Abstract
Chikungunya virus, a mosquito-borne alphavirus, recently caused the largest epidemic ever seen for this virus. Chikungunya disease primarily manifests as a painful and debilitating arthralgia/arthritis, and no effective drug or vaccine is currently available. Here we describe a recombinant chikungunya virus vaccine comprising a non-replicating complex adenovirus vector encoding the structural polyprotein cassette of chikungunya virus. A single immunisation with this vaccine consistently induced high titres of anti-chikungunya virus antibodies that neutralised both an old Asian isolate and a Réunion Island isolate from the recent epidemic. The vaccine also completely protected mice against viraemia and arthritic disease caused by both virus isolates.
Keywords: Chikungunya, Adenovirus, Vaccine, Arthritis
1. Introduction
Viruses of the genus Alphavirus in the family Togaviridae represent a group of globally distributed enveloped, positive-sense, single stranded RNA viruses, which are usually mosquito transmitted and comprise over 40 known members. Several alphaviruses cause rheumatic disease in humans, these include the Australasian Barmah Forest and Ross River viruses, the African o’nyong-nyong virus and the Afro-Asian chikungunya virus (CHIKV) [1, 2].
CHIKV has caused periodic but sporadic outbreaks in tropical Africa and Asia and has recently (2005–2007) caused the largest outbreak of this virus in recorded history. Over 260,000 cases (≈1/3 of the population) were reported in Réunion Island (France) [3] with 1.39 million cases reported in India [4]. Numerous imported cases have also been reported in Europe [5], Asia [6] and the United States [7]. No vaccine or effective drug for CHIKV disease is currently commercially available. The disease usually involves weeks to months of debilitating arthralgia/arthritis, and can involve myalgia, fever, headache, nausea, vomiting and/or a rash [8] with arthritis/arthralgia affecting 73–80% of patients [3]. Disease can persist for two years or more in some patients [9–11]. The word “chikungunya” is derived from the Makonde language (Tanzania) and means "that which bends up" referring to the severe joint pain-induced posture of afflicted individuals [12]. The recent epidemic involved the emergence of a new clade of CHIKV within the large East-, Central-, and South-African phylogroup [13]. Viruses within the new clade of CHIKV are efficiently transmitted by Aedes albopictus mosquito, whereas the usual vector for CHIKV is Aedes aegypti [14, 15]. The new viruses also appear to be associated with more severe disease in humans [12, 16]. Importantly, viruses within this new clade have been associated for the first time with human mortality [17–19].
The explosive Réunion Island epidemic, the rapid spread to India and other Asian countries [20] and the wide global distribution of the Ae. aegypti and Ae. albopictus mosquito vectors raise the concern that similarly rapid and severe CHIKV outbreaks could become more frequent and affect other regions of the world. Ae. albopictus has spread worldwide, having reached the United States, Brazil, Central America, Africa, and Europe [21, 22]. The Centers for Disease Control has thus identified imported cases of CHIKV disease and the potential dissemination of the virus by Ae. albopictus as a threat to the US population, particularly in urban areas [23, 24]. CHIKV is a biosafety level 3 (BSL3) pathogen and has been declared a Category C Priority Pathogen by the National Institute of Allergy and Infectious Disease (NIAID) in the United States.
The US Army has long recognized that CHIKV could be used as a biological weapon [25], and CHIKV is considered a possible agent for bioterrorism due to the potential for infection via aerosol [26]. Although CHIKV vaccine development began in the 1960s [27–31], earlier efforts toward the development of CHIKV vaccines were abandoned due to poor performance and safety, and changing budget priorities [32]. At present, no licensed vaccine or particularly effective drug is available for human use for any alphavirus.
To date a number of CHIKV vaccines have been developed, but none have been licensed. Formalin-killed CHIKV vaccines have been shown to be immunogenic in humans [33], non-human primates [34] and mice [35]. However, growth of large quantities of CHIKV for vaccine manufacture is complicated by the requirement for appropriate BSL3 containment. A live-attenuated CHIKV vaccine strain, known as TSI-GSD-218, induced neutralising responses and protected mice and monkeys against challenge [30]. However, in a phase II human trial, this vaccine caused side effects in several recipients that included arthralgia [36]. DNA-based CHIK vaccines encoding E1, E2 and capsid on three separate plasmids have been shown to be immunogenic in mice [37]. Unfortunately, DNA vaccines have so far not been particularly effective at generating antibody responses in humans [38], which is a concern as antibodies are believed to be required for protection against CHIKV infections [39]. Chimeric live-attenuated CHIK vaccines encoding CHIKV E1, E2 and capsid with the non-structural genes from Venezuelan Equine Encephalitis virus (VEEV), Eastern Equine Encephalitis virus (EEEV) or Sindbis virus (SINV) have been shown to be immunogenic and protective against nasal CHIKV challenge in mice [40]. However, manufacturing and safety remain significant hurdles given the known ability of alphaviruses to recombine and generate replication competent viruses [41]. Recently, the first alphavirus Virus-like particle (VLP) CHIKV vaccine was produced and tested in non-human primates [42]. Although this vaccine, based on the West African CHIKV genotype, provided protection, three doses were required.
Recombinant adenovirus vectors have been widely tested in humans and have been shown to be safe in an extensive series of human trials. They are also stable, immunogenic and relatively easy to manufacture [43]. The non-replicating Complex Adenovirus vaccine (CAdVax) vectors contain deletions in E1, E3 and most of the E4 regions (except orf6) of the adenovirus 5 (Ad5) genome. These deletions, coupled with multiple engineered transgene-expression sites, allow the insertions of multiple antigens at different locations, or a large antigen insert at the location of choice within the Ad5 genome. The technology has been used to generate recombinant dengue, influenza, Marburg, Ebola, West Nile and Rift Valley fever virus vaccines, and has been shown to be efficacious in murine, guinea pig, ferret and non-human primate models [44–50].
