Abstract
Cyclic stretching and growth factors like TGF-β have been used to enhance extracellular matrix (ECM) production by cells in engineered tissue to achieve requisite mechanical properties. In this study, the effects of TGF-β1 were evaluated during long-term cyclic stretching of fibrin-based tubular constructs seeded with neonatal human dermal fibroblasts. Samples were evaluated at 2, 5 and 7 weeks for tensile mechanical properties and ECM deposition. At 2 weeks, +TGF-β1 samples had 101% higher collagen concentration but no difference in ultimate tensile strength (UTS) or modulus compared to −TGF-β1 samples. However, at weeks 5 and 7, −TGF-β1 samples had higher UTS/modulus and collagen concentration, but lower elastin concentration compared to +TGF-β1 samples. The collagen was better organized in −TGF-β1 samples based on picrosirius red staining. Western blot analysis at weeks 5 and 7 showed increased phosphorylation of ERK in −TGF-β1 samples, which correlated with higher collagen deposition. The TGF-β1 effects were further evaluated by western blot for αSMA and SMAD2/3 expression, which were 16-fold and 10-fold higher in +TGF-β1 samples, respectively. The role of TGF-β1 activated p38 in inhibiting phosphorylation of ERK was evaluated by treating samples with SB203580, an inhibitor of p38 activation. SB203580-treated cells showed increased phosphorylation of ERK after 1 hour of stretching and increased collagen production after 1 week of stretching, demonstrating an inhibitory role of activated p38 via TGF-β1 signaling during cyclic stretching. One advantage of TGF-β1 treatment was the 4-fold higher elastin deposition in samples at 7 weeks. Further cyclic stretching experiments were thus conducted with constructs cultured for 5 weeks without TGF-β1 to obtain improved tensile properties followed by TGF-β1 supplementation for 2 weeks to obtain increased elastin content, which correlated with a reduction in loss of pre-stress during preconditioning for tensile testing. This study shows that a sequential stimulus approach -- cyclic stretching with delayed TGF-β1 supplementation -- can be used to engineer tissue with desirable tensile and elastic properties.
Keywords: Tissue Engineering, Tissue growth and remodeling, TGF-b, ERK, p38, Cyclic stretching, Collagen deposition
1. Introduction
Cardiovascular disease is the leading cause of death in Western countries with over 450,000 coronary bypass and 95,000 valvular replacement surgeries performed annually in the United States alone (Thom et al. 2006). Tissue-engineered vascular and valvular grafts have been proposed as a promising solution for replacement surgery (Isenberg et al. 2006; Yacoub and Nerem 2007). Under physiological cyclic loading, both tensile and elastic properties are important for in vivo success of engineered tissue constructs. Mechanical conditioning (primarily stretching) has been studied both for vascular and valvular graft as a mean to improve tensile properties of engineered tissue prior to implantation (Bilodeau and Mantovani 2006). Growth factors like TGF-β1 have also been shown to improve deposition of collagen (Clark et al. 1995; Neidert et al. 2002; Grouf et al. 2007) and elastin (Kucich et al. 2002; Long and Tranquillo 2003; Ross and Tranquillo 2003), which are major ECM components that provide tensile and elastic properties, respectively.
To date, most studies on the effects of TGF-β1, whether with native or engineered 3D tissue (Tuan et al. 1996; Grouf et al. 2007; Merryman et al. 2007) or cells in 2D culture (Lindahl et al. 2002; Bastiaansen-Jenniskens et al. 2008), have been limited to short duration (several days up to 3 weeks). These studies are meaningful to understand the short-term response of cells to growth factors. However, engineering a completely biological tissue in vitro currently requires long-term culture (often greater than 5 weeks) with additional mechanical stimulation, such as cyclic stretching, to develop the desired tissue mechanical and organizational properties. To date, no studies have investigated the translation of the short-term effects of TGF-β1 into long-term tissue development, with or without cyclic stretching.
TGF-β1 treatment of fibroblasts generally leads to transformation of the cells into α-smooth muscle actin (αSMA)-expressing myofibroblasts, with increased collagen production. In both native and engineered tissue, higher αSMA expression and collagen synthesis have been reported after 2-3 weeks (Grouf et al. 2007; Merryman et al. 2007). For a tissue-engineered construct, the organization of the deposited collagen is equally important in order to attain the desired tensile properties. Several groups have studied the effects of transformation of fibroblasts to myofibroblasts on collagen cross-linking and organization (Poobalarahi et al. 2006; Bastiaansen-Jenniskens et al. 2008)
In our previous study, we showed that incremental strain amplitude cyclic stretching (ICS) of fibrin-based engineered tissue fabricated with neonatal human dermal fibroblasts (nHDF) led to significantly higher ultimate tensile strength (UTS) and modulus compared to traditional constant strain amplitude cyclic stretching to which cells apparently adapt (Syedain et al. 2008). We also demonstrated that higher collagen deposition in the ICS samples correlated with increased phosphorylation of ERK. Regulation of other signaling proteins like SMAD-2 (Massague 2000; Massague et al. 2005), Akt (Kuang et al. 2007; Bujor et al. 2008) and p38 (Sato et al. 2002; Kuang et al. 2007) have also been shown to be important for collagen and elastin expression by fibroblasts.
Understanding the effects of mechanical or chemical conditioning is important for advancements in tissue engineering. This study was conducted to understand the long-term effects of TGF-β1 during cyclic stretching of tubular constructs prepared from nHDF in fibrin gel and the roles of ERK, p38, and SMAD-2. We examined the effects of TGF-β1 on collagen and elastin deposition and cell proliferation after 2 weeks of static culture. The constructs were then mounted in a cyclic distension bioreactor and conditioned for 3-5 weeks. Studies were conducted both in the presence and absence of TGF-β1 for the entire duration. In addition, subsequent studies were conducted to optimize the duration of stretching and TGF-β1 supplementation to fabricate a tissue with increased elastin content as well as physiological UTS and modulus.
