Summary
Frzb-1 is a secreted protein containing a domain similar to the putative Wnt-binding region of the frizzled family of transmembrane receptors. Frzb-1 is widely expressed in adult mammalian tissues. In the Xenopus gastrula, it is expressed and regulated as a typical Spemann organizer component. Injection of frzb-1 mRNA blocks expression of XMyoD mRNA and leads to embryos with enlarged heads and shortened trunks. Frzb-1 antagonizes the effects of Xwnt-8 ectopic expression in a non-cell-autonomous manner. Cultured cells transfected with a membrane-tethered form of Wnt-1 bind epitope-tagged Frzb-1 in the 10−10 M range. The results strengthen the view that the Spemann organizer is a source of secreted inhibitory factors.
Introduction
The Spemann organizer, or dorsal lip, of the Xenopus gastrula has been a fertile fishing ground for novel signaling molecules such as noggin, chordin, TGFβ family members, and cerberus (Smith and Harland, 1992; Sasai et al., 1994; Jones et al., 1995; Smith et al., 1995; Bouwmeester et al., 1996). Signals emanating from this relatively small group of cells impart dorsoventral and anteroposterior patterning to neighboring cells (Spemann and Mangold, 1924). The inductive properties of the organizer graft are dominant, but it is now emerging that the ventral side of the embryo contains signals that actively oppose the dorsalizing effect of the organizer. The best understood ventral signal is BMP-4, which promotes ventral development of ectoderm and mesoderm and is expressed in a broad ventrolateral domain of the gastrula (reviewed by De Robertis and Sasai, 1996). The organizer tissue secretes at least two proteins, noggin and chordin, that directly bind to BMP-4 and prevent BMP receptor activation (Piccolo et al., 1996; Zimmerman et al., 1996). Thus, dorsoventral patterning in the embryo is established, at least in part, by antagonistic proteins that bind to each other in the extracellular space. Xwnt-8, a second secreted signal with ventralizing effects that is expressed in ventrolateral gastrula mesoderm, has been also described (Christian and Moon, 1993).
In this study, we present a secreted protein initially expressed in the organizer region of the gastrula, Frzb-1, that has sequence similarity to the extracellular domain of the Drosophila gene frizzled. The protein encoded by frizzled is a seven transmembrane receptor with a large extracellular cysteine-rich domain (CRD). This gene is required for tissue polarity of cuticular hairs and bristles as well as for the correct orientation of ommatidia (Gubb and García-Bellido, 1982; Vinson et al., 1989; Zheng et al., 1995). It has recently been shown that Dfz2, a protein sharing structural homologies to frizzled, is able to function as a wingless receptor (Bhanot et al., 1996). Importantly, the extracellular CRD of Dfz2, which shares homology with Frzb-1, is sufficient to stabilize binding of wingless to cultured cells. At least ten different frizzled homologs have been isolated from rat, mouse, humans, and zebrafish, all of which share the general structure of an extracellular CRD followed by seven transmembrane domains (Chan et al., 1992; Wang et al., 1996). Several, but not all, of these vertebrate frizzled homologs are able to bind wingless protein in cell biological assays (Bhanot et al., 1996). Drosophila wingless is the homolog of vertebrate Wnt-1, the founding member of this growth factor family (Nusse and Varmus, 1992). Rat frizzled-1 (Rfz1) is able to specifically recruit Xwnt-8 to the plasma membrane in Xenopus animal cap explants in coinjection experiments (Yang-Snyder et al., 1996).
Wnt genes were discovered as oncogenes leading to mouse mammary tumors when activated by viral integration (Nusse and Varmus, 1982). At least fifteen different Wnt genes that play multiple roles in cell proliferation and differentiation in adult and embryonic tissues are known in the mouse (Nusse and Varmus, 1992; Parr and McMahon, 1994). Microinjection during early cleavage of some Wnt mRNAs (Xwnt-1, -3A, -8, -8b and Drosophila wingless) leads to a complete duplication of the body axis (McMahon and Moon, 1989; Sokol et al., 1991; Chakrabarti et al., 1992; Cui et al., 1995; Du et al., 1995). This duplication results from the formation of a second Nieuwkoop center, a group of cells located in the dorsal endoderm of the early blastula, which induces the overlying mesoderm to become organizer tissue (Smith and Harland, 1991). Despite Wnts being among the most potent experimental twinning agents known, the nature of the endogenous activity they mimic remains elusive, although possible candidates have been proposed (Ku and Melton, 1993; Cui et al., 1995). In the case of Xwnt-8, the phenotype observed by early expression resulting from mRNA injections (Nieuwkoop center dorsalizing activity) differs greatly from the effect of injection of DNA constructs that are expressed at the gastrula stage. Indeed, injection of Xwnt-8 DNA under the control of the cytoskeletal actin (CSKA) promoter results in ventralization, which diverts dorsoanterior fates such as head structures and notochord to more lateral derivatives such as muscle (Christian and Moon, 1993). This ventralizing activity is congruent with the expression pattern of endogenous Xwnt-8 in the ventrolateral mesoderm at the gastrula stage (Christian et al., 1991; Christian and Moon, 1993).
The Frzb-1 protein differs from frizzled proteins in that it lacks all transmembrane domains, resulting in a putative secreted Wnt-binding protein. We provide evidence that frzb-1 can block twinning by Xwnt-8 and Wnt-1 mRNAs in Xenopus and that this antagonism takes place in the extracellular space. In addition, frzb-1 mRNA can block the ventralizing activity of Xwnt-8 DNA constructs expressed at the gastrula stage. In embryo microinjection experiments, frzb-1 mRNA inhibits muscle and trunk formation, mimicking the effects of a dominant-negative Xwnt-8 construct (Hoppler et al., 1996). In cell biological experiments, epitope-tagged soluble Frzb-1 protein binds to cells expressing a Wnt-1/transmembrane chimera. The results suggest that Frzb-1 functions as a soluble antagonist of Wnt signals.
Results
Expression of frzb-1 in Xenopus and Mammals
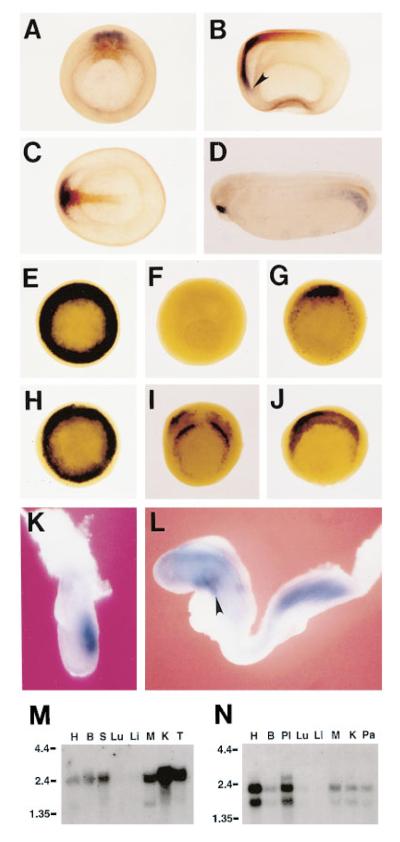
Xenopus frzb-1 was isolated during a differential screen for cDNAs enriched in the Spemann organizer carried out as reported earlier and originally named frezzled (Bouwmeester et al., 1996). The same gene was isolated by other means (Hoang et al., 1996; Wang et al., 1997 [this issue of Cell]), and the name frzb-1 was adopted here to uniformize the nomenclature. During development, frzb-1 transcripts are detectable in the dorsal lip of the early gastrula, starting at late blastula (not shown). As seen in Figure 1A, by midgastrula, frzb-1 transcripts (in blue) become localized to the anterior involuting endomesoderm of the organizer that will give rise to foregut and prechordal plate; this expression domain extends more anteriorly than that of chordin (shown in brown), which marks the future prechordal plate and notochord. frzb-1 expression in anterior endoderm extends to the prospective liver diverticulum, which is best seen at early neurula (Figure 1B, arrowhead). At this stage, chordin expression overlaps with that of frzb-1 in the prechordal plate (head mesoderm), but not in the notochord (Figure 1C). By early tailbud stage (Figure 1D), mesodermal expression in the posterior lateral plate becomes evident; this expression domain may be related to frzb-1 expressing cells that are weakly detected in the ventrolateral blastopore circumference at stage 10 1/2 (Figure 1G). frzb-1 expression in the anterior endodermal and prechordal region decreases at tailbud and is replaced by a burst of expression in the stomodeal-hypophyseal anlage (Figure 1D).