Herein we describe the application of the CAdVax technology to the design of a CHIKV vaccine. In this CAdVax-CHIK vaccine, a single insert encoding the structural polyprotein (comprising the envelope glycoproteins E1, E2 and capsid) of CHIKV was inserted in the right hand of the genome (as depicted in Fig. 1). The major advantage of this particular configuration of the CAdVax is that it prevents the generation of replication-competent adenovirus through homologous recombination in the packaging cell line, HEK293, a common problem of first generation Ad5 vectors. The antigen sequences are from a CHIKV isolate from the recent epidemic on Réunion Island, and the complete structural polyprotein gene was expressed in order to retain the native processing sequences. A single dose of the vaccine completely protected mice against viraemia and disease in recently developed adult wild-type mouse models of CHIKV-induced arthritis [51].
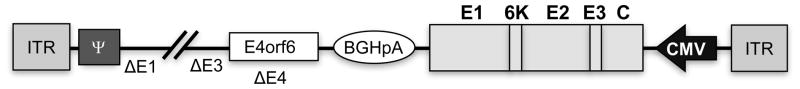
Figure 1. Genome structure of the recombinant CAdVax-CHIK vaccine.
The CAdVax-CHIK vaccine was designed to express the codon optimised CHIKV structural polyprotein gene (comprising Capsid, E3, E2, 6K, and E1). The gene is flanked by the immediate early human CMV promoter (CMV) and the bovine growth hormone polyadenylation signal (BGHpA) sequence. The CAdVax vector backbone has deletions (Δ) in E1, E3, and E4, except for E4orf6. ITR - inverted terminal repeat. ψ - adenovirus packaging signal.
2. Materials and Methods
2.1. Construction of CAdVax-CHIK
The coding sequence for the CHIKV, Réunion Island isolate, structural polypeptide (GenBank DQ443544.2, isolate LR2006 OPY1) was synthesized with codons optimized for expression in human cells by GeneArt (Regensburg, Germany). This gene, encoding capsid (C), E3, E2, 6K and E1, was subcloned into the 2pRAd plasmid shuttle vector as described previously [48–50, 52, 53]. Digestion with NruI and XbaI restriction enzymes followed by ligation was used to delete the CMV promoter and bovine growth hormone polyadenylation (BGHpA) signal from the 2pLAd plasmid shuttle vector. Using these shuttle vectors the vaccine was constructed as described previously [48–50, 52, 53]. Correct insertion was confirmed by sequencing, PCR, and restriction enzyme digestion. Vector stocks were generated on the HEK293 packaging cell line, isolated by plaque-purification, amplified, and then purified by caesium chloride gradient before final virus titration as described previously [52, 53].
2.2. Western analysis
BS-C-1 cells (ATCC CCL-26) were mock infected or infected with CAdVax-CHIK or a control CAdVax vector expressing dengue virus glycoproteins [45], at a multiplicity of infection (MOI) of 20 for 48 hours. Cell lysates prepared as described previously [47] were resolved on a 4–20% Tris-HCl gradient gel (BioRad) and transferred to PVDF membranes (Millipore). Membranes were probed with pooled polyclonal serum obtained from three C57BL/6 mice that had been infected with CHIKV (as described below) and bled 5–6 weeks later. Proteins in the immunoblot were detected using an HRP-conjugated secondary antibody (Kirkegaard & Perry Laboratories, Inc.) and TMB liquid substrate system (Sigma-Aldrich).
2.3. Vaccination of mice
Female CD-1 outbred mice (Charles River Laboratory, Raleigh, NC) or female C57BL/6 mice (ARC, Perth, Australia) 6–8 weeks old were vaccinated intraperitoneally (i.p.) once with 1 × 108 infectious units (i.u.) of the CAdVax-CHIK vaccine, or the indicated control CAdVax vaccine in 100 μl of PBS, or 100 μl of PBS. Mouse experiments were approved by the GenPhar Institutional Animal Care and Use committee or the Queensland Institute of Medical Research Animal Ethics committee.
2.4. ELISA assays
Monolayers of BS-C-1 cells were infected with CAdVax-CHIK at MOI 20 for 48 hours in serum-free medium. Cells were then harvested and lysates were prepared as described previously [47]. Lysate diluted in PBS (10 μg/ml) was absorbed onto Maxisorp microtiter plates (MaxiSorb, Nunc, Rochester, NY, USA) overnight at 37°C, and the ELISAs were performed as described previously [54]. Alternatively, inactivated and purified CHIKV (Asian isolate, GenBank: FJ457921, [51]) was coated onto the ELISA plates at 2 μg/ml in carbonate buffer pH 9 overnight, and the plates blocked with 5% FCS/PBS. Mouse serum was added at 3 fold serial dilutions and CHIKV-specific antibodies were detected with biotin-conjugated rat anti-mouse IgG2c (R19-15) and IgG1 (A85-1) (BD Pharmingen, Heidelberg, Germany), streptavidin-HRP (Biosource, Camarillo, CA, USA) and ABTS substrate (Sigma). End point titres were determined by calculating the dilution of serum that corresponded to an absorbance of two times the background obtained with naïve serum.
2.5. Neutralisation assays
Heat inactivated (56°C for 30 min) serum from each mouse was serial diluted in duplicate in 96 well plates and incubated with 200 50% cell culture infectivity dose (CCID50) of a Réunion Island (LR2006 OPY1) or Asian isolate (GenBank: FJ457921) of CHIKV for 2 hours at 37°C. Vero cells were then added (104/well) and the plates incubated for 5 days. The serum dilution giving 100% protection against cytopathic effect was determined using crystal violet staining.
