Abstract
Marinobactins A-E are a suite of amphiphilic siderophores which have a common peptidic head group that coordinates Fe(III), and a fatty acid which varies in length and saturation. As a result of the amphiphilic properties of these siderophores it is difficult to study siderophore-mediated uptake of iron, because the amphiphilic siderophores partition indiscriminately in microbial and other membranes. An alternative method to distinguish amphiphilic siderophore partitioning versus siderophore-mediated active uptake for Fe(III)-marinobactin E has been developed. In addition, a new member of the marinobactin family of siderophores is also reported, marinobactin F, which has a C18 fatty acid with one double bond and which is substantially more hydrophobic that marinobactins A-E.
Keywords: Amphiphilic siderophore, marine bacteria, marinobactins, iron, lipidic peptide
Introduction
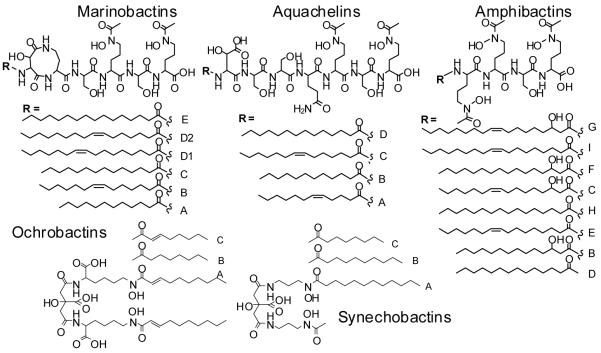
Siderophores are low molecular weight, high-affinity iron(III) chelates, produced by bacteria to solubilize and promote iron uptake under low iron conditions. The marinobactins, aquachelins, amphibactins, ochrobactins, and synechobactins are siderophores isolated from distinct genera of marine bacteria (e.g., Halomonas aquamarina, Marinobacter, Vibrio, Ochrobactrum and the cyanobacterium, Synechococcus PCC7002) [1-4] (Figure 1). Each suite of siderophores contains a unique head group that coordinates iron(III) and one of a series of fatty acid tails (C8-C18). The amphiphilic peptidic siderophores are strikingly similar compounds, consisting of an Orn-Ser-Orn carboxyl motif in which each Orn is hydroxylated and acetylated forming the hydroxamic acid moiety that coordinates Fe(III) [1,2,5]. The marinobactins, aquachelins, and most likely the amphibactin lipopeptides are characterized by low critical micelle concentrations (CMCs). At concentrations exceeding their CMC, the apo marinobactins form spherical micelles which shrink in diameter upon coordination of Fe(III). Upon addition of excess Fe(III), the Fe(III)-marinobactin micelles undergo a transition to form vesicles [1,6].
Figure 1.
Marine amphiphilic siderophores.
The vast majority of siderophores discovered to date, which have been largely from terrestrial bacteria, are water-soluble and non-amphiphilic. However, the mycobactins, produced by mycobacteria, and acinetoferrin, produced by Acinetobacter haemolyticus, are examples of amphiphilic siderophores that resemble the marine peptidic and citrate-derived siderophores, respectively [7,8]. The mycobactins and the carboxymycobactins, siderophores from mycobacteria, each have a 2-hydroxyphenoloxazoline-containing head groups but differ by the length and terminal modification of the fatty acid appendages. The mycobactins are cell-associated and have long fatty acid tails (C16-C21). Conversely, carboxymycobactins with terminal carboxyl and methylester groups and shorter fatty acid tails (C2-C8) are more hydrophilic and are secreted into the growth medium. The other peptidic siderophores known to contain hydrocarbon tails are the ornibactins, produced by Burkholderia cepacia, and corrugatin, produced by Pseudomonas corrugata, although within the amphiphilic siderophore spectrum these are quite hydrophilic due to short fatty acid appendages (i.e., up to C8) and longer peptides [9,10]. With relatively large head groups in relation to tail lengths, these siderophores will likely have very different colligative properties than the marinobactins, amphibactins, aquachelins, or the mycobactins.
