Abstract
TRAF6 is an E3 ubiquitin ligase that plays a pivotal role in the activation of NF-κB by innate and adaptive immunity stimuli. TRAF6 consists of a highly conserved carboxyl terminal TRAF-C domain which is preceded by a coiled coil domain and an amino terminal region that contains a RING domain and a series of putative zinc-finger motifs. The TRAF-C domain contributes to TRAF6 oligomerization and mediates the interaction of TRAF6 with upstream signaling molecules whereas the RING domain comprises the core of the ubiquitin ligase catalytic domain. In order to identify structural elements that are important for TRAF6-induced NF-κB activation, mutational analysis of the TRAF-C and RING domains was performed. Alterations of highly conserved residues of the TRAF-C domain of TRAF6 did not affect significantly the ability of the protein to activate NF-κB. On the other hand a number of functionally important residues (L77, Q82, R88, F118, N121 and E126) for the activation of NF-κB were identified within the RING domain of TRAF6. Interestingly, several homologues of these residues in TRAF2 were shown to have a conserved functional role in TRAF2-induced NF-κB activation and lie at the dimerization interface of the RING domain. Finally, whereas alteration of Q82, R88 and F118 compromised both the K63-linked polyubiquitination of TRAF6 and its ability to activate NF-κB, alteration of L77, N121 and E126 diminished the NF-κB activating function of TRAF6 without affecting TRAF6 K63-linked polyubiquitination. Our results support a conserved functional role of the TRAF RING domain dimerization interface and a potentially necessary but insufficient role for RING-dependent TRAF6 K63-linked polyubiquitination towards NF-κB activation in cells.
Keywords: TNF, TRAF6, NF-κB, ubiquitination
Introduction1
Tumor-necrosis-factor-receptor-associated factor 6 (TRAF6) is an E3 ubiquitin ligase that plays a central role in the propagation of extracellular signals by members of the tumor-necrosis-factor-receptor (TNFR) and interleukin-1-receptor/Toll-like receptor (IL1R/TLR) families (reviewed in). Gene targeting approaches in mice have uncovered a pleiotropic repertoire of TRAF6-dependent physiological processes which include bone metabolism, innate immune responses, thymic epithelial cell differentiation, peripheral T cell tolerance and dendritic cell maturation (reviewed in). Many of these processes depend on the ability of TRAF6 to mediate the activation of nuclear factor-κB (NF-κB) and mitogen activated protein kinases (MAPKs). In response to incoming signals, TRAF6 is recruited onto multisubunit complexes that assemble on the cytoplasmic receptor tails and mediates the formation of protein-conjugated or free K63-linked polyubiquitin chains. K63-linked polyubiquitin chains appear to play an important role in the process of signal-induced NF-κB and MAPK activation by serving as protein-interacting scaffolds that mediate the recruitment and activation of downstream kinases and associated molecules.
TRAF6 consists of an amino terminal RING domain which is followed by four zinc-finger motifs, a central coiled-coil region and a highly conserved carboxyl terminal domain, known as the TRAF-C domain. X-ray crystallography has revealed that the TRAF-C domain attains a trimeric mushroom head-like structure with three protein-binding pockets located on the periphery of the mushroom head. The E3 ligase activity of TRAF6 depends on the integrity of the really-interesting-gene (RING) domain and its ability to homodimerize and interact with the heterodimeric complex of E2 Ubc13 with Uev1A. Previous studies have demonstrated the functional importance of the RING domain towards NF-κB activation, by mutating primarily cysteine residues that constitute the core structural elements of the RING domain. However, limited information exists about the functional role of amino acid residues of the RING domain which are located outside the core scaffold that is formed by the cysteine residues. The TRAF-C domain mediates the interaction of TRAF6 with upstream signaling components and contributes to the trimerization of TRAF6 which is apparently essential for the signaling properties of TRAF6 towards the activation of NF-κB and MAPKs. Mutational analyses performed in previous studies have characterized primarily the role of TRAF-C residues that are involved in interactions with members of the TNFR family and signaling molecules. However, the functional importance of amino acid residues that are predicted to be involved in the trimerization of the TRAF-C domain has not been evaluated.
In this report we have utilized site directed mutagenesis to examine the dependence of TRAF6-induced activation of NF-κB on specific conserved amino acid residues of the RING domain as well as amino acid residues that are predicted to contribute to the trimerization of the TRAF-C domain. Our study revealed an independence of TRAF6-induced activation of NF-κB to a broad range of conserved single amino acid changes in the TRAF-C domain. On the other hand, a number of functionally important RING domain amino acid residues were identified and revealed a conserved functional importance of the RING dimerization interface and a potentially necessary but insufficient role of K63-linked polyubiquitination of TRAF6 in its ability to mediate NF-κB activation.