2.6. CHIKV challenge in C57BL/6 inbred mice
Six and a half weeks after vaccination mice were challenged as described previously [51] with 1 × 104 CCID50 of the Réunion Island or Asian isolate s.c. into the side of each hind foot towards the ankle. The extended time between vaccination and challenge was used to avoid any influence of vector-induced interferon α/β responses, given the sensitivity of CHIKV infection to these cytokines [51]. The height and width of the peri-metatarsal foot swelling was measured using electronic callipers, and height × width was used to represent the cross sectional area in mm2 as described [51]. Viraemia was measured as described [51] using blood taken from a tail vein and collected into MiniCollect serum separation tubes (Greiner Bio-One GmbH, Kremsmunster, Austria). Viral titres are expressed as log10 CCID50 per ml. Animals were monitored daily and no adverse events requiring euthanasia were reported during the experiments.
2.7. Statistics
Statistical analysis was performed using SPSS for Windows (version 17.0, 2010; SPSS, Chicago, IL).
3. Results
3.1 CAdVax-CHIK vaccine construction and expression of CHIKV structural proteins
In order to develop a safe and effective CHIKV vaccine, we constructed and characterized CAdVax-CHIK to express the antigenic structural proteins of this emerging arbovirus. The structural polyprotein cassette of a Réunion Island isolate of CHIKV was artificially synthesized and codon optimized for high-level expression in human cells. This gene was cloned into a CAdVax vector, with the cassette flanked by a CMV immediate early promoter and a bovine growth hormone polyadenylation signal (Fig. 1). Correct insertion was confirmed by sequencing, PCR, and restriction digest analyses (data not shown). The polyprotein comprises a N-terminal capsid protein (C) followed by glycoproteins E1 and E3/E2. The capsid protein is autoproteolitically cleaved from the polypeptide, and E1 and E3/E2 (known as ProE2 or pE2) are transported to the cellular membrane as a heterodimer. At a late stage of transport, E3/E2 is cleaved by furin, a host cellular protease [55], to yield functional E2, which is required for the formation of mature viruses that bud at the host cellular membrane [56]. In this design, the immune system is presented with de novo synthesized CHIKV antigens in the configuration seen during a natural infection, but without causing disease.
To analyze the CHIKV antigen synthesis, we first produced the CAdVax-CHIK according to previously established protocols [52, 53], and then infected BS-C-1 cells with the vaccine vector. The presence of CHIKV antigens in the cell lysates was analysed by western blotting using CHIKV-specific sera obtained from mice previously infected with a Reunion Island isolate of CHIKV [51]. Mature E1 (52 kDa) , E2 (56 kDa) and C (36 kDa) were readily identified in this lysate at the appropriate molecular masses. A small amount of the precursor protein, pE2 (65 kDa), was also seen in CAdVax-CHIK infected cells (Fig. 2). These proteins were not seen in the lysate of mock-infected cells or cells infected with a control CAdVax vector expressing an unrelated antigen (Fig. 2). The structural proteins were thus correctly proteolytically processed from the CHIKV polyprotein expressed by the CAdVax-CHIK vaccine.
Figure 2. Expression of the CHIKV structural proteins after infection of cells with the CAdVax-CHIK vector.
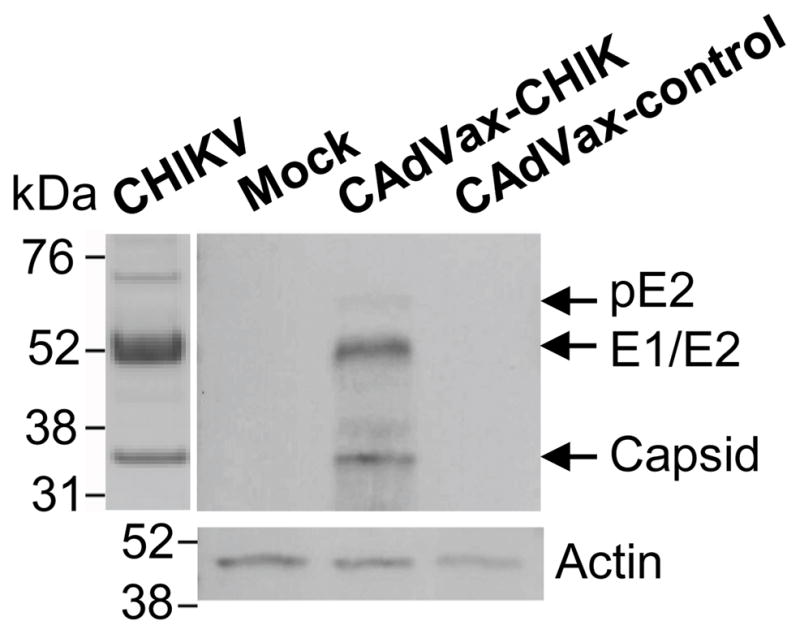
BS-C-1 cells were mock infected (Mock), infected with the CAdVax-CHIK vector or a control CAdVax vector expressing dengue antigens [45] (CAdVax-control). Cell lysates were then analysed by Western analysis using polyclonal sera from CHIKV infected C57BL/6 mice. Purified CHIKV (21) is shown in the first lane as a positive control.