The production of amphiphilic siderophores across many genera of bacteria, suggest that these organisms may have developed more sophisticated methods to counter siderophore diffusion than those bacteria producing freely soluble siderophores. This phenomenon might be particularly true for marine organisms where the majority of siderophores discovered to date are amphiphilic and where the environmental constraints of marine life might demand alternative iron scavenging strategies. We have previously studied the mechanisms of iron sequestration, mediated by the marinobactin siderophores, in model membranes. Both Fe(III)-marinobactin E (Fe(III)-ME) and apo-ME have high partition coefficients in large unilamellar phosphatidylcholine vesicles of 174 M−1 and 6387 M−1, respectively [5,11]. Experiments were also completed with the physiological mixture of marinobactin siderophores to better understand the utility of the suite of siderophores produced by this bacterium. Partition coefficients for the suite of siderophores varied over two orders of magnitude and showed a strong dependence on the length of the fatty acid tails (36 M−1 to 5818 M−1). These experiments suggested that producing a suite of siderophores would effectively create a concentration gradient of siderophores extending from the bacterium. This gradient could set up a shuttle system between the siderophores in solution (marinobactins with shorter fatty acid tails) and those more likely partitioned into the membrane (marinobactins with longer fatty acid tails). Certainly, partitioning of the marinobactins within the membrane would be facilitated by the lipidic tails and may therefore represent an early step in the iron uptake process mediated by the marinobactin siderophores. While much is known about Fe(III) uptake in mammalian cells [12-15], little is known about iron uptake mechanisms for oceanic organisms [16,17]. The paradigm of siderophore-mediated uptake of iron(III), for terrestrial bacteria, is that bacteria make and then secrete siderophores into the medium. The siderophores solubilize and chelate Fe(III), wherein the Fe(III)-siderophore complex is recognized by a siderophore-specific cell surface receptor that transports the Fe(III)-siderophore complex into the bacterium. The biosynthesis of these receptors, a type of gated protein channel, is co-regulated with siderophore biosynthesis in response to low iron levels. The uptake of Fe(III) through this mechanisms is, most typically, specific, saturable, and energy-dependant. Once transported, the Fe(III)-siderophore complex is shuttled from the periplasm to the cytoplasm by a permease within the cytoplasmic membrane [18]. For mycobacteria it is hypothesized that iron is acquired from mycobactins by a shuttle system in which the carboxymycobactins transport ferric iron to the membrane-associated mycobactins [12].
Traditional Fe(III)-siderophore uptake experiments generally employ a radiological label to track acquisition of iron or siderophore. Results from these types of experiments would be hard to decipher for the amphiphilic siderophores because the fatty acid appendage confers nonspecific adventitious partitioning into bacterial cells. We report herein results of an alternative method to distinguish amphiphilic siderophore partitioning versus siderophore-mediated active uptake of Fe(III) and siderophores, and we report the isolation of a new member of the marinobactin suite of siderophores which is substantially more hydrophobic.
Materials and Methods
Siderophore isolation
The marinobactin siderophores were isolated and purified as previously described [1,5,6]. Briefly, Marinobacter sp. strain DS40M6 was grown in artificial seawater medium (ASW) containing 15 g NaCl, 0.75 g KCl, 0.2 g MgS04-7H20, 0.1 g CaCl2-2H20, 1 g NH4Cl, 5 g sodium succinate, and 3 g Na2HPO4-7H20 per liter of doubly deionized water (typically 2 L growth culture per 4 L acid-washed Erlenmeyer flask). The culture medium was adjusted to pH 7.0 with phosphoric acid and autoclaved. A single colony of DS40M6 was streaked on a maintenance medium plate (ASW-agar), grown for 24 h, and the confluent plate resuspended in 5 mL of culture medium. The culture was inoculated with 2 mL resuspended bacteria and grown approximately 2 - 4 days on a rotary shaker (200 rpm) at ambient temperature.
The production of siderophores was assayed using the chromazurol S (CAS) solution assay containing CAS/Fe(III)/HDTMA (hexadecyl-trimethylammonium bromide) [1]. The assay was positive, i.e., showing the blue to pink color change, using equal volumes of broth culture and Fe(III)-CAS solution during growth or one part eluent to two parts Fe(III)-CAS during siderophore purification. The bacterial cells were harvested when equal volumes Fe(III)-CAS solution assay and bacterial supernatant (OD600 1.0) turned blue to pink within 1 minute. The siderophores were isolated from the supernatant following centrifugation of the culture (4800 × g, 20 min, 4 °C) and adsorption onto Amberlite XAD-2 resin (Supelco Inc). The resin was sequentially washed with two column volumes each of doubly deionized water (Barnstead, Nanopure®), 50% aqueous MeOH and then 100% MeOH. Siderophore containing fractions, corresponding to elution with 100% MeOH, were pooled, concentrated in vacuo, and applied onto a C4 reversed-phase HPLC column (Vydac, 10 mm ID × 250 mm L or 22 mm ID × 250 mm L). The siderophores were purified using a gradient of 60/40 (% A/B) to 0/100 over 40 min [A = 99.95% dH2O and 0.05% trifluoroacetic acid (TFA); B = 19.95% dH2O, 0.05% TFA, and 80% acetonitrile]. The absorbance of the eluent was monitored at 215 nm. Purity and identity of the siderophores was confirmed by analytical HPLC and electrospray mass spectrometry. A VG-Fisons Platform II (Micromass) quadrupole mass spectrometer coupled with a Michrom BioResources HPLC unit, for direct injection, was used with a source temperature of 70 °C and a cone voltage of 65 V. Marinobactin F was characterized using a source temperature (90 °C) and cone voltage (80 V) sufficient to produce fragmentation.