2. Materials and methods
2.1 Site directed mutagenesis
An expression vector containing either the cDNA that encodes the FLAG-tagged Mus musculus TRAF6 protein (pCRFLAGTRAF6, a kind gift of Dr H. Nakano) or the cDNA that encodes the FLAG-tagged Homo sapiens TRAF2 protein (pcDNA3FLAGTRAF2,) was used as a template for PCR-based site directed mutagenesis. In order to introduce mutations in the TRAF-C domain of TRAF6 the SmaI-ApaI fragment of pCRFLAGTRAF6 was mutagenized and used to replace the corresponding wild type fragment, with the exception of the L502G alteration for which the BlpI-ApaI fragment of pCRFLAGTRAF6 was mutagenized. In order to introduce mutations in the RING domain of TRAF6 the KpnI-XhoI fragment of pCRFLAGTRAF6 was mutagenized and used to replace the corresponding fragment of pCRFLAGTRAF6. The sequences of the mutagenic primers that were used for the TRAF6 constructs are: Y68Ffor (5′-AGCAAGTTTGAGTGTCCCA-3′), Y68Frev (5′-TGGGACACTCAAACTTGCT-3′), L77Afor (5′-TGATGGCTGCACGGGAAG-3′), L77Arev (5′-CTTCCCGTGCAGCCATCA-3′), Q82Afor (5′-AGCAGTGGCAACACCAT-3′), Q82Arev (5′-ATGGTGTTGCCACTGCT-3′), R88Afor (5′-CACGCCTTCTGCAAAGCCTG-3′), R88Arev (5′-CAGGCTTTGCAGAAGGCGTG-3′), E110Qfor (5′-AGTTGACAATCAAATACTG-3′), E110Qrev (5′-CAGTATTTGATTGTCAACT-3′), F118Afor (5′-TCAACTGGCACCCGACAAT-3′), F118Arev (5′-ATTGTCGGGTGCCAGTTGA-3′), N121Afor (5′-GTTTCCCGACGCTTTTGC-3′), N121Arev (5′-GCAAAAGCGTCGGGAAAC-3′), E126Qfor (5′-GCAAAGCGACAGATTCTT-3′), E126Qrev (5′-AAGAATCTGTCGCTTTGC-3′), Q356Gfor (5′-GGAAGCACAGGGGTGTAACGGG-3′), Q356Grev (5′-CCCGTTACACCCCTGTGCTTCC-3′), I362Gfor (5′-ACGGGATCTACGGTTGGAAG-3′), I362Grev (5′-CTTCCAACCGTAGATCCCGT-3′), W363Afor (5′-TAACGGGATCTACATTGCGAAGA-3′), W363Arev (5′-TCTTCGCAATGTAGATCCCGTTA-3′), K364Afor (5′-GGATCTACATTTGGGCGATTGG-3′), K364Arev (5′-CCAATCGCCCAAATGTAGATCC-3′), F388Afor (5′-GGAGCGTACACAGGCAGACC-3′), F388Arev (5′-GGTCTGCCTGTGTACGCTCC-3′), Y389Afor (5′-GGATTCGCAACAGGCAGACC-3′), Y389Arev (5′-GGTCTGCCTGTTGCGAATCC-3′), T390Afor (5′-GGATTCTACGCAGGCAGACC-3′), T390Arev (5′-GGTCTGCCTGCGTAGAATCC-3′), R392Afor (5′-GGCGCACCTGGGTACAAGC-3′), R392Arev (5′-GCTTGTACCCAGGTGCGCC-3′), E425Qfor (5′-CAATGCAAGGACAATATGACA-3′), E425Qrev (5′-TGTCATATTGTCCTTGCATTG-3′), W432Afor (5′-ACCTCCCCGCTCCCTTCCAG-3′), W432Arev (5′-CTGGAAGGGAGCGGGGAGGT-3′), P433Afor (5′-CACCTCCCCTGGGCTTTCC-3′), P433Arev (5′-GGAAAGCCCAGGGGAGGTG-3′), F434Afor (5′-GCCCGCACAGGGTACAATACGC-3′), F434Arev (5′-GCGTATTGTACCCTGTGCGGGC-3′), L443Gfor (5′-CCTTACAATTGGCGACCAGT-3′), L443Grev (5′-ACTGGTCGCCAATTGTAAGG-3′), Q445Afor (5′-TTCTCGACGCCTCTGAAGCACTT-3′), Q445Arev (5′-AAGTGCTTCAGAGGCGTCGAGAA-3′), R469Afor (5′-CCTTTCAGGCACCCACAATC-3′), R469Arev (5′-GATTGTGGGTGCCTGAAAGG-3′), L502Gfor (5′-GGATGATACATTAGGAGTG-3′), L502Grev (5′-CACTCCTAATGTATCATCC-3′). For the amplification of the fragments containing the desired mutants, two pairs of external primers were used. More specifically, the sequences of the external primers that were used for the construction of the mutants Y68F, L77A, Q82A, E110Q, N121A and E126Q, are T7 and mT6-566rev (5′-CAAGAAACCTGCCTCCTGGG-3′). For the creation of the constructs R88A and F118A, the external primers that were used are mT6(-170)for (5′-CACTGCTTACTGGCTTATC-3′) and mT6-566rev (5′-CAAGAAACCTGCCTCCTGGG-3′). The external primers and their sequences that were used for the amplification of the mutants Q356G, I362G, W363A, K364A, F388A, Y389A, T390A, R392A, E425Q, W432A, P433A, F434A, L443G, Q445A and R469A are T6-815for (5′-TGTTGGCCCAGGCTGTTCATAA-3′) and TRAF6rev (5′-ACGGGGGAGGGGCAAACAACA-3′), while for the mutant L502G, the external primer pair used is TRAF6for (5′-ATCCGGGAGCTGACTGCCAAAATG-3′) and TRAF6rev (5′-ACGGGGGAGGGGCAAACAACA-3′). For the creation of the double mutants K364A/R392A, K364A/E425Q and R392A/E425Q, the template DNAs that were used are the constructs K364A (in the case of the first two mutants) and R392A (in the case of the last mutant).