3.2 Antibody response kinetics after CAdVax-CHIK vaccination in outbred mice
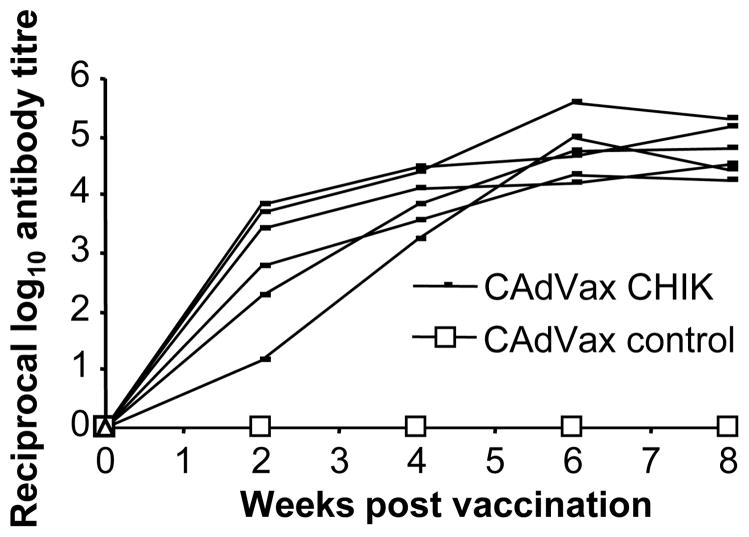
To determine the kinetics and potential genetic restriction of antibody responses induced by CAdVax-CHIK vaccination, outbred CD-1 mice were immunised once i.p. with 1 × 108 i.u. of CAdVax-CHIK or a control CAdVax vector and the antibody responses measured over time. Mouse sera were collected at 2 week intervals and antibody titres measured by ELISA against cell lysates of CAdVax-CHIK infected cells. Consistent IgG responses in all CAdVax-CHIK immunised mice were apparent at week 2 post vaccination and responses peaked at week 6, with a mean end point titre of ≈1/75,000 (Fig. 3). Due to the limited expression of adenovirus vector antigens both in vitro after CAdVax-CHIK infection and in vivo after CAdVax immunisation, no significant antibody reactivity to the ELISA antigen (a lysate of CAdVax-CHIK infected cells) was observed in sera from mice vaccinated once with the control CAdVax vector (Fig. 3, CAdVax control). These data illustrate that CAdVax-CHIK vaccination rapidly and consistently raised high titres of CHIKV antigen-specific antibodies in outbred mice after a single vaccination.
Figure 3. Antibody responses in outbred mice.
CD-1 mice (n=6) were immunised once i.p. with 1 × 108 i.u. of CAdVax-CHIK or a control CAdVax vector expressing avian influenza proteins [47]. Serum was taken at the indicated times post immunisation and tested by ELISA using lysates of CAdVax-CHIK infected BS-C-1 cells as antigen. The end point total IgG titres for individual mice are shown.
3.3 Antibody responses after CAdVax-CHIK vaccination in C57BL/6 mice
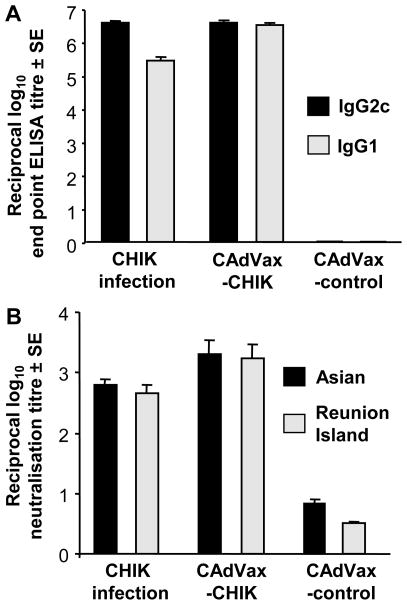
To measure antibody responses in a mouse strain suitable for protection studies [51], C57BL/6 mice were vaccinated once i.p. with 1 × 108 i.u. CAdVax-CHIK and anti-CHIKV antibody titres were evaluated by ELISA using purified CHIKV (Asian isolate [51]) as the ELISA antigen. CAdVax-CHIK vaccinated mice showed a mean end point titre of over 1 in 3.5 × 106 for anti-CHIKV IgG2c, similar to the mean end point titre seen in animals infected with 104 CCID50 of the Réunion Island isolate of CHIKV [51] (Fig. 4A). The mean IgG1 titre after CAdVax-CHIK vaccination was over 1 in 3 × 106, significantly higher than that seen after CHIKV infection (Fig. 4A). Mice vaccinated with the control CAdVax vector (Fig. 4A) or PBS (data not shown) generated no detectable CHIKV-specific IgG1 or IgG2c responses. CAdVax-CHIK vaccination thus provided strong and balanced IgG1/IgG2c anti-CHIKV antibody responses. IgG1/IgG2c ratios reflect the Th2/Th1 bias induced by the vaccine [57]. (C57BL/6 mice do not make IgG2a [58]).
Figure 4. Antibody isotype and neutralisation titres in vaccinated C57BL/6 mice.
(A) Mice (n=4-6 per group) were infected with the Réunion Island isolate of CHIKV or vaccinated once i.p. with 1 × 108 i.u. CAdVax-CHIK or a control CAdVax vaccine. Antibody titres at 5.5 weeks post infection were assessed by ELISA using purified CHIKV as the antigen target. For sera from mice vaccinated with CAdVax-CHIK and CAdVax-control anti-CHIKV, IgG2c and IgG1 titres were significantly different (p=0.003 and p=0.002, respectively. Mann Whitney U test). (B) Neutralisation titres for Asian and Réunion Island CHIKV isolates were determined using serum from mice vaccinated as above. The titres represent the mean serum dilution (± SE) giving 100% protection against viral cytopathic effects. Neuralisation titres were significantly different for sera from mice vaccinated with CAdVax-CHIK and CAdVax-control, p=0.032 for Asian and p=0.018 for Reunion Island (Mann Whitney U test).
Neutralising antibodies are considered to be important for protection against CHIKV [39]. Serum from CAdVax-CHIK vaccinated mice had similar neutralisation titres (≈1/2000) for both the Réunion Island and Asian isolates of CHIKV. These titres were slightly higher than those obtained after infection of unimmunized mice with the Réunion Island virus. Serum from CAdVax-control vaccinated mice had neutralisation titres of less than 1 in 6 (Fig. 4B). CAdVax-CHIK vaccination thus induced high titres of antibodies capable of neutralising both a Réunion Island and an Asian isolate of CHIKV; viruses from two genetically distant phylogroups [59].
