Abstract
We hypothesized that a whey protein diet would result in greater weight loss and improved body compositioncompared to standard weight loss diets. Weight change, body composition, and renin angiotensin aldosterone system activity in midlife adults was compared between diet groups. Eighteen subjects enrolled ina5 month study of8 weeks controlled food intake followed by 12 weeks ad libitum intake. Subjects were randomized to one of three treatment groups: control diet (CD) (55% carbohydrate: 15% protein: 30% fat), mixed protein (MP) (40% carbohydrate: 30% protein: 30% fat), or whey protein (WP) (40% carbohydrate: 15% mixed protein: 15% whey protein: 30% fat). Measurements included weight, metabolic measures, body composition by dual energy x-ray absorptiometry (DXA), and resting energy expenditure. No statistically significant differences in total weight loss or total fat loss were observed between treatments, however, a trend toward greater total weight loss (p = 0.08) and total fat loss (p=0.09) was observed in the WP group compared to the CD group. Fat loss in the leg and gynoid regions was greater (p < 0.05) in the WP group than the CD group. No RAAS mediated response was observed, but a decrease in systolic blood pressure was significantly greater (p <0.05) in the WP group compared to the CD group. In summary, increased whey protein intake did not result in statistically significant differences in weight loss or in total fat loss, but significant differences in regional fat loss and in decreased blood pressure were observed in the WP group.
Keywords: adults, fat loss, weight loss, whey protein, satiety, resting energy expenditure
1. Introduction
In the United States, there is a greater likelihood of obesity among men and women 40 – 59 years of age compared to men and women 20 – 39 years[1]. Obesity in midlife is associated with higher risk of metabolic disease[2,3]. Energy restriction diets of variouscompositions have been proposed to control weight gain and promote weight loss. Multiple studies have reported successful weight loss and maintenance of lean mass using energy restricted diets of moderate to high protein content (≥ 25% energy from protein)[4-6]. Generally, these diets have increased protein intake from meat, eggs, dairy, and nuts and decreased the proportion of energy from carbohydrates and fats.
In a single meal study of macronutrient intake, protein decreased postprandial appetite and subsequent energy intake compared to either carbohydrate or fat[7].Ad libitum intake of high protein dietsenhanced weight loss compared to diets with high carbohydrate content [8-9]. In a long term study, subjects following a moderate protein diet were more successful in maintaining significant weight loss and improved body composition after 12 months compared to subjects following an average protein diet plan [10].
Benefits associated with high protein reduced energy diets include enhanced satiety [11], increased thermogenesis [8], and improved insulin sensitivity [12,13]. These effects are thought to be associated with the branch chain amino acids (BCAA) found in complete proteins [13,14]. In addition,the BCAA leucine signals intracellular pathways regulating amino acid oxidation and gluconeogenesis [15]. Whey protein (100 grams) contains about twenty-four grams of BCAA [16], which have been associated with improved insulin sensitivity and satiety [17]. Some studies have investigated the use of whey protein in an ad libitum high protein weight loss diet [18, 19], but to our knowledge whey protein has not been compared against other protein sources in a weight loss protocol.
Ideal weight loss results in reduction of fat mass with minimal loss of lean tissue; therefore, a better understanding of the mechanisms governing adipose activity is required. Recently, adipose metabolism has been found to be influenced byhormones previously identified in blood pressure homeostasis as the renin-angiotensin aldosterone system(RAAS) [20,21]. With weight loss, decreases in renin, angiotensinogen, angiotensin converting enzyme (ACE), and aldosterone have been observed [22]. In a study of the pharmaceutical ACE inhibitors enalapril and captopril, a slight loss of body weight was noted in the treatment groups[23-25], althoughthe metabolic pathway is still unclear. Whether the decrease in RAAS activity is an effect of weight loss, or if the inhibition of the RAAS pathway also results in a loss of weight has not been specifically tested.
Recent studies have proposed that the RAAS system can be inhibited with bioactive peptides found in food sources. ACE inhibition by small peptide groups found in whey protein has been observed in vitro[26]. There have been mixed results in human clinical studies with some studies reporting an average decrease in systolic blood pressure of 7.0 mmHg [27-29] and another study showing no significant difference in blood pressure[30]. The specific mechanism of action by whey peptides has not been determined. However, it is believed to involve direct inhibition of ACE by binding with the zinc fingers of the molecule resulting in the inability of ACE to cleave angiotensin I into angiotensin II[31]. It is not clear whether a decrease in blood pressure after consuming whey protein is directly related to ACE inhibition or some other mechanism.
Whey protein may be a preferred protein choice for a weight loss diet because of its high BCAA content, the potential bioactivity with the RAAS of the adipose tissue, the potential to increase satiety, and its ease of incorporation into a diet plan. Wehypothesized that a whey protein diet would result in greater weight loss and improved body compositioncompared to standard weight loss diets. Using a randomized, parallel design of three diets, our objectives were to determine weight loss, body composition, insulin sensitivity, satiety, blood pressure, and RAAS activity in midlife adults who were following an energy restricted protocol to promote weight loss. Based upon the literature, increased consumption of whey protein may decrease ACE activity and may be related to maintenance of lean mass. Measures of changes in ACE activity, plasma renin activity, and aldosterone concentration were compared to changes in body composition for each diet group.
2. Methods and materials
2.1. Study design
A randomized, parallel design was used to compare the effects of diets containing varying protein quantities from differentprotein sources. After signing consent forms, subjects were randomized to acontrol diet (CD), mixedprotein diet (MP), or whey protein diet (WP) (Figure 1). Subjects were directed to maintain their daily activity habits and not increase physical activity during the study.
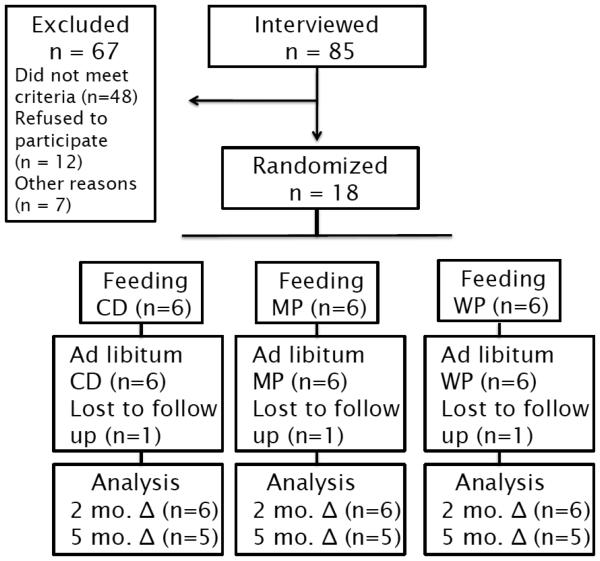
Fig 1.
