Abstract
Stroke is an important cause of neurologic morbidity in childhood. Population-based estimates of the annual incidence of childhood stroke range from 2 to 13 per 100,000 children. This article will review recent literature on both hemorrhagic and ischemic stroke in children with a focus on cerebral arteriopathy and vascular malformations as stroke risk factors. Additional risk factors include congenital heart disease, sickle cell disease, and hematologic abnormalities among others. Outcomes are variable and are related to the severity of presentation, associated illnesses, and other factors. More than half of children who have had a stroke have long-term neurological sequelae. Five-year recurrence risk is estimated to be 5–19%. Children with cerebrovascular abnormalities are at the highest risk of recurrence (66% at 5 years for ischemic stroke in one study). Furthermore, cerebral arteriopathy including arterial dissection may account for up to 80% of childhood stroke in otherwise healthy children. In many cases, evaluation and treatment of pediatric stroke is not evidence-based, and regional and geographic variations in practice patterns exist. Multicenter cohort studies and ultimately dedicated pediatric clinical trials will be essential to establish comprehensive evidence-based guidelines for pediatric stroke care.
Keywords: stroke, child, pediatric stroke, arteriopathy, vascular malformation, review
Stroke in children is at least as frequent as brain tumors [1] and is among the top ten causes of death in childhood [2]. Nevertheless, physicians and families often ask with amazement, “Children have strokes? Why?” Traditional adult stroke risk factors such as hypertension, diabetes, smoking, and hypercholesterolemia are uncommon in children. Instead, pediatric stroke risk factors include arteriopathy and vascular malformations, congenital heart disease, sickle cell disease, and hematologic abnormalities among others. The importance of vascular abnormalities as a cause of both ischemic and hemorrhagic stroke has become apparent in several recent studies. This paper will focus on “abnormal vasculature” in pediatric stroke. Recently cerebral arteriopathy has been recognized both as a major risk factor for arterial ischemic stroke in children [3, 4] and as a predictor of poor short-term outcome [5]. Vascular abnormalities (arteriovenous malformations (AVM), cavernous malformations, and aneurysms) are the major cause of hemorrhagic stroke in children [6–10]. Furthermore, vascular anomalies are sometimes discovered secondary to symptoms such as headache or seizure before a stroke has occurred. Pediatric neurologists and neurosurgeons must then determine the risks and benefits of various management strategies for these patients to balance the risk of future strokes with potential sequelae from the treatments themselves.
Epidemiology of Stroke in Children
The incidence of all stroke in children is reported as 2 to13 per 100,000 person-years in developed countries [8, 11–13]. Ischemic stroke typically refers to arterial ischemic stroke (AIS) and cerebral sinovenous thrombosis (CSVT) resulting in venous infarction. CSVT will not be discussed in detail. Hemorrhagic stroke (HS) typically includes spontaneous intracerebral (parenchymal and intraventricular) hemorrhage (ICH) and non-traumatic subarachnoid hemorrhage (SAH) [14, 15]. A study of a California-wide hospital pediatric discharge database found an incidence rate of 1.1 per 100,000 person-years for hemorrhagic stroke and 1.2 per 100,000 person-years for ischemic stroke [13]. Thus, nearly half of pediatric strokes are hemorrhagic.
Risk Factors for and Etiology of Stroke in Children
Arterial Ischemic Stroke
Numerous conditions have been associated with AIS in children. The most common etiologies in developed countries include cerebral arteriopathies, congenital or acquired cardiac disease, and serious systemic infection (meningitis, sepsis) [16]. Other stroke risks include hematologic disorders such as thrombophilias [17, 18]and sickle cell disease. Sickle cell disease is likely the most common risk factor for pediatric stroke world-wide since 10% of these patients have a clinically evident stroke [19] and at least another 20% have a “silent stroke” by age 20 [20, 21].
Our discussion of AIS etiology will focus on arteriopathy, including both intrinsic and acquired vascular disease. The frequency of cerebral arteriopathy in published reports varies from 53%–86% [3, 22], and the term cerebral arteriopathy has been used broadly to encompass the following abnormalities on vascular imaging: cervicocephalic arterial dissection, moyamoya, vasculitis, sickle cell disease arteriopathy, post varicella angiopathy, and idiopathic focal cerebral arteriopathy[3]. Additionally, several genetic syndromes have been associated with cervical and cerebral arteriopathy: neurofibromatosis type 1[23], Alagille syndrome [24, 25], Williams Syndrome [26], PHACES syndrome, and trisomy 21 [27, 28]. The distinction among various arteriopathies is not always apparent, and there is overlap in literature describing these vascular abnormalities [29]. Several major categories of arteriopathy are discussed below.
Focal Cerebral Arteriopathy of Childhood
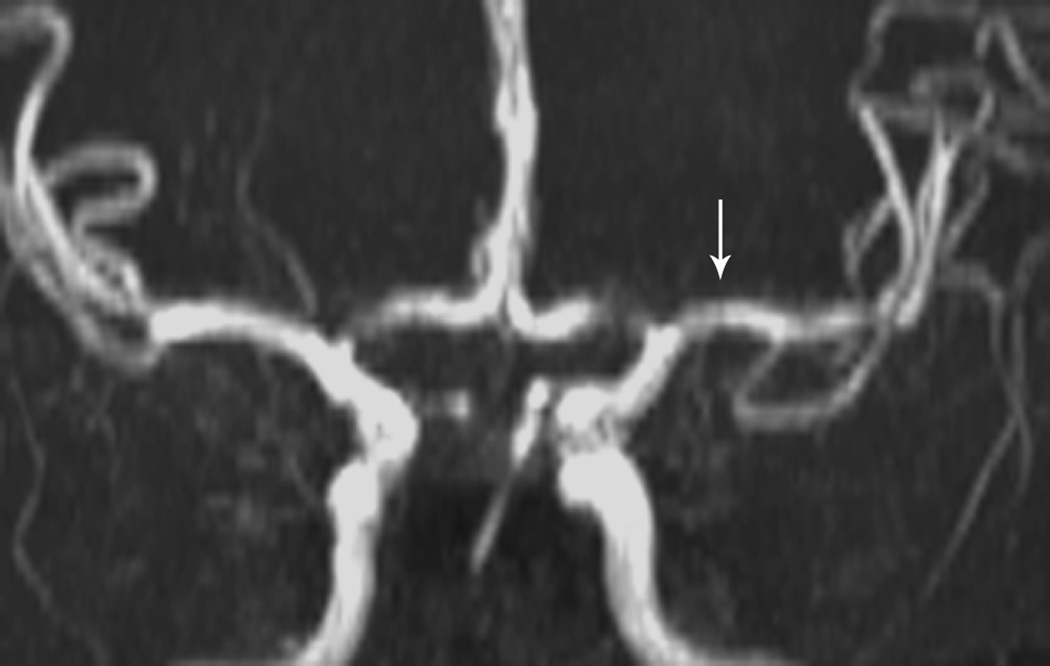
The term “focal cerebral arteriopathy of childhood” (FCA) describes children with idiopathic focal intracranial arterial stenosis (Figure 1) [3]. An analysis of 525 cases of childhood AIS in the International Pediatric Stroke Study (IPSS) found that FCA caused 25% of arteriopathy and was the most common cause of arteriopathy. In this study, the major predictor of FCA was recent upper respiratory infection, OR 2.36, (95% CI, 1.05–5.27). FCA is recent nomenclature that likely encompasses the previously identified entities of transient cerebral arteriopathy of childhood (TCA) and non-progressive central nervous system vasculitis. TCA is a monophasic and transient cerebral arteriopathy; [30] however, a diagnosis of TCA requires that follow-up imaging at 6 months post-stroke shows neither additional stenoses nor progression of the original stenosis. Sebire et al postulated that TCA cases were most likely precipitated by a preceding viral infection [30], which is further supported by the observation in the IPSS study [3].