3.4 CAdVax-CHIK vaccination protected C57BL/6 mice against viraemia and arthritis
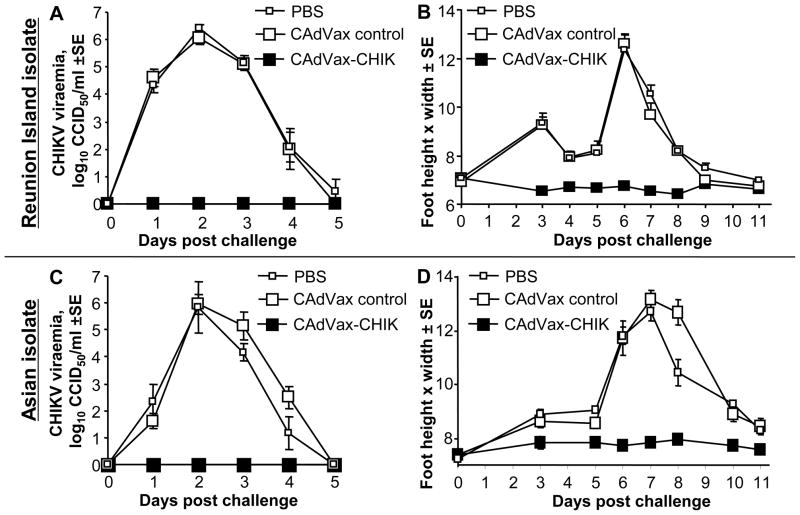
We have recently established an adult wild-type mouse model of CHIKV infection and arthritic disease, which manifests as a measurable self-limiting peri-metatarsal swelling [51]. To assess protection against viraemia and diseases mediated by the CAdVax-CHIK vaccine, vaccinated animals were challenged in the rear feet with 104 CCID50 of the Réunion Island and Asian isolates of CHIKV. Animals that received the control CAdVax vector or PBS both produced similar viraemias, which peaked at day 2. Animals from each of these two control groups also showed symptoms of arthritis, as seen by swelling of the foot, when challenged with the Réunion Island and Asian CHIKV isolates. CAdVax-CHIK vaccinated animals were completely protected against viraemia and foot swelling induced by both the Réunion Island (Fig. 5A,B) and Asian isolates (Fig. 5C, D). No CHIKV was detected in serum and no swelling was detected on any day in any animals that had received the CAdVax-CHIK vaccination (Fig. 5, black squares).
Figure 5. CHIK challenge of CAdVax-CHIK immunized C57BL/6 mice.
Mice (n=3–6 per group) were vaccinated with CAdVax-CHIK, a control CAdVax vaccine or PBS. At 6.5 weeks post-immunization, mice were challenged with CHIKV. (A) Viraemia after challenge with the Réunion Island isolate. Viraemia was significantly different between CAdVax-CHIK and CAdVax-control vaccinated groups on days 1–3 (all p<0.037, Mann Whitney U test). (B) Foot swelling after challenge with the Réunion Island isolate. Swelling (represented as a cross sectional area in mm2) was significantly different between CAdVax-CHIK and CAdVax-control vaccinated groups on days 3–8 (all p<0.02, Mann Whitney U test). (C) Viraemia after challenge with the Asian isolate. Viraemia was significantly different between CAdVax-CHIK and CAdVax-control vaccinated groups on days 1–4 (all p<0.014, Mann Whitney U test). (D) Foot swelling after challenge with the Asian isolate. Swelling was significantly different between CAdVax-CHIK and CAdVax-control vaccinated groups on days 3-10 (all p<0.04, Mann Whitney U test).
4. Discussion
CHIKV is an important emerging arbovirus causing severe rheumatic disease, with recent outbreaks in humans also associated for the first time with mortality. A safe and effective vaccine would play an important role in preventing future outbreaks in humans. Cells infected with the CAdVax-CHIK vaccine described herein expressed the structural polyprotein of CHIKV, which was correctly processed into E1, E2 and capsid. Herein we show that a single immunization with CAdVax-based CHIKV vaccine was able consistently to induce high titres of anti-CHIKV antibodies in both outbred (CD-1) and inbred (C57BL/6) strains of mice, illustrating that genetic background did not play a major role in determining the antibody response to the CAdVax-CHIK vaccine. Sera from CAdVax-CHIK vaccinated C57BL/6 mice were also able to neutralise two isolates (Asian and Réunion Island) of CHIKV from two different phylogroups. In addition, CAdVax-CHIK vaccination completely protected against CHIKV viraemia and arthritis induced by these isolates in a mouse model of infection and disease. Importantly, the animal model used in these challenge experiments is the only known mouse model using immune-competent wild-type adult animals that develop rheumatic disease after CHIKV infection, the main manifestation of CHIKV disease in humans [8]. Other models for CHIKV infection of mice use neonatal mice [60], IFNα/β receptor defective transgenic mice [61], or wild-type mice [40] and use lethality rather than rheumatic manifestations as measures of CHIKV-induced disease.
The Réunion Island isolate belongs to the large East-, Central-, and South-African phylogroup of CHIKV and the Asian isolate belongs to the genetically separate Asian phylogroup [13]. These phylogroups represent the majority of currently circulating viruses and show about a 3% amino acid sequence divergence [59]. The ability of CAdVax-CHIK vaccination to protect against these two isolates illustrates that the vaccine is effective against viruses from two distinct phylogroups. We have recently also observed cross neutralisation of CHIKV by anti-Ross River virus sera [51], suggesting neutralisation determinants are conserved even amongst distantly related arthrogenic alphaviruses [62]. The CAdVax-CHIK vaccine is thus likely to protect against most, if not all, strains of CHIKV.
