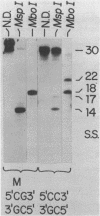
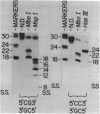
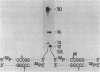
Abstract
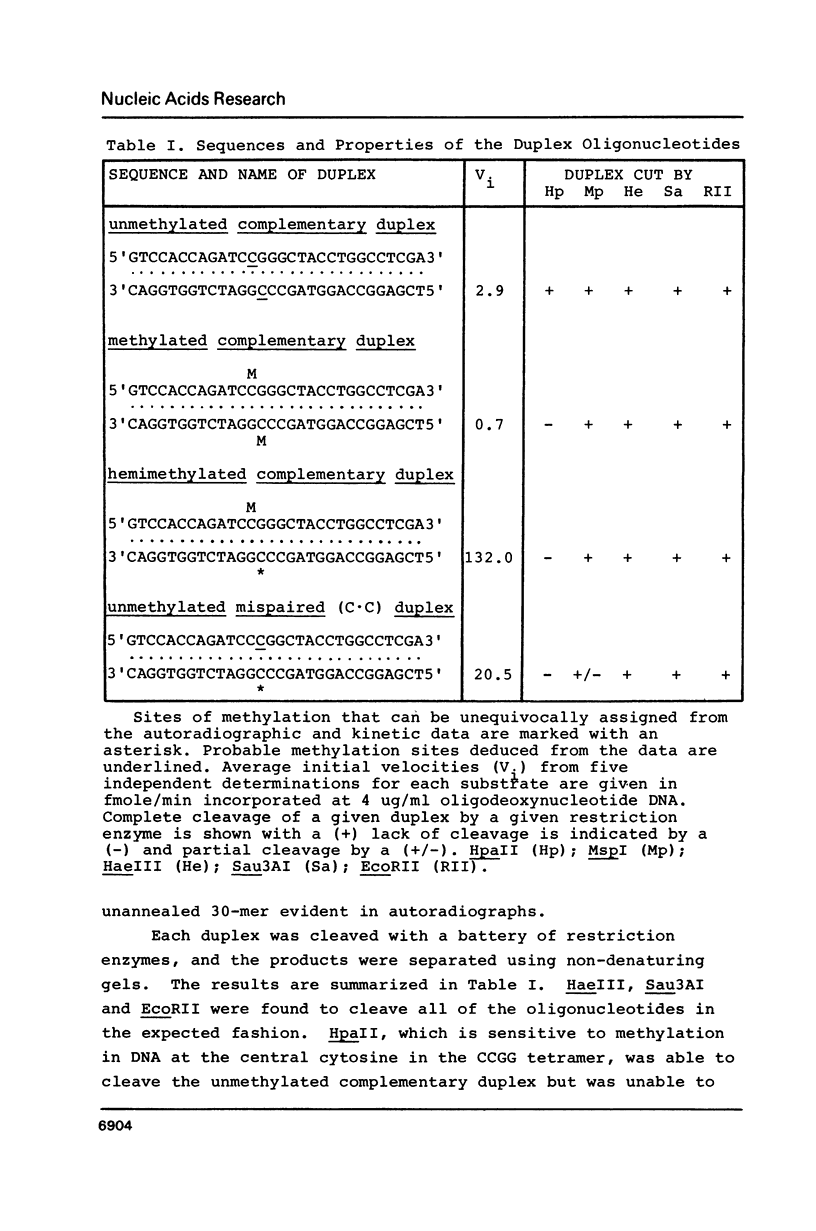
The presence of the C.C mispair in a defined duplex oligodeoxynucleotide enhanced its capacity to serve as a substrate for highly purified human DNA methyltransferase. Analysis of tritiated reaction products showed that the C.C mispair acted as a "methylation acceptor" in that it was itself rapidly methylated. The m5C.G base pair also enhanced the capacity of the oligodeoxynucleotide to serve as a substrate for the enzyme. However, this complementary base pair was found to act as a "methylation director". That is, the presence of the m5C in one strand induced the enzyme to rapidly methylate at the cytosine residue on the opposite strand in an adjacent C.G base pair.