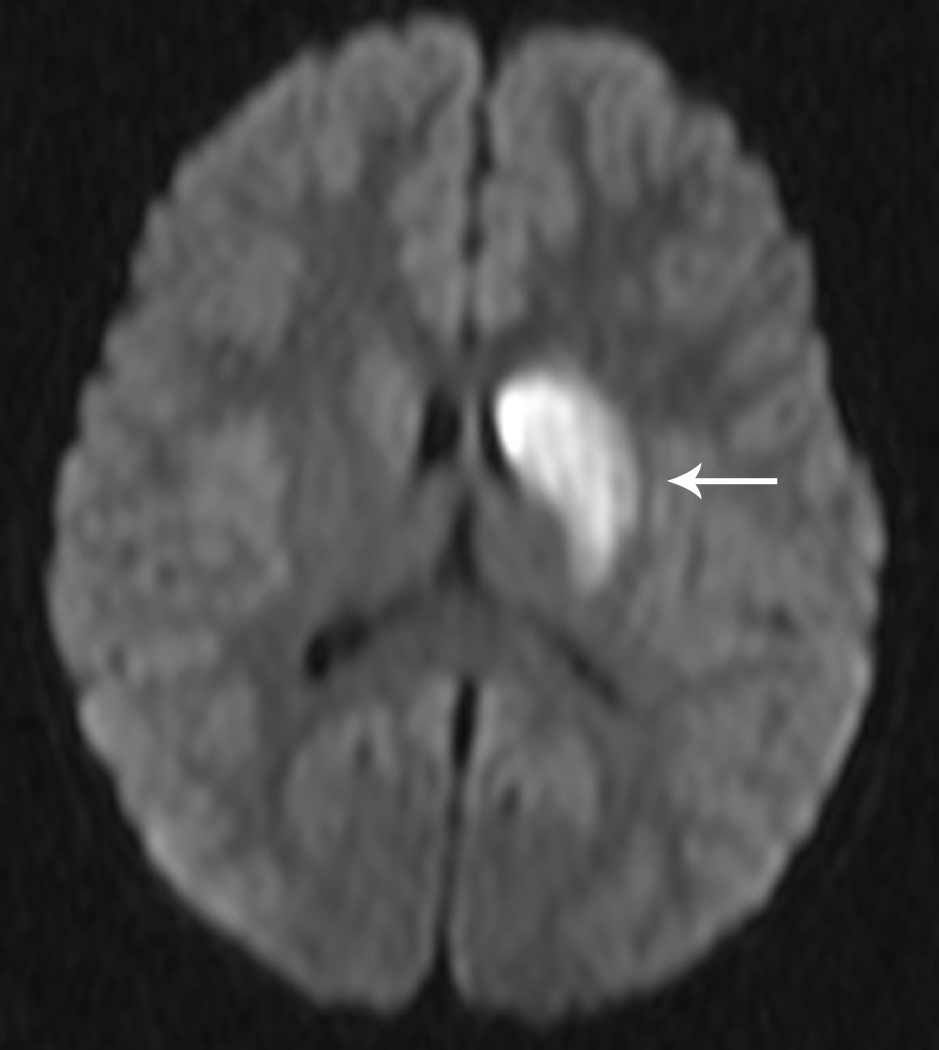
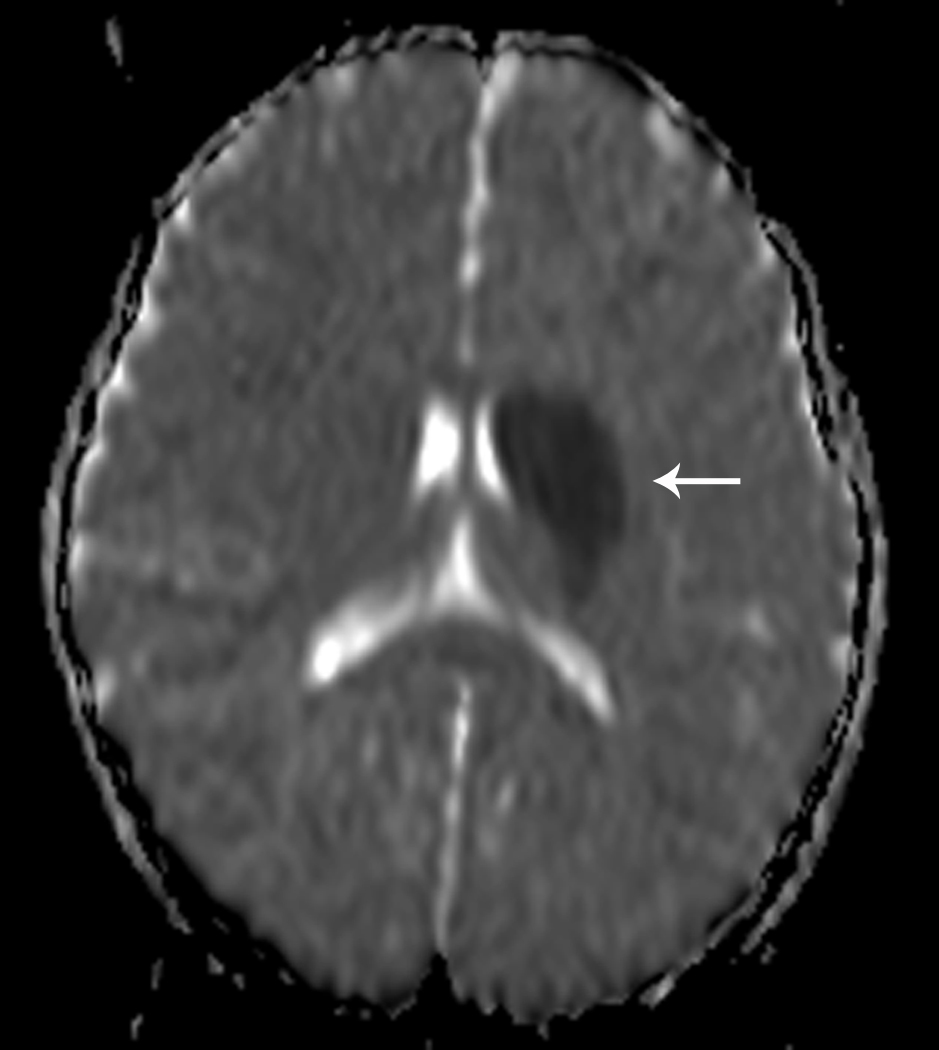
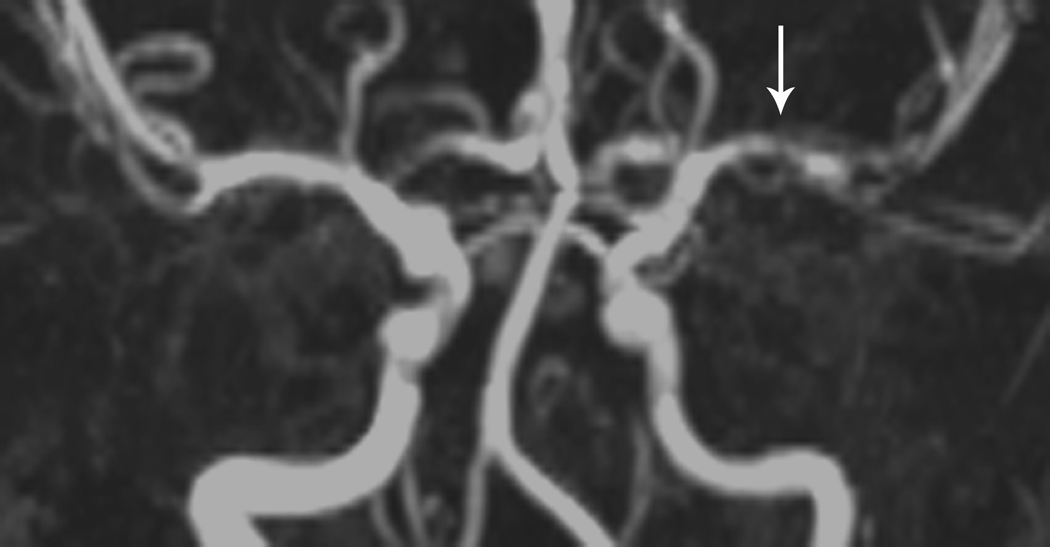
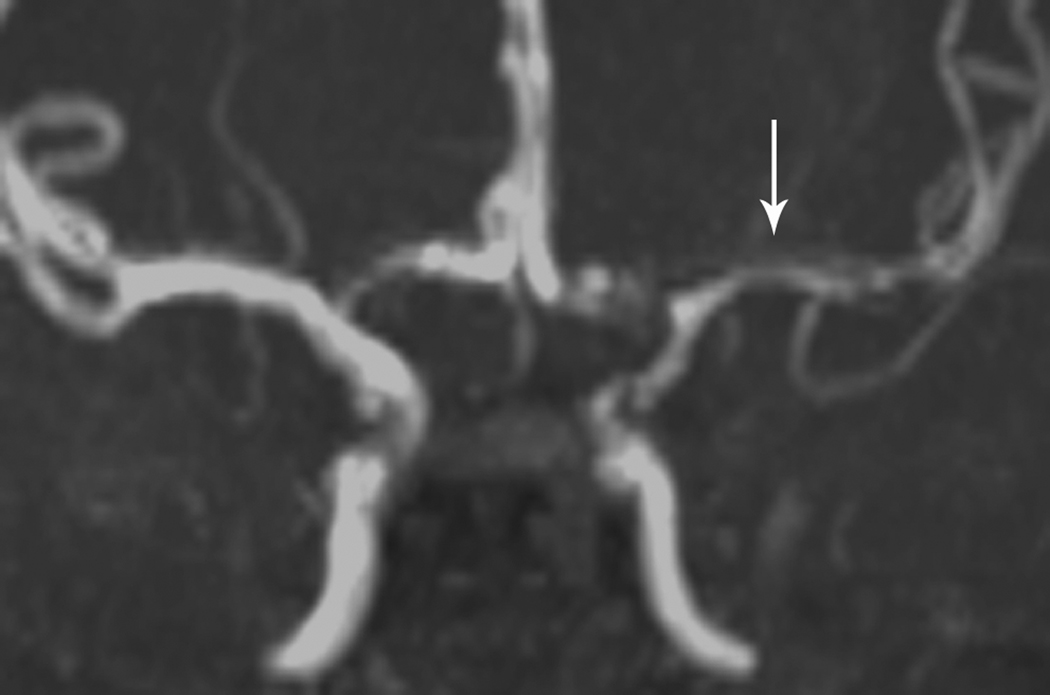
Fig 1. Focal Cerebral Arteriopathy.