Pre-existing anti-Ad5 antibodies have been shown to be associated with reduced responses to recombinant Ad5-based vaccines in humans [63]. As CAdVax is also based on Ad5, such pre-existing immunity may similarly impact vaccine efficacy. However, data from animal experiments and clinical trials show that an increase in Ad5 vector dose easily overcomes the effects of pre-existing immunity [64-68]. We have recently shown that simply increasing the dose for an Ebola CAdVax vaccine by one log, which is easily achievable with CAdVax, is sufficient to overcome pre-existing anti-vector (Ad5) immunity in cynomolgous macaques and protect animals from lethal infection by Ebola virus [49]. In addition, a dose of as little as 2 × 108 i.u. of CAdVax vaccine for Rift Valley fever was able to overcome pre-existing anti-vector immunity in mice [44]. We do not envisage that such dose increases will have any negative impact on the safety profile of the vector as biodistribution and toxicology studies conducted following Good Laboratory Practice guidelines (GLP; as described in the US Food and Drug Administration Code of Federal Regulations Title 21, Part 58) have shown that repeated administration of up to 2 × 1013 CAdVax virus particles per dose in New Zealand white rabbits was well tolerated by this highly sensitive animal model (data not shown).
Due to the favourable safety profile and prior success of the non-replicating CAdVax vector platform in development of viral vaccines, it is ideally suited for the development of a CHIKV vaccine for use in humans. In addition, the sporadic and unpredictable nature of CHIKV epidemics means that CHIKV vaccines may need to be rapidly manufactured. Adenovirus vectors can be rapidly and economically propagated and purified without the need for BSL3 containment [69], and they have a long shelf life if stored appropriately [70]. Future studies will seek to investigate the ability of CAdVax-CHIK to protect against virus challenge in a non-human primate model of CHIKV disease, such as that recently established in cynomolgus macaques [71]. Recent studies in a non-human primate model have shown that 1010 PFU of a Ebola virus CAdVax vaccine injected twice i.m. was sufficient for protection against disease and morality after Ebola virus challenge [49].
Acknowledgments
This work was funded by NIAID SBIR grant number R43 A1078625, SC Launch Matching grant number 2009-04-008, the Australian Centre for International and Tropical Health, and the National Health and Medical Research Council of Australia. We would like to thank Jessica McCoy and Yaenette Dixon-Mah for their help in conducting animal immunisations and immune analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suhrbier A, La Linn M. Clinical and pathologic aspects of arthritis due to Ross River virus and other alphaviruses. Curr Opin Rheumatol. 2004;16:374–9. doi: 10.1097/01.bor.0000130537.76808.26. [DOI] [PubMed] [Google Scholar]
- 2.Suhrbier A, Mahalingam S. The immunobiology of viral arthritides. Pharmacol Ther. 2009;124:301–8. doi: 10.1016/j.pharmthera.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–27. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 4.Mavalankar D, Shastri P, Raman P. Chikungunya epidemic in India: a major public-health disaster. Lancet Infect Dis. 2007;7:306–7. doi: 10.1016/S1473-3099(07)70091-9. [DOI] [PubMed] [Google Scholar]
- 5.Hochedez P, Hausfater P, Jaureguiberry S, Gay F, Datry A, Danis M, et al. Cases of chikungunya fever imported from the islands of the South West Indian Ocean to Paris, France. Euro Surveill. 2007:12. [Google Scholar]
- 6.Lim PL, Oh HM, Ooi EE. Chikungunya in Singapore: imported cases among travelers visiting friends and relatives. J Travel Med. 2009;16:289–91. doi: 10.1111/j.1708-8305.2009.00313.x. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Update: chikungunya fever diagnosed among international travelers--United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:276–7. [PubMed] [Google Scholar]
- 8.Brighton SW, Prozesky OW, de la Harpe AL. Chikungunya virus infection. A retrospective study of 107 cases. S Afr Med J. 1983;63:313–5. [PubMed] [Google Scholar]
- 9.de Andrade DC, Jean S, Clavelou P, Dallel R, Bouhassira D. Chronic pain associated with the Chikungunya Fever: long lasting burden of an acute illness. BMC Infect Dis. 2010;10:31. doi: 10.1186/1471-2334-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borgherini G, Poubeau P, Jossaume A, Gouix A, Cotte L, Michault A, et al. Persistent arthralgia associated with chikungunya virus: a study of 88 adult patients on reunion island. Clin Infect Dis. 2008;47:469–75. doi: 10.1086/590003. [DOI] [PubMed] [Google Scholar]
- 11.Larrieu S, Pouderoux N, Pistone T, Filleul L, Receveur MC, Sissoko D, et al. Factors associated with persistence of arthralgia among chikungunya virus-infected travellers: report of 42 French cases. J Clin Virol. 47:85–8. doi: 10.1016/j.jcv.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Charrel RN, de Lamballerie X, Raoult D. Chikungunya outbreaks--the globalization of vectorborne diseases. N Engl J Med. 2007;356:769–71. doi: 10.1056/NEJMp078013. [DOI] [PubMed] [Google Scholar]
- 13.Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3:e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsetsarkin KA, McGee CE, Volk SM, Vanlandingham DL, Weaver SC, Higgs S. Epistatic roles of E2 glycoprotein mutations in adaption of chikungunya virus to Aedes albopictus and Ae. aegypti mosquitoes. PLoS One. 2009;4:e6835. doi: 10.1371/journal.pone.0006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng LF, Chow A, Sun YJ, Kwek DJ, Lim PL, Dimatatac F, et al. IL-1beta, IL-6, and RANTES as biomarkers of Chikungunya severity. PLoS ONE. 2009;4:e4261. doi: 10.1371/journal.pone.0004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mavalankar D, Shastri P, Bandyopadhyay T, Parmar J, Ramani KV. Increased mortality rate associated with chikungunya epidemic, Ahmedabad, India. Emerg Infect Dis. 2008;14:412–5. doi: 10.3201/eid1403.070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suryawanshi SD, Dube AH, Khadse RK, Jalgaonkar SV, Sathe PS, Zawar SD, et al. Clinical profile of chikungunya fever in patients in a tertiary care centre in Maharashtra, India. Indian J Med Res. 2009;129:438–41. [PubMed] [Google Scholar]
- 19.Economopoulou A, Dominguez M, Helynck B, Sissoko D, Wichmann O, Quenel P, et al. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005-2006 outbreak on Reunion. Epidemiol Infect. 2009;137:534–41. doi: 10.1017/S0950268808001167. [DOI] [PubMed] [Google Scholar]
- 20.Hapuarachchi HC, Bandara KB, Sumanadasa SD, Hapugoda MD, Lai YL, Lee KS, et al. Re-emergence of Chikungunya virus in South-east Asia: virological evidence from Sri Lanka and Singapore. J Gen Virol. 2010;91:1067–76. doi: 10.1099/vir.0.015743-0. [DOI] [PubMed] [Google Scholar]
- 21.Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–27. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 22.Parola P, de Lamballerie X, Jourdan J, Rovery C, Vaillant V, Minodier P, et al. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis. 2006;12:1493–9. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanciotti RS. Chikungunya Virus in US Travelers Returning from India, 2006. Emerg Infect Dis. 2007;13:764–7. doi: 10.3201/eid1305.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turell MJ, Beaman JR, Tammariello RF. Susceptibility of selected strains of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) to chikungunya virus. J Med Entomol. 1992;29:49–53. doi: 10.1093/jmedent/29.1.49. [DOI] [PubMed] [Google Scholar]
- 25.James Martin Center for Nonproliferation Studies. [accessed Jan 2011];Chemical and Biological Weapons: Possession and Programs Past and Present. http://cns.miis.edu/cbw/possess.htm.