Flow diagram for the study design and subjects
CD – control diet; MP- mixed protein diet; WP – whey protein diet
For the first 8 weeks, all the food to be consumed was provided to the subjects. Subjects came daily to the General Clinical Research Center (GCRC) of the University of Minnesota to pick up meals. At daily visits, each subject completed a compliance questionnaire, a satiety questionnaire, and was weighed.
On completion of the 8-week feeding phase, subjects continued on their assigned diet for an additional 12-week ad libitumphase. Subjects were instructed by a registered dietitian on how to continue the assigned diet. Those subjects assigned to WP were given additional Designer Whey® protein powder and Pure Protein® bars. Subjects were expected to report daily intake on a secure, personal dietary analysis web program (www.nutrihand.com), which the principal investigator could evaluate for compliance. Every four weeks, each subject returned to the GCRC to be weighed and have blood drawn for fasting glucose and insulin levels. At baseline, 8, and 20 weeks, each subject came to the GCRC for evaluations, including indirect calorimetry to measure resting energy expenditure (REE), fasting glucose and insulin, RAAS activity, and a dual energy x-ray absorptiometry (DXA) scan tomeasure body composition. Concentrations of fasting glucose and insulin were used to calculate the homeostatic model assessment of insulin resistance(HOMA IR: = [fasting glucose (mmol/l) * fasting insulin (μU/ml)] / 22.5) [32] at these three clinical visits.
Approval of the study was obtained from the University of Minnesota Committee for the Use of Human Subjects and Research. The entire study was performed at the University of Minnesota GCRC.
2.2. Subjects
Healthy, midlife (40 – 60 yrs) adults were recruited from the Minneapolis-St. Paul metropolitan area through local posters and advertisement. Eligibility was determined by responses to a telephone interview and subjects were disqualified if they reported a current medical problem, were pregnant, or were using prescription medication. Subjects were asked about their willingness to comply with dietary treatments. Inclusion criteria included BMI 27 – 32 kg/m2, no recent weight changes, a fasting blood glucose measure < 126 mg/dL, and a desire to lose weight. Of 85 individuals screened by telephone interview, 27 were eligible for further screening at the GCRC. A total of 18 participants consented to the protocol and were enrolled in the study.
2.3. Resting Energy Expenditure
At the screening visit, eligible subjects had their REE measured with the ParvoMedics True One Metabolic Monitor (Provo, UT). All REE measures were completed in a fasting state after the subject had rested in a supine position for about thirty minutes. Fifteen minutes of astable, continuous measure were used to calculate the baseline REE for each subject. [33] The baseline REE was evaluated along with the activity level reported by each subject to determine total energy needs. To determine the activity level, each subject was asked if they regularly engaged in specific activities and what duration of time was spent in each given activity. From this evaluation, a custom reduced energy diet was tailored to promote 0.75 kg weight loss weekly. Each subject's usual food consumption prior to starting the study was assessed by the Diet History Questionnaire (NCI, 2003).
2.4. Dietary Treatments
The macronutrient composition for each dietary treatment is shown in Table 1. All three diets were similar in dietary fiber, cholesterol, calcium intake, and fatty acid balance. Ad libitum additions of salt were not controlled with these diets. Diets were designed to differ in thepercent of energy from protein and the protein source. The two high protein diets contained 30% energy from protein and 40% energy from carbohydrate compared to CD, which contained 15% energy from protein and 55% energy from carbohydrate. The CD and theMPdiets contained protein from the samesources, but in different quantities. The WPdiet contained the same quantity of protein by mass as the MP, but the entire increase of protein above the level of the CD came from the addition of Designer Whey®, a commercial whey protein isolate. Daily calcium intake was balanced across groups by providing supplemental calcium tablets (Caltrate 600) to subjects in the CD and MP groups.
Table 1.
Macronutrient composition of the dietary groups*
| Macronutrient | CD | MP | WP |
|---|---|---|---|
| Kilocalories | 1599.9 ± 2.0 | 1605.8 ± 0.5 | 1599.7 ± 2.8 |
| Protein (g) | 63.4 ± 0.2 | 124.1 ± 0.5 | 124.2 ± 0.4 |
| Carbohydrate (g) | 225.3 ± 0.2 | 163.9 ± 0.6 | 166.9 ± 0.5 |
| Fat, Total (g) | 54.9 ± 0.4 | 54.0 ± 0.2 | 54.7 ± 0.4 |
| Saturated Fat (g) | 16.1 ± 0.1 | 16.0 ± 0.2 | 16.2 ± 0.1 |
| Monounsaturated Fat (g) | 16.1 ± 0.1 | 16.1 ± 0.2 | 16.3 ± 0.1 |
| Polyunsaturated Fat (g) | 16.1 ± 0.1 | 15.9 ± 0.2 | 16.1 ± 0.1 |
| Dietary Fiber (g) | 25.0 ± 0.5 | 24.0 ± 1.0 | 23.2 ± 0.7 |
| Cholesterol (mg) | 239.8 ± 0.8 | 240.0 ± 1.6 | 268.5 ± 3.2 |
| Calcium (mg) | 1316.1 ± 41.0# | 1668.0 ± 90.9# | 1536.0 ± 47.8 |
Data are means (± SEM)
Calculated by Nutritionist V software
includes Caltrate 600 supplement
CD – control diet; MP- mixed protein diet; WP – whey protein diet
Experimental diets were formulated with commonly available food items, including whey protein, as shown in Table 2. A five day menu rotation was developed and then modified for each experimental group. The nutrient composition of the test diets was calculated with Nutritionist V nutrient analysis software (Hearst Corporation, 2005). The nutrient data for all food items used in the menus were obtained from the USDA standard reference database as included in Nutritionist V.
Table 2.