Partitioning and uptake of marinobactin E in vivo by Marinobacter sp. strain DS40M6
Marinobacter sp. DS40M6 was grown for 48 - 70 h in ASW medium (1 L growth per 4 L acid-washed Erlenmeyer flask) at 200 rpm on a rotary shaker at ambient temperature for in vivo partitioning and uptake studies. The cells were harvested (cellular density, OD600 0.50 - 0.75) by centrifugation in sterile acid washed polycarbonate centrifuge tubes (5000 × g, 10 min, 25 °C). The supernatant was decanted and the bacterial pellet was gently washed with sterile ASW medium to remove endogenous siderophore. The bacterial pellet was gently released from the centrifuge tube using a sterile disposable inoculation loop into roughly 400 mL sterile ASW medium. The resuspended bacteria were shaken in the centrifuge tube at 200 rpm for 30 min on a rotary shaker at ambient temperature.
The bacterial cell density was adjusted to an OD600 of 0.63 - 0.70 with sterile ASW medium. The resuspended bacterial solution (8 mL) was added to sterile 50 mL polypropylene conical tubes (Falcon). For active bacterial cells, the tubes were tilted at a 45° angle and shaken at 140 rpm for 30 min at ambient temperature. Treated cells consisted of metabolically inhibited cells (KCN-treated) and nonviable cells (glutaraldehyde-treated). For metabolically inhibited cells, the bacteria were tilted at a 45° angle and shaken as active cells for 20 min, wherein the bacteria were shaken an additional 10 min with 5 mM KCN. For nonviable cells, the bacteria were shaken for 30 min with 1% glutaraldehyde. Experiments were completed in duplicate.
A siderophore mixture (16 μL) consisting of a fixed ratio of apo-ME to Fe-ME was added to treated and active cells to a final concentration of 20 μM. The cells were recentrifuged following a 1.5 hour incubation at ambient temperature (140,000 × g, 30 min, 4 °C). The supernatant (2.5 mL) was loaded onto a C4 reversed-phase HPLC column (Vydac, 5 mm ID × 250 mm L ). The siderophores were separated using a gradient of 100/0 (% A/B) for 7 min to 0/100 over 25 min; and a hold at 0/100 for 3 min [A = 99.95% dH2O and 0.05% TFA; B = 19.95% dH2O, 0.05% TFA, and 80% acetonitrile]. Siderophore concentration in the supernatant was quantified by comparison to a standard curve generated for previously reported partition experiments [5]. Exposure to 0.05% TFA does not alter the ratio of Fe(III)- to apo-ME. Control experiments were completed with 20 μM siderophore mixture in medium incubated and centrifuged as described above. This experiment controlled for nonspecific adsorption of the siderophores to the reaction tubes and established the starting concentration of apo-ME to Fe-ME. No significant change in apo- to Fe(III)-ME was observed upon addition to the low iron medium. Additional controls were completed by incubating resuspended cells in fresh medium without addition of siderophore mixture as described above. This experiment controlled for siderophore production over the time-course of the experiment.
Viability tests were completed on treated and active cells by making 1:10 serial dilutions of the cell suspension, plating the cell suspension onto maintenance medium plates, and monitoring for colony formation.