- 26.Tesh RB. Arthritides caused by mosquito-borne viruses. Annu Rev Med. 1982;33:31–40. doi: 10.1146/annurev.me.33.020182.000335. [DOI] [PubMed] [Google Scholar]
- 27.Eckels KH, Harrison VR, Hetrick FM. Chikungunya virus vaccine prepared by Tween-ether extraction. Appl Microbiol. 1970;19:321–5. doi: 10.1128/am.19.2.321-325.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison VR, Binn LN, Randall R. Comparative immunogenicities of chikungunya vaccines prepared in avian and mammalian tissues. Am J Trop Med Hyg. 1967;16:786–91. [PubMed] [Google Scholar]
- 29.Harrison VR, Eckels KH, Bartelloni PJ, Hampton C. Production and evaluation of a formalin-killed Chikungunya vaccine. J Immunol. 1971;107:643–7. [PubMed] [Google Scholar]
- 30.Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE, Jr, Lupton HW. Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine. 1986;4:157–62. doi: 10.1016/0264-410x(86)90003-4. [DOI] [PubMed] [Google Scholar]
- 31.White A, Berman S, Lowenthal JP. Comparative immunogenicities of Chikungunya vaccines propagated in monkey kidney monolayers and chick embryo suspension cultures. Appl Microbiol. 1972;23:951–2. doi: 10.1128/am.23.5.951-952.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enserink M. Infectious diseases. Massive outbreak draws fresh attention to little-known virus. Science. 2006;311:1085. doi: 10.1126/science.311.5764.1085a. [DOI] [PubMed] [Google Scholar]
- 33.Edelman R, Ascher MS, Oster CN, Ramsburg HH, Cole FE, Eddy GA. Evaluation in humans of a new, inactivated vaccine for Venezuelan equine encephalitis virus (C-84) J Infect Dis. 1979;140:708–15. doi: 10.1093/infdis/140.5.708. [DOI] [PubMed] [Google Scholar]
- 34.Nakao E, Hotta S. Immunogenicity of purified, inactivated chikungunya virus in monkeys. Bull World Health Organ. 1973;48:559–62. [PMC free article] [PubMed] [Google Scholar]
- 35.Tiwari M, Parida M, Santhosh SR, Khan M, Dash PK, Rao PV. Assessment of immunogenic potential of Vero adapted formalin inactivated vaccine derived from novel ECSA genotype of Chikungunya virus. Vaccine. 2009;27:2513–22. doi: 10.1016/j.vaccine.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 36.Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, Mangiafico JA. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg. 2000;62:681–5. doi: 10.4269/ajtmh.2000.62.681. [DOI] [PubMed] [Google Scholar]
- 37.Muthumani K, Lankaraman KM, Laddy DJ, Sundaram SG, Chung CW, Sako E, et al. Immunogenicity of novel consensus-based DNA vaccines against Chikungunya virus. Vaccine. 2008;26:5128–34. doi: 10.1016/j.vaccine.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9:776–88. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Couderc T, Khandoudi N, Grandadam M, Visse C, Gangneux N, Bagot S, et al. Prophylaxis and Therapy for Chikungunya Virus Infection. J Infect Dis. 2009;200:516–23. doi: 10.1086/600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang E, Volkova E, Adams AP, Forrester N, Xiao SY, Frolov I, et al. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine. 2008;26:5030–9. doi: 10.1016/j.vaccine.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strauss JH, Strauss EG. Recombination in alphaviruses. Seminars in Virology. 1997;8:85–94. [Google Scholar]
- 42.Akahata W, Yang ZY, Andersen H, Sun S, Holdaway HA, Kong WP, et al. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16:334–8. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seregin SS, Amalfitano A. Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors. Expert Opin Biol Ther. 2009;9:1521–31. doi: 10.1517/14712590903307388. [DOI] [PubMed] [Google Scholar]
- 44.Holman DH, Penn-Nicholson A, Wang D, Woraratanadharm J, Harr MK, Luo M, et al. A complex adenovirus-vectored vaccine against Rift Valley fever virus protects mice against lethal infection in the presence of preexisting vector immunity. Clin Vaccine Immunol. 2009;16:1624–32. doi: 10.1128/CVI.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raviprakash K, Wang D, Ewing D, Holman DH, Block K, Woraratanadharm J, et al. A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus. J Virol. 2008;82:6927–34. doi: 10.1128/JVI.02724-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swenson DL, Wang D, Luo M, Warfield KL, Woraratanadharm J, Holman DH, et al. Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections. Clin Vaccine Immunol. 2008;15:460–7. doi: 10.1128/CVI.00431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holman DH, Wang D, Raja NU, Luo M, Moore KM, Woraratanadharm J, et al. Multi-antigen vaccines based on complex adenovirus vectors induce protective immune responses against H5N1 avian influenza viruses. Vaccine. 2008;26:2627–39. doi: 10.1016/j.vaccine.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 48.Wang D, Hevey M, Juompan LY, Trubey CM, Raja NU, Deitz SB, et al. Complex adenovirus-vectored vaccine protects guinea pigs from three strains of Marburg virus challenges. Virology. 2006;353:324–32. doi: 10.1016/j.virol.