Sample Menu (1600 Kcal)
| Meal | Menu Item Diet | CD (g) | MP (g) | WP (g) |
|---|---|---|---|---|
| Breakfast | Apple Juice | 200 | 100 | 70 |
| Wheat Chex Cereal | 37 | 25 | 25 | |
| 2% Milk | 162 | 180 | 200 | |
| Whole Wheat English Muffin | 62 | 23 | 30 | |
| w/ Butter | 10 | 3 | 0 | |
| w/ Canola Margarine | 0 | 0 | 6 | |
| Jam – Low Sugar | 4 | 3 | 3 | |
| Egg Beaters | 0 | 75 | 0 | |
| w/ Butter | 0 | 3 | 0 | |
| w/ 2% milk | 0 | 20 | 0 | |
| Cheddar Cheese, Low fat | 0 | 35 | 0 | |
| Designer Whey – Chocolate | 0 | 0 | 28 | |
|
| ||||
| Lunch | Rye Bread | 46 | 0 | 0 |
| Reduced Calorie Wheat Bread | 0 | 46 | 46 | |
| Corned Beef | 44 | 70 | 35 | |
| Mayonnaise, Regular | 14 | 6 | 0 | |
| Mayonnaise, Lite | 0 | 0 | 20 | |
| w/ Egg Yolk | 10 | 1.5 | 0 | |
| Romaine Lettuce | 25 | 20 | 20 | |
| Red Tomato | 37 | 30 | 30 | |
| Yellow Mustard | 4 | 3 | 3 | |
| Carrots, raw | 25 | 20 | 25 | |
| Celery, raw | 25 | 20 | 25 | |
| Blueberries | 187 | 80 | 75 | |
| GENISOY Deep Sea Soy Nuts | 0 | 35 | 0 | |
| Designer Whey – French Vanilla | 0 | 0 | 28 | |
|
| ||||
| Dinner | Chicken Breast, cooked | 56 | 135 | 55 |
| Egg Noodles, cooked | 187 | 75 | 0 | |
| Rotini, cooked | 0 | 0 | 85 | |
| Chicken Gravy | 75 | 50 | 75 | |
| w/ Olive oil | 3 | 4 | 4 | |
| Green Beans, cooked | 125 | 90 | 100 | |
| Whole Wheat Dinner Roll | 44 | 0 | 0 | |
| Reduced Calorie Wheat Bread | 0 | 25 | 30 | |
| w/ Butter | 0 | 3 | 8 | |
| w/ Canola Margarine | 5 | 0 | 0 | |
| Mandarin Oranges, canned | 175 | 90 | 70 | |
|
| ||||
| Snack | Milk Chocolate Chips | 30 | 0 | 0 |
| Walnuts | 14 | 14 | 15 | |
| Skim Milk | 150 | 240 | 220 | |
| Designer Whey – Chocolate | 0 | 0 | 28 | |
CD – control diet; MP- mixed protein diet; WP – whey protein diet
2.5. Dietary Compliance and Satiety Assessment
Subjects completed compliance questionnaires on a daily basis during the 8 week feeding phase. The first questionnaire asked the subject to report all quantities of provided food left uneaten, and to specify any additionalfood that had been consumed during the previous 24 hours. In the second questionnaire, satiety was evaluated by use of a Labeled Affective Magnitude scale (LAM) [34,35]. Similar to a Visual Analogue Scale (VAS), this scale contained phrases describing different degrees of hunger or fullness on a 100 mm vertical line (0mm = ‘Greatest Imaginable Hunger’, 50mm = ‘Neither hungry nor full’, 100mm = ‘Greatest Imaginable Fullness’). Subjects were instructed to mark the vertical line at the place that best described their current feeling of hunger or fullness.
During the 12 week ad libitum phase, compliance was monitored by daily online recording via the Nutrihand.com website. The online records were reviewed weekly by investigators with feedback provided to each subject by email. Records were evaluatedfor maintenance of restricted energy intake and assigned protein intake. The average weekly intake for each subject was calculated from the selection of consecutive days in each week with three or more meals recorded each day. The mean intake per treatment group was calculated using the average weekly intake of each subject.
2.6. Blood analyses
After fasting overnight, plasma samples were collected from subjects in EDTA vacutainer tubes for analysis of plasma renin concentrations. Serumsamples werecollected in vacutainer tubes for glucose, insulin, aldosterone, and ACE analysis. Samples were separated by centrifuge at 2000 × gand 5° C for 20 minutes and then individually labeled and stored at−80° C until analysis was completed.
Insulin was measured with a commercially available ELISA kit (LINCO Research, St. Charles, MO) with a range of 2-200μU/mL. The procedure used microtiter plates coated with an antibody for human insulin. Samples were added to the prescribed wells and additional antibodies were added to sandwich the captured insulin. Quantification of the captured insulin was accomplished by measuring the activity of horseradish peroxidase in tetramethylbenzidine with an ELISA reader. Glucose was measured with a spectrophotometric protocol developed by Morin et al [36]. A 2.5 mL glucose oxidase-preoxidase reagent was added induplicate to 50 μL serum samples, vortexed, and then incubated two minutes before measuring absorbance in the spectrophotometer (Varian Cray 50 2.0). ACE activity was measured with a commercially available spectrophotometric assay (ALPCO Immunoassays, Salem, NH) with a reference range of 27 – 68 U/L. The production of hippuric acid by ACE was stopped with the addition of HCl and the product then combined with cyanuric chloride to a measurable absorbance at 382 nm. [37] Plasmarenin activity was measured with a commercially available immunoradiometric assay (IRMA) (Diagnostic Systems Laboratories, Webster, TX) with lower and upper detection limits of 5 and 500 pg/mL, respectively. The assay measured the renin that was attached to two antibodies in a non-competitive enzyme reaction. [38] Aldosterone was measured with a commercially available radioimmunoassay (Diagnostic Systems Laboratories, Webster, TX) with lower and upper detection limits of 25 and 1600 pg/mL, respectively. This competitive radioimmunoassay measured the aldosterone bound to the antibody, which is inversely proportional to the aldosterone present in the original sample. [39]
2.7. Body Composition Measures
Body weight was measured on an electronic scale (Scale-Tronix 5005 Stand-On Scale) to the nearest ± 0.1 kgwith subjects wearing light clothing and no shoes. Height was measured to the nearest ± 1.0 cmon a stadiometer attached to the scale. BMI was calculated as kg/m2. DXA measures of total body, lumbar spine, and dual femur were completed at baseline, 8, and 20 weeks by a trained technician. The DXA software (Prodigy, General Electric Medical, Madison, WI) calculated total tissue, total fat tissue, total lean tissue, percent body fat, and bone mineral density from the total body scan.[40]In addition to total body measures, the DXA software identified five regions for additional analysis: trunk, arm, leg, gynoid, and android regions. The android region is defined as the waist area starting from a longitudinal line directly above the pelvis and progressing upward a height that is 20% of the measured distance from the pelvis to the neck base. The gynoid region is definedfrom a longitudinal line at the top of the pelvis and progressing downward a total height twice as high as the android region[41].
