Abstract
Background
Cost-effectiveness analyses of asthma controller regimens for adults exist, but similar evaluations exclusively for children are few.
Objective
To compare the cost-effectiveness of two commonly used asthma controllers, fluticasone and montelukast, with data from the Pediatric Asthma Controller Trial.
Methods
We compared the cost-effectiveness of low-dose fluticasone with montelukast in a randomized controlled multi-center clinical trial in children with mild-moderate persistent asthma. Analyses were also conducted on subgroups based on phenotypic factors. Effectiveness measures included a) the number of asthma-control days, b) the percentage of participants with an increase over baseline of FEV1≥12%, and c) the number of exacerbations avoided. Costs were analyzed from both a US health care payer's perspective and a societal perspective.
Results
For all cost-effectiveness measures studied, fluticasone cost less and was more effective than montelukast; e.g., fluticasone treatment cost $430 less in mean direct cost (P<0.01) and had 40 more asthma control days (P<0.01) during the 48 week study period. Considering sampling uncertainty, fluticasone cost less and was more effective at least 95% of the time. For the high eNO phenotypic subgroup (eNO≥25ppb) and more responsive PC20 subgroup (PC20<2 mg/mL), fluticasone was cost-effective compared with montelukast for all cost-effectiveness measures; whereas not all the effectiveness measures were statistically different for the other two phenotypic subgroups.
Conclusion
For children with mild-moderate persistent asthma, low dose fluticasone had lower cost and higher effectiveness compared with montelukast, especially in those with more airway inflammation as indicated by elevated levels of eNO and more responsivity to methacholine.
Keywords: Cost-effectiveness analysis, childhood asthma, fluticasone, montelukast, PACT
INTRODUCTION
Asthma is costly to treat with US direct health care costs of $14.7 billion in 2007.1 Given that nine million children in the US have asthma2 and that children account for 44% of all asthma hospitalizations,3 cost-effectiveness of therapies to treat childhood asthma is an important consideration.
In cost-effectiveness analysis (CEA) of medications for treating asthma, there are many publications based on data from outside the US.4–13 However, the US system has not been explored adequately, especially for pediatric asthma. Furthermore, although there have been many studies for asthma treatment in adults or combined analysis of adults and adolescents, there have been very few CEA done exclusively for children.14, 15 We used data collected from the Pediatric Asthma Controller Trial (PACT) 16 to compare cost effectiveness of two drugs commonly used in pediatric asthma, montelukast and low-dose fluticasone therapy.
METHODS
Study design
The PACT study was a randomized, controlled, double-blind trial conducted by the Childhood Asthma Research and Education (CARE) Network. Participants were recruited at five study centers (Denver and Tucson, CO; Madison, WI; San Diego, CA; St. Louis, MO) between October 2002 and January 2004. The PACT studied three treatment regimens in children with mild-moderate persistent asthma: 1) low-dose fluticasone 100 µg dry powder inhaler (DPI) twice daily (fluticasone monotherapy); 2) montelukast 5 mg in the evening; and 3) fluticasone 100 µg/salmeterol 50 µg DPI in the morning together with salmeterol 50 µg DPI in the evening (PACT combination regimen).16 Placebo Diskus and placebo oral drug were given to ensure double-blindness. All Diskus devices looked identical. Adherence to both inhaled medication and oral medication was assessed. Inclusion criteria were age 6 to less than 14 years, physician-diagnosed asthma, an FEV1 ≥80% predicted normal at screening and ≥70% predicted normal at randomization, a methacholine FEV1 PC20 ≤12.5 mg/mL, and ability to perform reproducible spirometry. Exclusion criteria and other design descriptions of PACT and primary study outcomes can be found in Sorkness et al.16 Results from PACT demonstrated the superiority of fluticasone monotherapy over montelukast for most asthma control, lung function and inflammatory marker outcomes. Since the combination regimen is not commercially available and was devised specifically for PACT, this CEA study will focus on the commercially available and commonly used inhaled corticosteroid, fluticasone, and the leukotriene receptor antagonist, montelukast.
Participants
This CEA studies only the two monotherapy treatment groups in PACT in which there were 96 children treated with fluticasone propionate 100 mcg twice daily (Flovent Diskus; GlaxoSmithKline, Research Triangle Park, NC) and 95 with montelukast 5 mg in the evening (Singulair; Merck, Whitehouse Station, NJ).16 Participants who did not complete the entire 48 weeks of PACT or did not have information on the variables of interest were excluded, resulting in a total of 154 participants for this CEA, 79 that received fluticasone and 75 that received montelukast. Data were collected from daily diaries, study visits, and phone contacts throughout the trial.