Figure 1.
frzb-1 mRNA Expression in Xenopus, Mouse, and Human
(A–D) Double-labeled in situ hybridization of Xenopus embryos showing frzb-1 (blue) and chordin expression (brown). (A) shows the vegetal view at stage 11; (B), lateral view of stage 13 embryo; (C), dorsal view of the same embryo; and (D), tailbud stage embryo. (E–J) Expression of frzb-1 in manipulated embryos. (E) shows the vegetal view of a Xenopus gastrula dorsalized by LiCl treatment; (F), Xenopus gastrula ventralized by UV irradiation; (G), vegetal view of an untreated embryo (stage 10 1/2); (H), Vegetal view of an embryo (stage 10 1/2) radially injected with siamois RNA; (I), frzb-1 expression in a stage 10 1/2 embryo radially injected with activated Xlim1 RNA; and (J), frzb-1 expression in a stage 10 1/2 embryo radially injected with goosecoid RNA.
(K) Mouse gastrula (midstreak stage) showing frzb-1 expression in the primitive streak.
(L) Mouse embryo (8.5 day) frzb-1 expression in the foregut diverticulum (arrowhead), nervous system, and posterior mesoderm.
(M–N) Northern blot of adult mouse tissue (M) and human tissue (N) showing frzb-1 expression in heart (H), brain (B), spleen (S), skeletal muscle (M), kidney (K), testis (T), placenta (Pl), and pancreas (Pa). frzb-1 is detected at very low levels in lung (Lu) and liver (Li).
To determine whether frzb-1 behaves as a typical organizer-specific gene, we treated Xenopus embryos with LiCl, which expands the organizer, or with ultraviolet light (UV), which prevents organizer formation (Kao and Elinson, 1988). As seen in Figures 1E and 1F, LiCl leads to radial expression of frzb-1, whereas UV treatment abolishes its expression. We next tested whether organizer-specific homeobox genes were able to activate frzb-1 expression. siamois mRNA (Lemaire et al., 1995) injected into the equatorial region of each blastomere at the four cell stage led to radial expression of frzb-1 (compare Figures 1G and 1H). In similar experiments, activated Xlim-1 mRNA (Taira et al., 1994) induced ectopic patches of expression (Figure 1I), and goosecoid mRNA led to an expansion of the frzb-1 domain (Figure 1J). Thus, frzb-1 appears to be a downstream target of organizer homeobox genes and is activated, directly or indirectly, by goosecoid, Xlim-1, and siamois.
In mouse, frzb-1 is expressed in the anterior 80% of the primitive streak in the 6.5 day gastrula (Figure 1K). At the head-fold stage (7.5 day), two domains of frzb-1 expression are found, one in the endomesoderm underlying the anterior neural plate and another in the primitive streak (not shown). At the early somite stage (8.5 day), expression in mouse is strong in the foregut and posterior mesoderm, and widespread expression in the forebrain and hindbrain regions becomes detectable (Figure 1L). In adult mouse tissues, frzb-1 expression, as determined by Northern blot, is widespread, and a strong signal is detected in heart, brain, spleen, skeletal muscle, kidney, and testis, while a weak one is detected in lung and liver (Figure 1M). Human frzb-1 is expressed strongly in placenta and heart, at intermediate levels in brain, skeletal muscle, kidney, and pancreas, and at low levels in lung and liver (Figure 1N).
We conclude from these expression studies that frzb-1 is a gene expressed during gastrulation in Xenopus and mice and that it behaves as a component of the Spemann organizer in Xenopus. In addition, frzb-1 is expressed in many adult tissues.
frzb-1 Encodes a Secreted Protein
The initial Xenopus clone obtained by differential screening was used to screen Xenopus and mouse gastrula cDNA libraries, and the longest clones were sequenced on both strands (see Experimental Procedures). The human GenBank EST database was searched, and overlapping ESTs were assembled into a contig to “virtually clone” the entire human Frzb-1 putative protein. These database studies also permitted mapping of the frzb-1 gene to human chromosome 2.
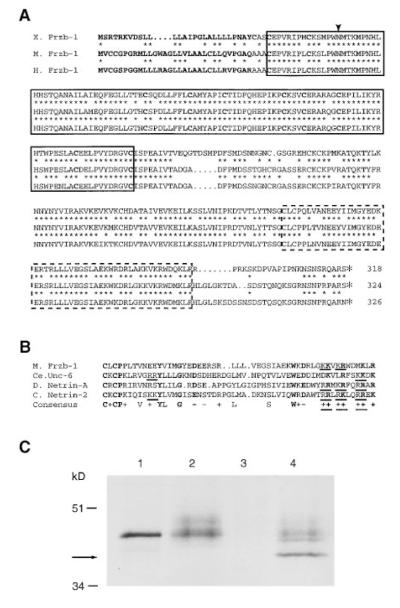
Figure 2A shows an alignment of the predicted Xenopus, mouse, and human Frzb-1 protein sequences. The proteins are conserved throughout their entire lengths; amino acid identity is 76% for Xenopus and mouse, 77% for Xenopus and human, and 92% for mouse and human. The Frzb-1 protein is just over 300 amino acids long and starts in a hydrophobic signal sequence predicted to be cleaved (von Heijne, 1986). It contains a conserved frizzled-like CRD but lacks the seven transmembrane domains present in the Drosophila and vertebrate frizzled gene family (Bhanot et al., 1996; Wang et al., 1996). All ten diagnostic cysteines of the CRD domain, as well as several other invariant amino acids, are conserved (Wang et al., 1996). Within this region, mouse frzb-1 shares 51% amino acid identity with Dfz2, 47% with Dfz1, and 47% with Rfz1.
Figure 2.