2. Materials and Methods
2.1. Cell Culture
Neonatal human dermal fibroblasts (nHDFs, Clonetics, Walkersville, MD) were maintained in DMEM/F12 culture medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (HyClone, Logan, UT), 100 U/ml penicillin (Invitrogen), 100 μg/ml streptomycin (Invitrogen), and 2.5 μg/ml amphotericin-β (Invitrogen) . Cells were passaged at 100% confluency and harvested for use from passage 7-9.
2.2. Tubular Construct Preparation and Culture
The details of sample preparation and culture have been previously described (Syedain et al. 2008). The final concentrations of the nHDF suspension were 6.6 mg/ml fibrinogen, 0.4 U/ml thrombin, 3.6mM Ca2+, and 500,000 cells/ml. Constructs were cast with initial length of 12.5 mm, thickness 3.4 mm and internal diameter of 8 mm. Constructs were cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 μg/ml insulin, 50 μg/ml ascorbic acid, 2.5 μg/ml ε-amphotericine-β, and ±1 ng/ml activated TGF-β1 (R&D Systems, Minneapolis MN, hereafter referred to as +TGF-β and −TGF-β). As before (Syedain et al. 2008), constructs were cultured for two weeks on the mandrel, after which they were transferred to a bioreactor for cyclic stretching.
2.3. Cyclic Distension (CD) Bioreactor
Constructs were transferred onto a distensible mandrel in a custom-built bioreactor described previously in detail (Isenberg and Tranquillo 2003). All constructs (stretched and static controls) were identically mounted on the latex tubes in the bioreactor, and the medium changed was performed at the same time. Constructs were randomly assigned to be stretched or serve as static controls. For all studies, a frequency of 0.5 Hz with 12.5% duty cycle was used, corresponding to a 0.25 sec stretch time in a 2 second cycle. Cyclic distension experiments were conducted with strain amplitude increased in 4 equal steps from 5% to 15% over 5 weeks. The medium in the CD bioreactor was changed 3 times per week with only 70% of the medium replaced.
In a separate treatment group (See Supplementary Figure 1 for illustration of the study design), denoted *TGF-β, after 3 weeks of cyclic stretching, the constructs were cultured for 2 more weeks in medium altered to include 1 ng/ml TGF-β1 and exclude ascorbic acid, which has been reported to inhibit TGF-β1 induced elastogenesis (Davidson et al. 1997).
After harvest, each construct was divided into four equal-length sections and then slit longitudinally to form four tissue strips, one for uniaxial tensile testing followed by biochemical analysis, and the others for western blotting, DNA quantification, and histology.
2.4. Uniaxial Tensile Testing
One tissue strip from each construct was tested for tensile properties in the circumferential direction as previously described (Syedain et al. 2008). The thickness of each strip was measured by laying the tissue on a flat surface and contacting it with a vertical displacement transducer connected with a 1 mm wide flat-end tip attached to a load cell. The tissue thickness was defined when a contact force of 5 mN was registered. Following 6 cycles of 0-10% strain at 2 mm/min, strips were stretched to failure at the same rate. True strain was calculated based on the change in length of the tissue over time. The stress was calculated as force divided by the initial cross-sectional area. A modulus (E) was determined by linear regression of the linear region of the stress-strain curve just prior to failure, which defined the UTS.
2.5. Histology
Tissue strips were fixed in 4% paraformaldehyde, infiltrated with a solution of 30% sucrose and 5% DMSO, frozen in OCT (Tissue-Tek), and sectioned into 9 μm cross-sections. Sections were then stained with Lillie's trichrome, Verhoeff's stain and picrosirius red stain. Images were taken using a color CCD camera from an Olympus IX70 microscope at 10x magnification. For picrosirius red staining, images were taken with the samples placed between crossed plane polarizers.
Elastin immunostaining was performed by blocking tissue section slides with 5% donkey serum, followed by elastin antibody (Elastin Inc) at 1:200 dilution and secondary cye2 antibody (Jackson Immuno, Inc) at 1:200 dilution. The images were taken on a fluorescent microscope using the rhodamine channel. For all staining, a sheep pulmonary valve leaflet was used as a positive control.
2.6. Collagen, Elastin, and Cell Quantification
Collagen content was quantified with the hydroxyproline assay assuming 7.46 mg of collagen per 1 mg of hydroxyproline (Stegemann and Stalder 1967). The insoluble elastin content was quantified using a ninhydrin-based assay as previously described (Starcher and Galione 1976). Tissue strip volume was calculated using the measured length, width, and thickness of the strips (as described above in uniaxial testing) and used to calculate collagen concentration. DNA content was quantified with a modified Hoechst assay (Robinson et al. 2008). Cell numbers were obtained from DNA contents assuming 7.6 pg of DNA per cell. Cell concentrations were calculated as the number of cells per unit volume using the dimensions of the strip.
2.7. Western Blotting Analysis
Tissue strips were flash frozen in liquid nitrogen at harvest. The proteins were extracted as previously described (Syedain et al. 2008). For each sample, 40 μg of total protein was boiled in a reducing sample buffer and separated by SDS-PAGE. The proteins were transferred to nitrocellulose (Whatman) using a wet transfer buffer (10% methanol, 2.2 g/L CAPS, pH 11). The blot was incubated in blocking solution (5% dry milk, 0.1% Tween-20 in PBS) for one hour and then incubated with primary antibody overnight at 4°C (mouse monoclonal phospho-ERK antibody p-Thr202/p-Tyr204, Cell Signaling, 1:1000; rabbit monoclonal phosphor-SMAD2 antibody p-Ser465/467, Cell Signaling, 1:1000). The blot was then probed with HRP-conjugated secondary anti-IgG (Amersham) at a dilution of 1:10000 and developed using enhanced chemiluminescence. The blot was then stripped of antibodies using Restore Plus (Pierce) and reprobed (rabbit polyclonal ERK antibody, Cell Signaling, 1:1000; rabbit polyclonal SMAD2/3 antibody, Cell Signaling, 1:1000). The phosphorylated protein to total protein ratio was determined by densitometry of scanned auto-radiograms.