Outer membrane preparation
The procedure for outer membrane protein preparations was adapted from literature [20-22]. A single colony of Marinobacter sp strain DS40M6 was streaked for on a maintenance medium plate, grown for 24 h, and the lawn was resuspended in 5 mL of culture medium. ASW medium (2 L) was inoculated with 1 mL resuspended bacteria and shaken at 200 rpm at ambient temperature. Approximately 7 h later, another flask of ASW medium (2 L), containing 5 mg ferric ammonium citrate (FAC) per 1 L medium, was inoculated in a similar fashion. Both cultures were incubated for an additional 36 h at ambient temperature with shaking at 200 rpm. Each bacterial culture (800 mL) was centrifuged (4000 × g, 30 min, 4 °C) in polycarbonate centrifuge tubes. The bacterial pellets from high and low iron medium were washed and individually resuspended in a total of 200 mL, 10 mM Tris-HCl (pH 8.0). Cell suspensions were sonicated on ice in 10 mL batches using a probe tip sonicator (Branson Sonifier 250, 3 min, 30 second intervals, constant duty cycle, output 4). The unbroken cells and debris were collected by centrifugation (17,210 × g, 30 min, 4 °C). The supernatant was decanted and recentrifuged to pellet the membranes (140,000 × g, 1.5 hrs, 4 °C). To solubilize the cytoplasmic membranes, each pellet was resuspended in 2 mL of 10 mM Tris-HCl (pH 8.0) buffer containing 2% Triton X-100 (140 rpm shaking speed, 30 min, 24 °C) [20]. The suspension was recentrifuged to pellet the outer membranes (140,000 × g, 2 hrs, 4 °C).
Outer membrane pellets from bacteria grown with or without FAC were resuspended in 1 mL of 10 mM Tris-HCl (pH 8.0). Membrane proteins were run on 12% SDS-PAGE polyacrylamide gels and visualized by silver staining. Visual comparison of proteins expressed under low iron growth conditions to those expressed under high iron growth conditions was completed.
Results and Interpretation
Bacterial partitioning and uptake of marinobactin E
To investigate partitioning and uptake of marinobactin by Marinobacter sp. strain DS40M6, cells from late log phase growth were harvested by centrifugation and resuspended in fresh ASW to eliminate siderophore contamination from the medium. A defined mixture of Fe(III)-ME and apo-ME was added to the resuspended cells, equilibrated, and ultracentrifuged to pellet the bacteria. The concentration of siderophore in the supernatant was determined by HPLC analysis. This procedure directly monitors the amount of both Fe(III)-ME and apo-ME remaining in the supernatant and indirectly accounts for uptake of Fe(III)- and apo- siderophore and partitioning of siderophore into the bacterium. The strength of this technique, over traditional uptake studies, is that the partitioning and uptake for both Fe-ME and apo-ME can be monitored, simultaneously.
The initial ratio of Fe(III)-ME to apo-ME, prior to the addition of cells, is established in the medium control (Figure 2). The procedure used to prepare the solution which is the “medium control” controls for changes in apo-ME concentration as a result of coordinating residual iron(III) in the medium. The ratio of apo-ME to Fe(III)-ME in the medium control is 1.7:1 (Figure 2). The concentration of both apo-ME and Fe(III)-ME diminished greatly with addition of active bacterial cells (late log phase). The largest change is seen for Fe(III)-ME, suggesting an active uptake process and not merely partitioning. The ratio of apo-ME to Fe-ME, following equilibration with active cells is 5.3:1. For dead cells, prepared by pretreatment with glutaraldehyde, an overall decrease in total siderophore concentration is observed. The large change in siderophore concentration likely results from passive adsorption or partitioning of the marinobactins onto the cell, resulting for an altered outermembrane. Interestingly, the starting ratio between apo-ME and Fe(III)-ME is not altered with addition of dead cells (1.8:1). Addition of the metabolic poison KCN, caused a similar decrease in apo-ME as was seen in active cells; however, Fe(III)-ME uptake was significantly less than seen in active cells (2.3:1). Control experiments with bacteria and no added siderophore showed that the bacterium neither released nor produced siderophore over the time period of the equilibration.
Figure 2.
