Abstract
The afferent vagus transmits sensory information from the gastrointestinal (GI) tract and other viscera to the brainstem via a glutamatergic synapse at the level of the nucleus of the solitary tract (NTS). Second order NTS neurons integrate this sensory information with inputs from other CNS regions that regulate autonomic functions and homeostasis. Glutamatergic and GABAergic neurons are responsible for conveying the integrated response to other nuclei, including the adjacent dorsal motor nucleus of the vagus (DMV). The preganglionic neurons in the DMV are the source of the parasympathetic motor response back to the GI tract. The glutamatergic synapse between the NTS and DMV is unlikely to be tonically active in regulating gastric motility and tone although almost all neurotransmitters tested so far modulate transmission at this synapse. In contrast, the tonic inhibitory GABAergic input from the NTS to the DMV appears to be critical in setting the tone of gastric motility and, under basal conditions, is unaffected by many neurotransmitters or neurohormones.
This review is based, in part, on a presentation by Dr Browning at the 2009 ISAN meeting in Sydney, Australia and discusses how neurohormones and macronutrients modulate glutamatergic transmission to NTS neurons and GABAergic transmission to DMV neurons in relation to sensory information that is received from the GI tract. These neurohormones and macronutrients appear to exert efficient “on-demand” control of the motor output from the DMV in response to ever-changing demands required to maintain homeostasis.
Keywords: vagus, brainstem, gastrointestinal, plasticity, receptor trafficking
Brainstem autonomic circuitry is critical for integrating homeostatic functions including, amongst other functions, the co-ordination of vago-vagal reflexes and regulation of food ingestion and the functions of the gastrointestinal (GI). Recent data from a number of laboratories, including our own, indicate that vagally-mediated GI functions are dramatically influenced by many GI hormones acting at sites within this brainstem circuitry.
Control of the gastrointestinal tract by vago-vagal reflexes
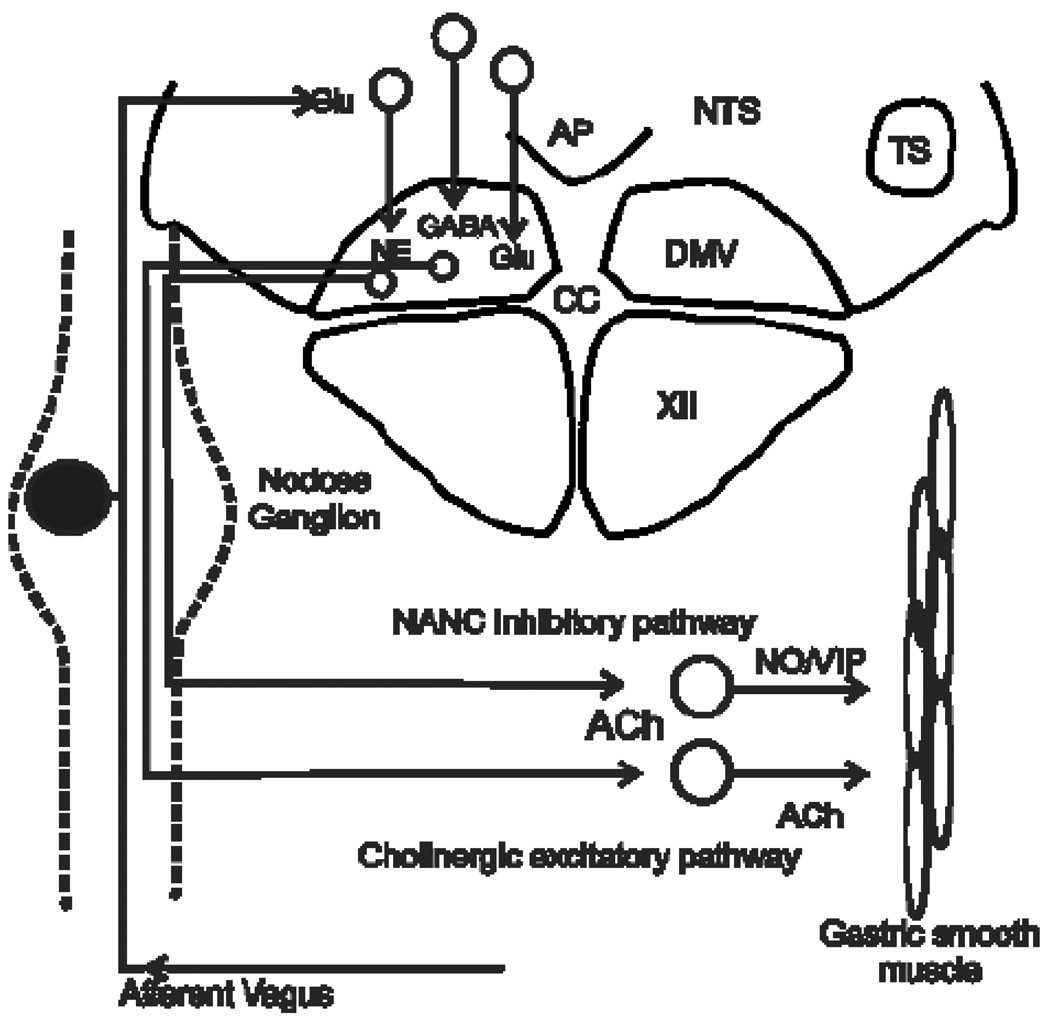
The central terminals of vagal afferent neurons transmit visceral sensory information to the brainstem through a glutamatergic synapse at the level of the NTS (Andresen & Kunze, 1994;Andresen & Yang, 1990;Hornby, 2001;Travagli et al., 2006;Jean, 2001). Second order NTS neurons display heterogeneous properties and contribute to the integration of synaptic input from vagal afferent axons as well as input from higher centers in the CNS that are involved in regulating autonomic functions and maintaining homeostasis (Bailey et al., 2006;Bailey et al., 2008;Bonham & Chen, 2005;Jin et al., 2004b;Travagli et al., 2006). These converging inputs provide information that is assimilated and integrated with metabolic and hormonal signals to shape the output from the NTS. Among other central autonomic areas, NTS neurons project to the adjacent DMV, where the parasympathetic preganglionic neurons that supply the vagal motor output to the GI tract are located. The cholinergic DMV neurons that control gastrointestinal function innervate postganglionic neurons located within the gastrointestinal tract. Parasympathetic postganglionic neurons are involved in two distinct pathways that control gastric functions. One is an excitatory cholinergic pathway that increases gastric tone, motility and secretion via activation of muscarinic cholinergic receptors. The other is an inhibitory non-adrenergic, noncholinergic (NANC) pathway that inhibits gastric functions mainly by releasing nitric oxide (NO) or vasoactive intestinal polypeptide (VIP) (reviewed in (Travagli et al., 2006). Either activation of the NANC pathway or inhibition of the tonic cholinergic pathway can inhibit gastric function (Figure 1).