In order to introduce mutations in the RING domain of TRAF2 the KpnI-XhoI fragment of pcDNA3FLAGTRAF2 was mutagenized and used to replace the corresponding wild type fragment. The TRAF2 constructs were created using the following mutagenic primers: Q46Afor (5′-CCTTCGCAGCGCAGTGTGG-3′), Q46Arev (5′-CCACACTGCGCTGCGAAGG-3′), F91Afor (5′- AGTTCGGCCGCACCAGATA-3′), F91Arev (5′-TATCTGGTGCGGCCGAACT-3′), N94Afor (5′-CCCAGATGCCGCTGCCCGC-3′), N94Arev (5′-GCGGGCAGCGGCATCTGGG-3′), E99Qfor (5′-CCCGCAGGCAGGTGGAGA-3′), E99Qrev (5′-TCTCCACCTGCCTGCGGG-3′). The external primers that were used for the amplification of the aforementioned mutants are T7 and hT2-737rev (5′-ATGGCCAGGTGCTCCCG-3′).
2.2 Protein Expression and Purification
Wild-type and mutant mouse TRAF6 (50-211) were cloned into pET28a vector (Novagen) between NheI and EcoRI sites using PCR methods as previously described. These N-terminally His6-tagged proteins were expressed in E. coli BL21 Codon-Plus (DE3) cells (Stratagene). Cells were induced with 0.4 mM IPTG when optical density (O.D. 600) reached 0.6, and grew at 20°C overnight. Harvested cells were lysed by sonication in lysis buffer containing 50 mM sodium phosphate (pH 7.4), 300 mM NaCl, 5% glycerol, 20 mM imidazole and 5 mM beta-mercaptoethanol. After centrifugation, the supernatants were purified by Ni affinity chromatography followed by size-exclusion chromatography (Superdex 200 10/300 GL, GE Healthcare) in buffer containing 20 mM Tris HCl (pH 7.5), 150 mM NaCl and 5 mM dithiothreitol (DTT).
2.3 Luciferase and β-galactosidase assays
HEK293FT cells were transfected with the pCRFLAGTRAF6 or pcDNA3FLAGTRAF2 expression vectors, along with a vector expressing the gene of β – galactosidase and a vector containing a luciferase gene under the control of three NF-κB binding sites (3xKBL). The cells were harvested approximately 16 hours post transfection and lysed in passive lysis buffer (Promega). The luciferase and β-galactosidase activities of the lysates were determined by the Luciferase Assay System (Promega) and the Galacto-light Plus Reporter Gene Assay System (Tropix), respectively, using a TD20/20 luminometer (Turner Designs). Relative Luciferase activities were determined by dividing the values of luciferase activities by the corresponding values of β-galactosidase activities. The mean and the standard error calculated using the Microsoft Office Excel 2007 software. Statistical evaluation of the results was performed with Student's t – test using the STATISTICA software. Statistically significant differences were considered those with a p value < 0, 05. The expression of TRAF6 and TRAF2 proteins was evaluated by Western blotting using the M5 anti-FLAG monoclonal antibody (Sigma).
2.4 Determination of TRAF6 polyubiquitination
HEK293FT cells were transfected with vectors expressing wild type or mutant TRAF6 along with a vector expressing ubiquitin. The cells were harvested approximately 16 hours posttransfection and lysed in RIPA buffer (25mM Tris-HCl pH 7.4, 150mM NaCl, 5mM EDTA, 1% Glycerol, 0.5% NP-40, 1mM DTT, 0.1% SDS, 1% sodium deoxycholate, 1mM PMSF 100mM, 1× protease inhibitor cocktail (Roche)). The cell lysates were subjected to immunoprecipitation with the OctA anti-FLAG antibody (Santa Cruz Biotechnology). The immunoprecipitated material was analyzed by SDS-PAGE and immunoblotting. FLAG-tagged proteins were detected with the M5 anti-FLAG monoclonal antibody (Sigma). Ubiquitination was detected with either an anti-ubiquitin antibody (Santa Cruz Biotechnology Inc.) or an antibody that recognizes K63-linked polyubiquitin chains.