8-year-old patient with MRI demonstrating acute stroke in the left basal ganglia that is bright on diffusion-weighted imaging (DWI) (Figure 1a) and dark on the apparent diffusion coefficient (ADC) map (Figure 1b). Magnetic resonance angiogram (MRA) of the circle of Willis shows a focal stenosis of the left middle cerebral artery (MCA) M1 segment (arrow) (Figure 1c). This appears worse 3 weeks later (Figure 1d) and better 1 year after stroke, though continued mild narrowing of the left MCA M1 segment is present (Figure 1e)
Post varicella angiopathy
Focal cerebral artery stenosis with a history of varicella infection in the preceding year has been termed post varicella angiopathy [30, 31]. The pathophysiology is incompletely understood but is thought to be due to invasion of the artery wall by the virus [30, 32].
Vasculitis
Central nervous system (CNS) vasculitis is termed primary when no other condition is present that causes blood vessel inflammation. It is termed secondary when vasculitis results from another process like intracranial infection, systemic vasculitis, collagen vascular disease, malignancy, or exposure to drugs or to certain medications. Furthermore, central nervous system vasculitis can be classified by size of the affected vessels: large, medium, or small vessel. Central nervous system vasculitis can cause ischemic or hemorrhagic stroke, and its diagnosis is often difficult, particularly when small vessels are involved [33]. CNS vasculitis should be considered in children who have one or more of the following: a protracted clinical presentation, a known risk factor for vasculitis (e.g. lupus), multifocal deficits and lesions, diffuse neurological deficits, or systemic symptoms like fever and weight loss [29]. Strokes that result from vasculitis may also occur abruptly. On neuroimaging, both white and gray matter can be affected [29]. In children with large and medium vessel disease, abnormalities on vascular imaging include multiple stenoses, but in a subset of children with small vessel disease, vascular imaging is normal. In these cases, a leptomeningeal and brain biopsy, preferably from a lesional site, may be required to confirm the diagnosis of angiography-negative primary CNS vasculitis [34]. In biopsy-proven cases of primary vasculitis, a lymphocytic transmural infiltrate of T cells plus other inflammatory cells has been reported [34]. Supportive laboratory evidence of vasculitis includes elevated sedimentation rate or C-reactive protein, hematological abnormalities, elevated anticardiolipin IgG, and elevated protein or mild pleocytosis on spinal fluid analysis [29, 34]. However, none of these laboratory studies is diagnostic and all can be normal. The course of CNS vasculitis and its treatment largely depend upon the underlying process. For example, children with underlying autoimmune disease like lupus may require systemic immunosuppression. Those with vasculitis secondary to infections may require specific antibacterial or antiviral agents [33]. The largest report of children with primary CNS vasculitis classified vasculitis into three groups: a progressive form in which further stroke episodes occurred; a non-progressive, monophasic form in which a large vessel stenosis occurs; and a small vessel form in which vascular imaging is negative [29, 34]. Patients who presented with neurocognitive changes, multifocal strokes, and bilateral or distal stenoses on vascular imaging were most likely to have the progressive form. Since most patients in whom vascular imaging is consistent with vasculitis do not undergo biopsy, it is difficult to distinguish cases of true focal vasculitis from those with focal vasculopathy. Hence the non-progressive form of primary vasculitis discussed by Benseler et al [29] may overlap with the focal cerebral arteriopathy of childhood.
Sickle cell arteriopathy
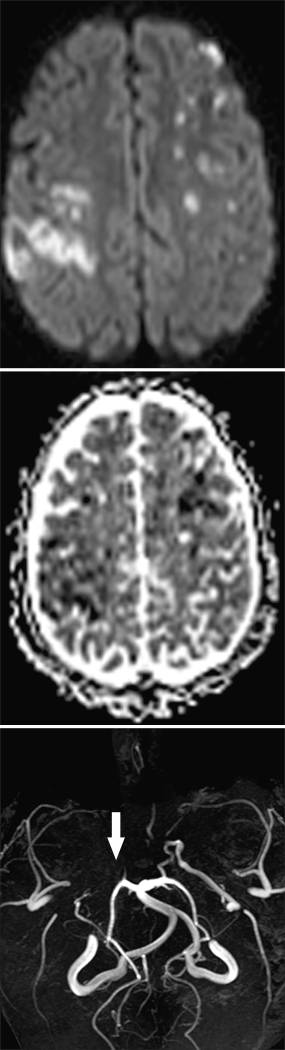
Children with sickle cell anemia have an approximately 100-fold increased risk of stroke compared to the general pediatric population [35]. While some children with sickle cell disease (SCD) have primary intracerebral hemorrhage, ischemic stroke is more common [19, 36]. Strokes in children with SCD can be “clinically silent” when no acute deficit accompanies the infarction. These “silent infarctions” are usually located in the deep white matter and may result from occlusion of small arteries [36]. Although “silent infarctions” are not evident when they occur, accumulation of injury results in neurocognitive dysfunction. Clinically evident infarctions, which often present with hemiparesis, also affect pediatric patients with SCD. Patients with symptomatic strokes often have a large vessel arteriopathy that affects the internal carotid system (Figure 2). This arteriopathy may progress to occlusive disease of the internal carotid arteries with resulting collateral formation, a pattern consistent with moyamoya syndrome [37]. Strokes due to large artery arteriopathy can be occlusive or can occur in the anterior watershed zones [33, 36], an observation consistent with large vessel disease. The pathophysiology of sickle cell arteriopathy is not completely understood, but several neuropathological studies have helped to elucidate some mechanisms of arterial injury. In one study of patients with SCD and stroke, thrombi were present in affected large arteries. There was also hyperplasia of the internal elastic membrane and scarring of the media [38]. The current recommendations for large vessel arteriopathy associated with SCD reserve revascularization procedures for children who have continued strokes despite maximal medical therapy, including long-term transfusion therapy and when transfusions cannot be continued, hydroxyurea [33]. Although large vessel arteriopathy associated with SCD has a propensity for the anterior circulation, aneurysms can also form, more commonly in the vertebrobasilar system [39].
Fig 2. Vasculopathy in Sickle Cell Disease.
14-year-old child with acute stroke in bilateral middle cerebral artery (MCA) watershed regions. Stroke is bright on DWI (top panel) and dark on ADC (center panel). MRA (bottom panel) shows severe cerebral vasculopathy with occlusion of the right MCA (arrow) and absence of the anterior cerebral arteries bilaterally. The majority of the intracranial blood flow is provided by the posterior cerebral arteries and the posterior division of the left MCA
Moyamoya disease and syndrome
The term “moyamoya” describes progressive occlusive disease of the distal internal carotid arteries. The posterior circulation can also be affected [33]. Moyamoya disease is the terminology used when the arteriopathy is idiopathic; moyamoya syndrome is used when the arteriopathy is secondary to an associated condition like cranial radiation or genetic syndromes such as neurofibromatosis type 1, trisomy 21, or Alagille syndrome [24, 40]. In a recent study of childhood arteriopathy, moyamoya was the second most common arteriopathy [3]. Although stroke from moyamoya more commonly presents with ischemic stroke in children, hemorrhagic stroke also occurs [33].