2.8. p38 Inhibitor Study Using the FlexCell System
nHDF were mixed with the fibrin-forming solution as described above, and 1.5 ml of the cell-populated gel was cast in each well of a FlexCell Tissue-Train plate (Flexcell Int., Hillsborough, NC). The gels were allowed to compact in response to the nHDF traction forces for 24 hr, after which the medium was changed to either DMEM with 10% FBS alone (n=8) or containing 1 ng/ml of TGF-β1 (n=8) and/or 5μM of the p38 inhibitor SB203580 (n=8), and the samples were stretched using the FlexCell FX-4000 tension system at 5% stretch for 4 days followed by 10% at 0.5 Hz up to 7 days. Static controls of samples identically cast in the FlexCell plate were kept in the incubator for the same duration. Half of the samples for each treatment were harvested after 1 hr of stretching for western blot analysis, while the remaining samples were incubated for 1 week with 3 medium changes. Each sample was split for collagen / cell quantification and protein extraction for western blotting, as described above.
2.9. Statistics
For all experiments, n= 6-8 for each treatment and time point. Statistical difference between groups was determined using one-way ANOVA with the Tukey post hoc test in GraphPad Prism® software for Windows. Any reference to a difference implies statistical significance at the level p < 0.05. In all cases where the error bars (plus or minus standard deviations) are non-overlapping, the differences are significant; hence, for clarity, no symbols are used. In cases where error bars are overlapping and the difference is statistically significant, paired symbols are used to indicate the difference.
3. Results
3.1. TGF-β1 supplemented samples possessed inferior mechanical properties
Constructs were evaluated after 2 weeks of static culture, when they were mounted in the bioreactor, and after 3 and 5 weeks of ICS (5 and 7 week of total culture time). Consistent with our previously reported study using the same tubular construct model (Syedain et al. 2008), after 7 weeks of culture, +TGF-β and −TGF-β samples had the same thickness (355±80 μm and 350±20 μm, respectively). As shown in Fig. 1a&b, at 2 weeks, there was no difference in tensile properties, even though +TGF-β samples had 101% higher collagen concentration (Fig. 1c). By week 5, both UTS and modulus were significantly higher for −TGF-β samples, and by week 7, the UTS and modulus were 192% and 107% higher, respectively, for −TGF-β samples. In addition, the UTS (1382±298 kPa) and modulus (2508±595 kPa) were comparable to sheep pulmonary valve leaflet values, also measured in the circumferential (aligned collagen) direction (UTS = 1472±445 kPa, modulus = 3221±711 kPa). See Supplementary Figure 2 for a representative stress-strain curve and a polarized light image of a construct showing circumferential alignment of the ECM fibers.
Figure 1.
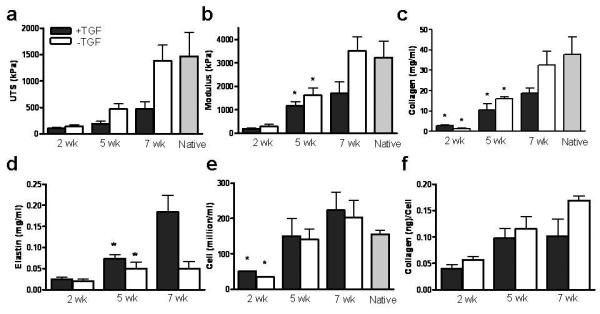
Time course study of fibrin-based constructs with and without TGF-β1 supplementation during incremental strain amplitude cyclic stretching a: UTS, b: Modulus, c: Collagen concentration, d: Elastin concentration, e: Cell concentration, and f: Collagen/Cell concentrations after 2, 5 & 7 weeks of incubation. In all cases where error bars are not overlapping, the means are different (p<0.05); for groups with overlapping error bars, a difference (p<0.05) between means is shown with paired symbols.
3.2. Improvement in mechanical properties of samples cultured without TGF-β1 correlated with increased collagen deposition and organization
Figure 2 shows trichrome and picrosirius staining under polarized light of samples at 2, 5 and 7 weeks. At both weeks 5 and 7, the picrosirius red stain was brighter for −TGF-β samples (Fig. 2). In trichrome staining, green color indicates collagen, which was visible in cross-sections of all samples at 5 and 7 weeks.
Figure 2.
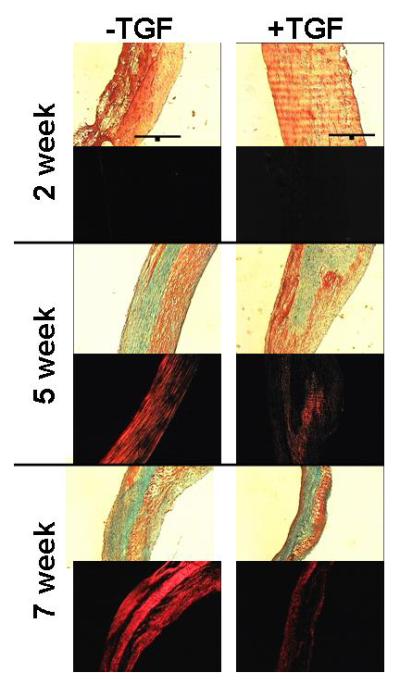
Trichrome and picrosirius red staining of constructs after 2, 5 and 7 weeks. In trichrome stained section, collagen stains green and fibrin (and other proteins) stain red. In picrosirius red stained sections, brighter intensity of red indicates more mature collagen. All images were taken at same magnification with a 200 μm scale bar (black line) shown in the 2 week trichrome images. The lumen of the constructs is on the left side of all sections. Collagen content increased over time as seen with increased green color using trichrome. The collagen maturation was greater in −TGF-β samples as seen with brighter red color using picrosirius red.