Effectiveness measures
Given the multiple domains of asthma, multiple measures of effectiveness were applied including asthma control days (ACD),5 improvement in FEV1, 17, 18 and the number of exacerbations avoided5, 19 for a comprehensive CEA. An ACD, the primary outcome in PACT, was defined as a day without albuterol rescue, prednisone for asthma, or non-study asthma medications as well as no daytime symptoms, nighttime awakenings, unscheduled health care visits, emergency department visits, hospitalizations for asthma, or school absenteeism for asthma. The improvement of FEV1 was measured by the percentage of participants who had ≥12% increase in FEV1 compared to baseline.17, 18 Thus, a value of 30 in the improvement of FEV1 means 30% of the participants had achieved an increase of FEV1≥12% at the last study visit. An asthma exacerbation was defined by prespecified criteria of worsening asthma by symptoms such as coughing, dyspnea, chest tightness and/or wheezing, or by a decrease in the patient’s PEF. 16
Cost measures
Both direct costs from a third-party payer’s perspective and societal costs from a societal perspective 20, 21 were measured for the 48-week study period. Since the PACT study was conducted in 2002–2004, costs in 2003 US dollars were used as an approximation.
Table 1 shows the unit costs used in this study. Unit costs for emergency department visits and regular physician costs were expressed in 2003 dollars previously reported. 18 The monetary value of lost productivity from missed school or work was estimated by the Human Capital approach,20–24 using the same value ($162.5 at the 1999 price level) previously reported 24 with the cost inflator being the index of the average weekly earnings.25 The drug costs were the average wholesale prices from the Drug Topics Red Book in 2003.26 Because Flovent Diskus (GlaxoSmithKline, fluticasone propionate Diskus, 100µg, DPI) was not yet available in the US at the time of the study period, the price of its then equivalent, Flovent Rotadisk, (GlaxoSmithKline, fluticasone propionate Rotadisk, 100µg, DPI) was used. Hospitalization costs were not included because there were no hospitalizations in these two study arms.
Table 1.
Estimates of unit costs (in 2003 US dollars)
| Direct Costs | Unit cost ($) |
|---|---|
| Emergency department visit | 296.95 |
| Physician office visit | 45.57 |
| Medication costs | |
| Fluticasone DPI treatment (per day) | 1.87 |
| Montelukast treatment (per day) | 2.93 |
| Indirect Costs | |
| Missed day of school or work | 164.92 |
The direct costs for a participant were the sum of the costs from asthma-related medication, emergency department visits, and regular physician visits, where each cost component was computed as the unit price times the corresponding quantity. The societal costs were the direct costs plus productivity losses from asthma-induced missed school or work. The mean cost and the mean effectiveness of a treatment group were the average cost and effectiveness across all participants in that group.
Cost-effectiveness analysis
CEA compares the effectiveness of different treatments relative to their costs. Suppose that hypothetical treatment A has better effectiveness; if it also has lower cost than hypothetical treatment B, then A is said to dominate B. However, if treatment A is not the dominant strategy, i.e., A has better effectiveness together with higher cost than treatment B, then the choice decision is made by comparing a decision maker’s willingness to pay (WTP) for one unit of effect with incremental cost-effectiveness ratio (ICER). ICER measures the additional cost for one additional unit of effect, i.e., ICER=Δcost/Δeffect, where Δ stands for difference between the two candidate therapies. If WTP is greater than ICER, then one is willing to pay the additional cost of treatment A to gain its additional effect, and treatment A is cost-effective. Note that unlike ACD, asthma exacerbation is an undesirable outcome; so for CEA regarding asthma exacerbation, the denominator in the ICER is defined as the number of asthma exacerbations avoided, i.e., the inverse of the difference in the number of exacerbations rather than the usual raw difference between the two treatments.
CEA was first done for the 154 participants, and then for subgroups based on phenotypic factors eNO and PC20, which were shown to have predictive values in treatment responses between fluticasone and montelukast. 27 We used the cutoffs of 25ppb for eNO and 2mg/mL for PC20 which were previously reported to discriminate between fluticasone and montelukast response. 27
Statistical Methods
The above deterministic CEA was used for the original sample. A probabilistic approach to account for sampling uncertainty was conducted by nonparametric bootstrap. The 95% confidence interval for an estimate of the ICER was defined by the 2.5% percentile through the 97.5% percentile of the corresponding values from all bootstrapped samples.
Two-sample t-tests were used to compare the group difference in mean between fluticasone and montelukast for variables that were continuous or could be treated as continuous. Two-sample z-tests for proportions were used to compare the distribution of dichotomous variables. For count variables, simple Poisson regression was used with the treatment group as the predictor. The Mann-Whitney U test is conducted to test the difference in median for baseline eNO and PC20.