Full text
PDF






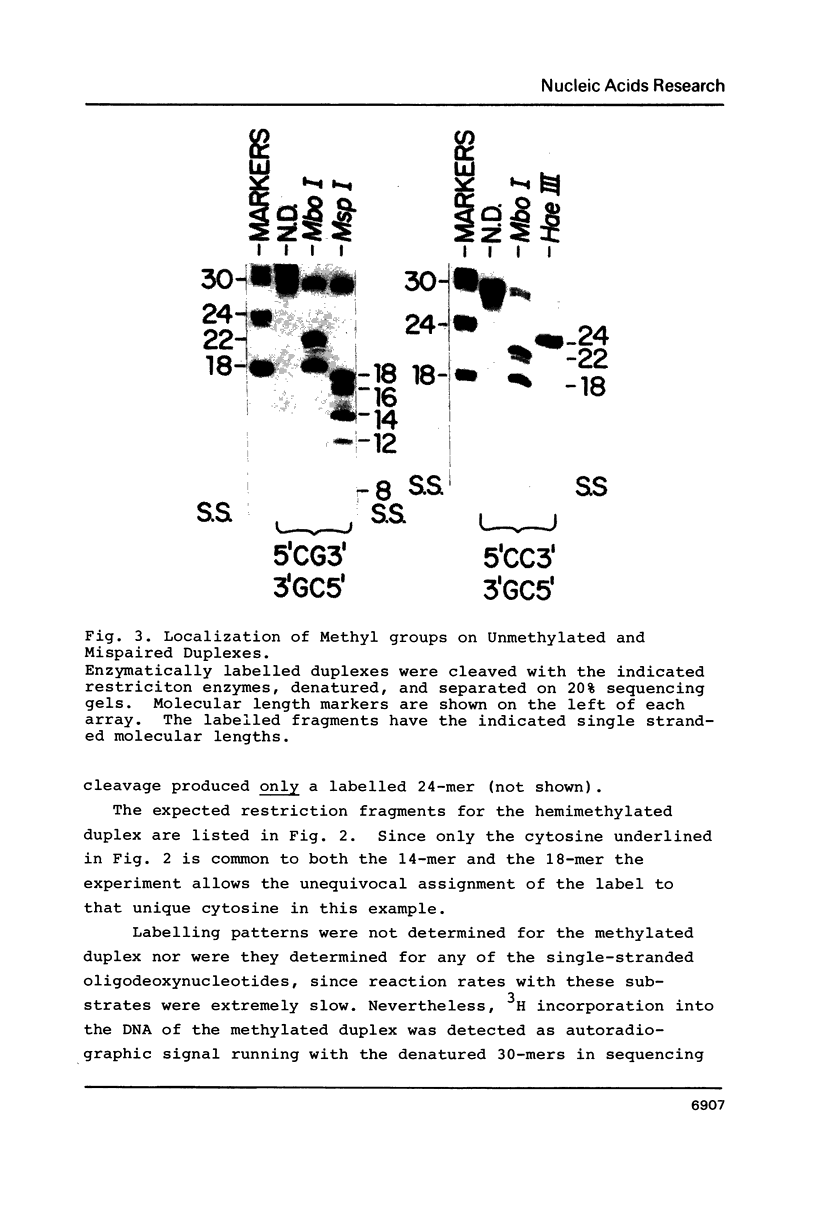
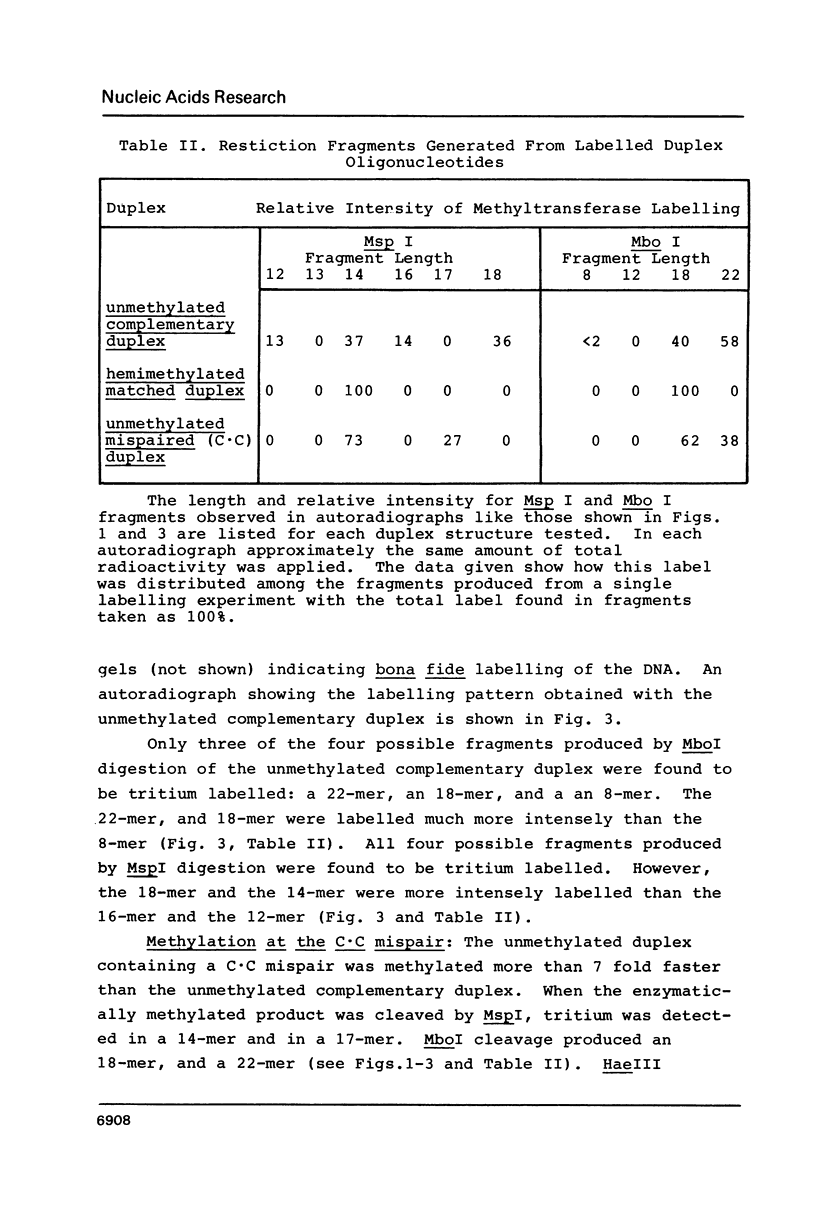
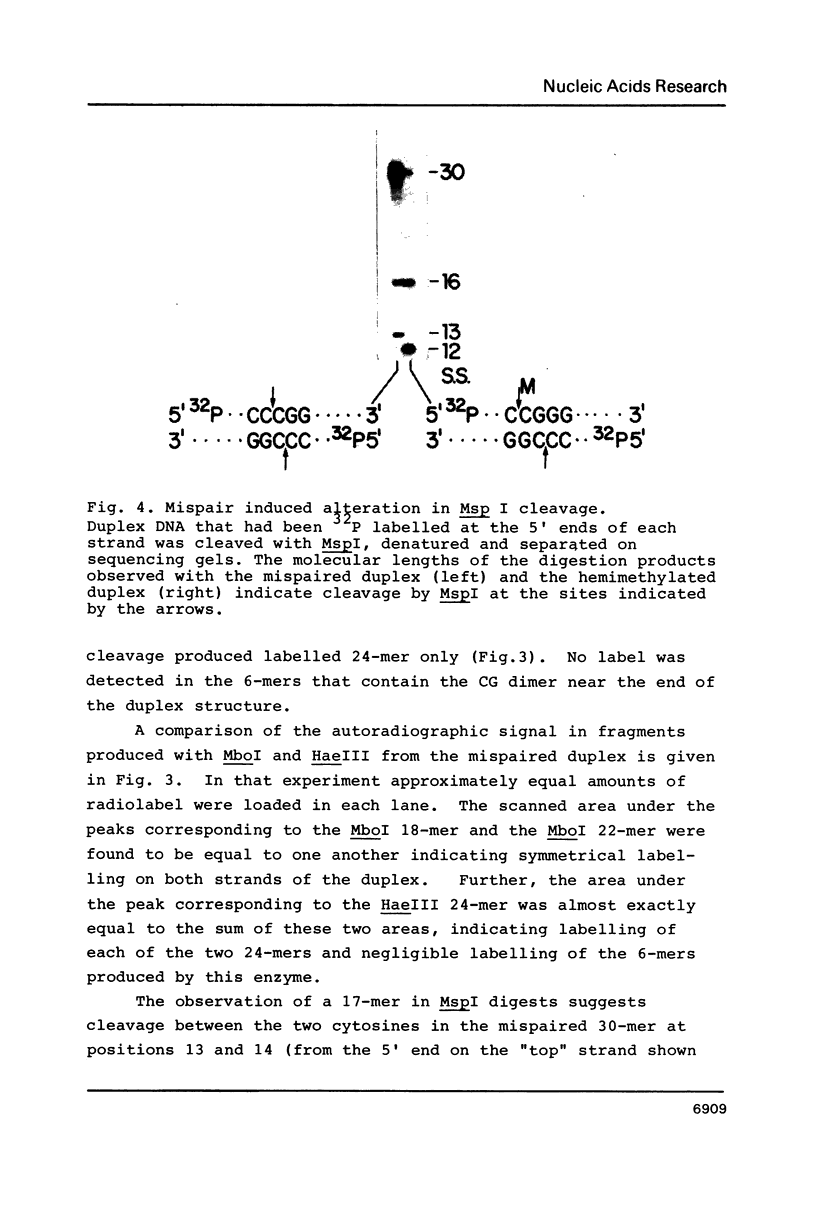







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker D. J., Hardy T. A., Smith S. S. The influence of the dT.dG mispair on the activity of the human DNA(cytosine-5)methyltransferase. Biochem Biophys Res Commun. 1987 Jul 31;146(2):596–602. doi: 10.1016/0006-291x(87)90570-5. [DOI] [PubMed] [Google Scholar]
- Bednarik D. P., Mosca J. D., Raj N. B. Methylation as a modulator of expression of human immunodeficiency virus. J Virol. 1987 Apr;61(4):1253–1257. doi: 10.1128/jvi.61.4.1253-1257.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Blakesley R. W., Wells R. D. 'Single-stranded' DNA from phiX174 and M13 is cleaved by certain restriction endonucleases. Nature. 1975 Oct 2;257(5525):421–422. doi: 10.1038/257421a0. [DOI] [PubMed] [Google Scholar]
- Borrello M. G., Pierotti M. A., Bongarzone I., Donghi R., Mondellini P., Della Porta G. DNA methylation affecting the transforming activity of the human Ha-ras oncogene. Cancer Res. 1987 Jan 1;47(1):75–79. [PubMed] [Google Scholar]