Although +TGF-β samples had 101% higher collagen concentration (2.85±0.2 mg/ml) at 2 weeks, −TGF-β samples had a 49% and 74% higher collagen concentration (18.7±2.4 mg/ml and 32.6±6.8 mg/ml) at 5 and 7 weeks, respectively. In comparison, the valve leaflet value was 37.8±8.4 mg/ml, not different from −TGF-β samples at 7 weeks.
Figure 1e shows the cell concentration, which showed no difference between treatment groups at both weeks 5 and 7. Hence, at 7 weeks, there was a greater collagen deposited per cell (by 66%) in −TGF-β samples (Fig. 1f).
3.3. TGF-β1 treatment increased elastin deposition
As shown in Fig. 1d, after 5 and 7 weeks, elastin content was 45% (0.07±0.01 mg/ml) and 366% (0.18±0.04 mg/ml) higher in +TGF-β samples, respectively.
3.4. TGF-β1 increased αSMA expression and phosphorylation of SMAD-2
Figure 3a shows a western blot for αSMA expression in samples at week 5. All +TGF-β samples showed a 16-fold higher αSMA expression. Upon harvest, +TGF-β samples coiled, a behavior not seen in −TGF-β samples (Fig. 3b).
Figure 3.
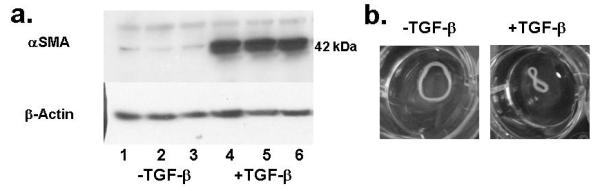
a: Western blot of αSMA expression by nHDF in constructs at week 5. β-actin bands indicate the same protein loading in all lanes, implying TGF-β1 induced expression of αSMA. b: constructs in PBS at week 5 after removal from latex tube of the cyclic distension bioreactor shows curling of +TGF-β samples.
Western blotting revealed a 10-fold higher expression of pSMAD-2 in +TGF-β samples at week 5 (Fig. 4a). The results show that the TGF-β1 dose administered to the samples had induced both phenotypic (αSMA-expressing cells) and signaling changes (increased pSMAD-2 expression).
Figure 4.
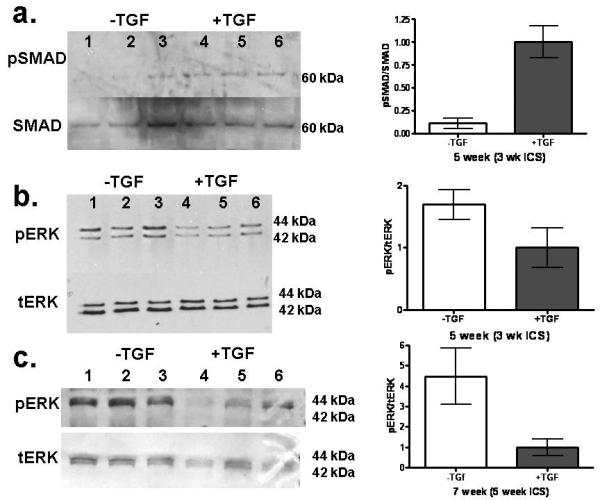
a: Western blotting for ERK 1/2 and SMAD activation of nHDF in constructs with and without TGF-β1 supplementation at 5 weeks. a: Western blot for SMAD and phosphorylated SMAD (pSMAD). Western Blot for levels of total ERK (tERK) and threonine 202/tyrosine204 phosphorylated ERK (pERK) at b: 5 weeks and c: 7 weeks. Band intensity was determined by densitometry and expressed as the ratio of phosphorylated protein band to total protein band and then normalized to the +TGF-β value. TGF-β is shown to increase fraction of SMAD that is phosphorylated but decrease the fraction of ERK that is phosphorylated. See Fig. 1 regarding paired symbols (n=3).
3.5. TGF-β1 decreased phosphorylation of ERK
Samples from week 5 and 7 were further tested for phosphorylation of ERK. The pERK signal was 70% and 350% higher in the −TGF-β samples at week 5 and 7, respectively (Fig. 4b,c). The inhibitory role of the TGF-β1 activated protein p38 on phosphorylation of ERK was evaluated by blocking activation of p38 using SB203580. When −TGF-β samples were stretched for 1 hour, pERK/tERK increased 4.4 fold compared to −TGF-β static controls. In contrast, when the +TGF-β samples were stretched, there was a modest increase compared to the +TGF-β static controls (pERK/tERK=1.2). However, when +TGF-β samples were treated with SB203580 and stretched, there was an 8.5 fold increase compared to the +TGF-β / SB203580 static controls (Fig. 5a,b). Further, collagen and cell concentrations were evaluated after 1 week of stretching and found to have a similar trend in collagen per cell as found for pERK, with more collagen produced by nHDF in −TGF-β and TGF-β/SB203580 samples compared to +TGF-β samples (Fig. 5d).
Figure 5.
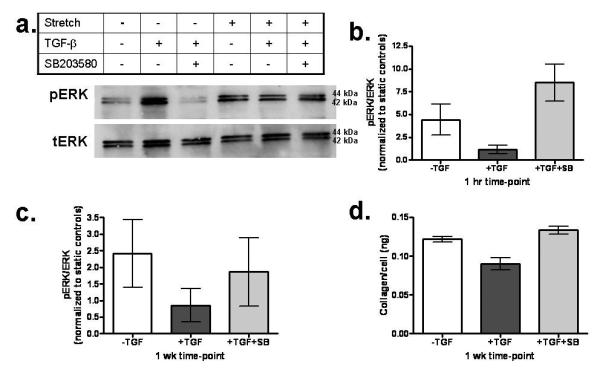
a: Western blotting for ERK activation of nHDF in constructs formed and stretched on FlexCell membranes. Treatment conditions were with and without TGF-β1 and p38 inhibitor SB203580, with and without cyclic stretching at 10% strain amplitude and 0.5 Hz. b: Ratio of pERK/tERK under cyclic stretching at 1 hour normalized to the same treatment with static incubation. c: Ratio of pERK/tERK under cyclic stretching at 1 week normalized to the same treatment with static incubation. d: Ratio of Collagen/Cell concentrations after 1 week.