Frzb-1 Is a Secreted Protein Containing a Frizzled-like CRD and a FUN Domain
(A) Deduced amino acid sequences of the Xenopus, mouse, and human Frzb-1 proteins. Amino acid identity between frog and mouse or frog and human is indicated by an asterisk. The predicted signal peptide and the 10 cysteines typical of the extracellular cysteinerich domain (CRD) of frizzled genes are shown in bold. The CRD is boxed, and the potential N-linked glycosylation site is indicated by an arrowhead. Outside the CRD, another region of Frzb-1 that shows homology to the Unc-6 and Netrin proteins, designated the FUN domain, is indicated by the dashed box.
(B) Alignment of the FUN domains of mouse Frzb-1, C. elegans Unc-6, Drosophila Netrin-A, and chick Netrin-2. Residues identical in all four proteins and conserved acidic or basic residues are shown in bold; conserved residues in 3 of the 4 proteins are included in the consensus sequence. Dibasic sets of residues indicative of possible proteolytic cleavage sites are underlined.
(C) [35S]Frzb-1 protein synthesized in vitro (rabbit reticulocyte, lane 1) or in vivo (culture supernatant of injected frog oocytes, lane 2) run on an SDS–PAGE gel. Treatment of the in vivo–synthesized protein with N-glycosidase A (lane 4) shows a shorter band (arrow), indicating signal peptide processing and possible carboxy-terminal cleavage of Frzb-1 (see text). Uninjected oocyte supernatant is shown in lane 3.
A stretch of 52 amino acids of the Frzb-1 proteins located close to the COOH terminus has moderate sequence identity (32% to 21%, Figure 2B) to the carboxy-terminal region of the Unc-6 and Netrin protein family, which is involved in axon guidance (Serafini et al., 1994). The role of this Frzb-1/Unc-6/Netrin conserved region, designated the FUN domain, in the function of these extracellular proteins is not known.
To test whether Frzb-1 is indeed a secreted protein, synthetic mRNA was microinjected into Xenopus oocytes. A diffuse band of 35S-labeled protein was found in the culture medium of injected oocytes (Figure 2C, compare lanes 2 and 3). When treated with N-glycosidase A, part of the secreted protein was converted to a faster migrating species (lane 4, see arrow), suggesting that Frzb-1 is N-glycosylated. The unglycosylated form of the oocyte protein migrated faster than the protein precursor synthesized in vitro in the rabbit reticulocyte system (Figure 2C, lanes 1 and 4), indicating that the Frzb-1 prepeptide is cleaved in vivo to a product estimated to be about 5 kDa shorter. This difference is somewhat more than that predicted from signal peptide cleavage (2.7 kDa). During the course of our studies, we placed Flag and myc epitope tags at the COOH terminusof Xenopus Frzb-1. Although the expected tagged proteins were found in the intracellular compartment of Xenopus oocytes, no epitope-tagged protein was secreted, while normal amounts of [35S]Frzb-1 were found in the culture medium of mRNA-injected oocytes (data not shown). This provides indirect evidence that the COOH end of Frzb-1 is cleaved proteolytically. There are multiple paired basic residues at the end of the FUN domain (underlined in Figure 2B) where cleavage could occur. Perhaps one of the roles of the FUN domain is related to maturation of the COOH end. We conclude that frzb-1 encodes a secreted protein conserved among the vertebrates that has sequence similarity to the extracellular domain of the frizzled receptor gene family.
frzb-1 Blocks Muscle and Trunk Formation
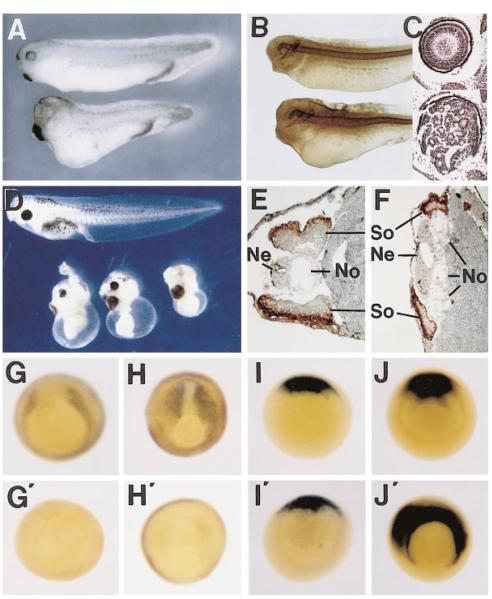
To test for biological activity, synthetic frzb-1 mRNA was microinjected into Xenopus embryos. When injected into the vegetal pole of each blastomere at the four cell stage, the embryos displayed a mild dorsalized phenotype (grade 6 of Kao and Elinson, 1988). The head, eyes, and cement gland were enlarged, and the axis was shortened (Figure 3A). Staining with the MZ-15 marker showed that the notochord was thickened (Figure 3B), and histological analysis showed in some embryos that a hyperplastic retina filled the enlarged eye, forming infoldings (Figure 3C, bottom panel). Since the vegetal pole contains Nieuwkoop center activity thought to result from Wnt pathway activity, we tested vegetal injections in particular detail (Figure 3A legend) and found that frzb-1 mRNA is unable to block Nieuwkoop center activity.
Figure 3.
frzb-1 mRNA Inhibits Skeletal Muscle Formation and Enhances Dorsoanterior Structures
(A) Top, control embryo. Bottom embryo was injected into the vegetal pole with 200 pg of synthetic frzb-1 mRNA into each of the four blastomeres and allowed to develop to stage 36. Note the enlarged cement gland and shortening of the body in this dorsalized embryo.
(B) Similar embryos after immunostaining with an antibody (MZ-15) labeling the notochord; the notochord is thickened in the frzb-1 dorsalized embryo.
(C) Histological section through the eye of a normal embryo (top) and of an embryo injected as in (A) (bottom). The retina of the dorsalized embryo is folded multiple times, filling the enlarged eye.
(D) Microinjection of frzb-1 mRNA into the marginal zone (400 pg/cell) leads to inhibition of trunk formation (bottom embryos).
(E) Uninjected tadpole sectioned at the trunk level after staining with the skeletal muscle marker 12/101.
(F) Sibling embryo injected with 250 pg/cell of frzb-1 mRNA into the marginal region of each of the four blastomeres. Note the decrease in the amount of 12/101 positive muscle and increase in notochord tissue ([So], somite; [No], notochord; [Ne], neural tube).
(G) In situ hybridization at stage 103/4 showing expression of XMyoD in prospective skeletal muscle cells.
(G’) frzb-1 mRNA inhibits XMyoD expression.
(H and H’) Expression of XMyoD at late gastrula (stage 12 1/2) is inhibited by frzb-1 mRNA microinjection.
(I and I’) Early expression (stage 10 1/2) of the organizer marker chordin is unaffected by frzb-1 ectopic expression.
(J and J’) Late gastrula expression of chordin mRNA (stage 12 1/2) is expanded into the lateral marginal zone in frzb-1-injected embryos.
Microinjection of frzb-1 mRNA into the marginal zone at high concentrations (ranging from 250 to 1000 pg/blastomere, four independent experiments) at the four cell stage resulted in embryos with greatly reduced trunk-tail structures and enlarged heads (Figure 3D). Sections through the affected trunk regions showed an enlargement of the notochord and a reduction of skeletal muscle, as indicated by staining with the widely used 12/ 101 monoclonal antibody (Figures 3E and 3F). A similar change of cell fates from somitic muscle into notochord and head structures has been described recently as the main phenotype of a dominant-negative form of Xwnt-8 in Xenopus embryos (Hoppler et al., 1996).