Cervicocephalic Arterial Dissection
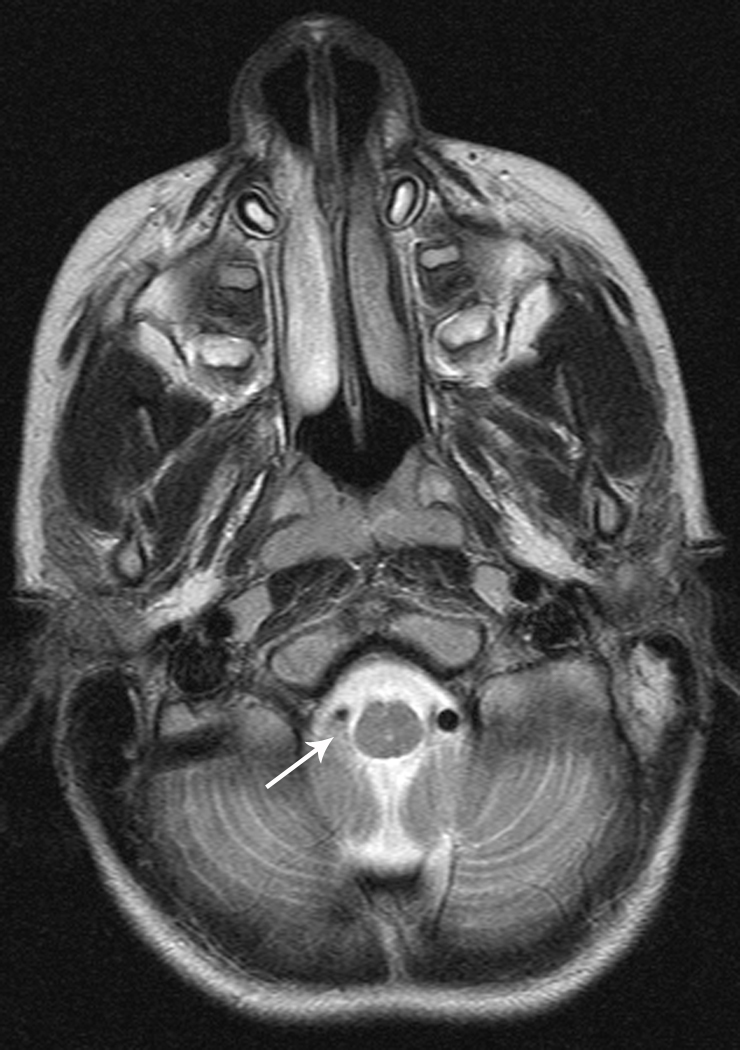
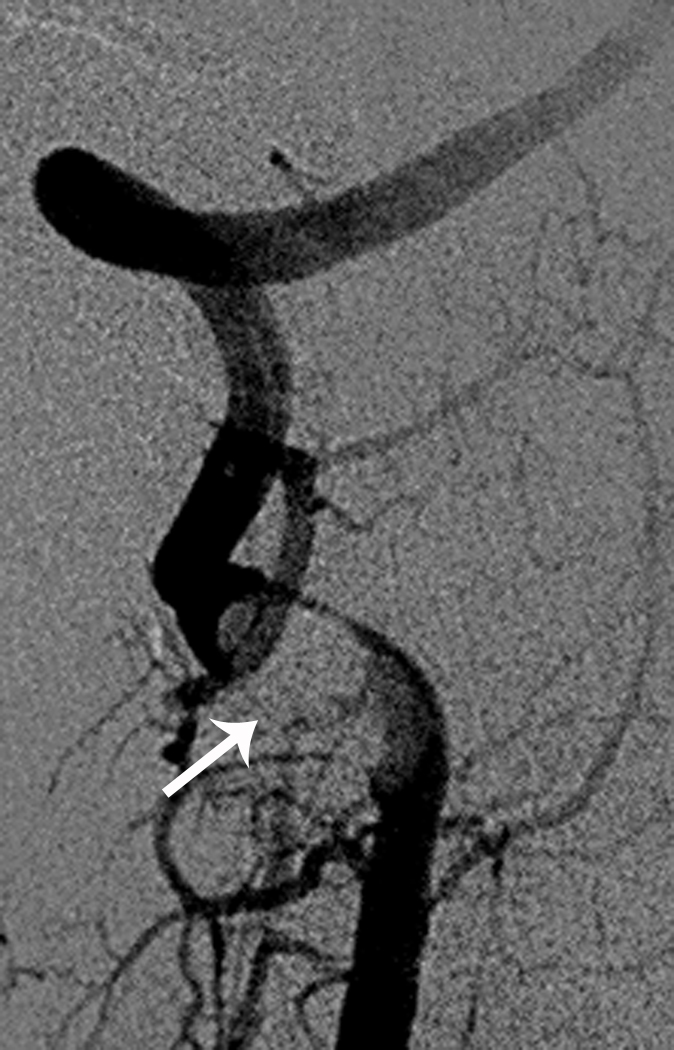
Arterial dissection accounts for 7.5% to 20% of childhood arterial ischemic strokes (Figure 3) [41, 42]. In a recent report published by the IPSS, 20% of AIS caused by arteriopathy was classified as arterial dissection [3]. In children, anterior circulation is involved more frequently than posterior circulation [41, 43]. In a systematic review of published studies and case reports, 118 pediatric patients with stroke caused by dissection were identified, and there was a significant male predominance for both anterior and posterior circulation dissection [43]. In this report, 60% of anterior circulation dissections were intracranial, particularly if the dissection was non-traumatic. Ten percent of children with dissection had recurrent dissection. Furthermore, about 14% had multiple ischemic events prior to definitive diagnosis of dissection, and 9% had recurrent ischemic events after the diagnosis. In another series of 16 consecutive patients, 12.5% had stroke recurrence [41].
Fig 3. Vertebral Artery Dissection.
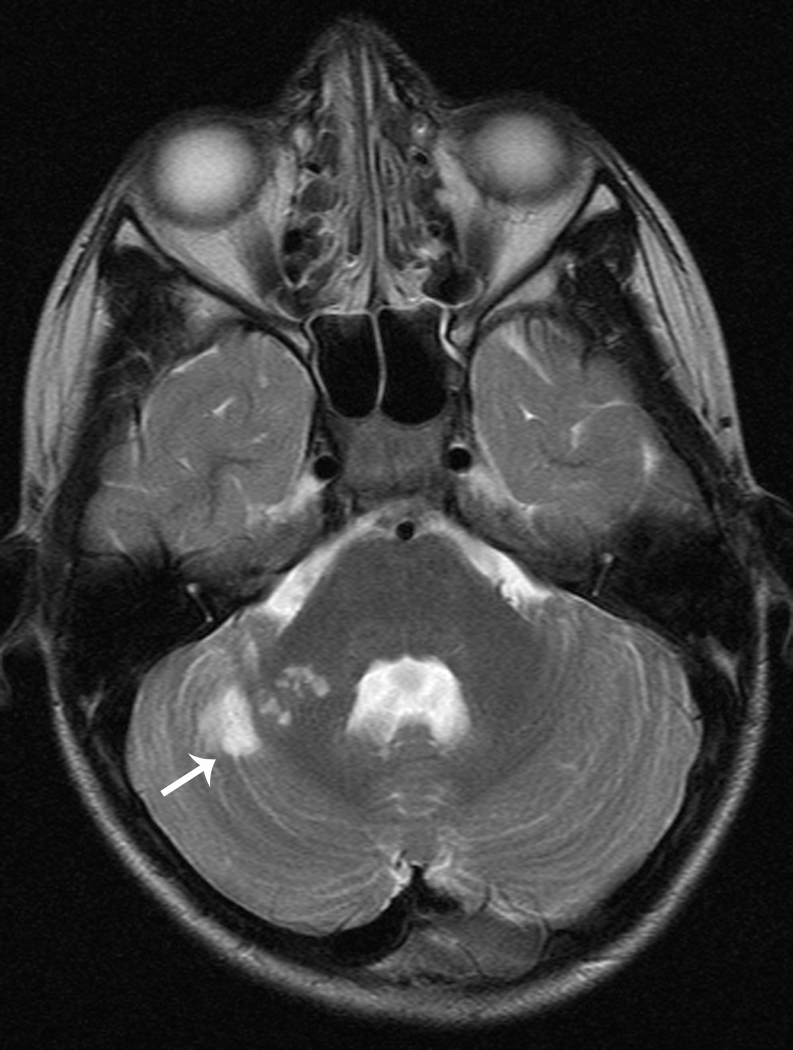
7-year-old boy with stroke in the right superior cerebellar artery territory on MRI (Figure 3a). Axial view with small right vertebral artery (Figure 3b). Cerebral angiogram (lateral view) with tapering of right vertebral artery and filling defect (Figure 3c)
Hemorrhagic Stroke
In a retrospective cohort study of 2.3 million children followed for more than a decade, there were 116 incident cases of non-traumatic childhood hemorrhagic stroke (HS), yielding an average annual incidence rate of 1.4 per 100,000 person-years (95% binomial exact confidence interval (CI), 1.2 – 1.7)[44]. Among these 116 cases of incident childhood HS, 35 (31%) had brain arteriovenous malformations (AVM), 15 (13%) had underlying cerebral aneurysms, 17 (15%) had cavernous malformations, 16 (14%) had medical etiologies, 3 (2.5%) had brain tumors, and 29 (25%) had HS of undetermined cause [9]. In this cohort, 69% had “vascular” causes for hemorrhagic stroke. In a recent, small prospective cohort (n=22) which excluded children with brain tumor, 91% had a vascular cause of hemorrhagic stroke [10].