3.6. Sequential cyclic stretching and TGF-β1 supplementation led to both improved mechanical properties and increased elastin deposition
The aforementioned results demonstrated strong evidence that TGF-β1 supplementation is detrimental to long-term growth and remodeling of engineered tissue during cyclic stretching, leading to inferior tensile properties. However, experiments also showed that TGF-β1 is beneficial for elastin deposition, which is an important ECM component of native tissue. Hence, subsequent experiments were conducted to combine the superior tensile properties of TC, achieved by omitting TGF-β1 during cyclic stretching, with increased elastin deposition by adding TGF-β1 at a late time-point, specifically at week 5. The subset with this treatment is denoted as the *TGF-β group.
Figure 6a shows increased elastin deposition in the 7-week samples that were treated with TGF-β only for the last 2 weeks of culture. Compared to –TGF-β samples at 7 weeks, the *TGF-β samples had 4-fold higher elastin, which corresponds to an elastin concentration (0.19±0.1 μg/ml) 10% of native tissue (1.8±0.4 μg/ml). Elastic fibers were observed in the *TGF-β samples using Verhoeff and elastin immuno-staining (Fig. 7). Tensile testing and biochemical analysis showed no decrease in tensile properties and collagen concentration by changing conditions from −TGF-β to +TGF-β for the last 2 weeks of culture, consistent with trichrome and picrosirius red staining.
Figure 6.
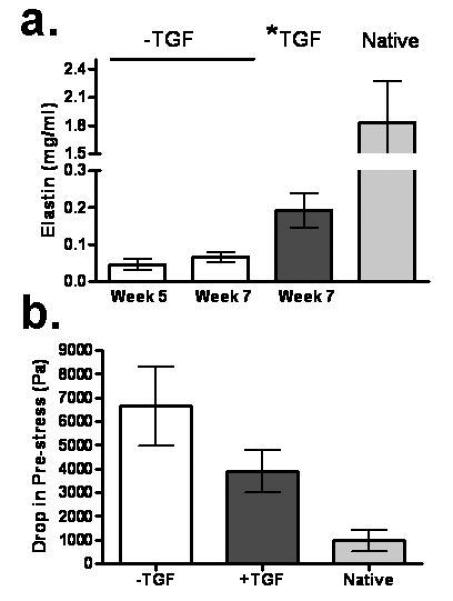
a: Elastin concentration comparison of −TGF-β samples at weeks 5 and 7 with the *TGF-β treated group and native tissue control. b: Drop in the pre-stress after 6 cycles of tensile preconditioning of the −TGF-β, *TGF-β and native tissue samples to 10% of their pre-loaded length. See Fig. 1 regarding paired symbols (n=3).
Figure 7.
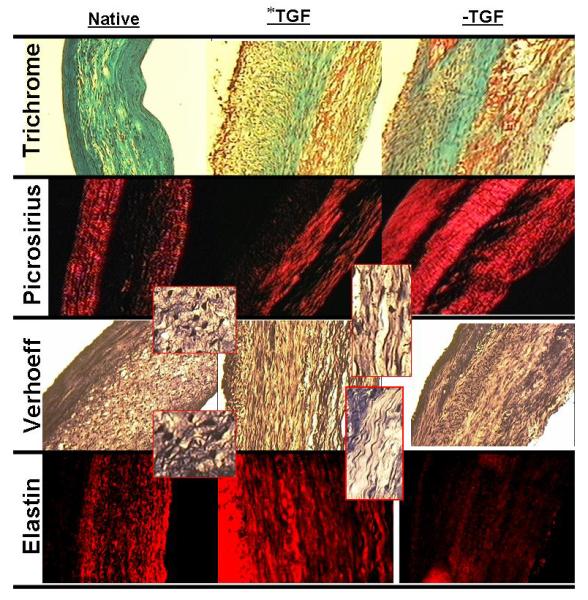
Trichrome, picrosirius red, Verhoeff, and elastin immunostaining for −TGF-β samples and *TGF-β samples at week 7 compared with native tissue control. Inset of Verhoeff stain shows magnified image of elastin fiber strands in native control and *TGF-β treated TC. All images were taken at same magnification with 200 μm scale bar (black line) shown in native trichrome images. The lumen of constructs and ventricular side of native leaflet is on the left side of all sections.
The functional role of deposited elastin was evaluated by analysis of preconditioning curves prior to stretching to failure. All samples were stretched to approximately 10% of their initial length (defined by a pre-load of 5 mN, with corresponding pre-stress that was the same for all samples because all constructs had the same thickness and length, yielding samples with the same cross-sectional area) at strain rate of 0.3%/sec for 6 cycles. The drop in stress at zero strain from the pre-stress after 6 cycles was calculated and found lowest for native tissue, followed by *TGF-β −TGF-β samples exhibiting the largest drop from the pre-stress (Fig. 6b and Supplementary Figure 3). This trend correlated with the elastin content of the three groups. Similarly, the drop in maximum stress (at 10% stretch) from cycle 1 to cycle 6 was evaluated and found to have the same trend, with the lowest drop for native tissue, followed by *TGF-β, and then −TGF-β (data not shown).