RESULTS
Among the 154 participants eligible for this analysis, there were no statistical differences in age, gender, height gained during the study, baseline FEV1, eNO and PC20 between the fluticasone and montelukast arms (Table 2).
Table 2.
Comparison of demographic and outcome features between fluticasone and montelukast
| Variable | Fluticasone (n=79) |
Montelukast (n=75) |
P Value |
|---|---|---|---|
| Age in years (mean± SD) | 9.7± 2.2 | 9.7± 2.2 | 0.94 a |
| Sex | |||
| Male, n (%) | 47 (59) | 48 (64) | 0.57 b |
| Female, n (%) | 32 (41) | 27 (36) | |
| Height increase in cm (SD) | 5.4 (1.8) | 5.8 (1.9) | 0.16 a |
| Baseline FEV1, mean (SD) | 1.86 (0.56) | 1.96 (0.53) | 0.24 a |
| Baseline eNO, ppb, median (Quartile 1, Quartile 3) | 24.5(13.0,48.5) | 29.4(12.7,55.4) | 0.82 c |
| Baseline Methacholine PC20, mg/mL, median (Quartile 1, Quartile 3) | 0.76(0.27,2.81) | 0.85(0.28,2.57) | 0.95 c |
| Outcomes (mean ± SD) | |||
| Treatment Exposure days | 336±17.6 | 338±19.8 | 0.49 a |
| Asthma control days during study period | 210±97 | 170±90 | 0.009 a |
| Percentage with an increase of FEV1≥12% | 73 | 41 | <.001 b |
| Number of exacerbations | 0.66±0.9 | 1.13±1.1 | 0.002 d |
| Emergency department visits | 0.10±0.3 | 0.35±0.6 | 0.002 d |
| Physician office visits | 2.19±1.9 | 2.08±1.8 | 0.64 d |
| Hospital days | 0 | 0 | N/A |
| Missed school days | 1.4±2.5 | 2.1±3.1 | <.001 d |
| Missed work days | 0.6±1.5 | 0.8±1.9 | 0.06 d |
by two-sample t-tests for difference in mean.
by two-sample z-tests for proportions.
by the Mann-Whitney U test for difference in median.
by simple Poisson regression with the treatment group as the predictor.
Effectiveness
The comparison of outcome features (Table 2) showed a significant higher effectiveness of fluticasone compared to montelukast with respect to ACD, percentage of participants with increase in FEV1 ≥ 12%, and number of asthma exacerbations (all P<0.01).
Costs
Based on the unit cost estimates for asthma care, the direct costs in the 48-week study period were $759 in 2003 dollars for fluticasone and $1189 for montelukast (p<.001). The societal costs were $1075 for fluticasone and $1673 for montelukast (p<.001). Since fluticasone had lower costs and higher effectiveness, fluticasone dominated montelukast with respect to all pairs of cost-effectiveness measures.
The ICER values, with 95% confidence intervals (Table 3) revealed direct cost savings from using fluticasone were $11 for one more ACD, $13 for one percentage point more of participants with a ≥12% improvement in FEV1, and $916 for one exacerbation avoided.
Table 3.
Incremental Cost Effectiveness Ratio for fluticasone vs. montelukast during the study period 1
|
ΔDC ΔACD |
ΔDC ΔFEV |
ΔDC Avoided Exacer 1 |
ΔSC ΔACD |
ΔSC ΔFEV |
ΔSC Avoided Exacer 1 |
|---|---|---|---|---|---|
| −11 (−6, −42) | −13 (−26, −9) | −916 (−2095, −531) | −15 (−55, −8) | −19 (−10, −38) | −1272 (−2616, −745) |
ICER=Δcost/Δeffect, where Δ stands for difference between fluticasone and montelukast. DC: direct costs; SC: societal costs. The negative sign of ICER resulted from the fact that fluticasone had lower costs and higher effectiveness than montelukast. The negative sign of 95% CI indicated that the dominance of fluticasone held at least 95% of the time.
