Abstract
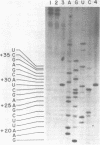
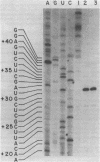
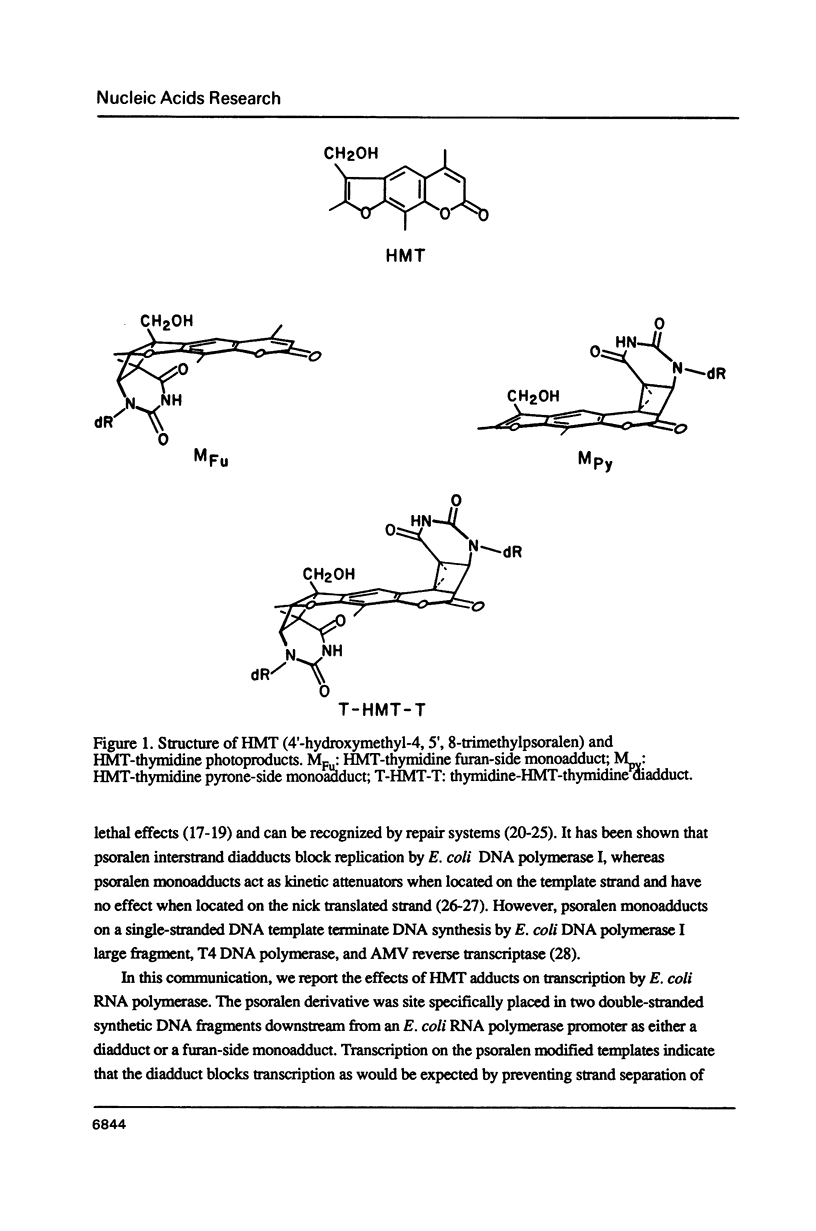
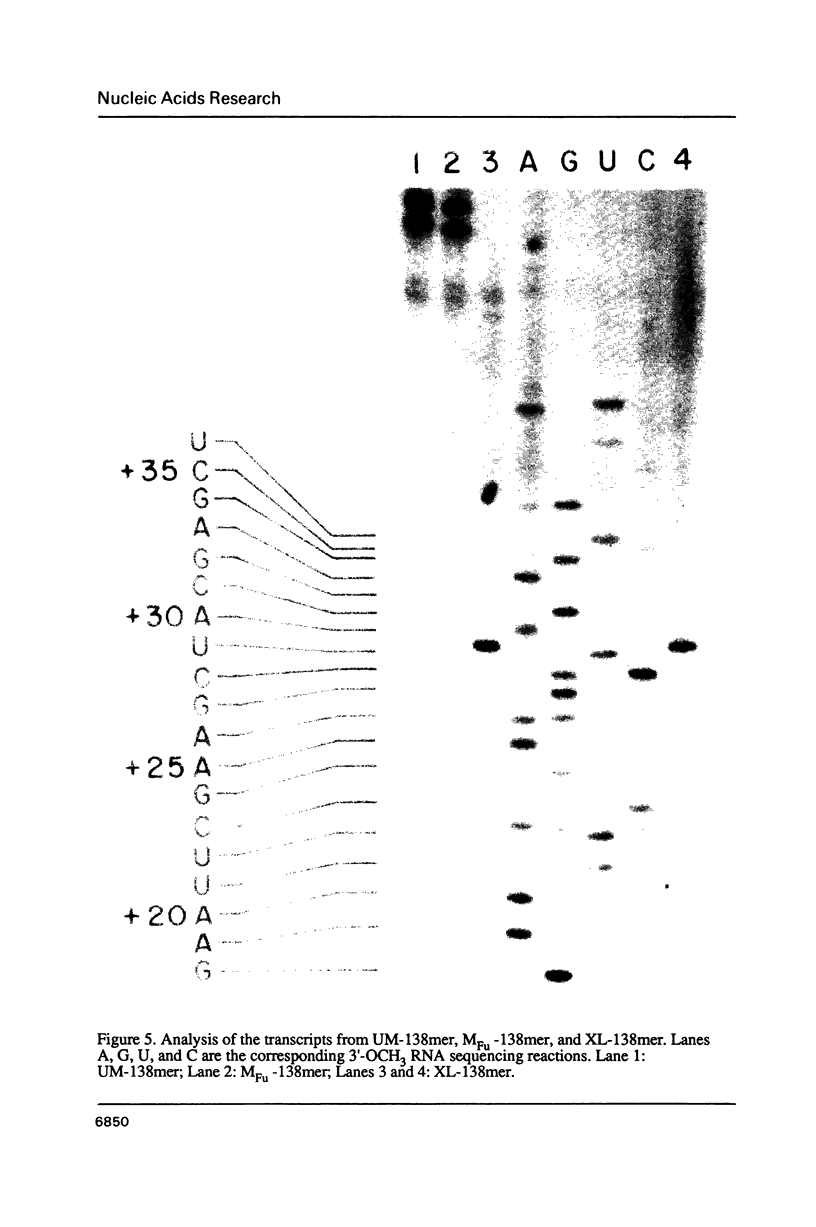
Synthetic DNA substrates containing a site-specifically engineered psoralen monoadduct or diadduct were used to characterize the response of the E. coli RNA polymerase elongation complex to these lesions. The psoralen derivative HMT (4'-hydroxymethyl-4,5', 8-trimethylpsoralen) was site specifically placed into two synthetic double-stranded DNA fragments each of which contained an E. coli RNA polymerase promoter at one end. The HMT molecule was attached to the middle of the DNA fragments as either a furan-side monoadduct or an interstrand diadduct. Transcription off the HMT crosslinked DNA templates showed that E. coli RNA polymerase terminated at the HMT diadduct site, i. e., one nucleotide before the modified thymidine residue on the template strand. The furan-side monoadduct when on the template strand also blocked transcription by the polymerase. However, no effect on transcription was observed when the monoadduct was located on the non-template strand.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohr V., Nielsen P. E. Psoralen-DNA crosslink repair in human lymphocytes. Comparison of alkaline elution with electron microscopy. Biochim Biophys Acta. 1984 Nov 22;783(2):183–186. doi: 10.1016/0167-4781(84)90012-5. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Meyer L. M., Pederson T. Small nuclear RNA U2 is base-paired to heterogeneous nuclear RNA. Science. 1982 Jul 30;217(4558):456–458. doi: 10.1126/science.6178162. [DOI] [PubMed] [Google Scholar]
- Cassier C., Chanet R., Moustacchi E. Mutagenic and recombinogenic effects of DNA cross-links induced in yeast by 8-methoxypsoralen photoaddition. Photochem Photobiol. 1984 Jun;39(6):799–803. doi: 10.1111/j.1751-1097.1984.tb08862.x. [DOI] [PubMed] [Google Scholar]
- Chan G. L., Doetsch P. W., Haseltine W. A. Cyclobutane pyrimidine dimers and (6-4) photoproducts block polymerization by DNA polymerase I. Biochemistry. 1985 Oct 8;24(21):5723–5728. doi: 10.1021/bi00342a006. [DOI] [PubMed] [Google Scholar]
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Cimino G. D., Shi Y. B., Hearst J. E. Wavelength dependence for the photoreversal of a psoralen-DNA cross-link. Biochemistry. 1986 May 20;25(10):3013–3020. doi: 10.1021/bi00358a042. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Beardsley G. P. Thymine glycol lesions terminate chain elongation by DNA polymerase I in vitro. Nucleic Acids Res. 1986 Jan 24;14(2):737–749. doi: 10.1093/nar/14.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H. Photobiological activity of 4-methylpsoralen and 4-methyl-4',5'-dihydropsoralen with respect to lethal and mutagenic effects on E. coli, and prophage induction. Photochem Photobiol. 1984 Jun;39(6):835–839. doi: 10.1111/j.1751-1097.1984.tb08868.x. [DOI] [PubMed] [Google Scholar]