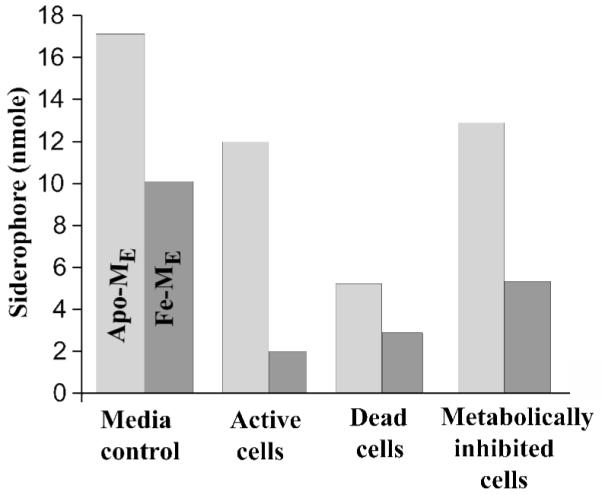
The in vivo partitioning and uptake study of apo-ME and Fe-ME with the Marinobacter sp. strain DS40M6. DS40M6 was grown to late log phase (OD600 0.65) in 2 L of ASW medium. The cells were centrifuged and resuspended in fresh ASW medium (OD600 0.68) and allowed to equilibrate for a total of 30 min. Descriptors of cells and conditions are as follows: medium control, ASW medium with 20 μM siderophore mixture (apo-ME and Fe-ME); active cells, 20 μM siderophore mixture added after 30 minute equilibration of cells; dead cells, 1% glutaraldehyde added 30 min prior to addition of 20 μM siderophore mixture; and metabolically inhibited cells, 5 mM KCN added 10 min prior to addition of 20 μM siderophore mixture. Siderophore-cell mixtures were incubated for 1.5 h. The cells were ultracentrifuged and the quantities of apo-ME and Fe-ME remaining in the supernatant were determined by HPLC.
Iron-repressible outer membrane protein production
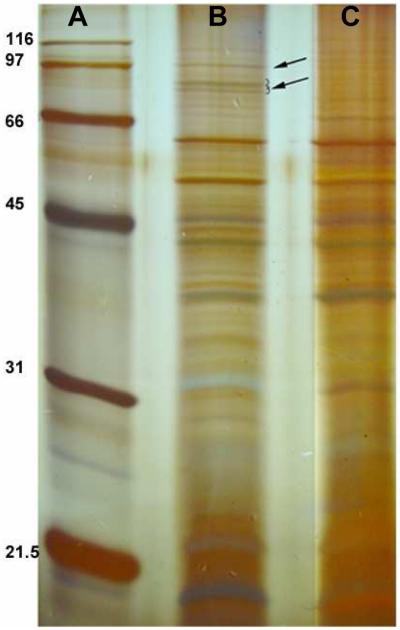
Two outer membrane proteins are expressed by the Marinobacter sp. strain DS40M6 under low iron, but not high iron, growth conditions. These proteins are of approximate molecular weights of 79 and 81 kDa. It was also observed that a 95 kDa protein was overexpressed in low iron conditions relative to high iron conditions (Figure 3). On average, iron-repressible outer membrane siderophore receptors have monomeric molecular weights of 80 kDa, and so run similarly on SDS-PAGE [23-25].
Figure 3.
SDS-PAGE of outer membrane proteins from the Marinobacter sp. strain DS40M6 grown with or without added Fe(III). Bacteria were grown for 40 h in ASW medium with (C) or without (B) 10 mg ferric ammonium citrate per 2 L growth. Bacteria were harvested and outer membrane proteins were isolated with modification of previously published protocols [20-22]. Proteins were separated on a 12% SDS-PAGE gel and visualized by silver staining. Broad-range molecular weight markers (kDa, Bio-Rad) (A) were used for comparison.
Characterization of Marinobactin F
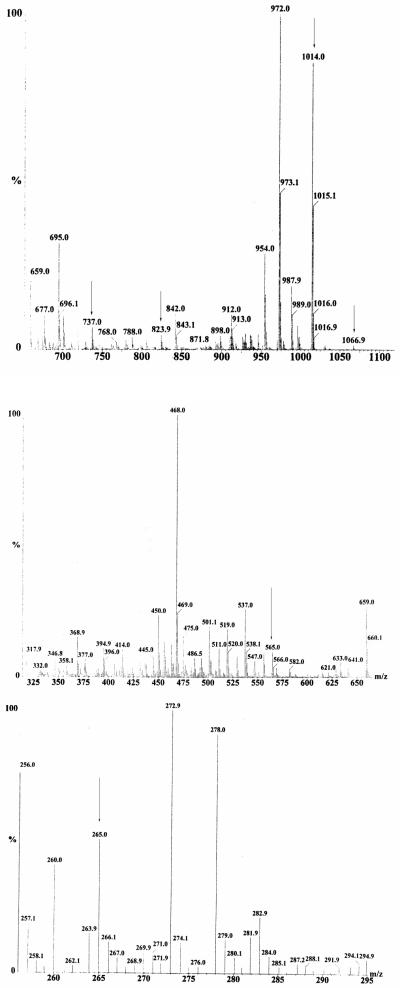
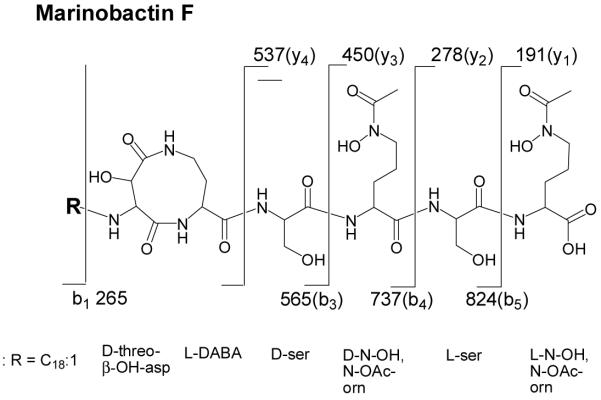
On some occasions during the isolation of marinobactin siderophores, an Fe(III)-CAS-reactive HPLC peak was observed eluting at 2% greater concentration of buffer B than apo-ME. This peak contains a mixture of molecular ion species at m/z 1014 (M+H)+ and 1067 (M+H)+. The m/z of the later species is 53 mass units higher than the former species, consistent with Fe(III) coordination. The ESI-mass spectrum was generated using a cone voltage and temperature sufficient to produce y and b fragments (Figure 4). The “y” and “b” nomenclature refers to the charge when retained by the COOH-terminal fragment or the NH2-terminal fragment of the peptide, respectively [26]. The b fragments observed in the mass spectrum are as follows (Figure 5): a b5 fragment of 824 (m/z), corresponding to loss of y1 191 (m/z); a b4 fragment of 737 (m/z), corresponding to loss of y2 278 (m/z); a b3 fragment of 565 (m/z), corresponding to loss of y3 450 (m/z); and a b1 fragment of 265 (m/z), corresponding to the mass of the fatty acid tail. Fragments y4 - y1 were seen in the mass spectrum, however no y5 fragment was observed, consistent with what was observed previously for characterization of marinobactins A - E [1].