To better dissect the basis of the observed phenotypes, the effect of frzb-1 microinjection was assayed at the gastrula stage using XMyoD and chordin as molecular readouts. XMyoD is expressed in the marginal zone lateral to the organizer, in the region fated to become skeletal muscle (Frank and Harland, 1991). Injection of frzb-1 mRNA eliminated or greatly reduced XMyoD expression at mid and late gastrula (Figures 3G’ and 3H’). The initial specification of the organizer is not affected by frzb-1 microinjection, as indicated by the chordin marker (Figures 3I and 3I’) and the formation of a normal dorsal lip (data not shown). However, by late gastrula (stage 12 1/2), chordin expression is greatly expanded in the marginal zone (Figure 3J’). This late expansion of dorsal regions (chordin marks cells fated to become notochord and prechordal plate) at the expense of lateral marginal zone cells that would have expressed XMyoD and formed somites provides a molecular basis for the phenotypes observed 1–2 days later at the tailbud stage (Figures 3A–3F). The main conclusion from these microinjection experiments is that Frzb-1 blocks the induction of XMyoD and the formation of trunk structures, diverting cells to more dorsal fates.
frzb-1 Blocks Early and Late Effects of Ectopic Xwnt-8
To test whether Xenopus frzb-1 was able to modulate the early effects of Wnt signaling, we first titrated the minimum amounts of Xwnt-8 and mouse Wnt-1 mRNA required to produce complete secondary axes (including eye structures) in most, but not all, injected embryos. As shown in Figures 4A and 4B, coinjection of frzb-1 mRNA together with Xwnt-8 mRNA into a ventral-vegetal blastomere at the 32 cell stage was able to block secondary axis formation. A 2-fold excess (2 pg of Xwnt-8 and 4 pg of frzb-1 mRNA) slightly inhibited (from 69% complete axes, n = 45, to 47%, n = 47) but a 10-fold mRNA excess abolished the formation of complete axes with eyes (n = 42, with 38% still containing partial axes without eyes). Higher concentrations of frzb-1 mRNA (20-, 50-, and 100-fold excess, n = 176) blocked the formation of all secondary axes when coinjected with Xwnt-8 mRNA (Figures 4A and 4B). Similar observations were made for 0.1 pg Wnt-1 mRNA, for which a 20-fold excess of frzb-1 mRNA significantly inhibited and higher amounts blocked secondary axis formation (data not shown).
Figure 4.
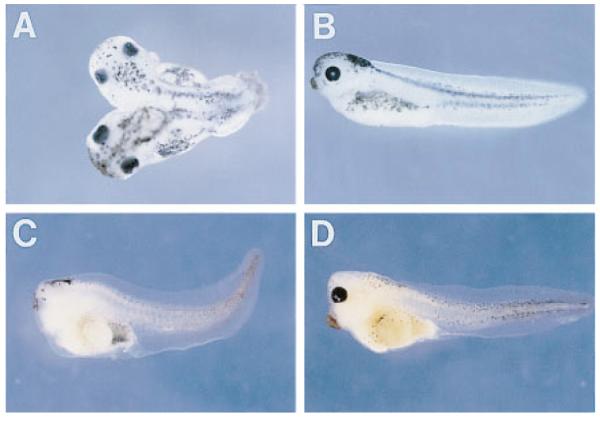
Frzb-1 Antagonizes the Early and Late Phenotypes of Xwnt-8
(A) Dorsal view of an embryo injected with Xwnt-8 RNA (4 pg) in a ventral-vegetal blastomere at the 32-cell stage.
(B) Lateral view of an embryo coinjected with Xwnt-8 (4 pg) and frzb-1 (160 pg) mRNA. Note that the formation of a secondary axis is blocked.
(C) Acephalous embryo resulting from the injection into the two dorsal blastomeres (four cell stage) of 80 pg of DNA construct expressing Xwnt-8 under the control of the cytoskeletal actin promoter.
(D) Rescue of the ventralization by coinjection of Xwnt-8 DNA construct with 400 pg of frzb-1 mRNA into the dorsal blastomeres. Note that eye development is restored and that the cement gland is enlarged.
Having shown that frzb-1 can antagonize the early twinning (dorsalizing) effects of Xwnt-8 mRNA injection, we next tested whether it could also antagonize the late effects of Xwnt-8 expression. Xenopus embryos injected into two dorsal blastomeres with CSKA Xwnt-8 DNA lack dorsoanterior structures, including eyes (Figure 4C) as reported by Christian and Moon (1993). When frzb-1 mRNA was coinjected, the ventralizing effect of Xwnt-8 was antagonized, leading to the development of moderately dorsalized embryos containing enlarged head structures and eyes (Figure 4D). We conclude that Frzb-1 can antagonize both the early and the late effects of Xwnt-8 signaling.
Frzb-1 Antagonizes Xwnt-8 Non-Cell-Autonomously
To test whether Frzb-1 can antagonize secondary axes caused by Xwnt-8 after secretion by injected cells, the experimental design shown in Figure 5 was used. frzb-1 mRNA was injected into each of the four animal blastomeres of eight-cell embryos, and subsequently, a single injection of Xwnt-8 mRNA was given to a vegetal-ventral blastomere at the 16–32 cell stage. In two independent experiments, we found that injection of frzb-1 alone (n = 13) caused mild dorsalization with enlargement of the cement gland (Figure 5D) in all embryos and that injection of Xwnt-8 alone (n = 53) led to induction of complete secondary axes in 67% of the embryos (Figure 5E). However, injection of frzb-1 into animal caps abolished the formation of complete axes induced by Xwnt-8 (n = 27), leaving only a residual 14% of embryos with very weak secondary axes (Figure 5F). The double-injected embryos retained the enlarged cement gland phenotype caused by injection of frzb-1 mRNA alone (compare Figures 5D and 5F). Because both mRNAs encode secreted proteins and were microinjected into different cells, we conclude that the antagonistic effects of Frzb-1 and Xwnt-8 took place in the extracellular space after these proteins were secreted.
Figure 5.
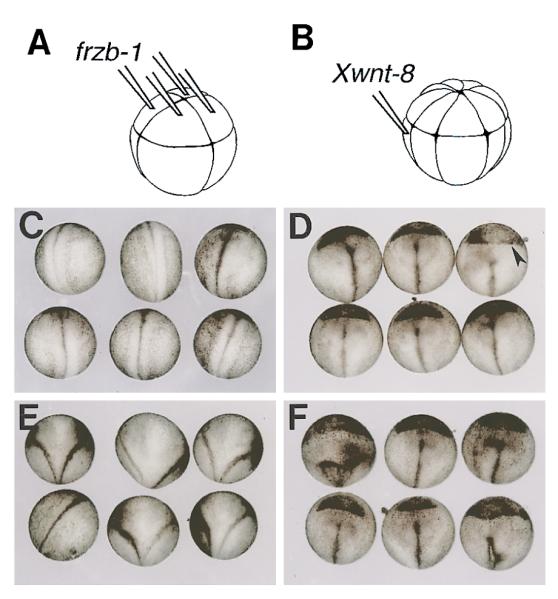
Noncell-Autonomous Antagonism of Xwnt-8 by frzb-1
(A) Experimental design showing embryos injected with frzb-1 synthetic mRNA into the four animal blastomeres at the 8-cell stage.