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 49.Pratt WD, Wang D, Nichols DK, Luo M, Woraratanadharm J, Dye JM, et al. Protection of nonhuman primates against two species of Ebola virus infection with a single complex adenovirus vector. Clin Vaccine Immunol. 2010;17:572–81. doi: 10.1128/CVI.00467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schepp-Berglind J, Luo M, Wang D, Wicker JA, Raja NU, Hoel BD, et al. Complex adenovirus-mediated expression of West Nile virus C, PreM, E, and NS1 proteins induces both humoral and cellular immune responses. Clin Vaccine Immunol. 2007;14:1117–26. doi: 10.1128/CVI.00070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gardner J, Anraku I, Le TT, Larcher T, Major L, Roques P, et al. Chikungunya virus arthritis in adult wild-type mice. J Virol. 2010;84:8021–32. doi: 10.1128/JVI.02603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubinchik S, Norris JS, Dong JY. Construction, purification and characterization of adenovirus vectors expressing apoptosis-inducing transgenes. Methods Enzymol. 2002;346:529–47. doi: 10.1016/s0076-6879(02)46075-2. [DOI] [PubMed] [Google Scholar]
- 53.Rubinchik S, Woraratanadharm J, Schepp J, Dong JY. Improving the transcriptional regulation of genes delivered by adenovirus vectors. Methods Mol Med. 2003;76:167–99. doi: 10.1385/1-59259-304-6:167. [DOI] [PubMed] [Google Scholar]
- 54.Wang D, Schmaljohn AL, Raja NU, Trubey CM, Juompan LY, Luo M, et al. De novo syntheses of Marburg virus antigens from adenovirus vectors induce potent humoral and cellular immune responses. Vaccine. 2006;24:2975–86. doi: 10.1016/j.vaccine.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 55.Ozden S, Lucas-Hourani M, Ceccaldi PE, Basak A, Valentine M, Benjannet S, et al. Inhibition of Chikungunya virus infection in cultured human muscle cells by furin inhibitors: impairment of the maturation of the E2 surface glycoprotein. J Biol Chem. 2008;283:21899–908. doi: 10.1074/jbc.M802444200. [DOI] [PubMed] [Google Scholar]
- 56.Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schroder WA, Le TT, Major L, Street S, Gardner J, Lambley E, et al. A physiological function of inflammation-associated SerpinB2 is regulation of adaptive immunity. J Immunol. 2010;184:2663–70. doi: 10.4049/jimmunol.0902187. [DOI] [PubMed] [Google Scholar]
- 58.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 1998;212:187–92. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 59.Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–77. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 60.Ziegler SA, Lu L, da Rosa AP, Xiao SY, Tesh RB. An animal model for studying the pathogenesis of chikungunya virus infection. Am J Trop Med Hyg. 2008;79:133–9. [PubMed] [Google Scholar]
- 61.Couderc T, Chretien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, et al. A Mouse Model for Chikungunya: Young Age and Inefficient Type-I Interferon Signaling Are Risk Factors for Severe Disease. PLoS Pathog. 2008;4:e29. doi: 10.1371/journal.ppat.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.La Linn M, Eble JA, Lubken C, Slade RW, Heino J, Davies J, et al. An arthritogenic alphavirus uses the alpha1beta1 integrin collagen receptor. Virology. 2005;336:229–39. doi: 10.1016/j.virol.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 63.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Appaiahgari MB, Saini M, Rauthan M, Jyoti, Vrati S. Immunization with recombinant adenovirus synthesizing the secretory form of Japanese encephalitis virus envelope protein protects adenovirus-exposed mice against lethal encephalitis. Microbes Infect. 2006;8:92–104. doi: 10.1016/j.micinf.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 65.Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77:6305–13. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cohen P. Immunity's yin and yang. A successful vaccine must first avoid being eliminated by pre-existing immunity before it can promote a protective immune response. IAVI Report. 2006;10:1–5. [PubMed] [Google Scholar]
- 67.Van Kampen KR, Shi Z, Gao P, Zhang J, Foster KW, Chen DT, et al. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine. 2005;23:1029–36. doi: 10.1016/j.vaccine.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 68.Xiang ZQ, Gao GP, Reyes-Sandoval A, Li Y, Wilson JM, Ertl HC. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J Virol. 2003;77:10780–9. doi: 10.1128/JVI.77.20.10780-10789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang DC, Zhang J, Toro H, Shi Z, Van Kampen KR. Adenovirus as a carrier for the development of influenza virus-free avian influenza vaccines. Expert Rev Vaccines. 2009;8:469–81. doi: 10.1586/erv.09.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Evans RK, Nawrocki DK, Isopi LA, Williams DM, Casimiro DR, Chin S, et al. Development of stable liquid formulations for adenovirus-based vaccines. J Pharm Sci. 2004;93:2458–75. doi: 10.1002/jps.20157. [DOI] [PubMed] [Google Scholar]
- 71.Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest. 2010;120:894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]