- Gamper H. B., Hearst J. E. A topological model for transcription based on unwinding angle analysis of E. coli RNA polymerase binary, initiation and ternary complexes. Cell. 1982 May;29(1):81–90. doi: 10.1016/0092-8674(82)90092-7. [DOI] [PubMed] [Google Scholar]
- Gamper H., Piette J., Hearst J. E. Efficient formation of a crosslinkable HMT monoadduct at the Kpn I recognition site. Photochem Photobiol. 1984 Jul;40(1):29–34. doi: 10.1111/j.1751-1097.1984.tb04549.x. [DOI] [PubMed] [Google Scholar]
- Garrett-Wheeler E., Lockard R. E., Kumar A. Mapping of psoralen cross-linked nucleotides in RNA. Nucleic Acids Res. 1984 Apr 11;12(7):3405–3423. doi: 10.1093/nar/12.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawalt P. C. Intragenomic heterogeneity in DNA damage processing: potential implications for risk assessment. Basic Life Sci. 1986;38:489–498. doi: 10.1007/978-1-4615-9462-8_51. [DOI] [PubMed] [Google Scholar]
- Ide H., Kow Y. W., Wallace S. S. Thymine glycols and urea residues in M13 DNA constitute replicative blocks in vitro. Nucleic Acids Res. 1985 Nov 25;13(22):8035–8052. doi: 10.1093/nar/13.22.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Rabkin S. D., Osborn A. L., King C. M., Strauss B. S. Effect of acetylated and deacetylated 2-aminofluorene adducts on in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7166–7170. doi: 10.1073/pnas.79.23.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocentini S. Impairment of RNA synthesis and its recovery in angelicin photosensitized mammalian cells. A probe for DNA damage and repair. Biochim Biophys Acta. 1978 Nov 21;521(1):160–168. doi: 10.1016/0005-2787(78)90259-9. [DOI] [PubMed] [Google Scholar]
- Ou C. N., Tsai C. H., Tapley K. J., Jr, Song P. S. Photobinding of 8-methoxypsoralen and 5,7-dimethoxycoumarin to DNA and its effect on template activity. Biochemistry. 1978 Mar 21;17(6):1047–1053. doi: 10.1021/bi00599a017. [DOI] [PubMed] [Google Scholar]
- Parsons B. J. Psoralen photochemistry. Photochem Photobiol. 1980 Dec;32(6):813–821. doi: 10.1111/j.1751-1097.1980.tb04061.x. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Crothers D. M. Kinetics and sequence specificity of drug-DNA interactions: an in vitro transcription assay. Biochemistry. 1986 Nov 18;25(23):7355–7362. doi: 10.1021/bi00371a017. [DOI] [PubMed] [Google Scholar]