(B) Injection of Xwnt-8 synthetic mRNA into a single ventrovegetal blastomere at the 16–32 cell stage.
(C) Dorsal view of uninjected siblings at neurula (stage 19). The anterior end is at the top, the neural tube is visible.
(D) Sibling embryos injected with frzb-1 (200 pg/cell) mRNA as in (A). Note the single neural tube and the considerable enlargement of the cement gland at the anterior end (arrowhead), indicating dorsalization.
(E) Embryos injected with Xwnt-8 (4 pg) synthetic mRNA as in B. The Y-shaped neural tube indicates the formation of a double axis.
(F) Sibling embryos injected successively with frzb-1 mRNA in the animal cap and later with Xwnt-8 mRNA into a ventro-vegetal blastomere to reveal extracellular antagonism. Note that the embryos show a single axis; the Xwnt-8 phenotype was antagonized, but the dorsalization by frzb-1 (enlarged cement gland) is still present.
Membrane-Anchored Wnt-1 Confers Frzb-1 Binding
To investigate a possible interaction between Frzb-1 and Wnts, the first step was to insert an HA epitope tag into a Xenopus frzb-1 construct driven by the CMV (cytomegalovirus) promoter. Frzb1–HA was tested in mRNA microinjection assays in Xenopus embryos and found to be biologically active (not shown). Conditioned medium from transiently transfected cells contained up to 10 μg/ml of Frzb1–HA (quantitated on Western blots using an HA-tagged protein standard kindly provided by Dr. A. Berk, UCLA).
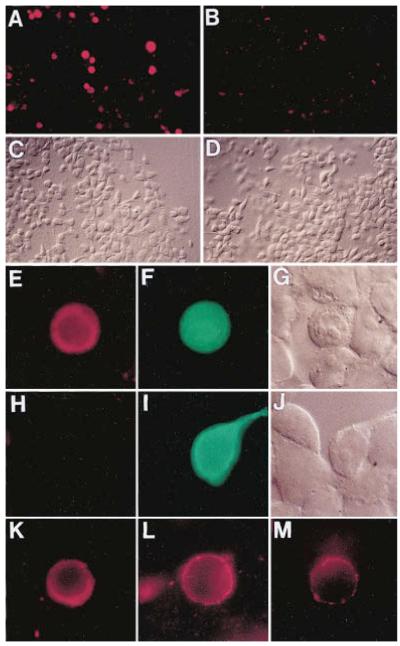
Transient transfection of 293 cells has been instrumental in demonstrating interactions between wingless and frizzled proteins (Bhanot et al., 1996). We therefore took advantage of constructs in which Wnt-1 was fused at the amino terminus of CD8, generating a transmembrane protein containing biologically active Wnt-1 exposed to the extracellular compartment (Parkin et al., 1993). A Wnt1CD8 cDNA construct (a generous gift of Dr. H. Varmus, NIH) was subcloned into the pcDNA (Invitrogen) vector and transfected into 293 cells. After incubation with Frzb1-HA-conditioned medium (overnight at 37°C), intensely labeled cells were observed by immunofluorescence (Figures 6A and 6C, four independent experiments). As a negative control, a construct containing 120 amino acids of Xenopus chordin, an unrelated secreted protein (Sasai et al., 1994), was used. Transfection of this construct produced background binding of Frzb1–HA to the extracellular matrix, both uniform and punctate (Figures 6B and 6D). Cotransfection of Wnt1CD8 with pcDNA-LacZ showed that transfected cells stained positively for Frzb1–HA and LacZ (Figures 6E–6G). Since Wnt1CD8 contains the entire CD8 molecule (Parkin et al., 1993), a CD8 cDNA was used as an additional negative control. After transfection with LacZ and full-length CD8, Frzb1–HA failed to bind to the transfected cells (Figures 6H–6J). Although most of our experiments were carried out at 37°C, Frzb1-HA-conditioned medium also stained Wnt1CD8-transfected cells after incubation at 4°C for 2 hr (Figure 6K).
Figure 6.
Frzb-1 Protein Binds to Cells Expressing a Wnt-1/Transmembrane Chimera
(A) Low magnification view of 293 cells transfected with a Wnt1CD8 construct expressing Wnt-1 tethered to the membrane. After incubation in conditioned medium containing 250 nM Frzb1–HA protein and staining with anti-HA, positive cells appear as red spots.
(B) Cells transfected with the plasmid coding for truncated Chordin protein (pΔ-Chd), used here as a negative control.
(C) The same field of view as in (A) in Nomarski optics.
(D) Nomarski view of the same cells as in (B).
(E) High power view of a culture co-transfected with Wnt1CD8 and LacZ in pcDNA3.
(F) The same cell that binds Frzb1–HA (red in panel E) also expresses LacZ from the cotransfected plasmid (in green).
(G) Nomarski view of the same field as in (E) and (F).
(H) Cells cotransfected with the full-length CD8 control as well as LacZ plasmid and treated with the Frzb1–HA medium shows no specific staining.
(I) The same cells as in panel (H) are shown after staining for β-galactosidase (in green).
(J) The same field of cells as in (H) and (I) is shown using Nomarski optics.
(K) Binding of Frzb1–HA (250 nM) performed at 4°C for 2.5 hr on Wnt1CD8-transfected cells stained with anti-HA (in red).
(L) Incubation of cells expressing Wnt1CD8 chimera with Frzb1–HA diluted down to 125 pM.
(M) Cell transfected with a mutant Wnt1CD8 (cysteine 369 mutated to tryptophan) that reduces its biological activity is able to bind diluted Frzb1–HA (125 pM). This experiment was carried out simultaneously to the one illustrated in (L) but exposure time was four times longer. Stained cells in (K)–(L) were GFP positive (data not shown). All pictures were taken on a Zeiss Axiophot microscope with 40X (A–D) or 100X objectives (E–M). Photos were taken with a Kodak Ektachrome P1600 film and printed photographically.
Attempts to biochemically quantitate the binding of Frzb-1 to Wnt1CD8-transfected cells were unsuccessful due to high background binding to control cultures, presumably due to binding to the extracellular matrix (Figure 6B). Thus, we were unable to estimate a KD for the affinity of the Frzb-1/Wnt-1 interaction. However, when serial dilutions of conditioned medium containing Frzb1–HA were performed (ranging from 2.5 × 10−7 to 1.25 × 10−10 M), staining of Wnt1CD8-transfected cells was found at all concentrations. Figure 6L shows a cell incubated with 125 pM Frzb1–HA. It is worth noting that at low concentrations the staining is weaker but localized in a punctate pattern on the cell membrane, reminiscent of that reported by Bhanot et al. (1996).
A point mutation in the last cysteine of Wnt-1 has been reported to abolish biological activity (McMahon and Moon, 1989). We tested this point mutation tethered to the membrane by a CD8 fusion (Wnt1CD8C369W; Parkin et al., 1993) in the expectation that it could serve as a negative control. However, we observed binding to cells transfected with the point mutation even at 125 pM Frzb1–HA (Figure 6M). One interpretation could be that the cysteine in question is required for signaling by Wnt-1 via its cognate receptor but not for Frzb-1 binding. However, microinjection of 200 pg of synthetic Wnt1CD 8C369W mRNA into a Xenopus ventral blastomere led to the formation of partial secondary axes. While the frequency and completeness of these axes were much lower than that of the nonmutant version, our unpublished results indicate that the Wnt-1 cysteine mutant retains a small amount of biological activity.