4. Discussion
Our findings showed that addition of TGF-β1 promoted collagen deposition during the first 2 weeks of static culture, consistent with ERK activation seen in nHDF monolayers treated with TGF-β1 (Pannu et al. 2007). However, when using cyclic stretching for longer culture times, which is required to attain the desired tensile properties of engineered cardiovascular tissues, the omission of TGF-β1 was more favorable for better tensile properties, collagen deposition, and collagen maturation. After 7 weeks of culture with ICS, tensile mechanical properties and collagen content were comparable to that of native pulmonary valve leaflet tissue, relevant for a tissue-engineered heart valve (Robinson et al. 2008; Syedain and Tranquillo 2009). A strong correlation between UTS and burst pressure for a fibrin-remodeled engineered vascular graft has been shown by Yao et al. (Yao et al. 2008); hence, the current UTS achieved with −TGF-β conditions should translate to burst pressure appropriate for physiological function, consistent with our recent findings (Syedain et al. 2010). The higher collagen content and higher collagen content per cell measured at week 7 in −TGF-β samples (Fig. 1) correlated with a higher ERK activation (higher pERK/tERK) measured at weeks 5 and 7 when compared to +TGF-β samples (Fig. 4), consistent with ERK activation preceding and overlapping with increased collagen deposition (Papakrivopoulou et al. 2004; Syedain et al. 2008).
However, one major benefit of using TGF-β1 was the increased elastin production. Hence, subsequent experiments were conducted where tissue samples were incubated without TGF-β1 for 5 weeks followed by the addition of TGF-β1 to induce elastin production for the last 2 weeks of cyclic stretching. The sequential approach produced tissue with substantial elastin (10% of native tissue) conferring increased elasticity without compromise of tensile mechanical properties.
The most relevant study for comparison was conducted by Grouf et al. who also seeded nHDF in fibrin gel formed as hemispherical constructs adherent to underlying tissue culture plastic (Grouf et al. 2007). They found that after 3 weeks of static culture with TGF-β1 there was a 33% increase in collagen content compared to −TGF-β samples. The results by Grouf et al. at 3 weeks are similar to our findings (Fig. 1c), as +TGF-β samples had 101% higher collagen after 2 weeks. However, in our experiments using cyclic stretching to promote tissue growth, after 5-7 weeks, the −TGF-β samples developed better mechanical properties (Fig 1a,b), more deposited collagen (Fig. 1c) and more mature collagen (Fig. 2). Although Grouf et al. used a higher TGF-β1 concentration (5 ng/ml) than in our study reported here (1 ng/ml), studies reported by other labs have shown effects of exogenous TGF-β1 on phenotypically similar valve interstitial cells at an even lower concentration (0.5 ng/ml) in the presence of the same or higher FBS concentrations than the 10% value used in our study (Walker et al. 2004; Merryman et al. 2007). Taking the nominal value of TGF-β1 in serum to be 5 ng/ml (the supplier (HyClone) reports a range of 3-13 ng/ml based on historical data), then the effective concentration of TGF-β1 in our sample culture medium was increased from 0.5 ng/ml to 1.5 ng/ml by supplementation with 1 ng/ml TGF-β1, so a response to a 3-fold increase in TGF-β1 concentration would be expected. (Supplementation with 1 ng/ml TGF-β1 would increase the concentration above serum level for any serum value below 10 ng/ml.). Studies have also shown production of TGF-β by cells when mechanically stretched (Gutierrez and Perr 1999; Yang et al. 2004); however, in this study, only in the presence of exogenous (pre-activated) TGF-β1 did significant elastin production occur, along with a 16-fold higher expression of pSMAD, indicating that any endogenously produced TGF-β (as well as the contribution from the FBS) had a negligible effect compared to the addition of the pre-activated TGF-β1. Our findings are thus consistent with previously published short-term studies and provide new insight into long-term culture using cyclic stretching.
An important difference between +TGF-β and −TGF-β samples was the intensity differences in picrosirius red staining. The brighter intensity in picrosirius red staining is reported to be correlated with higher collagen content, thicker and more aligned collagen fibers, or more cross-linked collagen (Junqueira et al. 1982; Dayan et al. 1989; Balguid et al. 2007). Picrosirius stain showed brighter staining in −TGF-β samples at both weeks 5 and 7 (Fig. 2). Others have shown that alginate gel seeded with nHDF and treated with TGF-β had lower cross-linking of collagen compared to −TGF-β samples(Bastiaansen-Jenniskens et al. 2008). Balguid et al. have shown a strong correlation between the intensity of picrosirius red stain and cross-linking in collagen deposited by myofibroblasts in a hybrid fibrin gel-polymer scaffold (Balguid et al. 2007), suggesting increased cross-linking in the −TGF-β samples.
To further explore the long-term culture effects of TGF-β1, we examined the phenotypic transformation of fibroblasts to myofibroblasts, which potentially influences cell-matrix interactions and thereby fibril bundling and maturation of deposited collagen, based on αSMA expression. We also examined the activation of the intracellular signaling proteins ERK and SMAD-2, which regulate complex but important pathways downstream of TGF-β1, involved in the production and organization of major ECM proteins like collagen and elastin, such as the MAPK and SMAD pathways (Massague 2000; Massague et al. 2005). +TGF-β samples had 16-fold higher expression of αSMA at 5 weeks. The higher αSMA expression and associated increased cell traction force may explain the construct coiling behavior upon removal form the mandrel, but further experiments are needed to distinguish this explanation from residual tension in the remodeled ECM. In addition to inhibiting stretching induced signaling through pERK, as supported by our findings, TGF-β1 may also modulate expression of collagen and other ECM genes and/or their extracellular assembly through altered cytoskeletal tension and associated motility, as reflected by altered αSMA levels (Chambers et al. 2003).
Western blot analysis showed a large increase in phosphorylation of SMAD-2 in +TGF-β samples (Fig. 4a). pERK, in contrast, was increased in −TGF-β samples (Fig 4b,c). It has been shown that pERK was obligatory for increased collagen expression when subjecting cardiac fibroblast to cyclic stretching (Papakrivopoulou et al. 2004). Though activation of SMAD-2 has not been shown to have a direct inhibition effect on ERK phosphorylation, there are other downstream proteins in the TGF-β1 activation pathway that can inhibit phosphorylation of ERK, such as p38 (Sato et al. 2002). To evaluate the role of p38, similarly prepared samples were cyclically stretched in the FlexCell system while treated with the p38 activation inhibitor SB203580 and/or TGF-β1. Addition of the inhibitor in the presence of TGF-β1 led to increased phosphorylation of ERK during stretching after 1 hour and increased collagen production by cells after 1 week (Fig 5). The SB203580 results provide direct evidence of an inhibitory effect of TGF-β1 on ERK activation induced by cyclic stretching that can explain the results found with the tubular constructs. Similar signaling results were obtained by Papakrivopoulou et al. (2004). Further evidence of p38 activation can be deduced from higher elastin deposition in the +TGF-β samples. One study demonstrated that activation of p38 leads to stabilization of elastin mRNA (Kuang and Goldstein 2005).