3. Results
3.1 Mutational analysis of the TRAF-C domain of TRAF6
The crystal structure of the TRAF-C domain of TRAF2, TRAF3 and TRAF6 has been determined and revealed extensive similarity in the 3-dimensional organization of these domains. These studies have predicted the direct involvement of several amino acid residues in the trimerization of theTRAF-C domain which is considered an essential component of the signaling capacity of TRAF6 towards the activation of NF-κB. Figure 1 shows the amino acid residue changes that were introduced in the TRAF-C domain of TRAF6 and tested for their effect on the ability of TRAF6 to activate NF-κB. Among these changes, eight single amino acid changes (I362G, K364A, R392A, E425Q, L443G, Q445A, R469A, L502G) and three double amino acids changes (K364A/R392A, K364A/E425Q, R392A/E425Q) targeted residues that are predicted to mediate homomeric interactions between TRAF-C domains (Fig. 1,). In addition, eight additional alterations of highly conserved residues of the TRAF-C domain of TRAF6 (Q356G, W363A, F388A, Y389A, T390A, W432A, P433A, F434A) were introduced and evaluated for their functional significance (Fig. 1). The amino acid alterations caused changes in the physicochemical properties of the corresponding wild type residues that included reduction of hydophobicity (I362G, L443G, L502G, W363A, F388A, W432A, F434A) and moderate (E425Q, Q445A, Q356G, T390A) or greater deviations in hydrophilicity (K364A, R392A).
Fig. 1.
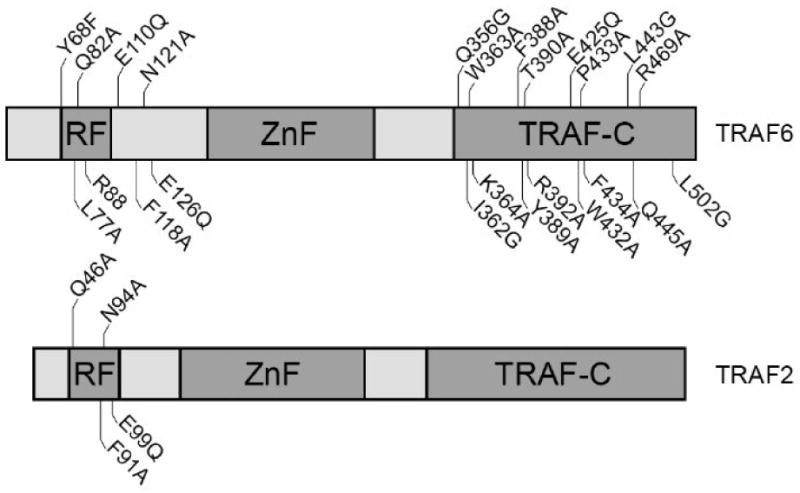
Schematic representation of the proteins TRAF6 and TRAF2. Previously defined domains are shown as darker rectangles. Vertical lines show the relative positions of the amino acid alterations that were introduced in both proteins. RF: RING finger, ZnF: Zinc fingers.
Interestingly, none of the changes that were introduced in the TRAF-C domain of TRAF6 resulted in a significant effect on the ability of TRAF6 to activate the transcription factor NF-κB (Fig. 2A). Similar results were obtained at low (obtained with transfection of 50 ng of expression vector) and high (obtained with transfection of 300ng of expression vector) levels of TRAF6 expression, indicating a relative independence of the observed results from the concentration of TRAF6. All the TRAF6 mutants were expressed at levels that were comparable to the expression level of wild type TRAF6 (Fig. 2B). These findings underscore an insignificant or minor contribution of highly conserved TRAF-C residues to TRAF6-induced NF-κB activation.
Fig. 2.
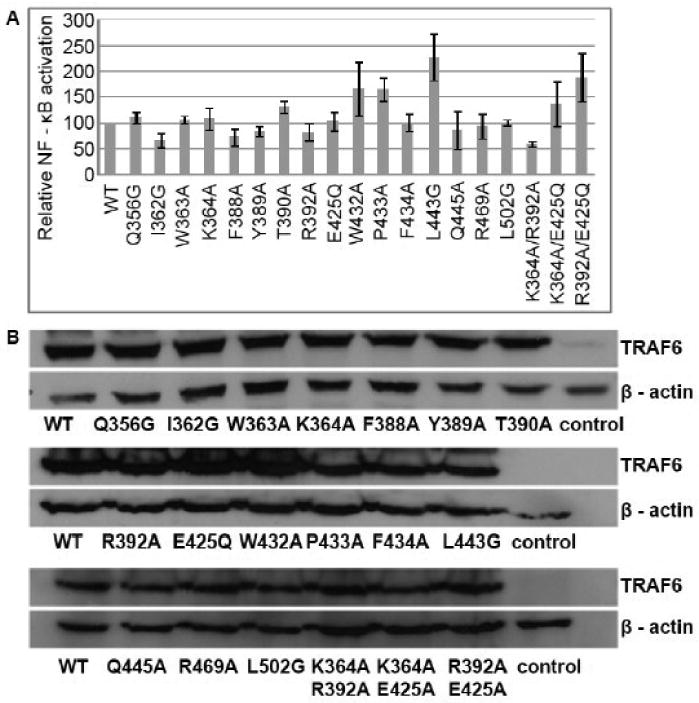
NF-κB activation potential of TRAF-C mutants of TRAF6. (A) Relative activity of a NF-κB-responsive luciferase reporter in response to expression of FLAG-tagged wild type or the indicated TRAF6 mutants in HEK293T cells. The results are the means ± SE of relative luciferase activity from at least three independent experiments. The asterisks designate the TRAF6 mutants that show a statistically significant difference in their ability to activate NF – κB relative to wild type TRAF6. (B) Representative Western blots showing the expression of FLAG-tagged wild type or mutated TRAF6 isoforms and β-actin in cell lysates analyzed in A.