- Busslinger M., Hurst J., Flavell R. A. DNA methylation and the regulation of globin gene expression. Cell. 1983 Aug;34(1):197–206. doi: 10.1016/0092-8674(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Cui T., Ratliff R. L. Circular dichroism measurements show that C.C+ base pairs can coexist with A.T base pairs between antiparallel strands of an oligodeoxynucleotide double-helix. Nucleic Acids Res. 1984 Oct 11;12(19):7565–7580. doi: 10.1093/nar/12.19.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y., Cedar H., Razin A. Substrate and sequence specificity of a eukaryotic DNA methylase. Nature. 1982 Feb 18;295(5850):620–622. doi: 10.1038/295620a0. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y., Naveh-Many T., Cedar H., Razin A. Sequence specificity of methylation in higher plant DNA. Nature. 1981 Aug 27;292(5826):860–862. doi: 10.1038/292860a0. [DOI] [PubMed] [Google Scholar]
- Hardy T. A., Baker D. J., Newman E. M., Sowers L. C., Goodman M. F., Smith S. S. Size of the directing moiety at carbon 5 of cytosine and the activity of human DNA(cytosine-5) methyltransferase. Biochem Biophys Res Commun. 1987 May 29;145(1):146–152. doi: 10.1016/0006-291x(87)91299-x. [DOI] [PubMed] [Google Scholar]