2.8. Statistical analyses
All data are presented as means (± SEM) of each dietary treatment group. The groups were compared using analysis of variance in Procedure General Linear Models (Proc GLM) (SAS version 9.2, SAS Institute, Inc. Cary, NC). Area under the curve (AUC) was measured for sodium and potassium intake during the ad libitum phase of the trial. The overall F-test is reported, with the results of pair-wise group comparisons and tests of within-group changes from baseline. Results were considered significant at p < 0.05.
3. Results
3.1. Subjects
Randomization of subjects to the three treatment groups was successful. At baseline, no statistically significant differences existed between treatment groups for age, weight, BMI, or percent body fat (Table 3). All subjects completed the 8 week feeding phase of the trial and one female from each group did not complete the 12 week ad libitum phase (Figure 1). Follow up with non-completers indicated the reasons for not returning for follow up clinical visits were life challenges and dissatisfaction with rate of weight loss.
Table 3.
Subject characteristics by group at baseline and change in study parameters at 2 and 5 months.
| CD | MP | WP | Overall p | |
|---|---|---|---|---|
| value | ||||
| N (F/M) | 6 (5/1) | 6(5/1) | 6(5/1) | |
| Age (years) | 51.3 ± 2.1 | 49.6 ± 3.5 | 49.2 ± 1.6 | 0.824 |
| Baseline | ||||
| BMI | 29.9 ± 0.6 | 30.3 ± 0.7 | 30.6 ± 0.6 | 0.712 |
| % Body Fat | 43.0 ± 2.5 | 42.7 ± 2.5 | 45.2 ± 2.9 | 0.777 |
| HOMA-IR | 0.89 ± 0.18 | 0.96 ± 0.28 | 0.99 ± 0.26 | 0.954 |
| Weight (kg) | ||||
| Baseline | 81.6 ± 1.9 | 84.4 ± 1.2 | 85.2 ± 4.2 | 0.626 |
| 2 mo. Δ | − 5.3 ± 0.76* | − 5.9 ± 0.59* | − 7.3 ± 0.73* | 0.167 |
| 5 mo. Δ | − 6.1 ± 0.82* | − 7.6 ± 1.72* | − 9.7 ± 1.27* | 0.198 |
| Systolic | ||||
| Baseline | 117.8 ± 4.3 | 117.7 ± 2.7 | 116.2 ± 3.2 | 0.933 |
| 2 mo. Δ | − 2.2 ± 5.1 | − 8.7 ± 3.9 | − 3.3 ± 3.8 | 0.541 |
| 5 mo. Δ | 3.2 ± 4.5a | − 3.6 ± 2.7a | − 7.2 ± 1.7b* | 0.106 |
| Diastolic | ||||
| Baseline | 67.7 ± 2.9 | 66.8 ± 2.4 | 66.2 ± 3.4 | 0.936 |
| 2 mo. Δ | 3.8 ± 4.2 | − 4.3 ± 2.1 | − 2.3 ± 3.8 | 0.255 |
| 5 mo. Δ | 1.2 ± 3.2 | − 2.6 ± 1.3 | − 6.0 ± 4.0 | 0.289 |
| Fasting REE | ||||
| Baseline | 1627 ± 139 | 1582 ± 152 | 1452 ± 81 | 0.613 |
| 2 mo. Δ | − 269 ± 93* | − 160 ± 125 | − 43 ± 69 | 0.296 |
| 5 mo. Δ | − 264 ± 237 | − 247 ± 184 | − 21 ± 88 | 0.583 |
| Total Body Fat mass (kg) | ||||
| Baseline | 34.65 ± 1.9 | 35.61 ± 2.1 | 37.78 ± 1.6 | 0.498 |
| 2 mo. Δ | − 4.29 ± 0.7* | − 5.17 ± 0.5* | −5.99 ± 0.7* | 0.211 |
| 5 mo. Δ | − 5.45 ± 1.1* | − 7.54 ± 1.4* | −8.77 ± 1.3* | 0.216 |
| Total Body Lean mass (kg) | ||||
| Baseline | 43.83 ± 2.6 | 45.67 ± 1.8 | 44.95 ± 4.9 | 0.927 |
| 2 mo. Δ | −1.12 ± 0.9 | − 0.32 ± 0.8 | −1.14 ± 0.4* | 0.680 |
| 5 mo. Δ | −0.32 + 0.4 | +0.43 + 1.1 | −1.09 + 0.1* | 0.300 |
Data are means ± SEM
marks significant within-group change from baseline (p< 0.05)
Row values displaying different lowercase letters are significantly different (p < .05, Proc GLM) 624CD – control diet; MP- mixed protein diet; WP – whey protein diet
3.2. Weight loss and body composition
Significant weight loss within each treatment group was observed (p < 0.05 for CD, MP, and WP ranging from 6 to almost 10 kg). Differences in weight loss between all three treatments were not significant. However, there was a trend (p = 0.08) toward greater weight loss in the WP group compared to the CD group.