Figure 1. Schematic diagram illustrating vago-vagal reflex control of the stomach.
The afferent vagus nerve relays sensory information from the GI tract centrally through cell bodies of sensory neurons in the paired nodose ganglia. Afferent axons carrying GI-related signals enter the dorsal medulla through the solitary tract (TS) and terminate within the NTS, where they use glutamate as their main neurotransmitter. Visceral afferent signals are integrated with inputs from other brainstem and higher CNS nuclei by NTS neurons. The resultant response is transmitted to the adjacent DMV, which contains the preganglionic parasympathetic motor neurons that provide the principal motor output to the stomach, and other central regions.
DMV neurons are cholinergic, and activate nicotinic cholinergic receptors on postganglionic neurons within the stomach. Two distinct postganglionic pathways are involved in regulating gastric activity. A cholinergic excitatory pathway increases motility and tone through activation of muscarinic cholinergic receptors on gastric smooth muscle. A non-adrenergic, non-cholinergic pathway decreases gastric tone and motility by releasing mainly nitric oxide (NO) and vasoactive intestinal polypeptide (VIP). Thus, either withdrawal of tonic cholinergic activity or via activation of the non-adrenergic, non-cholinergic (NANC) pathway can inhibit gastric functions.
Vagal reflexes are critical for the control and integration of visceral functions. As a result, GI pathologies and digestive disorders, including gastroparesis, dyspepsia, esophageal reflux, colitis, anorexia and bulimia nervosa, are often a consequence of, or are associated with, dysfunctional vagal reflexes (Yamano et al., 1997;Saito et al., 2006;Thumshirn, 2002;Ghia et al., 2007;Andrews & Sanger, 2002;Hornby & Abrahams, 2000;Faris et al., 2008).
DMV neurons that project to the stomach are remarkable in that they exhibit slow (1–2 Hz) spontaneous pacemaker-like activity (in vitro as well as in vivo; (Fogel et al., 1996;Zhang et al., 1992;Travagli et al., 1991) the rate of which can be modulated by synaptic inputs. Although NTS neurons have diverse biophysical and neurochemical properties, functional studies have determined that they provide primarily glutamatergic, noradrenergic and/or GABAergic input to gastric-projecting DMV neurons (reviewed in (Travagli et al., 2006). Gastric tone and motility are increased dramatically by microinjections of GABAergic antagonists into the dorsal vagal complex (DVC = NTS, DMV plus area postrema). In contrast, microinjections of antagonists against glutamate receptors or adrenoceptors have little effect (Sivarao et al., 1998;Travagli et al., 2006;Rogers et al., 2003). These data suggest the existence of a tonic GABAergic input from the NTS to DMV that regulates the firing rate of gastric-projecting DMV neurons and, therefore, gastric vagal motor output.
The observations above also imply that, even in periods of gastric inactivity, vagal efferent outflow to the stomach is continuously sculpted by afferent inputs, including sensory vagal, descending CNS and humoral inputs that modulate the activity of DMV neurons. Vagally-regulated GI functions must exhibit extraordinary adaptive plasticity to ensure an appropriate response to a variety of intrinsic and extrinsic factors, including food, stress and even time of day. An appropriate response is particularly important because even minor changes in neuronal input can influence the inherent pacemaker activity of DMV neurons. Changes in pacemaker activity would alter vagal motor output, causing major changes in gastric motility and tone as well as in responses to vago-vagal reflexes.