- Hare J. T., Taylor J. H. One role for DNA methylation in vertebrate cells is strand discrimination in mismatch repair. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7350–7354. doi: 10.1073/pnas.82.21.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähner D., Jaenisch R. Retrovirus-induced de novo methylation of flanking host sequences correlates with gene inactivity. Nature. 1985 Jun 13;315(6020):594–597. doi: 10.1038/315594a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Tomizawa J. I., Maxam A. M. Detection of 5-methylcytosine in DNA sequences. Nucleic Acids Res. 1978 May;5(5):1479–1485. doi: 10.1093/nar/5.5.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrali-Noy G., Weissbach A. Mammalian DNA methyltransferases prefer poly(dI-dC) as substrate. J Biol Chem. 1986 Jun 15;261(17):7600–7602. [PubMed] [Google Scholar]
- Razin A., Szyf M. DNA methylation patterns. Formation and function. Biochim Biophys Acta. 1984 Sep 10;782(4):331–342. doi: 10.1016/0167-4781(84)90043-5. [DOI] [PubMed] [Google Scholar]
- Santi D. V., Garrett C. E., Barr P. J. On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell. 1983 May;33(1):9–10. doi: 10.1016/0092-8674(83)90327-6. [DOI] [PubMed] [Google Scholar]