3.2 The NF-κB-activating function of TRAF6 depends on multiple residues of the RING domain
The RING domain of TRAF6 constitutes a core functional element of the protein that is essential for the ability of TRAF6 to mediate the activation of the NF-κB pathway. Our structural and functional analysis of the RING domain was extended beyond the core scaffold of cysteine residues that has been previously shown to play an essential role in TRAF6-induced activation of NF-κB. Eight amino acid alterations (Y68F, L77A, Q82A, R88A, E110Q, F118A, N121A, E126Q) were introduced in the RING domain of TRAF6 (Fig. 1) and evaluated for their functional significance. Interestingly, six (L77A, Q82A, R88A, F118A, N121A, E126Q) out of the eight alterations caused a significant reduction in TRAF6-induced activation of NF-κB (Fig. 3A, upper panel). All the RING domain mutants of TRAF6 were expressed at levels that were comparable to the expression level of wild type TRAF6 (Fig. 3A, lower panels). Molecular modeling revealed that five out of the six functionally significant TRAF6 RING-domain residues (Q82A, R88A, F118A, N121A, E126Q) are located at the dimerization interface of TRAF6 (Fig. 3B). R88A and F118A have been previously shown to disrupt the dimerization of TRAF6 RING domain. Gel filtration analysis revealed that Q82A compromised dramatically the dimerization capability if the TRAF6 RING domain, whereas N121A had a partial effect and E126Q had no apparent effect on the dimerization of the TRAF6 RING domain (Fig. 3C).
Fig. 3.
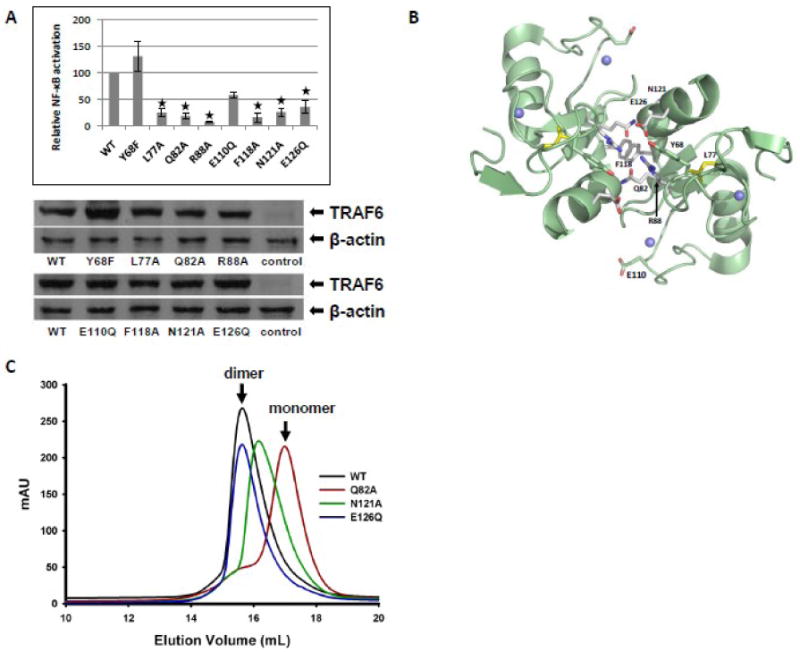
NF-κB activation and dimerization potential of RING domain mutants of TRAF6. (A) Relative activity of a NF-κB-responsive luciferase reporter in response to expression of FLAG-tagged wild type or the indicated TRAF6 mutants in HEK293T cells (upper panel). The results are the means ± SE of relative luciferase activity from at least three independent experiments. The asterisks designate the TRAF6 mutants that show a statistically significant difference in their ability to activate NF-κB relative to wild type TRAF6. Representative Western blots showing the expression of FLAG-tagged wild type or mutated TRAF6 isoforms and β-actin in cell lysates analyzed in A (lower panels). (B) Molecular modeling of the RING domain of TRAF6. A dimer of the RING domain is shown. Residues that were mutated are shown as sticks. Grey: residues on RING dimer interface. (C) Superimposed gel filtration profiles of wild-type (WT) and RING domain mutants of TRAF6 (50-211).