Although GABAergic transmission plays a prominent role in brainstem vagal circuits, several groups have been unable to demonstrate modulation of GABAergic synaptic transmition to DMV neurons even by neurotransmitters and neuromodulators known to affect vagal control of gastric functions. This resistance to modulation at such an important synapse led us to question its physiological relevance and benefit. We have begun to address this issue using the well-characterized µ-opioid receptor-effector system, which has a well-defined pharmacology and readily available receptor-selective agonists and antagonists (Browning et al., 2004;Browning et al., 2006). there is also evidence of the same type of effects for serotonin acting at the 5HT-1A receptor (Browning & Travagli, 2001), for oxytocin acting at the OT-1 receptor (Browning et al., 2009), for pancreatic polypeptides acting at Y1 and Y2 receptors (Browning & Travagli, 2009) and for norepinephrine acting at adrenoceptors (Browning and Travagli, unpublished data).
Plasticity at the NTS-DMV synapse depends on cAMP
We have shown that, under basal conditions, low resting levels of cAMP within NTS inhibitory nerve terminals mean that µ-opioid peptides do not modulate GABAergic synaptic transmission to DMV neurons regulating GI function (Browning & Travagli, 2001;Browning et al., 2004;Browning et al., 2002). We subsequently tested the effects of various compounds that raised cAMP levels (forskolin, the satiety peptides CCK or GLP-1, the stress-related neurotransmitters, TRH or CRF, to activate adenylate cyclase directly; IBMX to inhibit phosphodiesterase activity; 8-bromo cAMP, to provide a non-hydrolysable store of cAMP) and “uncovered” the ability of µ-opioid peptides to inhibit transmission at the GABAergic NTS-DMV synapse. In contrast, when we applied drugs that lowered cAMP levels (adenylate cyclase inhibitors, such as dideoxyadenosine) or inhibited the cAMP-PKA pathway (Rp-cAMPs or H89), the ability of adenylate cyclase activators to unmask the modulation of transmission at GABAergic synapses by µ-opioid peptides was abolished.
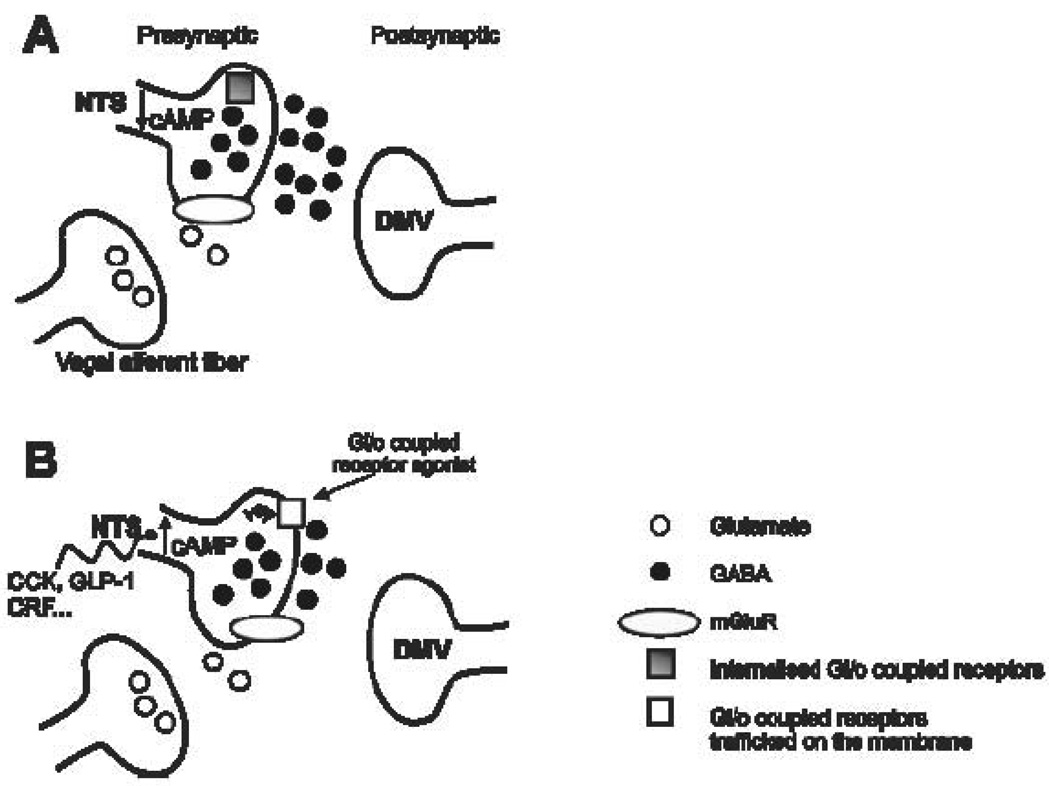
The effects of manipulating cAMP levels occurred within 5 minutes and disappeared relatively quickly, with the presynaptic inhibition mediated through µ-opioid and polypeptide Y1 & Y2 receptors lasting only about 60 minutes. The rapidity and transience of these effects suggest that de novo synthesis of new receptors was not involved in the activation of the cAMP-PKA pathway within GABAergic nerve terminals in the NTS. Instead, the likely mechanism for activation is the trafficking of pre-formed, internalized receptors to the nerve terminal membrane. This conclusion was supported by the effects on cAMP-PKA activation of the Golgi-disrupting agent, Brefeldin-A, or of low temperatures (Browning et al., 2004). Activation of the cAMP-PKA pathway also led to an increase in the occurrence of Gi/o coupled receptors such as µ-opioid receptors (Browning et al., 2004) and OT1 receptors (Figure 2) in terminals that were immunoreactive for GAD67, the GABA synthesizing enzyme, including in GABAergic terminals that closely apposed DMV neurons.