Figure 4.
Electrospray mass spectra for the Marinobacter sp. strain DS40M6 siderophore 1014 (M+H)+, named Marinobactin F.
Figure 5.
Structure of marinobactin F. The “y” and “b” nomenclature refers to the charge when retained by the COOH-terminal fragment or the NH2-terminal fragment of the peptide, respectively [28].
The new marinobactin siderophore identified here by mass spectrometry, marinobactin F (MF), contains the same head group as the other marinobactin siderophores and a C18 fatty acid tail with one unit of unsaturation (Figure 4). The limited quantity of MF precluded determining the position of the double-bond within the fatty acid appendage. Very low levels of this peptide are found in the supernatant and it is possible that more of this siderophore is associated with the bacterial pellet, resulting in the enhanced CAS activity of the pellet.
Discussion
Marinobacter sp. strain DS40M6 has been shown to produce a suite of siderophores with varying amphiphilicity (Figure 1). The physiological function of the fatty acid tails and the suites of siderophores is unknown; however, we have begun to investigate the in vivo Fe(III) uptake and siderophore partitioning. In contrast to model studies in which apo-ME partitioned better than Fe(III)-ME in phosphatidylcholine vesicles, in vivo studies showed that much more Fe(III)-ME was taken up by the source bacterial cells, Marinobacter sp. strain DS40M6 (Figure 2). This increase in uptake of Fe(III)-ME suggests an active transport process mediated by a receptor, such as one of the iron-repressible outer membrane proteins of DS40M6 observed in Figure 3. Dead cells of strain DS40M6, prepared by pretreatment of actively growing cells with glutaraldehyde, showed a large degree of total partitioning for apo-ME and Fe(III)-ME, but showed no difference from the medium control in the ratio of apo-ME:Fe(III)-ME. Metabolically inhibited cells showed that the Fe(III)-ME uptake is active transport. Also, the decrease in levels of apo-ME with active cells and KCN-treated cells, suggests that both apo-ME and Fe-ME actively partition into or associate with the bacterial cells.
Few siderophores have been found that are as lipophilic as marinobactins. As discussed in the introduction, the mycobactins and carboxymycobactins are a well-defined class of lipidic siderophores [27,28]. Similarities exist between the mycobactins and the marinobactins. Both types of siderophores have a similar structure assembly including production of a suite of siderophores with similar head groups and differing fatty acid tail lengths and both have potentially similar membrane affinity. However, there are strong differences in that iron accumulation by the mycobactins is thought to occur via diffusion through the cell envelope of Mycobacteria rather than recognition of a cell surface receptor as is found in Gram-negative bacteria like the Marinobacter sp. [18, 29] Furthermore, the interaction of the mycobactins with the Mycobacterial cellular envelope will differ significantly from the interaction of the marinobactins with Gram-negative outer membranes. Mycobacteria have a dense, mycolate-based, lipid matrix [30] in place of the outer membrane found in Gram-negative bacteria such as the Marinobacter sp.