Evaluation of Stroke
The American Heart Association (AHA) released consensus-based guidelines for the evaluation and management of stroke in infants and children in July 2008 [33]. There are also existing guidelines from the United Kingdom [45] as well as updated guidelines from the American College of Chest Physicians [46]. Only the AHA guidelines specifically address hemorrhagic stroke.
Evaluation of Ischemic Stroke
Thorough evaluation is crucial for each child with arterial ischemic stroke since multiple risk factors are common. For example, children in whom cardiac disease was a major stroke risk factor were found to have increased risk of prothrombotic conditions when compared to age-matched healthy controls [17]. Furthermore, cerebrovascular abnormalities may coexist with structural congenital heart disease. In the IPSS study, one third (45 of 135) of children with a prior history of cardiac disease were found to have an associated arteriopathy, suggesting that vascular imaging is still indicated in these children [3]. Many centers evaluate patients with ischemic stroke with vascular imaging of the head and neck, echocardiogram, and thrombophilia studies (Table), regardless of prior known risk factors.
Table.
Thrombophilia Evaluation for Pediatric Arterial Ischemic Stroke
| Tests* |
|---|
| Complete blood count |
| Prothrombin time (or international normalized ratio) |
| Activated partial thromboplastin time (APTT) |
| Prothrombin gene mutation 20210A |
| Activated protein C resistance |
| Factor V Leiden G1691A gene testing |
| Antiphospholipid antibody testing including: |
| Dilute Russell’s viper venom time |
| Beta 2 glycoprotein IgG and IgM |
| Anticardiolipin antibodies IgG and IgM |
| Lipoprotein (a) |
| Protein C activity |
| Protein S activity |
| Antithrombin III activity |
| Fasting homocysteine |
| Sickle cell screen or hemoglobin electrophoresis† |
Evaluation of Hemorrhagic Stroke
An appropriate diagnostic evaluation in children with hemorrhagic stroke has not been established. Thorough evaluation for vascular anomalies which account for 40–90% of hemorrhagic stroke in children is critical [6, 10, 47]. Coagulation studies and other basic laboratory tests are recommended in the AHA guidelines for management of intracerebral hemorrhage [48]. No studies have been done to examine the yield of an extensive evaluation for a bleeding diathesis in children with HS. Therefore, the AHA guidelines for the evaluation and management of stroke in infants and children simply note that an evaluation for hematologic disorders, coagulation defects and other risk factors may be appropriate [33].
If a hemorrhagic stroke is suspected, computed tomography (CT) is still considered the initial imaging study of choice by most authors because it is rapid, widely available, and clearly identifies acute hemorrhage [48]. Magnetic resonance imaging (MRI) sequences like susceptibility-weighted images clearly identify hemorrhage, but they are not universally available and require some experience and training to correctly identify the hemorrhage [49]. Given the high rate of vascular malformations, dedicated cerebrovascular imaging is critical. A recent study found that a combination of MRI, MRA, and MRV images accurately identified the cause of ICH in 25 of 38 children (66%) [50]. There were 2 false negative MRIs: one patient had a mycotic aneurysm, the other had a peripherally located AVM evident on conventional cerebral angiography (CCA). In the same study, CCA alone had a diagnostic yield of 61% that was statistically equivalent to the yield to the combination of MRI, MRA, and MRV [50]. However, in another series of children with non-traumatic ICH, the cause of bleeding was established in 97% of children who underwent CCA compared with 80% of children who did not have angiography [47]. CCA can be relatively safe, although fewer than 50% of children with ICH undergo CCA [7, 47]. In a series of 241 pediatric cerebral angiograms, the rate of major complications was only 0.5% [51]. In one study of 116 children with hemorrhagic stroke between 1993 and 2003 in a large California health maintenance organization, only 65% had vascular imaging [9]. A recent study documented vascular imaging in nearly 100% of non-neonates with hemorrhagic stroke [10] perhaps leading to a higher percentage of confirmed etiology for hemorrhage. When no cause for HS is found via non-invasive vascular imaging, CCA should be strongly considered [9, 33].
Sometimes vascular malformations are not evident, even for months after the acute hemorrhage. Therefore, when vascular imaging is normal or inconclusive in the acute setting, studies should be repeated once the clot has been reabsorbed. The timing and frequency of additional studies has not been studied and is center-dependent.
Treatment of Stroke in Children
Supportive Care
Maintaining normal body temperature with acetaminophen, cooling blankets, and fluids to maintain euvolemia is recommended [48]. Temperature elevation >37.5 °C, has been shown to increase the likelihood of poor outcome in adults with ICH [52]. When there is intraventricular hemorrhage, careful monitoring for and treatment of hydrocephalus is essential. Monitoring for signs of increased intracranial pressure due to AIS or HS is also important. Osmotherapy is recommended for elevated intracranial pressure. Corticosteroids are not recommended for cerebral edema due to ischemic or hemorrhagic stroke because randomized trials in adults have failed to show efficacy [53, 54]. Additionally, hyperglycemia which may result from corticosteroid use has been shown to be detrimental [55, 56].
Antithrombotic Therapy
Antithrombotic therapy includes both antiplatelet (typically aspirin) and anticoagulant (unfractionated heparin, low molecular weight heparin, and warfarin) medications. Neonates with arterial ischemic stroke are rarely treated; the Chest guidelines specifically recommend against treatment of neonates with first AIS without evidence of an ongoing cardioembolic source [46]. In non-neonates, antithrombotic therapy is usually recommended for secondary stroke prevention, except in SCD where transfusions are recommended to lower the percentage of sickle hemoglobin to <30% [33]. Treatment recommendations are provided by the AHA by stroke subtype, specifically noting that anticoagulation with low molecular weight heparin or warfarin should be considered for cervical artery dissection for a period of 3–6 months and for cardioembolic stroke [33]. Anticoagulation is not typically recommended in children with moyamoya disease or syndrome. For non-SCD-related childhood stroke, the Chest guidelines recommend initial anticoagulation or aspirin therapy at 1–5mg/kg/day until arterial dissection or cardioembolic causes have been excluded, followed by long-term aspirin therapy for a minimum of 2 years [46]. The choice of anticoagulation or antiplatelet therapy for children differs geographically, with centers in the United States using anticoagulation less often than centers in Canada, Europe, and Australia [5].
Thrombolytic therapy in children
Current standard of care for adult stroke involves using intravenous (IV) tissue plasminogen activator (tPA) for patients 18 years and older who meet strict inclusion criteria [57]. The role of thrombolysis for children under age 18 years is much more controversial. Time to presentation is a major limiting factor since IV thrombolysis typically must be started within 3 hours of symptom onset [57]. A recent scientific advisory (not a guideline) from the AHA suggested that the window of opportunity for tPA may be extended from 3 to 4.5 hours in non-diabetic, adult ischemic stroke patients [58]. The average child with a stroke presents for medical care more than 24 hours after symptom onset [59], and even with rapid presentation for care, limited awareness of pediatric stroke among healthcare providers may further delay neuroimaging [60]. Evidence for the safety and efficacy of thrombolysis for children with stroke is extremely limited, and existing studies of thrombolysis for systemic clots suggest a high risk of hemorrhagic complications [61]. A number of case reports document the use of tPA in children [62, 63], and a study of the National Inpatient Sample, a nationwide database, found that 46 children admitted for ischemic stroke between 2000 and 2003 received thrombolytic therapy[64]. Members of the International Pediatric Stroke Study compared case reports of tPA use in children with cases reported as part of the IPSS registry. They found that a publication bias exists and that children receiving tPA in their series were younger, had a longer time to treatment, and had worse outcomes than cases reported from other sources [65]. Despite anecdotal use of IV or intra-arterial tPA, the current AHA guidelines suggest that its use in children be limited to the confines of a clinical trial [33].
Surgical therapy for stroke
Craniectomy
Timely decompressive craniectomy may be both life-saving and function-sparing in children with large arterial ischemic stroke or intracerebral hemorrhage who display rapid deterioration in level of consciousness or progress to signs and symptoms of impending herniation [66–68].