- Piette J. G., Hearst J. E. Termination sites of the in vitro nick-translation reaction on DNA that had photoreacted with psoralen. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5540–5544. doi: 10.1073/pnas.80.18.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Decuyper-Debergh D., Gamper H. Mutagenesis of the lac promoter region in M13 mp10 phage DNA by 4'-hydroxymethyl-4,5',8-trimethylpsoralen. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7355–7359. doi: 10.1073/pnas.82.21.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Hearst J. Sites of termination of in vitro DNA synthesis on psoralen phototreated single-stranded templates. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Sep;48(3):381–388. doi: 10.1080/09553008514551381. [DOI] [PubMed] [Google Scholar]
- Piette J., Moore P. D. DNA synthesis on phi X174 template damaged by proflavine and light treatment. Photochem Photobiol. 1982 May;35(5):705–708. doi: 10.1111/j.1751-1097.1982.tb02633.x. [DOI] [PubMed] [Google Scholar]
- Prager A., Green M., Ben-Hur E. Inhibition of ornithine decarboxylase induction by psoralen plus near ultraviolet light in human cells: the role of monoadducts vs DNA crosslinks. Photochem Photobiol. 1983 May;37(5):525–528. doi: 10.1111/j.1751-1097.1983.tb04512.x. [DOI] [PubMed] [Google Scholar]
- Reisbig R. R., Hearst J. E. Escherichia coli deoxyribonucleic acid dependent ribonucleic acid polymerase transcriptional pause sites on SV40 DNA F1. Biochemistry. 1981 Mar 31;20(7):1907–1918. doi: 10.1021/bi00510a029. [DOI] [PubMed] [Google Scholar]
- Rinke J., Appel B., Digweed M., Lührmann R. Localization of a base-paired interaction between small nuclear RNAs U4 and U6 in intact U4/U6 ribonucleoprotein particles by psoralen cross-linking. J Mol Biol. 1985 Oct 20;185(4):721–731. doi: 10.1016/0022-2836(85)90057-9. [DOI] [PubMed] [Google Scholar]
- Saffran W. A., Cantor C. R. Mutagenic SOS repair of site-specific psoralen damage in plasmid pBR322. J Mol Biol. 1984 Sep 25;178(3):595–609. doi: 10.1016/0022-2836(84)90240-7. [DOI] [PubMed] [Google Scholar]
- Sagher D., Strauss B. Abasic sites from cytosine as termination signals for DNA synthesis. Nucleic Acids Res. 1985 Jun 25;13(12):4285–4298. doi: 10.1093/nar/13.12.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setyono B., Pederson T. Ribonucleoprotein organization of eukaryotic RNA. XXX. Evidence that U1 small nuclear RNA is a ribonucleoprotein when base-paired with pre-messenger RNA in vivo. J Mol Biol. 1984 Apr 5;174(2):285–295. doi: 10.1016/0022-2836(84)90339-5. [DOI] [PubMed] [Google Scholar]