What then leads to selectivity if the marinobactin siderophores exhibit such high membrane activity when it is likely that the heterotrophic bacteria producing these siderophores live among a community of other heterotrophic and autotrophic organisms [31]. It is clear that other membrane active molecules such as the membrane permeable N-acyl homoserine lactone signal molecules [32] produced and sensed by bacteria must operate under similar conditions. A uniformly membrane active compound would effectively dilute these molecules by partitioning into surrounding membranes and therefore would require higher synthesis levels by the bacterium. It is not yet known how the Marinobacter sp. strain DS40M6, producing the marinobactins, might counter this effect, nor is it known how the suite of siderophores partitions or facilitates iron uptake or if membrane associated micellar or vesicular siderophores increase the local iron concentration around this bacterium.
Our previous results have shown that the degree of partitioning directly correlates with the hydrophobicity of the fatty acid tail [2,5]. Here we have reported an additional marinobactin siderophore that is rarely found within the supernatant. Marinobactin F may be one of a highly hydrophobic set of marinobactins associated with the bacterial cell, possibly accounting for the high CAS reactivity of the bacterium. The ability of the Marinobacter sp. strain DS40M6, to sequester iron mediated by the marinobactins is likely altered by changing the typical affinity that siderophores have for the aqueous environment to that of the membrane environment. A membrane bound siderophore, constrained to move within the confines of the membrane, would limit a three dimensional search in space normally required for interaction with a specific receptor, to a two dimensional search within the membrane [33]. A critical aspect of siderophore mediated iron uptake, particularly in the oceanic environment, is the prevention of siderophore diffusion. To limit or prevent diffusion, the bacteria might: 1) anchor the siderophore within the membrane; 2) maintain a sheltered sub-environment such as a polysaccharide matrix or fence, thus limiting release of siderophores; or, 3) engage in siderophore piracy and utilize siderophores from vastly different bacteria [16, 34]. Thus the Marinobacter sp., in producing the marinobactins, could employ two such diffusion-countering strategies because the lipophilic marinobactins are likely particle reactive and membrane reactive, both would contribute to increased residence time in the vicinity of the bacterium.
Acknowledgements
Support from NIH GM38130 (AB) and NSF CHE-0221978 (Center for Environmental BioInorganic Chemistry, a NSF Environmental Molecular Science Institute) is gratefully acknowledged. We thank California Sea Grant for a graduate student traineeship for JSM.
Abbreviations
- CMC
critical micelle concentration
- ME
Marinobactin E
- ASW
artificial seawater medium
- CAS
chromazurol S
- HDTMA
hexadecyl-trimethylammoniumbromide
- HPLC
high performance liquid chromatography
- TFA
trifluoroacetic acid
Footnotes
We dedicate this paper to the memory of Ed Stieffel
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martinez JS, Zhang GP, Holt PD, Jung H-T, Carrano CJ, Haygood MG, Butler A. Science. 2000;287:1245–1247. doi: 10.1126/science.287.5456.1245. [DOI] [PubMed] [Google Scholar]
- 2.Martinez JS, Carter-Franklin JN, Mann EL, Martin JD, Haygood MG, Butler A. Proc. Nat’l Acad. Sci., USA. 2003;100:3754–3759. doi: 10.1073/pnas.0637444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin JD, Ito Y, Homann VV, Haygood MG, Butler A. J. Biol. Inorg. Chem. 2006;11:633–641. doi: 10.1007/s00775-006-0112-y. [DOI] [PubMed] [Google Scholar]
- 4.Ito Y, Butler A. Limnol & Oceanogr. 2005;50:1918–1923. [Google Scholar]