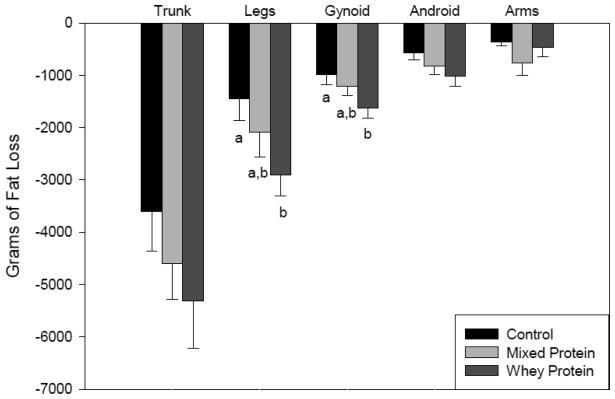
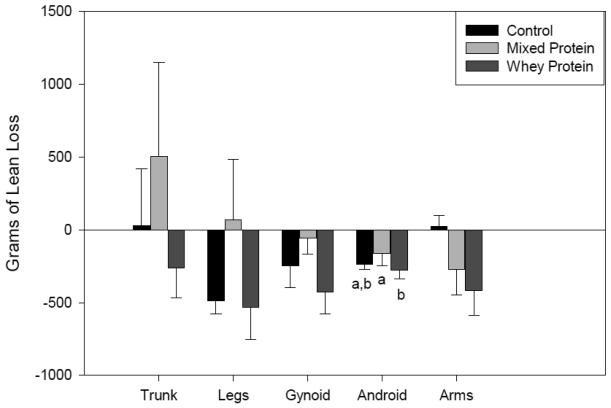
Body composition improved with a reduction in fat mass in all treatment groups. There was an observed trend of greater total fat mass loss (p = 0.09) in the WP group compared to the CD group, especially in the leg and gynoid regions (Figure 2). In addition, there weresome regional differencesin lean mass changesbetween treatments (Figure 3) with the WP group showing slightly greater loss of lean mass in association with greater total body weight reduction. In comparison by fat : lean ratio, the CD group showed a loss of 5.6 : 1g compared to a loss of 8.4 : 1 g with the WP group. The difference in total lean mass loss between groups at week 20 was not significantly different.
Fig. 2.
Fat loss by Region and by Diet at Five Months. Values measured by DXA and reported as mean (± SEM). Different lower case letters indicate significance between groups as determined by GLM.
Fig. 3.
Lean Tissue Change by Region and by Diet at Five Months. Values measured by DXA and reported as mean (± SEM). Different lower case letters indicate significance between groups as determined by GLM.
3.3. RAAS activity, blood pressure and metabolic measures
Plasma renin and ACE activities, and aldosterone concentration are reported in Table 4. Renin activity wasnot significantly different between groups and did not change over the five month protocol. ACE activity in the CD groupwas higher at baseline compared to the other treatment groups but the difference was not statistically significant. By week 8, all the groups had similar ACE measures with a significant decrease within the CD group (p < 0.05). Over the 20 weeks, ACE activity decreased in the WP group, but was not statistically different from other groups. Aldosterone concentration was similar at baseline between treatments, and a trend toward decreasing aldosterone concentration (p = 0.08) was observed in the CD and WP groups at week 20.
Table 4.
Renin –Angiotensin Aldosterone System concentrations by group
| CD | MP | WP | p value | |
|---|---|---|---|---|
| N (F/M) | 6 (5/1) | 6(5/1) | 6(5/1) | |
| Renin (ng/L) | ||||
| Baseline | 7.2 ± 0.5 | 7.2 ± 1.3 | 7.7 ± 1.8 | 0.941 |
| 2 mo. | 8.4 ± 0.8 | 8.5 ± 1.6 | 8.3 ± 0.7 | 0.984 |
| 5 mo. | 9.5 ± 1.4 | 8.4 ± 1.8 | 9.0 ± 1.2 | 0.871 |
| ACE (U/L) | ||||
| Baseline | 61.2 ± 3.2 | 50.3 ± 4.4 | 50.0 ± 5.9 | 0.187 |
| 2 mo. | 51.7 ± 2.1* | 50.0 ± 5.4 | 48.0 ± 6.0 | 0.867 |
| 5 mo. | 52.2 ± 7.0 | 54.4 ± 5.7 | 46.8 ± 4.6 | 0.651 |
| Aldosterone (ng/L) | ||||
| Baseline | 46.5 ± 6.4 | 53.8 ± 8.7 | 54.5 ± 11.1 | 0.789 |
| 2 mo. | 48.9 ± 8.6 | 77.8 ± 21.5 | 73.1 ± 18.6 | 0.461 |
| 5 mo. | 49.0 ± 4.4a | 67.8 ± 11.1b | 42.8 ± 4.4a | 0.077 |
Data are means ± SEM
marks significant within-group change from baseline (p< 0.05)
Row values displaying different lowercase letters are significantly different (p < .05, Proc GLM)
CD – control diet; MP- mixed protein diet; WP – whey protein diet
Systolic blood pressure decreased in all three treatments during the first 8 weeks. At the conclusion of the study, there was a significant difference (p < 0.04) in change of systolic blood pressure between the CD and WP treatment. There were no significant changes in diastolic blood pressure between treatments.
No significant differences were observed between groups for fasting glucose or insulin measures at any time point in the study. HOMA IR values at baseline were similar between groups and the decreasing values observed with all treatments were not significantly different at weeks 8 or 20.
3.4. Dietary Results
Protein intake between diets was significantly different throughout the 20 weeks. As designed,protein intake during the feeding phase forthe CD group averaged 0.8 g/kg/day, while protein intake of the MP and WP groups averaged 1.4 g/kg/day. A comparison of the essential amino acid compositionaverage between the MP and WP treatment during the feeding phase showed an increase by mass in tryptophan (48%), threonine (63%), isoleucine(27%), leucine (45%), cystine (46%), and valine (21%) with daily intake in the WP treatment. This increase is based primarily on the additional whey protein consumed in the diet.
During the ad libitum phase,protein intake of the CD group increased to 1.1 g/kg/day and protein intake of the MP group was 1.3 g/kg/day, while the WP group maintained 1.4 g/kg/day. Subjects maintained a protein and carbohydrate intake that was significantly different (p < 0.05) between the CD treatment and both high protein treatments. No significant difference in AUC analysis for sodium and potassium intake was observed. However, the self-reported online dietary records did not ask subjects to report added salt. Ninety-three percent of the subjects reported ad libitumintake for more than six weeks, and 53% of the subjects reported all weeks of ad libitum intake.
3.5. Satiety
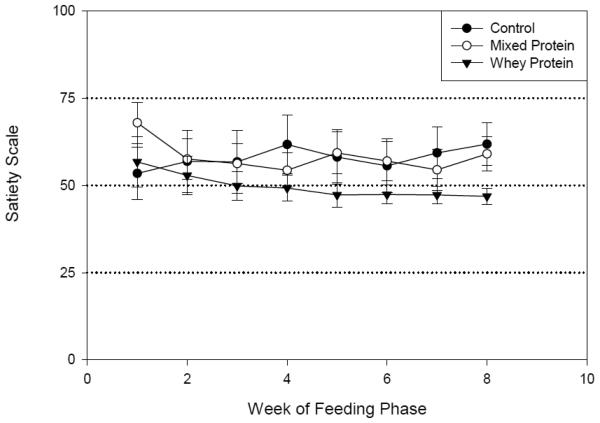
Average satiety responsesduring the feeding phase showed that even on a reduced calorie diet most subjects reported “fullness” (>50 mm). Average weekly responses did not show a significant difference between the groups. However, the WPgroup reported the least variability during the entire 8 weeksas shown in Figure 4. By the week 8, a trend (p=0.08) toward differences between the WP and the other treatments was observed at the ‘Neither hungry nor full’ level.