- Shames B., Kalma S., Heimer Y. M., Tal J. Differential inhibition of cellular RNAs by photosensitized trioxalen. Nucleic Acids Res. 1983 Sep 24;11(18):6453–6464. doi: 10.1093/nar/11.18.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. B., Hearst J. E. Wavelength dependence for the photoreactions of DNA-psoralen monoadducts. 1. Photoreversal of monoadducts. Biochemistry. 1987 Jun 30;26(13):3786–3792. doi: 10.1021/bi00387a008. [DOI] [PubMed] [Google Scholar]
- Shi Y. B., Hearst J. E. Wavelength dependence for the photoreactions of DNA-psoralen monoadducts. 2. Photo-cross-linking of monoadducts. Biochemistry. 1987 Jun 30;26(13):3792–3798. doi: 10.1021/bi00387a009. [DOI] [PubMed] [Google Scholar]
- Shi Y., Hearst J. E. Thermostability of double-stranded deoxyribonucleic acids: effects of covalent additions of a psoralen. Biochemistry. 1986 Oct 7;25(20):5895–5902. doi: 10.1021/bi00368a009. [DOI] [PubMed] [Google Scholar]
- Song P. S., Tapley K. J., Jr Photochemistry and photobiology of psoralens. Photochem Photobiol. 1979 Jun;29(6):1177–1197. doi: 10.1111/j.1751-1097.1979.tb07838.x. [DOI] [PubMed] [Google Scholar]
- Tessman J. W., Isaacs S. T., Hearst J. E. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. Conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985 Mar 26;24(7):1669–1676. doi: 10.1021/bi00328a015. [DOI] [PubMed] [Google Scholar]
- Turner S., Noller H. F. Identification of sites of 4'-(hydroxymethyl)-4,5',8-trimethylpsoralen cross-linking in Escherichia coli 23S ribosomal ribonucleic acid. Biochemistry. 1983 Aug 16;22(17):4159–4164. doi: 10.1021/bi00286a026. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. W., Dervan P. B. Chromomycin, mithramycin, and olivomycin binding sites on heterogeneous deoxyribonucleic acid. Footprinting with (methidiumpropyl-EDTA)iron(II). Biochemistry. 1983 May 10;22(10):2373–2377. doi: 10.1021/bi00279a011. [DOI] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Hearst J. E., Sancar A. Construction of DNA substrates modified with psoralen at a unique site and study of the action mechanism of ABC excinuclease on these uniformly modified substrates. J Biol Chem. 1986 Oct 25;261(30):14135–14141. [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Holbrook S. R., Hearst J. E., Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen W. P., Jeppesen C., Nielsen P. E. Repair in Escherichia coli of a psoralen-DNA interstrand crosslink site specifically introduced into T410A411 of the plasmid pUC 19. Photochem Photobiol. 1986 Jul;44(1):47–51. doi: 10.1111/j.1751-1097.1986.tb03562.x. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel P. H., Bear D. G., Morgan W. D., McSwiggen J. A. Protein-nucleic acid interactions in transcription: a molecular analysis. Annu Rev Biochem. 1984;53:389–446. doi: 10.1146/annurev.bi.53.070184.002133. [DOI] [PubMed] [Google Scholar]