Figure 2. Schematic diagram illustrating the cAMP-dependent trafficking of Gi/o coupled receptors on GABAergic NTS terminals.
GABAergic NTS neurons provide a tonic inhibitory input onto vagal efferent DMV motoneurons. Glutamate released from vagal afferent (monosynaptic) terminals activates mGluR present on GABAergic terminals, decreasing cAMP levels within the nerve terminal (A). This, in turn, results in internalization of Gi/o coupled receptors, such as µ-opioid, oxytocin (OT-1), 5-hydroxytryptamine 5-HT1A, neuropeptide Y1 and Y2 receptors, removing their ability to modulate inhibitory synaptic transmission between NTS and DMV. Exposure to neurohormones such as the satiety peptides cholecystokinin (CCK) and glucagon-like peptide-1 (GLP-1) or the stress neurotransmitter corticotrophin releasing factor (CRF) activates adenylate cyclase and increases cAMP levels within the nerve terminal, overcoming the effects of metabotropic glutamate receptor (mGluR) activation (B). This results in trafficking of Gi/o coupled receptors to the nerve terminal, allowing their activation to modulate inhibitory synaptic transmission to DMV neurons.
These observations led us to hypothesize that the ability of neurotransmitters or neuromodulators to affect inhibitory transmission to DMV neurons that regulate GI functions is dictated by the level of cAMP (or the level of activity in the cAMP-PKA pathway) in the presynaptic GABAergic nerve terminals. Under basal or resting conditions, when cAMP levels are low, receptors negatively coupled to adenylate cyclase are concentrated inside the synaptic terminal such that neurotransmitters or modulators cannot bind to them and affect GABA transmission. When the cAMP level increases, or the cAMP-PKA pathway is activated in the nerve terminal, the internalized receptors move rapidly and transiently to the membrane of the terminal, permitting other transmitters to bind to their receptors and modulate GABA release.
If this hypothesis is correct, it raises a number of other questions, including: what mechanisms keep levels of cAMP in GABAergic NTS nerve terminals low, and why are GABAergic terminals regulated by cAMP levels but not glutamatergic terminals? To answer these questions we investigated whether the afferent (sensory) vagus plays a role in regulating cAMP levels within GABAergic NTS nerve terminals, since input from higher centers in the CNS is not required for vagally-mediated GI reflexes.
Vagal afferents control cAMP levels in GABAergic terminals in the NTS
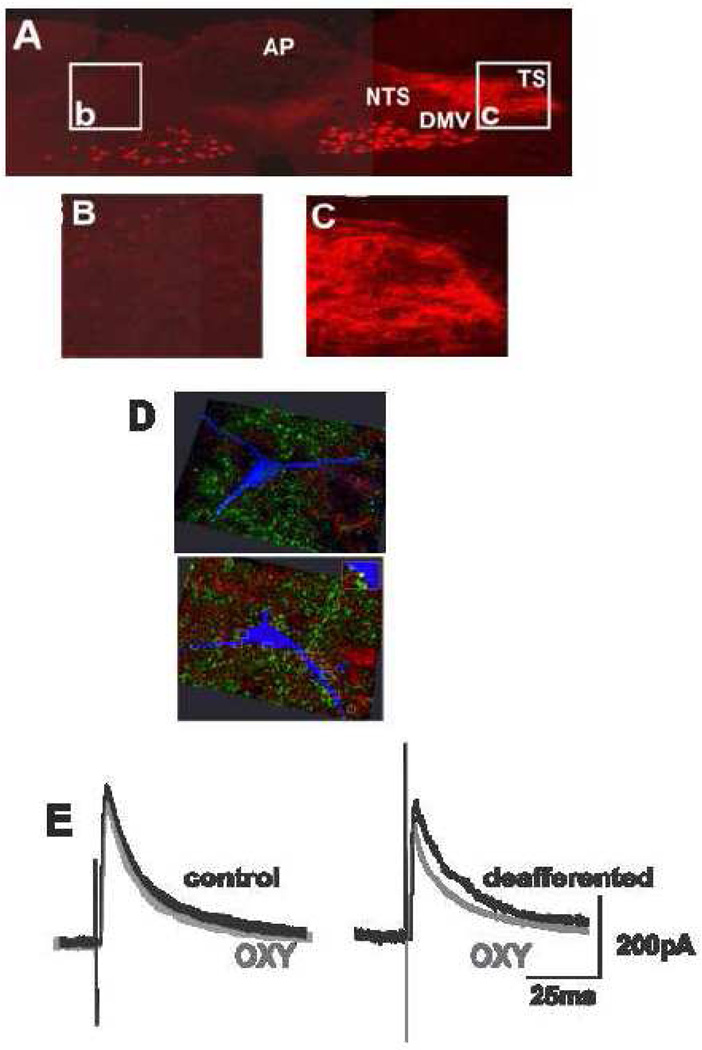
We subsequently showed that GABAergic transmission to DMV neurons involved in regulating GI function could be inhibited without increasing cAMP levels (Browning et al., 2006) if we selectively removed vagal afferent inputs either by perivagal application of capsaicin (Holzer et al., 1991) or by surgical rhizotomy. Furthermore, brainstem levels of cAMP were increased by vagal deafferentation as was the co-localization of µ-opioid receptors and markers of GABAergic transmission in nerve terminals in the NTS (Browning et al., 2006) (see also Figure 3, demonstrating the co-localization of OT-1 receptors on GABAergic nerve terminals within the NTS).