Uncertainty analysis
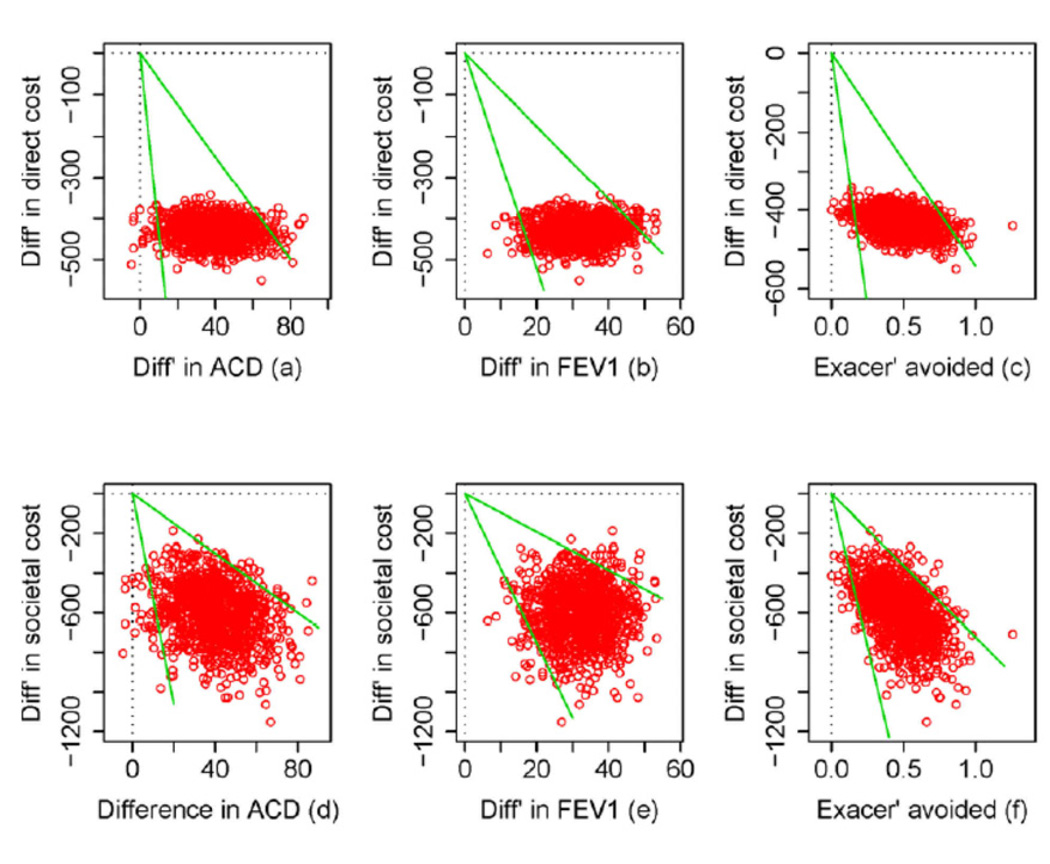
Scatter-plots of cost difference vs. effectiveness difference on the cost-effectiveness plane revealed that for all the cost-effectiveness measures, fluticasone had lower costs and higher effectiveness in at least 95% of the bootstrapped samples (See Figure E1 in the Online Repository at http://www.jacionline.org). Thus, the probability of fluticasone being cost-effective compared with montelukast was at least 95% considering sampling uncertainty.
Subgroup cost effectiveness analyses
Higher eNO levels and lower PC20 values predicted superiority of fluticasone over montelukast for pediatric asthma control in the PACT trial; e.g., increased baseline eNO levels predicted the expected treatment benefit with fluticasone over montelukast regarding the gain in ACDs. 27 Based on those findings, a subgroup analysis of CEA was conducted to examine whether the cost-effectiveness of fluticasone still held for these phenotype subgroups.
For the high eNO subgroup (eNO≥25ppb), fluticasone dominated montelukast at least 95% of the time by bootstrap analysis for each of the cost-effectiveness measures (Table 4). For the low eNO subgroup (eNO<25ppb), fluticasone dominated montelukast with respect to improvement in FEV1; but there was no statistical difference in effectiveness between fluticasone and montelukast regarding ACD or the number of exacerbations avoided.
Table 4.
Incremental Cost Effectiveness Ratio in subgroups based on baseline levels of eNO and PC20 1
| Group |
ΔDC ΔACD |
ΔDC ΔFEV |
ΔDC Avoided Exacer 1 |
ΔSC ΔACD |
ΔSC ΔFEV |
ΔSC Avoided Exacer 1 |
|---|---|---|---|---|---|---|
| A. low eNO | 71# | −12* (−45, −7) | −1940 # | 95# | −17* (−61, −2) | −2605 # |
| B. high eNO | −5* (−9, −4) | −14* (−36, −8) | −640* (−1617, −389) | −8* (−13, −5) | −21* (−56, −11) | −964* (−2138, −597) |
| C. low PC20 | −5 * (−9, −4) | −10 * (−17, −7) | −621* (−1532, −400) | −10 * (−17, −8) | −18 * (−10, −35) | −1125 * (−2516, −661) |
| D. high PC20 | 12 # | −33 # | −6157 # | 7 # | −20 # | −3707 # |
A. Fluticasone N = 43 and Montelukast N = 35
B. Fluticasone N = 36 and Montelukast N = 40
C. Fluticasone N = 54 and Montelukast N =51
D. Fluticasone N = 25 and Montelukast N =24
fluticasone dominated montelukast at least 95% of the time in bootstrap analysis.
no statistically significant difference in effectiveness between fluticasone and montelukast.