All six functionally important amino acids of the TRAF6 RING domain are conserved in TRAF2, which is another member of the TRAF family with ubiquitin ligase activity. In order to investigate whether the functional significance of specific residues of the TRAF6 RING domain is conserved, four corresponding amino acid changes (Q46A, F91A, N94A, E99Q) were introduced in the RING domain of TRAF2 and tested for their effect on TRAF2-induced activation of NF-κB. Remarkably, all four amino acid alterations compromised significantly the ability of TRAF2 to mediate NF-κB activation (Fig. 4A, upper panel), without affecting the expression level of the protein (Fig. 4A, lower panel). Molecular modeling revealed that all four of the TRAF2 RING-domain residues that have been altered are located at the dimerization interface TRAF2 RING domain (Fig. 4B). However, our previous experiments have shown that although F91A affects dramatically the dimerization capability of TRAF2 RING domain, Q46A and N94A do not have an apparent effect on the dimerization of TRAF2 RING domain.
Fig. 4.
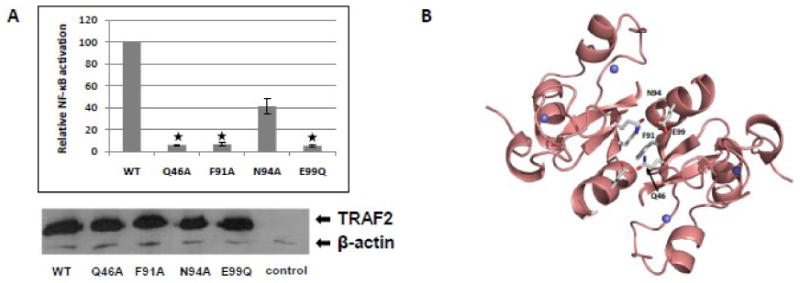
NF-κB activation potential of RING domain mutants of TRAF2. (A) Relative activity of a NF-κB-responsive luciferase reporter in response to expression of FLAG-tagged wild type or the indicated TRAF2 mutants in HEK293T cells (upper panel). The results are the means ± SE of relative luciferase activity from at least three independent experiments. The asterisks designate the TRAF2 mutants that show a statistically significant difference in their ability to activate NF – κB relative to wild type TRAF2. Representative Western blot showing the expression of wild type or mutated TRAF2 isoforms and β-actin in cell lysates analyzed in A (lower panel). (B) Molecular modeling of the RING domain of TRAF2. A dimer of the RING domain is shown. Residues that were mutated are shown as sticks. Grey: residues on RING dimer interface.
Taken together, our results have identified an essential and conserved signaling role for multiple RING domain residues of TRAF6 and TRAF2 that do not belong to the core cysteine scaffold. Although, most of these residues are located at the RING-domain dimerization interface they are likely to mediate their effect on TRAF6-induced-NF-κB activation by RING-domain dimerization dependent and independent mechanisms.
3.3 RING domain-dependent TRAF6 polyubiquitination is potentially necessary but insufficient for NF-κB activation
The identification of functionally important residues in the RING domain of TRAF6 prompted an investigation into the signaling mechanism that is affected by the amino acid changes. The RING domain of TRAF6 is a core element of the ubiquitin ligase activity of the protein, which can mediate its self-ubiquitination by K63-linked polyubiquitin chains. Previous studies have shown that mutations that affect the cysteine scaffold of the TRAF6 RING domain diminish dramatically TRAF6 self ubiqitination by K63-linked polyubiquitin chains. Therefore, the effect of RING domain amino acid changes on TRAF6 polyubiquitination was evaluated by immunoprecipitating exogenously expressed TRAF6 and assessing its ubiquitination status. As expected Q82A, R88A and F118A which compromised NF-kappaB activation resulted also in a dramatically diminished TRAF6 polyubiquitination (Fig. 5A) as well as K63-linked TRAF6 polyubiquitination, as assessed by an antibody that recognizes specifically K63-linked polyubiquitin chains (Fig. 5B). Surprisingly, the L77A, N121A and E126Q TRAF6 mutants which were defective in their NF-kappaB-activating ability, were polyubiquitinated to a similar extent as wild type TRAF6 (Fig. 5A). In addition, the extent of K63-linked polyubiquitination of L77A, N121A, E126Q and wild type TRAF6 was similar (Fig. 5B). Taken together our results indicate that RING-domain-dependent TRAF6 polyubiquitination by K63-linked chains may be necessary albeit insufficient for activation of the NF-κB pathway, suggesting that additional RING-dependent conditions must be fulfilled for the activation of this pathway by TRAF6.
Fig. 5.
