmann MS, Hoffmann RG, et al. Adiposity, fat distribution, and cardiovascular risk. Ann Intern Med. 1989;110:867–872. doi: 10.7326/0003-4819-110-11-867. [DOI] [PubMed] [Google Scholar]
- 27.Murano I, Barbatelli G, Parisani V, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J. Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Olefsky JM, Glass CK. Macrophages, Inflammation, and Insulin Resistance. Annual Review of Physiology. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 29.Ido Y, Carling D, Ruderman N. Hyperglycemia-Induced Apoptosis in Human Umbilical Vein Endothelial Cells. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg GR, Michell BJ, van Denderen BJW, et al. Tumor necrosis factor [alpha]-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metabolism. 2006;4:465–474. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Song P, Xu J, et al. Activation of Protein Phosphatase 2A by Palmitate Inhibits AMP-activated Protein Kinase. Journal of Biological Chemistry. 2007;282:9777–9788. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- 32.Yoshizaki T, Milne JC, Imamura T, et al. SIRT1 Exerts Anti-Inflammatory Effects and Improves Insulin Sensitivity in Adipocytes. Mol. Cell. Biol. 2009;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan F, Cacicedo JM, Ruderman N, et al. SIRT1 Modulation of the Acetylation Status, Cytosolic Localization, and Activity of LKB1: Possible Role in AMP-Activated Protein Kinase Activation. J. Biol. Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaidhu MP, Anthony NM, Patel P, et al. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol. 2010;298:C961–C971. doi: 10.1152/ajpcell.00547.2009. [DOI] [PubMed] [Google Scholar]
- 35.Kola B, Christ-Crain M, Lolli F, et al. Changes in Adenosine 5'-Monophosphate-Activated Protein Kinase as a Mechanism of Visceral Obesity in Cushing's Syndrome. J Clin Endocrinol Metab. 2008;93:4969–4973. doi: 10.1210/jc.2008-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbasi F, Chu JW, Lamendola C, et al. Discrimination Between Obesity and Insulin Resistance in the Relationship With Adiponectin. Diabetes. 2004;53:585–590. doi: 10.2337/diabetes.53.3.585. [DOI] [PubMed] [Google Scholar]
- 37.Jocken JWE, Blaak EE. Catecholamine-induced lipolysis in adipose tissue and skeletal muscle in obesity. Physiology & Behavior. 2008;94:219–230. doi: 10.1016/j.physbeh.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Tomas E, Tsao T-S, Saha AK, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: Acetylâ "CoA carboxylase inhibition and AMP-activated protein kinase activation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gauthier M-S, Miyoshi H, Souza SC, et al. AMP-activated Protein Kinase Is Activated as a Consequence of Lipolysis in the Adipocyte: Potential Mechanism and Physiological Relevance. J. Biol. Chem. 2008;283:16514–16524. doi: 10.1074/jbc.M708177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koh H-J, Hirshman MF, He H, et al. Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem J. 2007;403:473–481. doi: 10.1042/BJ20061479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gauthier MS, Tao R, Luo ZJ, et al. Inhibition of AMP-Activated Protein Kinase Activation During the Stimulation of Lipolysis Augments Energy Depletion, Oxidative Stress, and Inflammation in 3T3-L1 Adipocytes. Obesity. 2008;16:S73–S73. [Google Scholar]
- 42.Gauthier MS, O'Brien E, Bigornia SJ, et al. Decreased AMP-Activated Protein Kinase Activity is Associated with Markers of Inflammation and Infiltration of Immune Cells in Visceral and Subcutaneous Adipose Tissue, and with Whole-Body Insulin Resistance in Obese Patients. Obesity. 2009;17:S93–S93. [Google Scholar]