This table shows that fluticasone dominated montelukast in the high eNO subgroup and the more responsive PC20 subgroup during the 48-week study period.
In the more responsive PC20 subgroup (PC20<2 mg/mL), fluticasone dominated montelukast for all of the cost-effectiveness measures considered. In the less responsive PC20 subgroup (PC20≥2 mg/mL), there was no significant difference in effectiveness between fluticasone and montelukast. The insignificance of effectiveness difference in the low eNO and less responsive PC20 phenotypic subgroups may be partly due to the modest sample sizes in the subgroup analysis.
Sensitivity analyses
A participant’s success in improving FEV1 was defined as an improvement ≥12%. For sensitivity analysis, we varied this definition between 10 and 15 percent, and fluticasone still dominated montelukast at least 95% of the time. A sensitivity analysis with respect to the unit cost of health care utilization in Table 1 was conducted by replacing the unit cost estimate one at a time with 0.5, 1.5 or 2 times its original estimate. Sensitivity analysis on drug costs was done by simultaneously changing both fluticasone and montelukast drug costs to 0.9 or 1.1 times their original values with fluticasone consistently dominating montelukast.
Since the metered dose inhaler (MDI) formulation of fluticasone is widely used, a sensitivity analysis was conducted to compare fluticasone in the MDI formulation with montelukast. For cost comparison purposes, the MDI formulation of fluticasone used in sensitivity analysis was Flovent HFA (by GlaxoSmithKline), and the cost of fluticasone also included the cost of a spacer device. The MDI formulation of fluticasone had lower direct and societal costs compared with monetlukast. If the effectiveness of the MDI formulation is assumed to be the same as that of the DPI formulation although they may not be exactly the same, then fluticasone in the MDI formulation can be considered cost-effective. For instance, it would cost 12 dollars less in direct costs for fluticasone in the MDI formulation compared with montelukast for each additional ACD.
A sensitivity analysis that included all the 191 randomized subjects in the cost-effectiveness analysis was conducted by extrapolating missing values. For each variable of interest, missing values were extrapolated by the average of all the participants in each drug arm who had recorded values on that variable. With 191 subjects included, results were similar to those from the original cost-effectiveness analysis. Again fluticasone had lower direct and societal costs and higher effectiveness, and was cost-effective using the intent-to-treat approach. For instance, fluticasone cost 16 dollar less in societal costs for each additional ACD. Other extrapolation methods were also used, and the cost-effectiveness of fluticasone was robust.
DISCUSSION
The present CEA studies the cost-effectiveness of fluticasone vs. montelukast treatment for children with mild to moderate persistent asthma using data from the PACT clinical trial.16 The fluticasone treatment was shown to be cost-effective compared with montelukast both from point estimates and bootstrap simulations for all six cost-effectiveness measures analyzed.
To our knowledge, this report is the first to formally perform a comprehensive CEA comparing fluticasone and montelukast in mild-moderate childhood asthma based on a randomized trial conducted in the US. Previous CEA of asthma treatments compared other controller regimens.5–10, 19, 24, 28–30 Two studies,31, 32 though not formal CEA, compared the efficacy of fluticasone with montelukast relative to their costs using retrospective insurance claims data. With claims data on patients 4–17 years in 2001–2003, Stempel et al.32 found annualized asthma-related costs of $861 for fluticasone and $1616 for montelukast. Pathak et al.31 identified the annual treatment charges to be $572 (in 1999 dollars) for fluticasone and $902 for montelukast in patients 4–45 years of age. Both studies found fewer hospitalizations for those treated with fluticasone. The present study extends prior claims data based retrospective analyses of heterogeneous populations with no clinical outcome measures by showing conclusively that fluticasone was cost-effective compared with montelukast using data from a prospective clinical trial. Sensitivity analyses show that the cost-effectiveness of fluticasone over montelukast was robust in a wide range of settings, ensuring the generaliziblity of our study.
The present CEA also is the first to conduct subgroup analyses based on asthma phenotypic characteristics, and the cost-effectiveness of fluticasone over montelukast was substantiated for phenotypes indicating higher degrees of airway inflammation and hyper-responsiveness. This phenotypic subgroup analysis has similar implications as in Knuffman et al.27 in that baseline eNO levels greater than 25 ppb and PC20 value less than 2 mg/mL were more likely to show superiority of fluticasone over montelukast.