Figure 3. Deafferentation increases co-localization of oxytocin receptors on GABAergic nerve terminals within the brainstem and allows modulation of inhibitory synaptic transmission between the NTS and DMV.
A, B,C: Anatomical confirmation of successful deafferentation surgery (rootlet rhizotomy) in a rat that received an injection of rhodamine dextran into the corpus. Coronal section through the rostral dorsal vagal complex (DVC). Note that rhodamine dextran-labeled vagal afferent terminals are absent from the solitary tract (TS) or NTS (A,B) on the deafferented side of the brainstem (left). In contrast, a dense vagal afferent innervation is present the intact side of the brainstem (right) receives (A,C). Also note that both right and left DMV contain labelled preganglionic motoneurons, confirming that the surgery is selective for vagal afferents.
D: Brainstem sections containing the DMV were immunohistochemically processed to reveal the oxytocin-1 receptor with a red fluorophore and GAD67 (γ-aminodecarboxylase; the enzyme required for GABA synthesis, hence used as a marker for GABAergic nerve terminals), with a green fluorophore. Note that, in the deafferented brainstem (upper micrograph) oxytocin-1 receptors (red) and GAD (green) are often co-localized (yellow; arrows) and that some of the GAD terminals containing oxytocin receptors appose a Neurobiotin-filled DMV neuron (blue). In contrast, in a vagally-intact brainstem (lower micrograph), few, if any, terminals contain co-localized GAD and oxytocin-1 receptors.
E: Whole cell patch clamp recordings of DMV neurons voltage clamped at −50mV from control (left) and deafferented (right) brainstems. electrical stimulation of the adjacent NTS evoked GABAergic inhibitory currents in the recorded neurons.. In vagally-intact brainstems, oxytocin (100nM) did not affect the amplitude of evoked GABAergic currents (left) whereas, in deafferented brainstems, the amplitude of evoked GABAergic currents in DMV neurons was decreased by the application of oxytocin.
Adapted, in part, from Browning et al. 2004 & (Wan et al., 2008).
These data suggest that vagal afferent inputs do indeed regulate cAMP levels within GABAergic nerve terminals in the NTS and therefore regulate the ability of transmitters to modulate inhibitory synaptic transmission to GI-related DMV neurons. cAMP within nerve terminals are not only important modulators of neurotransmitter release, but under certain circumstances, may actually dominate the release of neurotransmitter. cAMP can facilitate synaptic transmission by acting at multiple sites, from regulating the availability of intracellular calcium, to the sequence of events leading to exocytosis (Chavis et al., 1998;Ingram et al., 1998;Chavez-Noriega & Stevens, 1994;Leenders & Sheng, 2005). The stimulus-dependent insertion of proteins and receptors into the plasma membrane occurs commonly in many systems, including the CNS (Brismar et al., 1998); (Passafaro et al., 1996;Quick et al., 1997;Shuster et al., 1999). In fact, previously silent synapses have been shown to release neurotransmitters spontaneously following activation of the cAMP/PKA pathway (Chavis et al., 1998). Increasing the intracellular levels of cAMP is also likely to increase the sensitivity of the synapse to activation of receptors negatively coupled to adenylate cyclase (such as µ-opioid, 5-HT1A, pancreatic polypeptide Y1 and Y2 as well as oxytocin OT1 receptors).
As mentioned above, vagal afferent axons use glutamatergic synapses to convey sensory input to the brainstem (Andresen & Kunze, 1994;Andresen & Yang, 1990;Hornby, 2001;Travagli et al., 2006;Jean, 2001). Transmission of this visceral sensory information relies upon the activation of ionotropic NMDA and non-NMDA receptors. Vago-vagal neurocircuits also express metabotropic glutamate receptors (mGluR), indeed mRNA for all 8 mGluR identified to date (mGluR1–8) have been identified in vagal afferent neurons (Page et al., 2005;Hoang & Hay, 2001) and their peripheral terminals and may play prominent roles in gastric and gastroesophageal vagal afferent sensitivity (Page et al., 2005;Young et al., 2008;Young et al., 2007a). Activation of group II mGluR (mGluR2 & 3) and group III mGluR (mGluR4,7,&8), for example, decreases vagal afferent sensitivity and relaxation of the lower esophageal sphincter (Young et al., 2008;Page et al., 2005). In contrast, activation of group I mGluR (mGluR1&5) increases vagal afferent activity and lower esophageal sphincter relaxation; antagonists of mGluR5 in particular may be of benefit in the treatment of esophageal reflux conditions since they appear to exert few, if any, central side effects (Young et al., 2007b).