Fig. 4.
The weekly average satiety score during the eight week feeding phase. The Labeled Affective Magnitude (LAM) Scale consisted of labeled terms on a 100 mm scale with 0 mm being ‘Extremely Hungry’, 50 mm being ‘Neither Hungry nor Full’, and 100 mm being ‘Extremely Full’. Data shown are means (± SEM). Week 8 difference between groups p = .08.
4. Discussion
In our study design incorporating custom reduced energy diets, all subjects showed similar weight loss and circulating hormone activity even though dietary composition was significantly different. Therefore, the hypothesis that a whey protein diet would result in greater weight loss and improved body compositioncompared to standard weight loss diets was rejected in this study. Although the measured outcomes were not statistically different, there was a trend in the WP group toward greater weight loss, greater fat loss, and reduced blood pressure compared to the other treatment groups. There was no evidence to support a RAAS mediated effect as a possible mechanism to explain the blood pressure changes in the WP group.
Some previous weight loss studies [42,43] have reported decreases in plasma renin and ACE activities as well as aldosterone concentrations when subjects have achieved greater than 3% weight loss. It is important to note thatall of our subjects achieved greater than 3% weight loss, and showedno significant differencesamong the three diets in RAAS activity as assessed by renin and ACE activity, and aldosterone level. Therefore, our results fail to identify a weight loss mechanism related to a decrease in circulating ACEactivity and the consumption of whey protein at the levels provided (15% of energy). In a previous study, when a high protein diet (2.0g/kg/day) was fed in a weight maintenance diet to normal weight subjects, increased plasma renin activity and aldosteronewere observed[44]. Two of our treatments were high protein diets, which may have contributed to sustaining RAAS measures even during weight loss. However, the CD group did not show any significant decrease in RAAS with the comparatively lower protein levels. It is possible that the sample size of each group in the pilot study was not sufficient to identify a RAAS mediating effect. It is important to note that dietary sodium and potassium differences between groups may have confounded examination of this issue. Additionally, we only examined circulating RAAS biomolecules. Therefore, we cannot rule out the possibility of RAAS related adipose and muscle-specific mechanisms related to weight loss under these conditions.
Consumption of prepared milk proteins in other studies has resulted in decreased blood pressure in moderately hypertensive subjects[28,29,45]. All of the subjects in our study were normotensive; therefore, no elevated RAAS levels were observed at baseline, which might have negated the ability to demonstrate a RAAS mediating effect by whey protein during the course of the study. At the end ofour study, subjects in the WP group showed a significant decrease in systolic blood pressure compared to the CD group with no significant change in RAAS activity.
Changes in circulating ACE activity did not correlate with the observed decrease in blood pressure. In contrast to our results, Harp et al reported a decrease in blood pressure and ACE activity with weight loss[42]. However, Harp et al also observed that decreases in plasma renin activity and aldosterone were more strongly associated with weight loss than was ACE activity. A trend of decreasing aldosterone concentration (p = 0.08) was observed in the CD and WP groups compared to the MP group. However, the similar decrease in aldosterone concentration in the CD and WP groups was not associated with similar weight loss between groups.
High protein reduced energy diets have been shown to maintain lean tissue while promoting decreases in fat tissue[5]. In this pilot study,there were no significant differences between groups for change in lean tissue. On average, the MP group gained lean tissue and the WP group lost lean tissue. Because of the high availability of essential amino acids and BCAA, the observed decrease in lean tissue for the WP group was surprising. The measure of lean tissue loss in the WP group was comparable to the measure of lean tissue loss in the CD group, yet the WP group lost more weight than the CD group. Since exercise was not part of the protocol, these changes in lean tissue reflect the diet effect on lean tissue without an exercise program. If an exercise program were added to the weight loss protocol, a greater retention of lean tissue might be anticipated[46].
Trends of decreased fat tissue were observed with the MP and WP diets compared to the CD diet. These results agree with earlier studies by Layman et althat demonstrated improved body composition among those subjects assigned to a high protein diet [5,10]. Differences in body composition between treatments were most clearly observed in measures of the leg and gynoid regions. It is not clear whether or to what extent the difference in amino acid composition between treatmentswas related to the differences observed in fat loss and lean tissue gain between the two high protein groups.
The strengths of this pilot study include the control feeding and parallel design, which provided opportunity to evaluate the effect of the different diets. The three treatments offered comparison between a standard diet and two variations of a high protein diet. The comparison between high protein diets allowed the assessment of outcome differences when different sources of protein are favored in the diet composition.
One limitation of this pilot study was small sample size. A parallel-arms design with 6 subjects in each arm was 80% power to detect an effect size larger than 1.8 between groups, at the .05 level. This corresponds to a difference between groups of at least 5.6 kg in weight, 5.6 kg in total body fat mass, or 6.5 ng/L in renin. Therefore, the hypothesis of this study was rejected since these differences were not observed.
These preliminary results based on our feasibility study establish the basis for further studies regarding the effectiveness of incorporating whey protein into custom reduced-energy diets for weight loss at midlife. The trend in the WP group toward greater weight loss, greater fat loss, and reduced blood pressure compared to the other treatment groups warrants further investigation in a larger sample.
Acknowledgement
Funding for this work was provided by grants from the National Center for Research Resources, National Institutes of Health, M01-RR00400, and from USDA National Needs Graduate Fellowship Competitive Grant No. 2005-38420-15786 from the National Institute of Food and Agriculture, and from Next Proteins, LLC.