This study has several limitations. The unit cost estimates were taken from sources on pediatric asthma, but the inflators were based on the entire population rather than exclusively on children to adjust costs to the year 2003. Though this method was not ideal, sensitivity analysis showed the results were robust to a wide range of unit costs.
As to the societal cost, the monetary loss of productivity from missed school or work would vary greatly depending on the estimation method. We employed the Human Capital approach and used a published estimate, which assumed the loss to be a caregiver’s earnings. Methods to more accurately measure the societal cost of pediatric asthma are left for future study.
The PACT study found no significant difference in height growth between the two treatment groups.16 So no steroid effect on growth was considered in the cost-effectiveness analysis. The incorporation of potential steroid effect on growth into cost-effectiveness analysis was left for future research.
Rescue treatment in PACT included telephone contact with the study physician who would recommend starting oral corticosteroids if indicated by the study protocol. These contacts as well as use of oral corticosteroids occurred significantly more often in the montelukast group. It would be expected that this expeditious intervention reduced urgent care and emergent visits that would have occurred more frequently in the montelukast group than the fluticasone group if study physicians had not been available; this process could have caused an underestimation of the cost-effectiveness of fluticasone compared with montelukast.
Conclusions and Recommendations
Fluticasone is cost-effective compared with montelukast for children with mild to moderate persistent asthma. This CEA demonstrated that fluticasone dominated montelukast as it led to more ACD, a higher proportion of participants with 12% of FEV1 improvement, and fewer asthma exacerbations, yet at lower direct and societal costs.
Our study demonstrates the cost-effectiveness of low-dose fluticasone compared with montelukast in addition to the previously demonstrated clinical benefits in three clinically relevant asthma domains (asthma control days, lung function, and exacerbations) and further supports the NAEPP guidelines based on effectiveness that recommend inhaled corticosteroid monotherapy as the preferred asthma controller option for mild to moderate persistent asthma in children.
Figure 1. Confidence Interval for the Incremental Cost Effectiveness Ratio: 1 (a–c) direct costs; (d–f) societal costs.
1 The difference in mean cost is the cost of the fluticasone arm minus that of the montelukast arm. The green rays show 95% confidence interval for Incremental Cost-Effectiveness Ratio as indicated in Table 3.
Acknowledgments
Funding/Support: This study was supported by: Grants 5U10HL064287, 5U10HL064288, 5U10HL064295, 5U10HL064307, 5U10HL0643055, 5U10HL064313 from the National Heart, Lung, and Blood Institute. This study was carried out in part in the General Clinical Research Centers at Washington University School of Medicine (M01 RR00036) and National Jewish Health and University of Colorado Denver School of Medicine (M01 RR00051) and Colorado CTSA grant 1 UL1 RR025780 from NCRR/HIH.
Abbreviations Used
- ACD
asthma-control days
- CEA
cost-effectiveness analysis
- DPI
dry powder inhaler
- FEV1
forced expiratory volume in one second
- ICER
incremental cost effectiveness ratio
- MDI
metered dose inhaler
- PACT
Pediatric Asthma Controller Trial
- WTP
willingness to pay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest: D. T. Mauger has received research support from GlaxoSmithKline. R. S. Zeiger is a consultant for Aerocrine, AstraZeneca, Genentech, GlaxoSmithKline, MedImmune, Merck, and Sunovion and has received research support from Aerocrine, Genentech, GlaxoSmithKline, and Merck. C. A. Sorkness is a consultant for GlaxoSmithKline, AstraZeneca, and Novartis and has received research support from Schering-Plough, Pharmaxis, Sandoz/Compleware, and the National Heart, Lung, and Blood Institute (NHLBI). R. F. Lemanske is a speaker for Merck, Washington University, the Medicus Group, the Park Nicolet Institute, the ACAAI (American College of Allergy, Asthma & Immunology), the LA Allergy Society, the Michigan Allergy/Asthma Society, the Medical College of Wisconsin, the Fund for Medical Research and Education (Detroit), Children’s Hospital of Minnesota, the Toronto Allergy Society, the AAAAI (American Academy of Allergy, Asthma & Immunology), Beaumont Hospital (Detroit), the University of Illinois, the Canadian Society of Allergy and Clinical Immunology, and New York Presbyterian; is a consultant and speaker for AstraZeneca; is a consultant for Map Pharmaceuticals, Gray Consulting, Smith Research, the Merck Childhood Asthma Network, Novartis, Quintiles/Innovax, RC Horowitz & Co, International Meetings and Science, and Scienomics; is the author of Up-to-Date; and is a textbook editor for Elsevier. F. D. Martinez is on the advisory board for and receives lecture fees from Merck; is a consultant for GlaxoSmithKline and MedImmune; receives lecture fees from Pfizer; and has received research support from the National Institutes of Health. S. J. Szefler is a consultant for GlaxoSmithKline, Genentech, Merck, Boehringer-Ingelheim, Novartis, and Schering-Plough and has received research support from the National Institutes of Health (NIH)/NHLBI’s Childhood Management Program, the NHLBI’s Childhood Asthma Research and Education, NIH/NHLBI’s Asthma Clinical Research Network, the NIH/NIAID’s Inner City Asthma Consortium, GlaxoSmithKline, NIH/NHLBI Asthma Met, and a National Institute of Environmental Health Sciences/US Environmental Protection Agency Childhood Environmental Health Center grant. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.National Heart, Lung, and Blood Institute Chartbook on Cardiovascular, Lung and Blood Diseases, U.S. Department of Health and Human Services, National Institute of Health. 2007 http://www.nhlbi.nih.gov/resources/docs/07-chtbk.pdf.