Although we have been unable to provide biochemical evidence for direct binding between Wnts and Frzb-1, a cell biological assay indicates that Frzb1–HA can bind, directly or indirectly, to Wnt-1 on the cell membrane in the 10−10 M range.
Discussion
Frzb-1 is a novel secreted factor isolated during a screen for transcripts enriched in the Spemann organizer (Bouwmeester et al., 1996). The amino-terminal part of the Xenopus protein has homology to the extracellular CRD of the receptor Dfz2, a domain that is sufficient to stabilize binding of Drosophila wingless in cultured cells (Bhanot et al., 1996). The large frizzled family of proteins has, in addition to the CRD, seven transmembrane domains characteristic of serpentine membrane receptor proteins (Perrimon, 1996; Wang et al., 1996). Frzb-1, however, has no transmembrane domains and is secreted into the culture medium by microinjected oocytes and transfected human cells, suggesting that Frzb-1 might act as a soluble Wnt-binding protein.
Frzb-1 Antagonizes Wnt Signaling in Xenopus
Two very different activities of Wnts were inhibited by frzb-1 mRNA in microinjected Xenopus embryos. First, the formation of secondary axes by Xwnt-8 and Wnt-1 mRNA was antagonized by coinjecting frzb-1 mRNA. In this experimental condition, the injected Wnt mRNA mimics the formation of a Nieuwkoop signaling center in the early cleavage stage embryo (McMahon and Moon, 1989; Smith and Harland, 1991; Sokol et al., 1991). This effect can be blocked by injecting frzb-1 into neighboring cells, showing that the two secreted proteins can antagonize each other in a non-cell-autonomous manner (Figure 5). Second, frzb-1 was able to reverse the ventralizing effects of pCSKA-Xwnt-8 (Christian and Moon, 1993). This DNA construct is expressed at the gastrula stage and leads to the opposite effect, ventralization with loss of head structures, of that of injected Xwnt-8 mRNA (which is expressed during cleavage stages and leads to formation of duplicated dorsal axes). Thus, frzb-1 mRNA can antagonize two separate activities mediated by Xwnt-8.
We have been unable to purify Wnts, which are insoluble and difficult to isolate (Nusse and Varmus, 1992; Burrus and McMahon, 1995). This has prevented us from demonstrating direct biochemical binding to Frzb-1. To circumvent this obstacle, we prepared biologically active Frzb-1 protein tagged with the HA epitope. This protein was shown to bind to cells expressing Wnt-1 tethered to the cell membrane (Parkin et al., 1993), even at a concentration of 10−10 M. These experiments do not eliminate the possible requirement of additional proteins for binding, either in the conditioned medium or on the cell surface. Although purified components were not used, the results suggest an interaction, direct or indirect, between Wnts and Frzb-1. The model in Figure 7A proposes a direct interaction between soluble Frzb-1 and Wnts, which would result in inhibition of binding to a frizzled-like transmembrane receptor. In this model, Frzb-1 would act as a competitive inhibitor unable to signal by itself.
Figure 7.
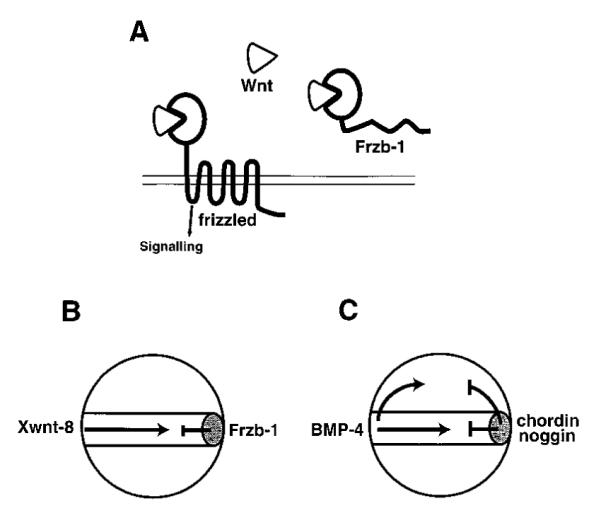
Speculative Model for the Mechanism of Frzb-1 Function
(A) Frzb-1 competes with frizzled receptors for the binding of Wnt factors. Frzb-1 and frizzled share a homologous cysteine-rich domain (PacMan shape) that is responsible for Wnt binding.
(B) Diagram of the possible function of Frzb-1 during Xenopus gastrulation. Frzb-1 is secreted by the Spemann organizer (gray oval) and antagonizes Xwnt-8, which is involved in patterning mesoderm in the ventrolateral marginal zone.
(C) Other organizer-specific inhibitory factors involved in patterning the mesoderm and neuroectoderm. BMP-4 is secreted by the ventral side of the embryo and is antagonized by chordin and noggin which directly bind to it in the extracellular space of ectoderm and mesoderm. In this view, the Spemann organizer would be a source of inhibitory secretory proteins.
Two studies have recently reported loss-of-functionof Wnt signaling using dominant-negative constructs of Xwnt-8 (Hoppler et al., 1996) and dishevelled (Sokol, 1996). Xenopus embryos injected with synthetic mRNAs developed with enlarged head structures and reduced trunks containing enlarged notochords and reduced somites (Hoppler et al., 1996; Sokol, 1996). An important observation was that dnXwnt-8 blocks XMyoD expression at gastrula stage (Hoppler et al., 1996). Expression of organizer genes such as goosecoid and chordin was unaffected at early gastrula stage (Hoppler et al., 1996; Sokol, 1996; this study). Our observation that chordin expression is expanded at late gastrula supports the hypothesis that (assuming Frzb-1 acts by inhibiting Xwnt-8) an important function of Xwnt-8 in the embryo may be to prevent the expansion of organizer tissue into the lateral marginal zone (Christian and Moon, 1993). Overall, the phenotype resulting from frzb-1 overexpression (Figure 3) is remarkably similar to the loss-of-function caused by dnXwnt-8 or dn-dishevelled.
It is worth noting that, as is the case for dnXwnt-8 and dn-dishevelled, xfrzb-1 microinjection does not inhibit formation of the Nieuwkoop center. This provides further support for the notion that Nieuwkoop center formation may be triggered by activation of downstream components of the Wnt pathway (Miller and Moon, 1996; Schneider et al., 1996) without requiring the secretion of a Wnt ligand, as has been proposed previously (Hoppler et al., 1996; Yost et al., 1996).
Frzb-1 Is Widely Expressed in Vertebrates
frzb-1 mRNA is abundantly expressed in a wide variety of mouse and human adult tissues, suggesting that Frzb-1 may be involved in multiple processes mediated by members of the Wnt family of signaling molecules. The action of Frzb-1 need not always be inhibitory. For example, one might envisage that Frzb-1 could function as a long-range transporter of Wnt proteins, helping to overcome the tendency of Wnts to bind to the extracellular matrix. Perhaps such a task might be shared with membrane-bound frizzled proteins, helping to explain the intriguing non-cell-autonomous effects of Drosophila frizzled (Vinson and Adler, 1987; Zheng et al., 1995).