- 5.Xu G, Martinez JS, Groves JT, Butler A. J. Am. Chem. Soc. 2002;124:13408–13415. doi: 10.1021/ja026768w. [DOI] [PubMed] [Google Scholar]
- 6.Owen T, Pynn R, Martinez JS, Butler A. Langmuir. 2005;21:12109–12114. doi: 10.1021/la0519352. [DOI] [PubMed] [Google Scholar]
- 7.Ratledge C, Dover L. Annu. Rev. Microbiol. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- 8.Luo M, Fadeev EA, Groves JT. J. Am. Chem. Soc. 2005;127:1726–1736. doi: 10.1021/ja044230f. [DOI] [PubMed] [Google Scholar]
- 9.Meyer JM, Van V. Tran, Stintzi A, Berge O, Winkelmann G. BioMetals. 1995;8:309–317. doi: 10.1007/BF00141604. [DOI] [PubMed] [Google Scholar]
- 10.Risse D, Beiderbeck H, Tara K, Budzikiewicz H, Gustin D. Z. Natur. J. Biosci. 1998;53:295–304. [Google Scholar]
- 11.Martinez JS. PhD Dissertation. University of California Santa Barbara: 2002. [Google Scholar]
- 12.Gobin J, Horowitz MA. J. Exp. Med. 1996;183:1527. doi: 10.1084/jem.183.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raymond KN, Dertz EA. In: Iron Transport in Bacteria. Crosa J, editor. ASM Press; Washington D.C.: 2004. pp. 3–17. [Google Scholar]
- 14.Boukhalfa H, Crumbliss AL. BioMetals. 2002;15:325–339. doi: 10.1023/a:1020218608266. [DOI] [PubMed] [Google Scholar]
- 15.Luo M, Lin H, Fischbach M, Liu D, Walsh C, Groves J. ACS Chemical Biology. 2006;1:29–32. doi: 10.1021/cb0500034. [DOI] [PubMed] [Google Scholar]
- 16.Granger J, Price NM. Limnol. Oceanogr. 1999;44:541–555. [Google Scholar]
- 17.Price NM, Morel FMM. Science. 2003;300:944–947. doi: 10.1126/science.1083545. [DOI] [PubMed] [Google Scholar]
- 18.Winkelmann G. Handbook of microbial iron chelates. CRC Press Inc.; Boca Raton, Florida: 1991. [Google Scholar]
- 19.Schwyn B, Neilands JE. Biochemistry. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 20.Jalal MAF, van der Helm D. FEBS Lett. 1989;243:366–370. doi: 10.1016/0014-5793(89)80163-2. [DOI] [PubMed] [Google Scholar]
- 21.Nelson M, Carrano CJ, Szaniszlo PJ. BioMetals. 1992;5:37–46. doi: 10.1007/BF01079696. [DOI] [PubMed] [Google Scholar]
- 22.Reid RT, Butler A. Limnol and Oceanogr. 1991;36:1783–1792. [Google Scholar]
- 23.Yamamoto S, Akiyama T, Okujo N, Matsuura S, Shinoda S. Microbiol. Immunol. 1995;39:759–766. doi: 10.1111/j.1348-0421.1995.tb03268.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhou SH, van der Helm D, Adjimani J. Biometals. 1993;6:25–35. doi: 10.1007/BF00154229. [DOI] [PubMed] [Google Scholar]
- 25.Bergeron RJ, Weimer WR, Dionis JB. J. Bacteriol. 1988;170:3711–3717. doi: 10.1128/jb.170.8.3711-3717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roepstorff P, Fohlman J. Biomedical Mass Spectrometry. 1984;11:601. doi: 10.1002/bms.1200111109. [DOI] [PubMed] [Google Scholar]
- 27.De Voss JJ, Rutter K, Schroeder BG, Barry CE., III J. Bacteriol. 1999;181:4443–4451. doi: 10.1128/jb.181.15.4443-4451.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratledge C. In: The Mycobacteria, A sourcebook. Kubica GP, Wayne LG, editors. Marcel Dekker; New York, NY: 1984. pp. 603–627. (Microbiology Series). [Google Scholar]
- 29.Sritharan M. World J. Microbiol. Biotechnol. 2001;16:769–780. [Google Scholar]
- 30.Brennan PJ. Mycobacterium and other actinomycetes. In: Ratledge C, Wilkinson SG, editors. Microbial Lipids. 1988. pp. 203–298. [Google Scholar]
- 31.Campbell L, Nolla HA, Vaulot D. Limnol. Oceanogr. 1994;39:954–961. [Google Scholar]
- 32.Pearson JP, Van Delden C, Iglewski BH. J. Bacteriol. 1999;181:1203–1210. doi: 10.1128/jb.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Epand RM. Biopolymers. 1997;43:15–24. doi: 10.1002/(SICI)1097-0282(1997)43:1<15::AID-BIP3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Stintzi A, Barnes C, Xu J, Raymond KN. Proc. Natl. Acad. Sci. USA. 2000;97:10691–10696. doi: 10.1073/pnas.200318797. [DOI] [PMC free article] [PubMed] [Google Scholar]