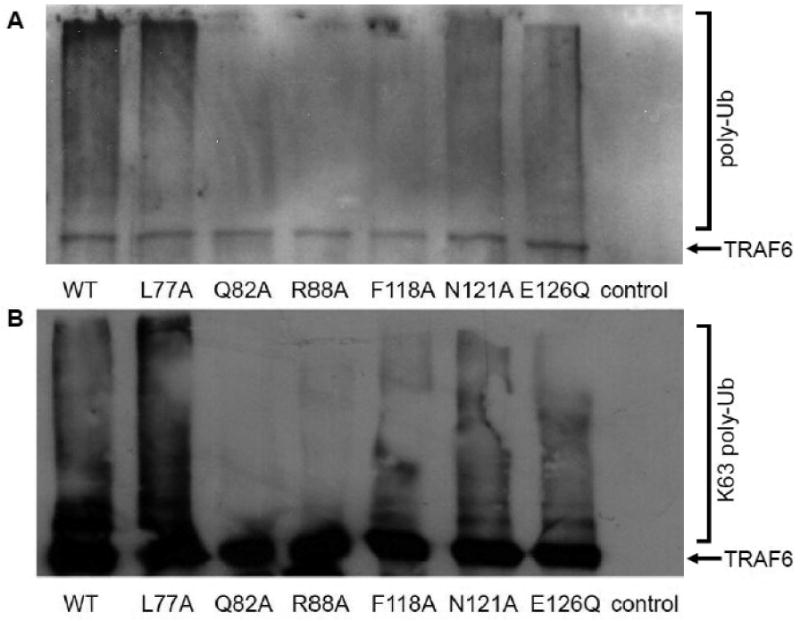
Analysis of TRAF6 polyubiquitination. HEK293T cells were mock transfected (control) or transfected with FLAG-tagged wild type (WT) or the indicated mutated forms of TRAF6 along with a vector expressing ubiquitin. The TRAF6 proteins were immunoprecipitated 16 hours post transfection and analyzed by immunoblotting using the anti-FLAG M5 monoclonal antibody or antibodies that recognize ubiquitin or K63-linked polyubiquitin chains. (A) Representative Western blot showing poyubiquitinated (poly-Ub) and non-ubiquitinated (TRAF6) TRAF6 proteins. (B) Representative Western blot showing poyubiquitinated by K63-linked chains (K63 poly-Ub) and non-ubiquitinated (TRAF6) TRAF6 proteins.
4. Discussion
An extensive mutational analysis of the TRAF-C and RING domains of TRAF6 was undertaken in order to identify amino acid residues that play an essential role in TRAF6-induced activation of NF-κB. Our study targeted primarily conserved residues with potential functional importance that has been suggested by structural studies, and revealed a number of novel aspects of the structural and functional profile of TRAF6.
First, multiple single and even double changes of highly conserved TRAF-C residues did not affect the ability of TRAF6 to mediate robust activation of NF-κB. These findings would be consistent with a highly compact structure of the TRAF-C domain that is stabilized by multiple amino acid interactions and retains its functionality despite single or double alterations of presumably critical amino acid residues. Alternatively, our findings indicate an insignificant or at best minor contribution of the TRAF-C domain to TRAF6-induced NF-κB activation. The latter possibility is in agreement with recent evidence involving a large deletion of the TRAF-C domain of TRAF6.
Second, mutational analysis of the RING domain of TRAF6 and TRAF2 revealed the conserved functional importance of multiple residues located outside the core cysteine scaffold of the RING domain. A recent study has indicated the functional importance of the dimerization capability of the TRAF6 RING domain. Interestingly, five (Q82, R88, F118, N121, E126) out of the six functionally important TRAF6 RING domain residues and all four functionally important TRAF2 RING domain residues (Q46, F91, N94, E99) that were characterized in the present study are predicted to lie at the dimerization interface of the corresponding RING domains. Nevertheless, although some of the functionally important mutations affect RING domain dimerization (TRAF6Q82A, TRAF6R88A, TRAF6F118A, TRAF2F91A), others did not cause an apparent effect on the dimerization of TRAF6 (E126Q) or TRAF2 (Q46A, N94A) RING domains as determined by gel filtration experiments. Although, it is possible that RING domain dimerization is also affected by TRAF6E126Q, TRAF2Q46A and TRAF2N94A mutations albeit in a subtle manner that cannot be detected by gel filtration, our results suggest strongly that dimerization-independent properties of the RING domain dimerization interface contribute to TRAF6-induced NF-κB activation. Such properties may include intreractions with other proteins that mediate TRAF6-induced NF-κB activation. This possibility will be explored in the future by analyzing the profile of proteins that interact with wild type and RING-mutants of TRAF6.