- 2.Summary health statistics for U.S. children: national health interview survey, 2002. Series 10, Number 221. [PubMed] [Google Scholar]
- 3.Asthma and Allergy Foundation of America. http://www.aafa.org. [Google Scholar]
- 4.Simonella L, Marks G, Sanderson K, Andrews G. Cost-effectiveness of current and optimal treatment for adult asthma. Intern Med J. 2006 Apr;36(4):244–250. doi: 10.1111/j.1445-5994.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 5.Miller E, FitzGerald JM. Budesonide/formoterol as maintenance and reliever treatment compared to fixed dose combination strategies - a Canadian economic evaluation. Can J Clin Pharmacol. 2008 Summer;15(2):e165–e176. [PubMed] [Google Scholar]
- 6.Bisgaard H, Price MJ, Maden C, Olsen NA. Cost-effectiveness of fluticasone propionate administered via metered-dose inhaler plus babyhaler spacer in the treatment of asthma in preschool-aged children. Chest. 2001 Dec;120(6):1835–1842. doi: 10.1378/chest.120.6.1835. [DOI] [PubMed] [Google Scholar]
- 7.Ericsson K, Bantje TA, Huber RM, Borg S, Bateman ED. Cost-effectiveness analysis of budesonide/formoterol compared with fluticasone in moderate-persistent asthma. Respir Med. 2006 Apr;100(4):586–594. doi: 10.1016/j.rmed.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Pieters WR, Lundbäck B, Sondhi S, Price MJ, Thwaites RMA. Cost-Effectiveness Analysis of Salmeterol/Fluticasone Propionate 50/500g vs Fluticasone Propionate 500g in Patients with Corticosteroid-Dependent Asthma V: Results. PharmacoEconomics. 1999;16:29–34. [Google Scholar]
- 9.Johansson G, Price MJ, Sondhi S. Cost-Effectiveness Analysis of Salmeterol/Fluticasone Propionate 50/100g vs Fluticasone Propionate 100g in Adults and Adolescents with Asthma III: Results. PharmacoEconomics. 1999;16:15–21. [Google Scholar]
- 10.Lundback B, Jenkins C, Price MJ, Thwaites RM. Cost-effectiveness of salmeterol/fluticasone propionate combination product 50/250 microg twice daily and budesonide 800 microg twice daily in the treatment of adults and adolescents with asthma. International Study Group. Respir Med. 2000 Jul;94(7):724–732. doi: 10.1053/rmed.2000.0876. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Liu Z, et al. Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over. Health Technol Assess. 2008 May;12(19):iii–iv. 1–360. doi: 10.3310/hta12190. [DOI] [PubMed] [Google Scholar]
- 12.Barnes NC, Thwaites RM, Price MJ. The cost-effectiveness of inhaled fluticasone propionate and budesonide in the treatment of asthma in adults and children. Respir Med. 1999 Jun;93(6):402–407. doi: 10.1053/rmed.1999.0577. [DOI] [PubMed] [Google Scholar]
- 13.Briggs AH, Bousquet J, Wallace MV, Busse WW, Clark TJ, Pedersen SE, et al. Cost-effectiveness of asthma control: an economic appraisal of the GOAL study. Allergy. 2006 May;61(5):531–536. doi: 10.1111/j.1398-9995.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 14.Feenstra TL, Rutten-Van Molken MP, Jager JC, Van Essen-Zandvliet LE. Cost effectiveness of guideline advice for children with asthma: a literature review. Pediatr Pulmonol. 2002 Dec;34(6):442–454. doi: 10.1002/ppul.10177. [DOI] [PubMed] [Google Scholar]
- 15.Murphy KR. Cost-effectiveness of pediatric asthma treatments. J Allergy Clin Immunol. 2003 Jan;111(1):202. doi: 10.1067/mai.2003.50. [DOI] [PubMed] [Google Scholar]
- 16.Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007 Jan;119(1):64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor RD, Nelson H, Borker R, Emmett A, Jhingran P, Rickard K, et al. Cost effectiveness of fluticasone propionate plus salmeterol versus fluticasone propionate plus montelukast in the treatment of persistent asthma. Pharmacoeconomics. 2004;22(12):815–825. doi: 10.2165/00019053-200422120-00004. [DOI] [PubMed] [Google Scholar]