Surgical evacuation of parenchymal hemorrhage
The Surgical Trial in Intracerebral Hemorrhage (STICH) demonstrated that in adult patients with spontaneous supratentorial ICH, emergent surgical evacuation of hematoma within 72 hours of bleeding onset did not improve outcome beyond best medical management [69]. The application of this trial to children is confounded because clinical equipoise was required for enrollment in STICH, so patients whom the local investigator felt would benefit most from surgery were not enrolled in the trial. Young patients with lobar hemorrhages with clinical deterioration due to mass effect have been reported to show benefit from early surgery in a small retrospective series [70], therefore such patients were unlikely to have been enrolled in the STICH trial. Children may require more urgent intervention to reduce intracranial pressure and thereby prevent brain herniation because they do not have cerebral atrophy that permits expansion of the hematoma. Further, in adult series, patients with cerebellar hemorrhage >3 cm in diameter benefit from early evacuation of the hematoma because the location of the hemorrhage often leads to tonsillar herniation, hydrocephalus, or brainstem compression [71, 72]. Cerebellar hemorrhage in children may also require urgent evacuation. In a recent small series, 3 of 22 children with ICH had decompressive craniectomy, and all were functionally independent [10], but decompressive craniectomy has not been studied in a randomized setting.
Treatment of Vascular Malformations
Treatments for vascular malformations include surgery, endovascular procedures, gamma knife radiosurgery, and proton beam therapy. The appropriate treatment for each patient is determined by the location and vascular anatomy of the lesion. A team of neurovascular specialists including neurology, neurosurgery, neuroradiology, interventional radiology, and radiation oncology is often needed to provide optimal care.
Outcome after Pediatric Stroke
Arterial Ischemic Stroke Outcome
Population-based and hospital-based series have reported a case fatality rate for ischemic stroke of 16–20%; however, these estimates are based on data from 1970–2003 [13, 73]. Mortality from childhood stroke is decreasing over time [74]. Neurological sequelae including motor and cognitive deficits are present in more than half of children after AIS [75, 76]. Young age, male sex, and bihemispheric infarction predict poor outcome [77].
Only 3 pediatric stroke studies have used survival analysis to calculate recurrence risk. In a retrospective cohort of 181 neonates and children with AIS from Kaiser Permanente in California, the 5-year cumulative recurrence risk for neonatal AIS was 1.2% while the risk in older children was 19% (95% CI, 12–30%) [16]. Similarly, a UK single-center study reported an 18% 5-year recurrence risk for childhood AIS, (95% CI, 11–25%)[78]. A German study reported only 5%, CI not provided [79].
Hemorrhagic Stroke Outcome
One study pooled data from non-population-based studies and reported an average mortality of 25% in children with HS [15], but individual study estimates range from 7% [80] to 54% [81]. A more modern and population-based study found a case fatality rate for HS of 5.2% [44].
Only one population-based study exists that has looked at recurrence risk after pediatric HS. In this California study, the 5-year cumulative recurrence rate was 10% (95% CI, 5–18%) [44]. Interestingly, no children with idiopathic HS had a recurrent stroke, while 13% of children with structural lesions and medical etiologies recurred over 5 years. All of the recurrences in children with medical etiologies of HS occurred within a week of the original diagnosis.
Several studies in children specifically addressed features that predict poor neurologic outcome after HS. Meyer-Heim et al [6] found that infratentorial location, Glasgow Coma Score (GCS) ≤ 7 at admission, aneurysm, age <3 years at the time of HS, and underlying hematological disorder all predicted more severe outcome. A recent retrospective study showed that ICH volume predicts poor outcome in children [82]. This finding was confirmed in a prospective series, and altered mental status within 6 hours of hospital presentation was an added risk factor for poor short-term neurological outcome [10]. This is not surprising; in the adult literature, volume of ICH and GCS <9 at the time of presentation are strong predictors of 30-day mortality [83].
Little information exists on cognitive outcomes after HS in children. One retrospective cohort study of 56 Dutch children <16 years of age at onset of HS who received care at a single medical center between 1978 and 1998 [14] had long-term follow up (mean 10.3 years) on all 36 surviving patients;10-year survival after HS was 64%. Mean Full Scale Intelligence Quotient (IQ) of the 31 subjects who reported for cognitive testing was not below average (IQ 106, SD 20, n=28) or left-shifted, but the standard deviation was large, indicating a range of IQs. Furthermore, 15 of 31 patients (48%) had signs of cognitive deficits when their performance was compared to their pre-morbid academic abilities or to those of their parents. Moderate to severe cognitive deficits were present in 7 patients (23%).
Summary
Pediatric stroke is a heterogenous disorder. Arteriopathies and vascular malformations are amongst the most common causes of arterial ischemic stroke and hemorrhagic stroke in children. Furthermore, cerebral arteriopathy is a predictor of poor short-term outcome after AIS, and vascular cause of hemorrhage is a predictor of recurrence after HS, if untreated. Additional studies are needed to evaluate treatments for stroke in children. This will require multicenter and multispecialty collaboration. These studies are important as long-term neurological deficits are present in at least 50% of pediatric stroke survivors.
Acknowledgments
Lauren Beslow is supported by National Institutes of Health T32NS007413 and by the L. Morton Morley Funds of the Philadelphia Foundation.
Lori Jordan is supported by National Institutes of Health K23NS062110.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Heideman RL, Packer RJ, Albright LA, Freeman CR, Rorke LB. Tumors of the central nervous system. In: Pizzo PA, editor. Principles and Practice of Pediatric Oncology. 3rd edn. Philadelphia, PA: Lippencott; 1997. pp. 633–697. [Google Scholar]
- 2.National Center for Health Statistics. Deaths, percentage of total deaths, and death rates for the 10 leading causes of death in selected age groups, by race and sex: United States. [Accessed 16 June 2006];2002 Updated 2005. www.cdc.gov/nchs/data/nvsr/nvsr53/nvsr53_17.pdf.
- 3.Amlie-Lefond C, Bernard TJ, Sebire G, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: Results of the international pediatric stroke study. Circulation. 2009;119:1417–1423. doi: 10.1161/CIRCULATIONAHA.108.806307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganesan V, Prengler M, McShane MA, Wade AM, Kirkham FJ. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003;53:167–173. doi: 10.1002/ana.10423. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg NA, Bernard TJ, Fullerton HJ, Gordon A, Deveber G for the International Pediatric Stroke Study Group. Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: A multicentre, observational, cohort study. Lancet Neurol. 2009 Oct 2; doi: 10.1016/S1474-4422(09)70241-8. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Meyer-Heim AD, Boltshauser E. Spontaneous intracranial haemorrhage in children: Aetiology, presentation and outcome. Brain Dev. 2003;25:416–421. doi: 10.1016/s0387-7604(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 7.Lin CL, Loh JK, Kwan AL, Howng SL. Spontaneous intracerebral hemorrhage in children. Kaohsiung J Med Sci. 1999;15:146–151. [PubMed] [Google Scholar]