Abbreviations
- ACE
Angiotensin Converting Enzyme
- AUC
Area Under the Curve
- BCAA
Branch Chain Amino Acids
- BMI
Body Mass Index
- DXA
Dual Energy X-ray Absorptiometry
- GCRC
General Clinical Research Center
- HOMA IR
Homeostatic Model Assessment Insulin Resistance
- LAM
Labeled Affective Magnitude
- RAAS
Renin-Angiotensin Aldosterone System
- REE
Resting Energy Expenditure
- SEM
Standard Error of the Mean
- VAS
Visual Analogue Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary data presented as a poster at the North American Research Conference on Complementary and Alternative Medicine, May 2009.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and Trends in Obesity Among US Adults, 1999 – 2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Hu F, Willett W, Li T, Sampfer M, Colditz G, Manson J. Adiposity as compared with the physical activity in predicting mortality among women. N Eng J Med. 2004;351:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 4.Baba NH, Sawaya S, Habbal Z, Azar S, Hashim SA. High Protein vs High Carbohydrate Hypoenergetic Diet for the Treatment of Obese Hyperinsulinemic Subjects. Inter J Obes. 1999;23:1202–1206. doi: 10.1038/sj.ijo.0801064. [DOI] [PubMed] [Google Scholar]
- 5.Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, Christou DD. A Reduced Ratio of Dietary Carbohydrate to Protein Improves Body Composition and Blood Lipid Profiles during Weight Loss in Adult Women. J Nutr. 2003;133:411–417. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- 6.Clifton PM, Keogh JB, Noakes M. Long-term effects of a high-protein weight lossDiet. Am J Clin Nutr. 2008;87:23–29. doi: 10.1093/ajcn/87.1.23. [DOI] [PubMed] [Google Scholar]
- 7.Marmonier C, Chapelot D, Louise-Sylvestre J. Effects of macronutrient content and energy density of snacks consumed in a satiety state on the onset of the next meal. Appetite. 2000;30:161–168. doi: 10.1006/appe.1999.0302. [DOI] [PubMed] [Google Scholar]
- 8.Halton TL, Hu FB. The effects of high protein diets on the thermogenesis, satiety, and weight loss: A critical review. J Am Col Nutr. 2004;23:373–85. doi: 10.1080/07315724.2004.10719381. [DOI] [PubMed] [Google Scholar]
- 9.Weigle D, Breen P, Matthys C, et al. A High Protein Diet Induces Sustained Reductions in Appetite, Ad Libitum Caloric Intake, and Body Weight Despite Compensatory Changes in Diurnal Plasma Leptin and Ghrelin Concentrations. Am J Clin Nutr. 2005;82:41–48. doi: 10.1093/ajcn.82.1.41. [DOI] [PubMed] [Google Scholar]
- 10.Layman DK, Evans EM, Erickson D, Seyler J, Weber J, Bagshaw D, Griel A, Psota T, et al. A Moderate Protein Diet Produces Sustained Weight Loss and Long-term Changes in Body Composition and Blood Lipids in Obese Adults. J Nutr. 2009;139:514–521. doi: 10.3945/jn.108.099440. [DOI] [PubMed] [Google Scholar]
- 11.Apolzan J, Carnell N, Mattes R, Campbell W. Inadequate Dietary Protein Increases Hunger and Desire to Eat in Younger and Older Men. J Nutr. 2007;137:1478–1482. doi: 10.1093/jn/137.6.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An Increase in Dietary Protein Improves the Blood Glucose Response in Persons with Type 2 Diabetes. Am J Clin Nutr. 2003;78:734–741. doi: 10.1093/ajcn/78.4.734. [DOI] [PubMed] [Google Scholar]
- 13.Layman DK, Shiue H, Sather C, Erickson DJ, Baum J. Increased Dietary Protein Modifies Glucose and Insulin Homeostasis in Adult Women during Weight Loss. J Nutr. 2003;133:405–410. doi: 10.1093/jn/133.2.405. [DOI] [PubMed] [Google Scholar]
- 14.Schaafsma G. Health Issues of Whey Protein: 1. Protection of Lean Body Mass. Current Topics in Nutraceutical Research. 2006;4:113–122. [Google Scholar]
- 15.Layman DK, Baum JI. Dietary Protein Impact on Glycemic Control during Weight Loss. J Nutr. 2004;134:968S–973S. doi: 10.1093/jn/134.4.968S. [DOI] [PubMed] [Google Scholar]
- 16.Cribb PJ. U.S. Whey Proteins in Sports Nutrition. Applications Monograph Sports Nutrition. US Dairy Export Council. 2005;4:1–12. [Google Scholar]
- 17.Nilsson M, Holst JJ, Bjorck IM. Metabolic Effects of Amino Acid Mixtures and Whey Protein in Healthy Subjects: Studies Using Glucose-Equivalent Drinks. Am J Clin Nutr. 2007;85:996–1004. doi: 10.1093/ajcn/85.4.996. [DOI] [PubMed] [Google Scholar]
- 18.Frestedt JL, Zenk JL, Kuskowski MA, Ward LS, Bastian ED. A Whey-Protein Supplement Increases Fat Loss and Spares Lean Muscle in Obese Subjects: A Randomized Human Clinical Study. Nutrition and Metabolism. 2008;5:1–8. doi: 10.1186/1743-7075-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treyzon L, Chen S, Hong K, Yan E, Carpenter CL, Thames G, Bowerman S, Wang H, et al. A Controlled Trial of Protein Enrichment of Meal Replacements for Weight Reduction with Retention of Lean Body Mass. Nutrition Journal. 2008;7:23. doi: 10.1186/1475-2891-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engeli S, Negrel R, Sharma A. Physiology and Pathophysiology of the adipose tissue reninangiotensin system. Hypertension. 2000;35:1270–1277. doi: 10.1161/01.hyp.35.6.1270. [DOI] [PubMed] [Google Scholar]
- 21.Gorzelniak K, Engeli S, Janke J, Luft F, Sharma A. Hormonal regulation of the human adipose tissue renin-angiotensin system: Relationship to obesity and hyper tension. J Hyper. 2002;20:965–973. doi: 10.1097/00004872-200205000-00032. [DOI] [PubMed] [Google Scholar]