- 18.Borker R, Emmett A, Jhingran P, Rickard K, Dorinsky P. Determining economic feasibility of fluticasone propionate-salmeterol vs montelukast in the treatment of persistent asthma using a net benefit approach and cost-effectiveness acceptability curves. Ann Allergy Asthma Immunol. 2005 Aug;95(2):181–189. doi: 10.1016/S1081-1206(10)61209-4. [DOI] [PubMed] [Google Scholar]
- 19.Johansson G, Andreasson EB, Larsson PE, Vogelmeier CF. Cost effectiveness of budesonide/formoterol for maintenance and reliever therapy versus salmeterol/fluticasone plus salbutamol in the treatment of asthma. Pharmacoeconomics. 2006;24(7):695–708. doi: 10.2165/00019053-200624070-00008. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan SD, Buxton M, Andersson LF, Lamm CJ, Liljas B, Chen YZ, et al. Cost-effectiveness analysis of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol. 2003 Dec;112(6):1229–1236. doi: 10.1016/j.jaci.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Buxton MJ, Sullivan SD, Andersson LF, Lamm CJ, Liljas B, Busse WW, et al. Country-specific cost-effectiveness of early intervention with budesonide in mild asthma. Eur Respir J. 2004 Oct;24(4):568–574. doi: 10.1183/09031936.04.00108703. [DOI] [PubMed] [Google Scholar]
- 22.Rice DP. Estimating the cost of illness. Am J Public Health Nations Health. 1967 Mar;57(3):424–440. doi: 10.2105/ajph.57.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haddix A, Teutsch S, Corso P. Prevention Effectiveness: A Guide to Decision Analysis and Economic Evaluation. New York: Oxford University Press; 2003. [Google Scholar]
- 24.Weiss K, Buxton M, Andersson FL, Lamm CJ, Liljas B, Sullivan SD. Cost-effectiveness of early intervention with once-daily budesonide in children with mild persistent asthma: results from the START study. Pediatr Allergy Immunol. 2006 May;17 Suppl 17:21–27. doi: 10.1111/j.1600-5562.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 25.Bureau of Labor Statistics. www.bls.gov.
- 26.Drug Topics Redbook: Thomson Healthcare. 2003 [Google Scholar]
- 27.Knuffman JE, Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Martinez FD, et al. Phenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthma. J Allergy Clin Immunol. 2009 Feb;123(2):411–416. doi: 10.1016/j.jaci.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pieters WR, Wilson KK, Smith HC, Tamminga JJ, Sondhi S. Salmeterol/fluticasone propionate versus fluticasone propionate plus montelukast: a cost-effective comparison for asthma. Treat Respir Med. 2005;4(2):129–138. doi: 10.2165/00151829-200504020-00007. [DOI] [PubMed] [Google Scholar]
- 29.Doull I, Price D, Thomas M, Hawkins N, Stamuli E, Tabberer M, et al. Cost-effectiveness of salmeterol xinafoate/fluticasone propionate combination inhaler in chronic asthma. Curr Med Res Opin. 2007 May;23(5):1147–1159. doi: 10.1185/030079907x187982. [DOI] [PubMed] [Google Scholar]
- 30.Akazawa M, Stempel DA. Single-inhaler combination therapy for asthma: a review of cost effectiveness. Pharmacoeconomics. 2006;24(10):971–988. doi: 10.2165/00019053-200624100-00005. [DOI] [PubMed] [Google Scholar]
- 31.Pathak DS, Davis EA, Stanford RH. Economic impact of asthma therapy with fluticasone propionate, montelukast, or zafirlukast in a managed care population. Pharmacotherapy. 2002 Feb;22(2):166–174. doi: 10.1592/phco.22.3.166.33548. [DOI] [PubMed] [Google Scholar]
- 32.Stempel DA, Kruzikas DT, Manjunath R. Comparative efficacy and cost of asthma care in children with asthma treated with fluticasone propionate and montelukast. J Pediatr. 2007 Feb;150(2):162–167. doi: 10.1016/j.jpeds.2006.10.069. [DOI] [PubMed] [Google Scholar]