Functional tissue-engineered constructs require mechanical stiffness, strength, and elasticity. This study showed that while TGF-β1 can lead to increased elastin production, it is detrimental to the development of engineered tissue when using long-term cyclic stretching. However, this study also demonstrated that use of TGF-β1 following extended cyclic stretching in its absence can yield both physiological UTS and modulus and substantial elastin content with evidence of increased tissue elasticity. A similar sequential approach to use of mechanical and chemical stimuli has been reported for tissue engineered bone (Lima et al. 2007) and ligaments (Moreau et al. 2008). This study demonstrates a rationalized approach to maximize the benefits of mechanical (cyclic stretching) and chemical (TGF-β1) stimuli that can be applied to engineered connective tissue and potentially improved by optimized regimens for both.
Supplementary Material
Acknowledgements
The authors like to thank Ricky Chow, Naomi Ferguson, Sandy Johnson, Justin Weinbaum and Stephen Stephens for technical assistance. Funding was provided by NIH BRP HL71538 and R01 HL083880 to R.T.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflict of interest.
References
- Balguid A, Rubbens MP, Mol A, Bank RA, Bogers AJ, van Kats JP, de Mol BA, Baaijens FP, Bouten CV. The role of collagen cross-links in biomechanical behavior of human aortic heart valve leaflets--relevance for tissue engineering. Tissue Eng. 2007;13(7):1501–11. doi: 10.1089/ten.2006.0279. [DOI] [PubMed] [Google Scholar]
- Bastiaansen-Jenniskens YM, Koevoet W, De Bart AC, Zuurmond AM, Bank RA, Verhaar JA, DeGroot J, van Osch GJ. TGFbeta affects collagen cross-linking independent of chondrocyte phenotype but strongly depending on physical environment. Tissue Eng Part A. 2008;14(6):1059–66. doi: 10.1089/ten.tea.2007.0345. [DOI] [PubMed] [Google Scholar]
- Bilodeau K, Mantovani D. Bioreactors for tissue engineering: focus on mechanical constraints. A comparative review. Tissue Eng. 2006;12(8):2367–83. doi: 10.1089/ten.2006.12.2367. [DOI] [PubMed] [Google Scholar]
- Bujor AM, Pannu J, Bu S, Smith EA, Muise-Helmericks RC, Trojanowska M. Akt blockade downregulates collagen and upregulates MMP1 in human dermal fibroblasts. J Invest Dermatol. 2008;128(8):1906–14. doi: 10.1038/jid.2008.39. [DOI] [PubMed] [Google Scholar]
- Chambers RC, Leoni P, Kaminski N, Laurent GJ, Heller RA. Global expression profiling of fibroblast responses to transforming growth factor-beta1 reveals the induction of inhibitor of differentiation-1 and provides evidence of smooth muscle cell phenotypic switching. Am J Pathol. 2003;162(2):533–46. doi: 10.1016/s0002-9440(10)63847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Nielsen LD, Welch MP, McPherson JM. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-beta. J Cell Sci. 1995;108(Pt 3):1251–61. doi: 10.1242/jcs.108.3.1251. [DOI] [PubMed] [Google Scholar]
- Davidson JM, LuValle PA, Zoia O, Quaglino D, Jr., Giro M. Ascorbate differentially regulates elastin and collagen biosynthesis in vascular smooth muscle cells and skin fibroblasts by pretranslational mechanisms. J Biol Chem. 1997;272(1):345–52. doi: 10.1074/jbc.272.1.345. [DOI] [PubMed] [Google Scholar]
- Dayan D, Hiss Y, Hirshberg A, Bubis JJ, Wolman M. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry. 1989;93(1):27–9. doi: 10.1007/BF00266843. [DOI] [PubMed] [Google Scholar]
- Grouf JL, Throm AM, Balestrini JL, Bush KA, Billiar KL. Differential effects of EGF and TGF-beta1 on fibroblast activity in fibrin-based tissue equivalents. Tissue Eng. 2007;13(4):799–807. doi: 10.1089/ten.2006.0206. [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, Perr HA. Mechanical stretch modulates TGF-beta1 and alpha1(I) collagen expression in fetal human intestinal smooth muscle cells. Am J Physiol. 1999;277(5 Pt 1):G1074–80. doi: 10.1152/ajpgi.1999.277.5.G1074. [DOI] [PubMed] [Google Scholar]
- Isenberg BC, Tranquillo RT. Long-Term Cyclic Distention Enhances the Mechanical Properties of Collagen-Based Media-Equivalents. Annals of Biomedical Engineering. 2003;31:937–949. doi: 10.1114/1.1590662. [DOI] [PubMed] [Google Scholar]
- Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98(1):25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Montes GS, Sanchez EM. The influence of tissue section thickness on the study of collagen by the Picrosirius-polarization method. Histochemistry. 1982;74(1):153–6. doi: 10.1007/BF00495061. [DOI] [PubMed] [Google Scholar]
- Kuang PP, Goldstein RH. Regulation of elastin gene transcription by proteasome dysfunction. Am J Physiol Cell Physiol. 2005;289(3):C766–73. doi: 10.1152/ajpcell.00525.2004. [DOI] [PubMed] [Google Scholar]
- Kuang PP, Zhang XH, Rich CB, Foster JA, Subramanian M, Goldstein RH. Activation of elastin transcription by transforming growth factor-beta in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L944–52. doi: 10.1152/ajplung.00184.2006. [DOI] [PubMed] [Google Scholar]
- Kucich U, Rosenbloom JC, Abrams WR, Rosenbloom J. Transforming growth factor-beta stabilizes elastin mRNA by a pathway requiring active Smads, protein kinase C-delta, and p38. Am J Respir Cell Mol Biol. 2002;26(2):183–8. doi: 10.1165/ajrcmb.26.2.4666. [DOI] [PubMed] [Google Scholar]
- Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15(9):1025–33. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl GE, Chambers RC, Papakrivopoulou J, Dawson SJ, Jacobsen MC, Bishop JE, Laurent GJ. Activation of fibroblast procollagen alpha 1(I) transcription by mechanical strain is transforming growth factor-beta-dependent and involves increased binding of CCAAT-binding factor (CBF/NF-Y) at the proximal promoter. J Biol Chem. 2002;277(8):6153–61. doi: 10.1074/jbc.M108966200. [DOI] [PubMed] [Google Scholar]
- Long JL, Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22(4):339–50. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1(3):169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19(23):2783–810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Merryman WD, Lukoff HD, Long RA, Engelmayr GC, Jr., Hopkins RA, Sacks MS. Synergistic effects of cyclic tension and transforming growth factor-beta1 on the aortic valve myofibroblast. Cardiovasc Pathol. 2007;16(5):268–76. doi: 10.1016/j.carpath.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau JE, Bramono DS, Horan RL, Kaplan DL, Altman GH. Sequential biochemical and mechanical stimulation in the development of tissue-engineered ligaments. Tissue Eng Part A. 2008;14(7):1161–72. doi: 10.1089/ten.tea.2007.0147. [DOI] [PubMed] [Google Scholar]
- Neidert MR, Lee ES, Oegema TR, Tranquillo RT. Enhanced fibrin remodeling in vitro with TGF-beta1, insulin and plasmin for improved tissue-equivalents. Biomaterials. 2002;23(17):3717–31. doi: 10.1016/s0142-9612(02)00106-0. [DOI] [PubMed] [Google Scholar]
- Pannu J, Nakerakanti S, Smith E, ten Dijke P, Trojanowska M. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J Biol Chem. 2007;282(14):10405–13. doi: 10.1074/jbc.M611742200. [DOI] [PubMed] [Google Scholar]
- Papakrivopoulou J, Lindahl GE, Bishop JE, Laurent GJ. Differential roles of extracellular signal-regulated kinase 1/2 and p38MAPK in mechanical load-induced procollagen alpha1(I) gene expression in cardiac fibroblasts. Cardiovasc Res. 2004;61(4):736–44. doi: 10.1016/j.cardiores.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Poobalarahi F, Baicu CF, Bradshaw AD. Cardiac myofibroblasts differentiated in 3D culture exhibit distinct changes in collagen I production, processing, and matrix deposition. Am J Physiol Heart Circ Physiol. 2006;291(6):H2924–32. doi: 10.1152/ajpheart.00153.2006. [DOI] [PubMed] [Google Scholar]
- Robinson PS, Johnson SL, Evans MC, Barocas VH, Tranquillo RT. Functional tissue-engineered valves from cell-remodeled fibrin with commissural alignment of cell-produced collagen. Tissue Eng Part A. 2008;14(1):83–95. doi: 10.1089/ten.a.2007.0148. [DOI] [PubMed] [Google Scholar]
- Ross JJ, Tranquillo RT. ECM gene expression correlates with in vitro tissue growth and development in fibrin gel remodeled by neonatal smooth muscle cells. Matrix Biol. 2003;22(6):477–90. doi: 10.1016/s0945-053x(03)00078-7. [DOI] [PubMed] [Google Scholar]
- Sato M, Shegogue D, Gore EA, Smith EA, McDermott PJ, Trojanowska M. Role of p38 MAPK in transforming growth factor beta stimulation of collagen production by scleroderma and healthy dermal fibroblasts. J Invest Dermatol. 2002;118(4):704–11. doi: 10.1046/j.1523-1747.2002.01719.x. [DOI] [PubMed] [Google Scholar]
- Starcher BC, Galione MJ. Purification and comparison of elastins from different animal species. Anal Biochem. 1976;74(2):441–7. doi: 10.1016/0003-2697(76)90224-4. [DOI] [PubMed] [Google Scholar]
- Stegemann H, Stalder K. Determination of hydroxyproline. Clinica Chimica Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Syedain ZH, Meier LA, Bjork JW, Lee A, Tranquillo RT. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials. 2010;32(3):714–22. doi: 10.1016/j.biomaterials.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syedain ZH, Tranquillo RT. Controlled cyclic stretch bioreactor for tissue-engineered heart valves. Biomaterials. 2009;30(25):4078–84. doi: 10.1016/j.biomaterials.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syedain ZH, Weinberg JS, Tranquillo RT. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci U S A. 2008;105(18):6537–42. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr., Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- Tuan TL, Song A, Chang S, Younai S, Nimni ME. In vitro fibroplasia: matrix contraction, cell growth, and collagen production of fibroblasts cultured in fibrin gels. Exp Cell Res. 1996;223(1):127–34. doi: 10.1006/excr.1996.0065. [DOI] [PubMed] [Google Scholar]
- Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res. 2004;95(3):253–60. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- Yacoub M, Nerem R. Introduction. Bioengineering the heart. Philos Trans R Soc Lond B Biol Sci. 2007;362(1484):1253–5. doi: 10.1098/rstb.2007.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Crawford RC, Wang JH. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech. 2004;37(10):1543–50. doi: 10.1016/j.jbiomech.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Yao L, Liu J, Andreadis ST. Composite fibrin scaffolds increase mechanical strength and preserve contractility of tissue engineered blood vessels. Pharm Res. 2008;25(5):1212–21. doi: 10.1007/s11095-007-9499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