- 22.Engeli S, Bohnke J, Grozelniak K, et al. Weight Loss and the Renin-Angiotensin-Aldosterone System. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 23.Enalapril in Essential Hypertension: A Comparative Study with Propranolol. Enalapril in Hyperension Study Group (UK) Brit J Clin Pharm. 1984;18:51–56. doi: 10.1111/j.1365-2125.1984.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGrath B, Matthews P, Louis W, et al. Double-blind Study of Dilevalol and Captopril, Both in Combination with Hydrochlorothiazide, in Patients with Moderate to Severe Hypertension. J Cardio Pharm. 1990;16:831–838. doi: 10.1097/00005344-199011000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Santos EL, Souza K, Guimaraes PB, Reis FC, Silva SM, Costa-Neto CM, Luz J, et al. Effect of Angiotensin Converting Enzyme Inhibitor Enalapril on Body Weight and Composition in Young Rats. International Immunopharmacology. 2008;8:247–253. doi: 10.1016/j.intimp.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Pihlanto-Leppala A, Koskinen P, Piilola K, Tupasela T, Korhonen H. Angiotensin I-Converting Enzyme Inhibitory Properties of Whey Protein Digests: Concentrations and Characterizations of Active Peptides. J Dairy Research. 2000;67:53–64. doi: 10.1017/s0022029999003982. [DOI] [PubMed] [Google Scholar]
- 27.Kawase M, Hashimoto H, Hosoda M, Morita H, Hosono A. Effect of Administration of Fermented Milk Containing Whey Protein Concentrate to Rats and Healthy Men on Serum Lipids and Blood Pressure. J Dairy Science. 2000;83:255–263. doi: 10.3168/jds.S0022-0302(00)74872-7. [DOI] [PubMed] [Google Scholar]
- 28.Seppo L, Jauhiainen T, Poussa T, Korpela R. A Fermented Milk High in Bioactive Peptides has a Blood Pressure-Lowering Effect in Hypertensive Subjects. Am J Clin Nutr. 2003;77:326–330. doi: 10.1093/ajcn/77.2.326. [DOI] [PubMed] [Google Scholar]
- 29.Aihara K, Kajimoto O, Hirata H, Takahashi R, Nakamura Y. Effect of Powdered Fermented Milk with Lactobacillus helveticus on Subjects with High-Normal Blood Pressure or Mild Hypertension. Journal of Am. Coll Nutr. 2005;24:257–265. doi: 10.1080/07315724.2005.10719473. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y, Skurk T, Hennig M, Hauner H. Effect of a milk drink supplemented with whey peptides in patients with mild hypertension. European J Nutr. 2007;46:21–27. doi: 10.1007/s00394-006-0625-8. [DOI] [PubMed] [Google Scholar]
- 31.Sturrock ED, Natesh R, van Rooyen JM, Acharya KR. Structure of angiotensin I-converting enzyme. Cell Mol Life Sci. 2004;61:2677–2686. doi: 10.1007/s00018-004-4239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muniyappa R, Sihoon L, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J PhysiolEndocrinolMetab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 33.Rocha E, Alves V, Fonesca R. Indirect calorimetry: Methodology, instruments, and clinical application. Current Opinion in Clinical Nutrition and Metabolic Care. 2006;9:247–256. doi: 10.1097/01.mco.0000222107.15548.f5. [DOI] [PubMed] [Google Scholar]
- 34.Schutz H, Cardello A. A Labeled Affective Magnitude (LAM) Scale for Assessing Food Liking/Disliking. J Sensory Studies. 2001;16:117–159. [Google Scholar]
- 35.Cardello A, Schutz H. Research Note Numerical Scale-Point Locations for Constructing the LAM (Labeled Affective Magnitude) Scale. J Sensory Studies. 2004;19:341–346. [Google Scholar]
- 36.Morin LG, Prox J. Single Glucose Oxidase-Peroxidase Reagent for Two-Minute Determination of Serum Glucose. ClinChem. 1973;19:959–962. [PubMed] [Google Scholar]
- 37.Hurst P, Lovell-Smith CJ. Optimized assay for serum angiotensin converting enzyme activity. ClinChem. 1981;27:2048–52. [PubMed] [Google Scholar]
- 38.Miles LE. Properties, variants, and applications of the immunoradiometric assay method. International Journal of Clinical and Laboratory Research. 1975;5:59–72. doi: 10.1007/BF02910016. [DOI] [PubMed] [Google Scholar]
- 39.Yalow R, Berson S, Odell WD, Daughaday WH. Principles of Competitive Protein Binding Assays. JB Lippincott Co.; 1971. [Google Scholar]
- 40.Williams J, Wells J, Wilson C, Haroun D, Lucas A, Fewtrell M. Evaluation of lunar prodigy dual-energy X-ray absorptiometry for assessing body composition in healthy persons and patients by comparison with the criterion 4-component model. Am J ClinNutr. 2006;83:1047–54. doi: 10.1093/ajcn/83.5.1047. [DOI] [PubMed] [Google Scholar]
- 41.Campbell L, Wallman K, Green D. The effects of intermittent exercise on physiological outcomes in an obese population: Continuous versus interval walking. J of Sports SciMed. 2010;9:24–30. [PMC free article] [PubMed] [Google Scholar]
- 42.Harp JB, Henry SA, DiGirolamo M. Dietary Weight Loss Decreases Serum Angiotensin Converting Enzyme Activity in Obese Adults. Obesity Research. 2002;10:985–990. doi: 10.1038/oby.2002.134. [DOI] [PubMed] [Google Scholar]
- 43.Ho JT, Keogh JB, Bornstein SR, Ehrhart-Bornstein M, Lewis JG, Clifton PM, Torpy DJ. Moderate Weight Loss Reduces Renin and Aldosterone but does not Influence Basal or Stimulated Pituitary Adrenal Axis Function. Horm Metab Res. 2007;39:694–699. doi: 10.1055/s-2007-985354. [DOI] [PubMed] [Google Scholar]
- 44.Daniels BS, Hostetter TH. Effects of dietary protein intake on vasoactive hormones. Am J Physiol. 1990;258:R1095–R1100. doi: 10.1152/ajpregu.1990.258.5.R1095. [DOI] [PubMed] [Google Scholar]
- 45.Hata Y, Yamamoto M, Ohni M, Nakajima K, Nakamura Y, Takano T. A Placebo-Controlled Study of the Effect of Sour Milk on Blood Pressure in Hypertensive Subjects. Am J Clin Nutr. 1996;64:767–771. doi: 10.1093/ajcn/64.5.767. [DOI] [PubMed] [Google Scholar]
- 46.Layman D, Evans E, Baum J, Seyler J, Erickson D, Boileau R. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. 2005;135:1903–1910. doi: 10.1093/jn/135.8.1903. [DOI] [PubMed] [Google Scholar]