When overexpressed, Wnt proteins cause cancer (Nusse and Varmus, 1982, 1992). If Frzb-1 is an antagonist of Wnts in vivo, it might function as a tumor suppressor gene. In this context, it is of interest that human frzb-1 maps to chromosome 2q31–33. Loss of one copy of the 2q arm has been observed with high incidence in lung carcinomas, colorectal carcinomas, and neuroblastomas, leading to the proposal that the 2q arm carries a tumor suppressor gene (Tsuchiya et al., 1992; Kohno et al., 1994).
These considerations suggest that the activities of Frzb-1 may be more diverse than those we have been able to demonstrate in the Xenopus assays. Indeed, the frzb-1 gene product has been independently isolated by an entirely different methodology, involving the biochemical purification of protein fractions containing chondrogenic activities (Hoang et al., 1996). However, pure recombinant Frzb-1 protein has not yet been shown to have chondrogenic activity.
The Spemann Organizer as a Source of Antagonists
In Xenopus, frzb-1 expression is regulated as a typical component of the Spemann organizer (Figure 1). At early gastrula, frzb-1 is expressed in the dorsal marginal zone, whereas Xwnt-8 is expressed in the ventrolateral marginal zone. This complementary pattern of expression makes it attractive to propose that the function of Frzb-1 during early gastrula is to antagonize the ventralizing activity of endogenous Xwnt-8 (Figure 7B). This inhibition would explain the phenotypic effects of frzb-1 in microinjected embryos. The antagonism is proposed to occur within the embryonic mesoderm or marginal zone; we have been unable to detect any effects of injected frzb-1 mRNA in the differentiation of animal cap explants, which form epidermis (L. L. et al., unpublished data). In this respect, Frzb-1 differs from chordin and noggin, which are able to pattern both ectoderm and mesoderm (Figure 7C). Recent results show that chordin and noggin function by binding and inactivating a ventral signal provided by BMP-4 (Piccolo et al., 1996; Zimmerman et al., 1996). The general theme emerging from these studies is that the organizer, a small group of cells with potent inductive activities, is the source of secreted factors such as noggin, chordin, and Frzb-1 that bind to and antagonize ventral signals. In this view, patterning of the embryo would result in part from inhibitory interactions between proteins in the extracellular space.
Experimental Procedures
Embryo Manipulation
Xenopus embryos were obtained and microinjected as described (Sasai et al., 1994). Mouse embryos were obtained from intercrosses of B6SJL/F1 hybrids (Jackson Lab).
Plasmid Constructions
Xenopus frzb-1 was subcloned in the pCS2+ vector to generate pFrzb-1. The control pΔ-Frzb-1 plasmid was generated by deleting most of the CRD region of frzb-1. An internal HA tag (pFrzb1-HA-i) was inserted in pFrzb-1 by PCR adding the amino acid sequence YPYDVPDYA after Ser158. The control plasmid pΔ-Chd consisted of the first 120 aa of Xenopus chordin cloned in pCS2+.
The Wnt1CD8 EcoRI/HindIII insert was subcloned from a pSP36T vector (Parkin et al., 1993) into pcDNA1/Amp (used for Figure 6A) and later into pcDNA3.1 (used in Figures 6E–6G, 6K, and 6L). The Wnt1CD8C369W EcoRI/HindIII insert was subcloned from the pSP36T vector (Parkin et al., 1993) into pcDNA3.1 (used in Figure 6M). The full-length cDNA of human CD8 was placed under control of the human β-actin promoter in a vector also containing the SV40 replication origin (a gift from C. Miceli). For GFP expression, a redshifted but not humanized variant, cloned in pcDNA3, was used. The pcDNA1/Amp, pcDNA3.1, and pcDNA3.1/His/LacZ constructs were purchased from Invitrogen.
Screening of Libraries and Databases
After rescreening a Xenopus dorsal lip library, a total of 48 clones were obtained, and 1 was sequenced. A cDNA library of mouse embryo (6.5–7.5 days) was screened at low stringency with an xfrzb-1 probe, 11 clones were obtained, and the longest one was sequenced on both strands. In addition, the 5′ and 3′ ends of the others were also sequenced, confirming that a single coding sequence is present.
To virtually clone the human frzb-1 gene, the GenBank EST database was searched for similarities with the mouse sequence. By searching the STS database with the full-length contig, the location of frzb-1 on chromosome 2 was uncovered. EST R36531 has been mapped by the Whitehead Institute Center for Genome Research to 857.8 centirays from the top of the chromosome 2 linkage group, which corresponds approximately to 2q31–33.
RNA Expression Studies
Xenopus whole-mount in situ hybridization was performed as described previously (Sasai et al., 1994). The same protocol with modifications was used for mouse in situ hybridization. Mouse and human adult Northern blots of poly(A) mRNA were purchased from Clonetech and probed according to instructions of the manufacturer. After frzb-1 hybridization, the blots were stripped and reprobed with mouse β-actin. Every lane showed the presence of β-actin mRNA at similar levels, except for mouse heart and skeletal muscle, in which the signal was much more intense.
RNA Synthesis
Production of synthetic capped mRNA was carried out as in Sasai et al. (1994). To obtain sense mRNA, pFrzb-1, pΔ-Frzb-1, and pFrzb1-HA-i were digested with NotI and transcribed with SP6 RNA polymerase. To generate Xwnt-8 sense mRNA, pSPXwnt-8 (Christian et al., 1991) was digested with BamHI and transcribed by SP6 RNA polymerase. To produce Wnt-1 sense mRNA, mouse pWnt1 (a gift from A. McMahon) was digested with EcoRI and transcribed with T7 RNA polymerase. To produce Wnt1CD8 and Wnt1CD 8C369W sense mRNA, the pSP-Wnt1CD8 and pSP-Wnt1CD8C369W plasmids (a gift from H. Varmus) were digested with EcoRI and transcribed by SP6 RNA polymerase. All RNAs produced were quantified by gel electrophoresis and OD measurement.
Antibodies
Detection of the epitope tag was carried out with anti-HA (Babco) mouse monoclonal antibody. Secondary antibody and luminescent detection for Western blots were from Amersham. Staining was carried out with the MZ-15 and 12/101 antibodies as in Sasai et al. (1994). Antibodies used for cell staining are as follows: mouse monoclonal anti-HA (ascites fluid from Babco), rabbit anti-β-galactosidase (Cappel), donkey anti-mouse IgG coupled to Cy3, and donkey anti-rabbit coupled to FITC (both preadsorbed for multiple labeling from Jackson Immunochemicals). The immunocytochemical binding assay on 293 cells was adapted from Bhanot et al. (1996).
Cell Culture
Embryonic human kidney cells (293T) were cultured in D-MEM containing 10% FCS. Transfection was carried out overnight at 50%–70% confluency by standard calcium phosphate technique. Conditioned medium was obtained by culturing the transfected cells in D-MEM plus 10% FCS from 24 hr after adding the DNA to the fifth day, when collection occurred. For cotransfection experiments, the reporter to experimental plasmid ratio was 1:5.