- 8.Giroud M, Lemesle M, Madinier G, Manceau E, Osseby GV, Dumas R. Stroke in children under 16 years of age. clinical and etiological difference with adults. Acta Neurol Scand. 1997;96:401–406. doi: 10.1111/j.1600-0404.1997.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 9.Jordan LC, Johnston SC, Wu YW, Sidney SS, Fullerton HJ. The importance of cerebral aneurysms in childhood hemorrhagic stroke: A population-based study. Stroke. 2008;40:400–405. doi: 10.1161/STROKEAHA.108.518761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beslow LA, Licht DJ, Smith SE, et al. Predictors of outcome in childhood intracerebral hemorrhage: A prospective consecutive cohort study. Stroke. 2009 doi: 10.1161/STROKEAHA.109.568071. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the national institute of neurological disorders and stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116–123. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 12.Chung B, Wong V. Pediatric stroke among hong kong chinese subjects. Pediatrics. 2004;114:e206–e212. doi: 10.1542/peds.114.2.e206. [DOI] [PubMed] [Google Scholar]
- 13.Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: Ethnic and gender disparities. Neurology. 2003;61:189–194. doi: 10.1212/01.wnl.0000078894.79866.95. [DOI] [PubMed] [Google Scholar]
- 14.Blom I, De Schryver EL, Kappelle LJ, Rinkel GJ, Jennekens-Schinkel A, Peters AC. Prognosis of haemorrhagic stroke in childhood: A long-term follow-up study. Dev Med Child Neurol. 2003;45:233–239. doi: 10.1017/s001216220300046x. [DOI] [PubMed] [Google Scholar]
- 15.Lynch JK, Han CJ. Pediatric stroke: What do we know and what do we need to know? Semin Neurol. 2005;25:410–423. doi: 10.1055/s-2005-923535. [DOI] [PubMed] [Google Scholar]
- 16.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: The importance of cerebrovascular imaging. Pediatrics. 2007;119:495–501. doi: 10.1542/peds.2006-2791. [DOI] [PubMed] [Google Scholar]
- 17.Strater R, Vielhaber H, Kassenbohmer R, von Kries R, Gobel U, Nowak-Gottl U. Genetic risk factors of thrombophilia in ischaemic childhood stroke of cardiac origin. A prospective ESPED survey. Eur J Pediatr. 1999;158 Suppl 3:S122–S125. doi: 10.1007/pl00014336. [DOI] [PubMed] [Google Scholar]
- 18.Nowak-Gottl U, Strater R, Heinecke A, et al. Lipoprotein (a) and genetic polymorphisms of clotting factor V, prothrombin, and methylenetetrahydrofolate reductase are risk factors of spontaneous ischemic stroke in childhood. Blood. 1999;94:3678–3682. [PubMed] [Google Scholar]
- 19.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 20.Miller ST, Sleeper LA, Pegelow CH, et al. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;342:83–89. doi: 10.1056/NEJM200001133420203. [DOI] [PubMed] [Google Scholar]
- 21.Miller ST, Macklin EA, Pegelow CH, et al. Silent infarction as a risk factor for overt stroke in children with sickle cell anemia: A report from the cooperative study of sickle cell disease. J Pediatr. 2001;139:385–390. doi: 10.1067/mpd.2001.117580. [DOI] [PubMed] [Google Scholar]
- 22.Danchaivijitr N, Cox TC, Saunders DE, Ganesan V. Evolution of cerebral arteriopathies in childhood arterial ischemic stroke. Ann Neurol. 2006;59:620–626. doi: 10.1002/ana.20800. [DOI] [PubMed] [Google Scholar]
- 23.Rea D, Brandsema JF, Armstrong D, et al. Cerebral arteriopathy in children with neurofibromatosis type 1. Pediatrics. 2009 Aug 24; doi: 10.1542/peds.2009-0152. epub. [DOI] [PubMed] [Google Scholar]
- 24.Emerick KM, Krantz ID, Kamath BM, et al. Intracranial vascular abnormalities in patients with alagille syndrome. J Pediatr Gastroenterol Nutr. 2005;41:99–107. doi: 10.1097/01.mpg.0000162776.67758.2f. [DOI] [PubMed] [Google Scholar]
- 25.Kamath BM, Spinner NB, Emerick KM, et al. Vascular anomalies in alagille syndrome: A significant cause of morbidity and mortality. Circulation. 2004;109:1354–1358. doi: 10.1161/01.CIR.0000121361.01862.A4. [DOI] [PubMed] [Google Scholar]
- 26.Wollack JB, Kaifer M, LaMonte MP, Rothman M. Stroke in williams syndrome. Stroke. 1996;27:143–146. doi: 10.1161/01.str.27.1.143. [DOI] [PubMed] [Google Scholar]
- 27.Heyer GL, Dowling MM, Licht DJ, et al. The cerebral vasculopathy of PHACES syndrome. Stroke. 2008;39:308–316. doi: 10.1161/STROKEAHA.107.485185. [DOI] [PubMed] [Google Scholar]
- 28.Jea A, Smith ER, Robertson R, Scott RM. Moyamoya syndrome associated with down syndrome: Outcome after surgical revascularization. Pediatrics. 2005;116:e694–e701. doi: 10.1542/peds.2005-0568. [DOI] [PubMed] [Google Scholar]
- 29.Benseler SM, Silverman E, Aviv RI, et al. Primary central nervous system vasculitis in children. Arthritis Rheum. 2006;54:1291–1297. doi: 10.1002/art.21766. [DOI] [PubMed] [Google Scholar]
- 30.Sebire G, Fullerton H, Riou E, deVeber G. Toward the definition of cerebral arteriopathies of childhood. Curr Opin Pediatr. 2004;16:617–622. doi: 10.1097/01.mop.0000144441.29899.20. [DOI] [PubMed] [Google Scholar]
- 31.Sebire G, Meyer L, Chabrier S. Varicella as a risk factor for cerebral infarction in childhood: A case-control study. Ann Neurol. 1999;45:679–680. doi: 10.1002/1531-8249(199905)45:5<679::aid-ana22>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 32.Linnemann CC, Jr, Alvira MM. Pathogenesis of varicella-zoster angiitis in the CNS. Arch Neurol. 1980;37:239–240. doi: 10.1001/archneur.1980.00500530077013. [DOI] [PubMed] [Google Scholar]
- 33.Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: A scientific statement from a special writing group of the american heart association stroke council and the council on cardiovascular disease in the young. Stroke. 2008;39:2644–2691. doi: 10.1161/STROKEAHA.108.189696. [DOI] [PubMed] [Google Scholar]
- 34.Benseler SM, deVeber G, Hawkins C, et al. Angiography-negative primary central nervous system vasculitis in children: A newly recognized inflammatory central nervous system disease. Arthritis Rheum. 2005;52:2159–2167. doi: 10.1002/art.21144. [DOI] [PubMed] [Google Scholar]
- 35.Earley CJ, Kittner SJ, Feeser BR, et al. Stroke in children and sickle-cell disease: Baltimore-washington cooperative young stroke study. Neurology. 1998;51:169–176. doi: 10.1212/wnl.51.1.169. [DOI] [PubMed] [Google Scholar]
- 36.Switzer JA, Hess DC, Nichols FT, Adams RJ. Pathophysiology and treatment of stroke in sickle-cell disease: Present and future. Lancet Neurol. 2006;5:501–512. doi: 10.1016/S1474-4422(06)70469-0. [DOI] [PubMed] [Google Scholar]
- 37.Dobson SR, Holden KR, Nietert PJ, et al. Moyamoya syndrome in childhood sickle cell disease: A predictive factor for recurrent cerebrovascular events. Blood. 2002;99:3144–3150. doi: 10.1182/blood.v99.9.3144. [DOI] [PubMed] [Google Scholar]
- 38.Rothman SM, Fulling KH, Nelson JS. Sickle cell anemia and central nervous system infarction: A neuropathological study. Ann Neurol. 1986;20:684–690. doi: 10.1002/ana.410200606. [DOI] [PubMed] [Google Scholar]
- 39.Preul MC, Cendes F, Just N, Mohr G. Intracranial aneurysms and sickle cell anemia: Multiplicity and propensity for the vertebrobasilar territory. Neurosurgery. 1998;42:971–997. doi: 10.1097/00006123-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360:1226–1237. doi: 10.1056/NEJMra0804622. [DOI] [PubMed] [Google Scholar]
- 41.Rafay MF, Armstrong D, Deveber G, Domi T, Chan A, MacGregor DL. Craniocervical arterial dissection in children: Clinical and radiographic presentation and outcome. J Child Neurol. 2006;21:8–16. doi: 10.1177/08830738060210010101. [DOI] [PubMed] [Google Scholar]
- 42.Chabrier S, Husson B, Lasjaunias P, Landrieu P, Tardieu M. Stroke in childhood: Outcome and recurrence risk by mechanism in 59 patients. J Child Neurol. 2000;15:290–294. doi: 10.1177/088307380001500504. [DOI] [PubMed] [Google Scholar]
- 43.Fullerton HJ, Johnston SC, Smith WS. Arterial dissection and stroke in children. Neurology. 2001;57:1155–1160. doi: 10.1212/wnl.57.7.1155. [DOI] [PubMed] [Google Scholar]
- 44.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Recurrent hemorrhagic stroke in children: A population-based cohort study. Stroke. 2007;38:2658–2662. doi: 10.1161/STROKEAHA.107.481895. [DOI] [PubMed] [Google Scholar]
- 45.Pediatric Stroke Working Group. Stroke in childhood: clinical guidelines for diagnosis, management and rehabilitation. [Accessed 26 October 2009];2004 http://www.rcplondon.ac.uk/pubs/books/stroke/stroke_guidelines_2ed.pdf.