Finally, the analysis of polyubiquitination of TRAF6 mutants uncovered novel functional properties of specific RING domain residues of TRAF6. It is believed that TRAF6-associated K63-linked polyubiquitin chains serve as scaffolds for the recruitment and activation of downstream kinases and signaling molecules, including the IKK complex, by a proximity induced mechanism. Whereas Q82A, R88A and F118A TRAF6 mutants were defective in both K63-polyubiquitination and NF-κB activation, L77A, N121A and E126Q TRAF6 mutants underwent normal K63-linked polyubiquitination but failed to mediate optimal activation of NF-κB. It is possible that although L77A, N121A and E126Q mutants allow TRAF6 polyubiqitination, the overall conformation of the protein is not permissive for the proper assembly of higher order complexes and the activation of IKK. A recent study has shown that TRAF6 is capable of synthesizing unanchored K63-linked polyubiquitin chains which play an essential role in IKK activation in vitro. Therefore, it is also possible that the defective signaling capacity of L77A, N121A and E126Q TRAF6 mutants is due to their inability to synthesize unanchored polyubiquitin chains despite the fact that they can undergo polyubiquitination. These issues will be addressed in future experiments through the identification of structural and possibly protein-protein interaction deviations that compromise the NF-κB activating capability of L77A, N121A and E126Q TRAF6 mutants. Interestingly, the dimerization capability of the TRAF6 RING domain could be correlated, at least in part, with the polyubiquitination profile of TRAF6 indicating that RING domain dimerization is necessary for TRAF6 polyubiquitination in accordance with previous observations.
Our mutational analysis of TRAF6 expanded the functional features of the structural framework that has been developed by crystallography, by revealing novel RING domain-dependent properties of TRAF6.
Conclusions
Mutational analysis of TRAF6 and TRAF2 RING domains supports dimerization dependent and independent functions of the RING domain that contribute to NF-κB activation and a potentially necessary but insufficient role of RING-dependent K63-linked polyubiquitination of TRAF6 for NF-κB activation in cells. In addition, alteration of highly conserved residues in the TRAF-C domain of TRAF6 did not affect significantly its ability to induce NF-κB activation, suggesting a tightly folded structure for this domain or its insignificant contribution to TRAF6-induced NF-κB activation.
Acknowledgments
This work was co-financed in part by E.U.-European Social Fund (75%) and the Greek Ministry of Development-GSRT (25%) under the project ΠENEΔ2003 and in part by the Sixth Research Framework Programme of the European Union, Project INCA (LSHC-CT-2005-018704). DAAV is supported by the National Institutes of Health, an NCI Comprehensive Cancer Center Support CORE grant and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Abbreviations: TRAF, tumor-necrosis-factor-receptor-associated factor; IL1/TLR, interleukin-1-receptor/Toll-like receptor; TNFR, tumor-necrosis-factor-receptor; NF-κB, nuclear factor κB; MAPK, mitogen activated protein kinase; RING, really interesting gene; Ubc, ubiquitin conjugating enzyme; IKK, IκB kinase.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chung JY, Lu M, Yin Q, Lin SC, Wu H. Adv Exp Med Biol. 2007;597:122. doi: 10.1007/978-0-387-70630-6_10. [DOI] [PubMed] [Google Scholar]
- 2.Ha H, Han D, Choi Y. Curr Protoc Immunol. 2009;Chapter 11(Unit11):9D. doi: 10.1002/0471142735.im1109ds87. [DOI] [PubMed] [Google Scholar]
- 3.Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. Cell. 2000;103:351. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 4.Xia ZP, Sun L, Chen X, Pineda G, Jiang X, Adhikari A, Zeng W, Chen ZJ. Nature. 2009;461:114. doi: 10.1038/nature08247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye H, Arron JR, Lamothe B, Cirilli M, Kobayashi T, Shevde NK, Segal D, Dzivenu OK, Vologodskaia M, Yim M, Du K, Singh S, Pike JW, Darnay BG, Choi Y, Wu H. Nature. 2002;418:443. doi: 10.1038/nature00888. [DOI] [PubMed] [Google Scholar]
- 6.Yin Q, Lin SC, Lamothe B, Lu M, Lo YC, Hura G, Zheng L, Rich RL, Campos AD, Myszka DG, Lenardo MJ, Darnay BG, Wu H. Nat Struct Mol Biol. 2009;16:658. doi: 10.1038/nsmb.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaye KM, Devergne O, Harada JN, Izumi KM, Yalamanchili R, Kieff E, Mosialos G. Proc Natl Acad Sci U S A. 1996;93:11085. doi: 10.1073/pnas.93.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Matsuzawa A, Brown SA, Zhou J, Guy CS, Tseng PH, Forbes K, Nicholson TP, Sheppard PW, Hacker H, Karin M, Vignali DA. Proc Natl Acad Sci U S A. 2008;105:20197. doi: 10.1073/pnas.0810461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park YC, Burkitt V, Villa AR, Tong L, Wu H. Nature. 1999;398:533. doi: 10.1038/19110. [DOI] [PubMed] [Google Scholar]
- 10.Ni CZ, Welsh K, Leo E, Chiou CK, Wu H, Reed JC, Ely KR. Proc Natl Acad Sci U S A. 2000;97:10395. doi: 10.1073/pnas.97.19.10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S. Nature. 465:1084. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin Q, Lamothe B, Darnay BG, Wu H. Biochemistry. 2009;48:10558. doi: 10.1021/bi901462e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang KZ, Galson DL, Auron PE. J Cell Biochem. 110:763. doi: 10.1002/jcb.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]