Centrally, group II and III mGluR are located mainly at presynaptic sites in the DVC and are negatively coupled to adenylate cyclase. Because metabotropic receptors operate through signaling mechanisms that involve G-proteins, the effects of activation of these receptors on sensory integration and synaptic transmission may be longer-lasting (Hay & Lindsley, 1995;Hay et al., 1999;Glaum & Miller, 1992;Glaum & Miller, 1993;Jin et al., 2004a;Andresen & Yang, 1990;Chen et al., 2002;Chen & Bonham, 2005). Thus, mGluR on GABAergic terminals in the NTS could be critical for regulating cAMP levels and, therefore, the modulation of synaptic transmission. We have demonstrated that the level of cAMP within GABAergic terminals in the NTS depends upon ongoing activation of group II, but not group III, mGluR (Browning & Travagli, 2007). If we applied group II mGluR antagonists to brainstem slice preparations, cAMP levels were elevated sufficiently for µ-opioid receptors to translocate to the terminal membrane such that their activation could inhibit synaptic transmission to GI-related DMV neurons. When we removed glutamatergic inputs by disrupting vagal afferents, these group II mGluR no longer experienced tonic activation and, by consequence, it was no longer necessary to exogenously increase cAMP levels in order to modulate inhibitory synaptic transmission. Although modulating transmission in this way may appear rather unwieldy, this mechanism could be convenient and metabolically “cheap”, providing visceral afferent inputs with ‘on-demand’ control the efferent motor response (Browning & Travagli, 2006;Browning & Travagli, 2007). The principal neurotransmitter that vagal afferent inputs use to control NTS neurons is glutamate. By activating metabotropic, rather than ionotropic, receptors with the same neurotransmitter, vagal afferent inputs can regulate the tonic GABAergic input onto DMV neurons, thereby setting the “tone” of vagal efferent motor output. Using this mechanism, visceral inputs themselves can easily “gate” the efferent output in response to homeostatic demands.
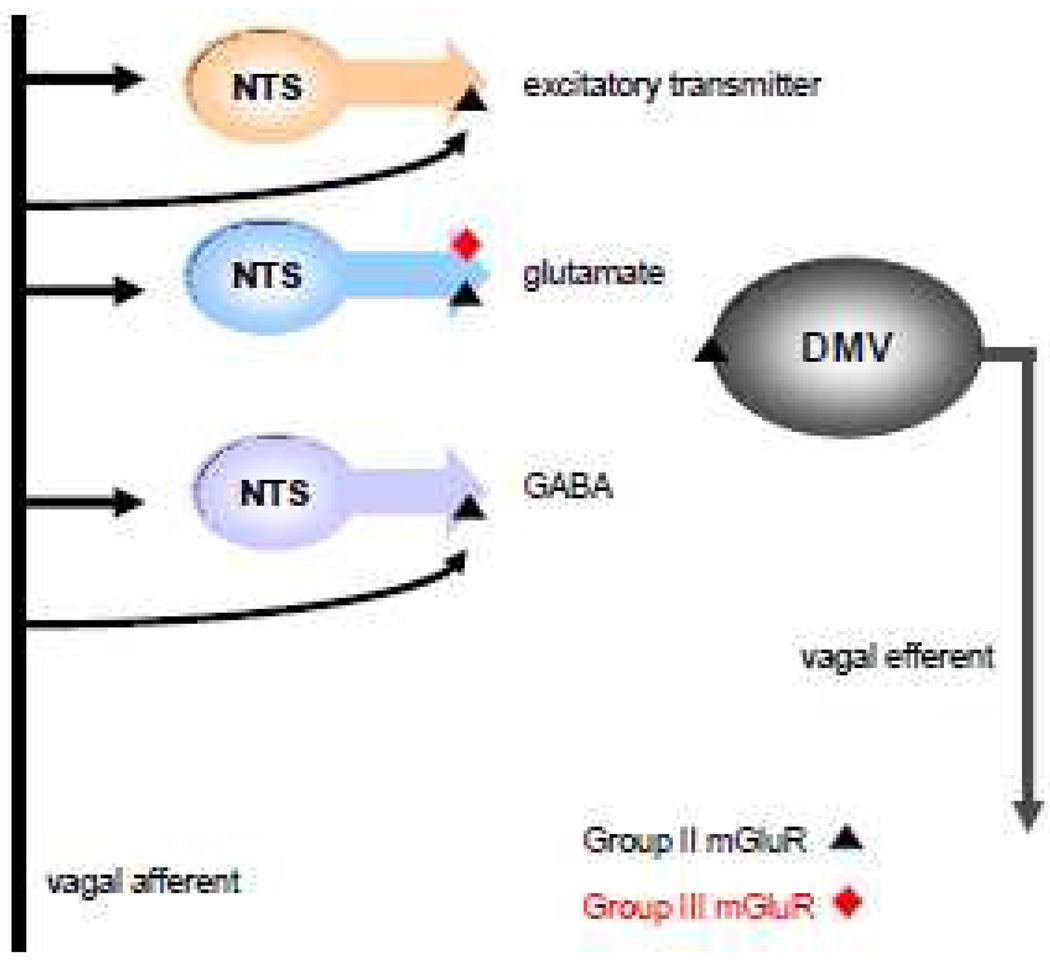
In contrast to GABAergic terminals in the NTS, glutamatergic terminals display both functional group II and group III mGluR (Browning & Travagli, 2007). Thus, the main difference between glutamatergic and GABAergic terminals in the NTS is probably in the monosynaptic input that they receive from vagal afferent axons. There is no tonic activation of either group II or group III mGluR on glutamatergic NTS terminals because they do not appear to receive a direct monosynaptic input from vagal afferents. As a result, there is no suppression of cAMP levels within glutamatergic NTS nerve terminals and their excitatory synaptic transmission to GI-related DMV neurons can be modulated, even in the basal state. Hence, there is likely to be a fundamental difference in vagal afferent-driven glutamatergic transmission to DMV neurons and vagal afferent-driven GABAergic transmission to DMV neurons, with the anatomical organization of vagal brainstem neurocircuitry underlying the two types of transmission being quite distinct (see Figure 4).