- 46.Monagle P, Chalmers E, Chan A, et al. Antithrombotic therapy in neonates and children: American college of chest physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133(6 Suppl):887S–968S. doi: 10.1378/chest.08-0762. [DOI] [PubMed] [Google Scholar]
- 47.Al-Jarallah A, Al-Rifai MT, Riela AR, Roach ES. Nontraumatic brain hemorrhage in children: Etiology and presentation. J Child Neurol. 2000;15:284–289. doi: 10.1177/088307380001500503. [DOI] [PubMed] [Google Scholar]
- 48.Broderick JP, Adams HP, Jr, Barsan W, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A statement for healthcare professionals from a special writing group of the stroke council, american heart association. Stroke. 1999;30:905–915. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- 49.Kidwell CS, Chalela JA, Saver JL, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292:1823–1830. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 50.Liu AC, Segaren N, Cox TS, et al. Is there a role for magnetic resonance imaging in the evaluation of non-traumatic intraparenchymal haemorrhage in children? Pediatr Radiol. 2006;36:940–946. doi: 10.1007/s00247-006-0236-9. [DOI] [PubMed] [Google Scholar]
- 51.Burger IM, Murphy KJ, Jordan LC, Tamargo RJ, Gailloud P. Safety of cerebral digital subtraction angiography (DSA) in children. complication rate analysis in 241 consecutive diagnostic angiograms. Stroke. 2006;37:2535–2539. doi: 10.1161/01.STR.0000239697.56147.77. [DOI] [PubMed] [Google Scholar]
- 52.Schwarz S, Hafner K, Aschoff A, Schwab S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2000;54:354–361. doi: 10.1212/wnl.54.2.354. [DOI] [PubMed] [Google Scholar]
- 53.Poungvarin N, Bhoopat W, Viriyavejakul A, et al. Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. N Engl J Med. 1987;316:1229–1233. doi: 10.1056/NEJM198705143162001. [DOI] [PubMed] [Google Scholar]
- 54.Tellez H, Bauer RB. Dexamethasone as treatment in cerebrovascular disease. 1. A controlled study in intracerebral hemorrhage. Stroke. 1973;4:541–546. doi: 10.1161/01.str.4.4.541. [DOI] [PubMed] [Google Scholar]
- 55.Passero S, Ciacci G, Ulivelli M. The influence of diabetes and hyperglycemia on clinical course after intracerebral hemorrhage. Neurology. 2003;61:1351–1356. doi: 10.1212/01.wnl.0000094326.30791.2d. [DOI] [PubMed] [Google Scholar]
- 56.Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? results of a long-term follow up study. BMJ. 1997;314:1303–1306. doi: 10.1136/bmj.314.7090.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tissue plasminogen activator for acute ischemic stroke. the national institute of neurological disorders and stroke rt-PA stroke study group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 58.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP, Jr American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: A science advisory from the american heart Association/American stroke association. Stroke. 2009;40:2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gabis LV, Yangala R, Lenn NJ. Time lag to diagnosis of stroke in children. Pediatrics. 2002;110:924–928. doi: 10.1542/peds.110.5.924. [DOI] [PubMed] [Google Scholar]
- 60.Srinivasan J, Miller SP, Phan TG, Mackay MT. Delayed recognition of initial stroke in children: Need for increased awareness. Pediatrics. 2009;124:e227–e234. doi: 10.1542/peds.2008-3544. [DOI] [PubMed] [Google Scholar]
- 61.Monagle P, Chan A, Massicotte P, Chalmers E, Michelson AD. Antithrombotic therapy in children: The seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126(3 Suppl):645S–687S. doi: 10.1378/chest.126.3_suppl.645S. [DOI] [PubMed] [Google Scholar]
- 62.Carlson MD, Leber S, Deveikis J, Silverstein FS. Successful use of rt-PA in pediatric stroke. Neurology. 2001;57:157–158. doi: 10.1212/wnl.57.1.157. [DOI] [PubMed] [Google Scholar]
- 63.Benedict SL, Ni OK, Schloesser P, White KS, Bale JF., Jr Intra-arterial thrombolysis in a 2-year-old with cardioembolic stroke. J Child Neurol. 2007;22:225–227. doi: 10.1177/0883073807300296. [DOI] [PubMed] [Google Scholar]
- 64.Janjua N, Nasar A, Lynch JK, Qureshi AI. Thrombolysis for ischemic stroke in children: Data from the nationwide inpatient sample. Stroke. 2007;38:1850–1854. doi: 10.1161/STROKEAHA.106.473983. [DOI] [PubMed] [Google Scholar]
- 65.Amlie-Lefond C, Deveber G, Chan AK, et al. Use of alteplase in childhood arterial ischaemic stroke: A multicentre, observational, cohort study. Lancet Neurol. 2009;8:530–536. doi: 10.1016/S1474-4422(09)70106-1. [DOI] [PubMed] [Google Scholar]
- 66.Ruf B, Heckmann M, Schroth I, et al. Early decompressive craniectomy and duraplasty for refractory intracranial hypertension in children: Results of a pilot study. Crit Care. 2003;7:R133–R138. doi: 10.1186/cc2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robertson SC, Lennarson P, Hasan DM, Traynelis VC. Clinical course and surgical management of massive cerebral infarction. Neurosurgery. 2004;55:55–61. doi: 10.1227/01.neu.0000126875.02630.36. [DOI] [PubMed] [Google Scholar]
- 68.Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 69.Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (STICH): A randomised trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 70.Rabinstein AA, Atkinson JL, Wijdicks EF. Emergency craniotomy in patients worsening due to expanded cerebral hematoma: To what purpose? Neurology. 2002;58:1367–1372. doi: 10.1212/wnl.58.9.1367. [DOI] [PubMed] [Google Scholar]
- 71.Broderick JP, Diringer MN, Hill MD, et al. Determinants of intracerebral hemorrhage growth: An exploratory analysis. Stroke. 2007;38:1072–1075. doi: 10.1161/01.STR.0000258078.35316.30. [DOI] [PubMed] [Google Scholar]
- 72.Mathew P, Teasdale G, Bannan A, Oluoch-Olunya D. Neurosurgical management of cerebellar haematoma and infarct. J Neurol Neurosurg Psychiatry. 1995;59:287–292. doi: 10.1136/jnnp.59.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lanthier S, Carmant L, David M, Larbrisseau A, de Veber G. Stroke in children: The coexistence of multiple risk factors predicts poor outcome. Neurology. 2000;54:371–378. doi: 10.1212/wnl.54.2.371. [DOI] [PubMed] [Google Scholar]
- 74.Fullerton HJ, Chetkovich DM, Wu YW, Smith WS, Johnston SC. Deaths from stroke in US children, 1979 to 1998. Neurology. 2002;59:34–39. doi: 10.1212/wnl.59.1.34. [DOI] [PubMed] [Google Scholar]
- 75.Hogan AM, Kirkham FJ, Isaacs EB. Intelligence after stroke in childhood: Review of the literature and suggestions for future research. J Child Neurol. 2000;15:325–332. doi: 10.1177/088307380001500509. [DOI] [PubMed] [Google Scholar]
- 76.Ganesan V, Hogan A, Shack N, Gordon A, Isaacs E, Kirkham FJ. Outcome after ischaemic stroke in childhood. Dev Med Child Neurol. 2000;42:455–461. doi: 10.1017/s0012162200000852. [DOI] [PubMed] [Google Scholar]
- 77.deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol. 2000;15:316–324. doi: 10.1177/088307380001500508. [DOI] [PubMed] [Google Scholar]
- 78.Ganesan V, Prengler M, Wade A, Kirkham FJ. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation. 2006;114:2170–2177. doi: 10.1161/CIRCULATIONAHA.105.583690. [DOI] [PubMed] [Google Scholar]
- 79.Strater R, Becker S, von Eckardstein A, et al. Prospective assessment of risk factors for recurrent stroke during childhood--a 5-year follow-up study. Lancet. 2002;360:1540–1545. doi: 10.1016/S0140-6736(02)11520-0. [DOI] [PubMed] [Google Scholar]
- 80.Visudhiphan P, Chiemchanya S, Wattanasirichaigoon D. Strokes in thai children : Etiology and outcome. Southeast Asian J Trop Med Public Health. 1996;27:801–805. [PubMed] [Google Scholar]
- 81.Livingston JH, Brown JK. Intracerebral haemorrhage after the neonatal period. Arch Dis Child. 1986;61:538–544. doi: 10.1136/adc.61.6.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jordan LC, Kleinman JT, Hillis AE. Intracerebral hemorrhage volume predicts poor neurologic outcome in children. Stroke. 2009;40:1666–1671. doi: 10.1161/STROKEAHA.108.541383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 84.Kenet G, Sadetzki S, Murad H, et al. Factor V leiden and antiphospholipid antibodies are significant risk factors for ischemic stroke in children. Stroke. 2000;31:1283–1288. doi: 10.1161/01.str.31.6.1283. [DOI] [PubMed] [Google Scholar]
- 85.Manco-Johnson MJ, Grabowski EF, Hellgreen M, et al. Laboratory testing for thrombophilia in pediatric patients. on behalf of the subcommittee for perinatal and pediatric thrombosis of the scientific and standardization committee of the international society of thrombosis and haemostasis (ISTH) Thromb Haemost. 2002;88:155–156. [PubMed] [Google Scholar]