Acknowledgments
Correspondence regarding this paper should be addressed to E. M. D. R. We wish to thank Drs. H. Varmus, R. Moon, and A. McMahon for generous gifts of Wnt cDNAs that permitted us to enter a new area of research and Drs. R. Moon, W. Risau, and F. Luyten for communication of unpublished results. Additional materials were kindly provided by Drs. E. Agius, A. Berk, J. Brockes, I. Dawid, A.-C. Fluckiger, J. B. Gurdon, R. Harland, P. Lemaire, C. Miceli, R. Rupp, and F. Watts. We are in debt to Drs. G. Weinmaster, A. Hainski, B. Brizuela, K. Lyons, and an anonymous reviewer for critical reading of this manuscript and to Nathalie Kertesz for excellent technical assistance. T. B. is supported by a Human Frontier Science Project Organization long-term fellowship. S. P. is an awardee of a Comitato Promotore Telethon fellowship. E. M. D. R. is an Investigator of the Howard Hughes Medical Institute. This work was supported by NIH grant HD21502-11.
Footnotes
References
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Kim S-H, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- Burrus LW, McMahon AP. Biochemical analysis of murine Wnt proteins reveals both shared and distinct properties. Exp. Cell Res. 1995;220:363–373. doi: 10.1006/excr.1995.1327. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A, Matthews G, Colman A, Dale L. Secretory and inductive properties of Drosophila wingless protein in Xenopus oocytes and embryos. Development. 1992;115:355–369. doi: 10.1242/dev.115.1.355. [DOI] [PubMed] [Google Scholar]
- Chan SDH, Karpf DB, Fowlkes ME, Hooks M, Bradley MS, Vuong V, Bambino T, Liu MYC, Arnaud CD, Strewler GJ, Nissenson RA. Two homologs of the Drosophila polarity gene frizzled (fz) are widely expressed in mammalian tissues. J. Biol. Chem. 1992;267:25202–25207. [PubMed] [Google Scholar]
- Christian JL, McMahon JA, McMahon AP, Moon RT. Xwnt-8, a Xenopus Wnt-1/int-1 related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development. 1991;111:1045–1055. doi: 10.1242/dev.111.4.1045. [DOI] [PubMed] [Google Scholar]
- Christian JL, Moon RT. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 1993;7:13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- Cui Y, Brown JD, Moon RT, Christian JL. Xwnt-8b: a maternally expressed Xenopus Wnt gene with a potential role in establishing the dorsoventral axis. Development. 1995;121:2177–2186. doi: 10.1242/dev.121.7.2177. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380:37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- Du SJ, Purcell SM, Christian JL, McGrew LL, Moon RT. Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol. Cell. Biol. 1995;15:2625–2634. doi: 10.1128/mcb.15.5.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D, Harland RM. Transient expression of XMyoD in non-somitic mesoderm of Xenopus gastrulae. Development. 1991;113:1387–1393. doi: 10.1242/dev.113.4.1387. [DOI] [PubMed] [Google Scholar]
- Gubb D, García-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- Hoang B, Moos M, Jr., Vukicevic S, Luyten FP. Primary structure and tissue distribution of FRZB, a novel protein related to Drosophila Frizzled, suggest a role in skeletal morphogenesis. J. Biol. Chem. 1996;271:26131–26137. doi: 10.1074/jbc.271.42.26131. [DOI] [PubMed] [Google Scholar]
- Hoppler S, Brown JD, Moon RT. Expression of a dominant negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 1996;10:2805–2817. doi: 10.1101/gad.10.21.2805. [DOI] [PubMed] [Google Scholar]
- Jones CM, Kuehn MR, Hogan BLM, Smith JC, Wright CVE. Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation. Development. 1995;121:3651–3662. doi: 10.1242/dev.121.11.3651. [DOI] [PubMed] [Google Scholar]
- Kao KR, Elinson RP. The entire mesodermal mantle behaves as a Spemann’s organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev. Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Kohno T, Morishita K, Takano H, Shapiro DN, Yokota J. Homozygous deletion at chromosome 2q33 in human small-cell lung carcinoma identified by arbitrarily primed PCR genomic fingerprinting. Oncogene. 1994;9:103–108. [PubMed] [Google Scholar]
- Ku M, Melton DA. Xwnt-11: a maternally expressed Xenopus wnt gene. Development. 1993;119:1161–1173. doi: 10.1242/dev.119.4.1161. [DOI] [PubMed] [Google Scholar]
- Lemaire P, Garrett N, Gurdon JB. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell. 1995;81:85–94. doi: 10.1016/0092-8674(95)90373-9. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989;58:1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- Miller JR, Moon RT. Signal transduction through β-catenin and specification of cell fate during embryogenesis. Genes Dev. 1996;10:2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- Parkin NT, Kitajewski J, Varmus HE. Activity of Wnt-1 as a transmembrane protein. Genes Dev. 1993;7:2181–2193. doi: 10.1101/gad.7.11.2181. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Wnt genes and vertebrate development. Curr. Opin. Genet. Dev. 1994;4:523–528. doi: 10.1016/0959-437x(94)90067-d. [DOI] [PubMed] [Google Scholar]
- Perrimon N. Serpentine proteins slither into the wingless and hedgehog fields. Cell. 1996;86:513–516. doi: 10.1016/s0092-8674(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of Chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P. β-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech. Dev. 1996;57:191–198. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991;67:753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith WC, McKendry R, Ribisi S, Jr., Harland RM. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell. 1995;82:37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Sokol S, Christian JL, Moon RT, Melton DA. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991;67:741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signaling pathways during Xenopus development. Curr. Biol. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Spemann H, Mangold H. Über Induktion von Embryonalanlagen durch Implantation Artfremder Organisatoren. Roux’s Arch. Entw. Mech. 1924;100:599–638. [Google Scholar]
- Taira M, Otani H, Saint-Jeannet JP, Dawid I. Role of the LIM class homeodomain protein Xlim-1 in neural and muscle induction by the Spemann organizer in Xenopus. Nature. 1994;372:677–679. doi: 10.1038/372677a0. [DOI] [PubMed] [Google Scholar]
- Tsuchiya E, Nakamura Y, Weng SY, Nakagawa K, Tsuchiya S, Sugano H, Kitagawa T. Allelotype of non-small cell lung carcinoma—comparison between loss of heterozygosity in squamous cell carcinoma and adenocarcinoma. Cancer Res. 1992;52:2478–2481. [PubMed] [Google Scholar]
- Vinson CR, Adler PN. Directional non-cell autnomoy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338:263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucl. Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Macke JP, Abella BS, Andreasson K, Worley P, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J. Biol. Chem. 1996;271:4468–4476. doi: 10.1074/jbc.271.8.4468. [DOI] [PubMed] [Google Scholar]
- Wang S, Krinks M, Lin K, Luyten FP, Moos M., Jr. Frzb, a secreted protein expressed in the Spemann Organizer, binds and inhibits Wnt-8. Cell. 1997 doi: 10.1016/s0092-8674(00)81922-4. this issue. [DOI] [PubMed] [Google Scholar]
- Yang-Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr. Biol. 1996;6:1302–1606. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Zheng L, Zhang J, Carthew RW. frizzled regulates mirror-symmetric pattern formation in the Drosophila eye. Development. 1995;121:3045–3055. doi: 10.1242/dev.121.9.3045. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesús-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]