- Saper M. A., Eldar H., Mizuuchi K., Nickol J., Appella E., Sussman J. L. Crystallization of a DNA tridecamer d(C-G-C-A-G-A-A-T-T-C-G-C-G). J Mol Biol. 1986 Mar 5;188(1):111–113. doi: 10.1016/0022-2836(86)90487-0. [DOI] [PubMed] [Google Scholar]
- Selker E. U., Stevens J. N. Signal for DNA methylation associated with tandem duplication in Neurospora crassa. Mol Cell Biol. 1987 Mar;7(3):1032–1038. doi: 10.1128/mcb.7.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M. H. The inheritance of methylation patterns in vertebrates. Cell. 1981 May;24(2):285–286. doi: 10.1016/0092-8674(81)90317-2. [DOI] [PubMed] [Google Scholar]
- Woodcock D. M., Crowther P. J., Diver W. P. The majority of methylated deoxycytidines in human DNA are not in the CpG dinucleotide. Biochem Biophys Res Commun. 1987 Jun 15;145(2):888–894. doi: 10.1016/0006-291x(87)91048-5. [DOI] [PubMed] [Google Scholar]
- Zucker K. E., Riggs A. D., Smith S. S. Purification of human DNA (cytosine-5-)-methyltransferase. J Cell Biochem. 1985;29(4):337–349. doi: 10.1002/jcb.240290407. [DOI] [PubMed] [Google Scholar]