Figure 4. Proposed schematic representation of the location of groups II and III mGluR within gastric brainstem circuits.
Within the rat brainstem, GABAergic, glutamatergic and other excitatory nerve terminals impinging upon gastric-projecting DMV neurons express presynaptic group II mGluR. These receptors are also present on a subpopulation of DMV neurons. Glutamatergic nerve terminals also express Group III mGluR. GABAergic but not glutamatergic terminals receive monosynaptic glutamatergic input from vagal afferent terminals that is tonically active. Previous studies (Browning & Travagli, 2007) suggest that non-glutamatergic excitatory terminals impinging upon gastric-projecting DMV neurons may also receive a tonically-active monosynaptic input from vagal afferent terminals. Adapted from Browning & Travagli, J. Neuroscience 2007.
Plasticity of vagal brainstem circuits and functional dyspepsia
Functional dyspepsia (FD), or the presence of gastroduodenal symptoms in the absence of any organic, systemic or metabolic explanation, includes a constellation of gastrointestinal symptoms such as impaired gastric emptying, reduced gastric compliance, early satiety and weight loss (Tack et al., 2006). Although the pathophysiology of FD is incompletely understood, there are several indications that vago-vagal reflex control of the upper GI tract is impaired. For example, it has been suggested that FD may from either inadequate vagal sensory stimulation (Lunding et al., 2008;Holtmann et al., 1998;Greydanus et al., 1991) or from excessive or hypersensitive vagal sensory stimulation as a consequence of either insult or injury (Holzer, 2006), including abnormal acid challenge (Holzer, 2003;Holzer et al., 2003).
Although there is variability among subgroups of patients, it has long been known that food ingestion and/or psychosocial factors, such as stress, can aggravate the symptoms of dyspepsia. Interestingly, neurotransmitters, neuromodulators and neurohumoral agents that are positively coupled to adenylate cyclase, including GLP-1, CCK and CRF, are released in response to both food ingestion and stress; and these neuroactive chemicals ultimately cause an increase in brainstem cAMP levels (Berthoud et al., 2006;Drucker, 2006;Dufresne et al., 2006). Stress-related changes in gastric motility have been identified as one of the main pathophysiological mechanisms underlying dyspeptic symptoms and stressful life events initiate or exacerbate these symptoms in most dyspepsia patients (Tack & Lee, 2005;Mimidis & Tack, 2008). In light of these findings, we have developed the following working hypothesis based on our data and information from other laboratories: vagal afferent terminals release glutamate on NTS neurons that regulate GI function. At “rest” or during periods of minimal feed-back from the GI tract, not enough glutamate is released from vagal terminals to evoke “full-scale” vago-vagal reflexes. However, enough glutamate is released to activate mGluRs present at the NTS-DMV synapse since they have an affinity for glutamate that is several orders of magnitude greater than the affinity of ionotropic receptors. mGluR activation tonically inhibits vagal circuits by decreasing cAMP levels. As a result, the metabolically “inexpensive” neurotransmitter, glutamate, causes a profound and long-lasting dampening of the activity in vago-vagal circuitry.
After ingestion of food or a stressful event, an increase in adenylate cyclase activity induced by circulating hormones or local neurotransmitters activates brainstem vagal circuits. The tonic inhibition induced by mGluR is overcome by the increase in cAMP levels and/or activation of the cAMP-PKA pathway. As a result, Gi/o coupled-receptors move to the neuronal membrane of GABA-releasing terminals in the NTS. These trafficked receptors, together with the increase in cAMP, allow neurotransmitters/modulators to modulate the tonic GABAergic input from the NTS to DMV neurons. As a result, the vagal efferent outflow to the viscera can now undergo the adaptive changes that are necessary to prepare the GI tract for the appropriate ingestive and digestive responses. In functional dyspepsia patients, however, these adaptive changes do not occur and disrupted coupling in the sensory-motor vagal circuitry leads to altered gastric motility. In fact, these circuits may be in a constant “state of activation” in functional dyspepsia due to the enhanced release (or coupling) of stress- or meal-related hormones. These hormones increased cAMP levels in vago-vagal circuits, overcoming the dampening of effects mGluR on the GABAergic synapses. By consequence, signals from an otherwise harmless situation, such as meal ingestion, that usually induce plasticity and adaptability are processed inappropriately and gastric functions are disrupted.
Plasticity at the vagal afferent-NTS synapse depends on glucose
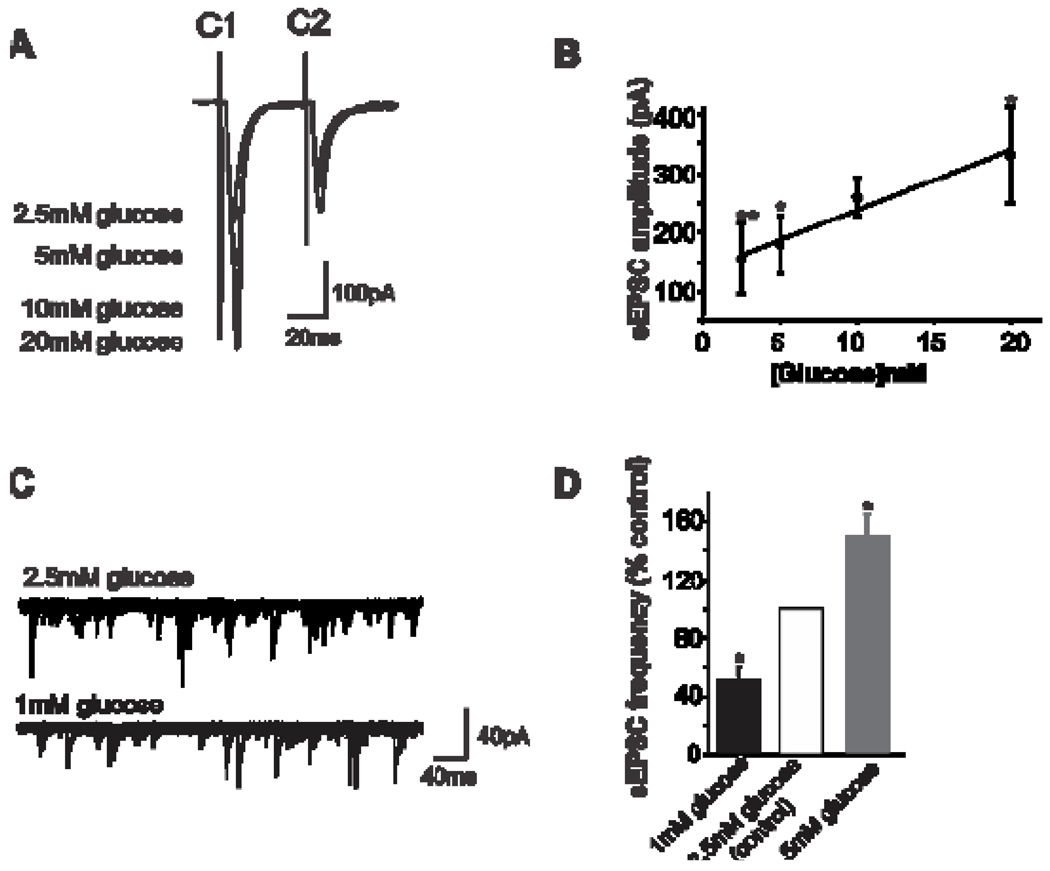
Plasticity within GI vagal brainstem circuits may not be restricted to the GABAergic synapse between the NTS and DMV. Recent evidence suggests that the first synapse in vago-vagal reflexes, i.e., the synapse between vagal afferent terminals and NTS neurons may be open to modulation (Wan & Browning, 2008a;Wan & Browning, 2008b). In fact, glutamatergic transmission from vagal afferent terminals increases or decreases with an elevation or reduction in extracellular glucose levels, respectively (Wan & Browning, 2008a). This effect of glucose to regulate excitatory synaptic transmission appears to involve alterations in either the number and/or the function of presynaptic 5-HT3 receptors, which act to facilitate glutamate release (Wan & Browning, 2008b) (Figure 5).
Figure 5. Glucose modulates glutamatergic synaptic transmission.
A: Representative traces showing that electrical stimulation of the solitary tract evoked paired glutamatergic excitatory postsynaptic currents (EPSCs), marked C1 and C2, in an NTS neuron voltage clamped at −60 mV. Glucose modulated the amplitude of the evoked EPSC was in a concentrationdependent manner.
B: Graphical representation showing that glucose concentration correlates with EPSC amplitude. *P<0.05 vs 10mM glucose; **P<0.05 vs 20mM glucose.
C: Six consecutive traces from an NTS neuron voltage clamped at −60mV showing that The frequency, but not the amplitude, of spontaneous EPSCs was decreased by lowering the extracellular glucose concentration from 2.5mM to 1.0 mM.
D: Graphical representation showing that glucose concentration correlates with the frequency of spontaneous EPSC. Note that “physiological” changes in the concentration of extracellular glucose also alter glutamatergic synaptic transmission to NTS neurons. *P<0.05 vs control.
Adapted from Wan & Browning, 2008
While they are undoubtedly lower than peripheral glucose levels, central glucose levels do appear to change in concert with blood glucose levels although over a much narrower range (Silver & Erecinska, 1994;Dunn-Meynell et al., 2009). Furthermore, the area postrema is entirely outside the blood-brain barrier and the NTS and DMV have a ‘leaky’ blood-brain barrier because of their fenestrated capillaries (Cottrell & Ferguson, 2004;Gross et al., 1990). Circulating factors can therefore more easily access neurons within the DVC and glucose levels within the NTS or DMV may actually be higher than in other central nuclei (Routh, 2002). Our preliminary evidence supports the idea that more “physiological” levels of extracellular glucose modulate glutamatergic transmission from vagal afferent terminals (Figure 5).
Plasticity of vagal brainstem circuits and gastric emptying
Vagal afferents regulating the GI tract are likely to be excited by glucose acting at a variety of central and peripheral sites. These would include peripheral vagal terminals within the intestine (Raybould & Zittel, 1995;Raybould et al., 2003), nerve cell bodies in the nodose ganglion (Grabauskas et al., 2006) and central vagal terminals within the brainstem (Wan & Browning, 2008a). Rather than redundancy, these multiple sites of action may provide a large safety margin for signal processing. Alternatively, glucose acting at multiple sites could amplify peripheral signals to produce a rapid and robust response in motor output. Gastric relaxation and a delay in gastric emptying result from glucose within the intestinal tract (Gentilcore et al., 2007;Rayner et al., 2001) so vagal afferent activation by glucose is likely to cause an inhibitory efferent motor response.
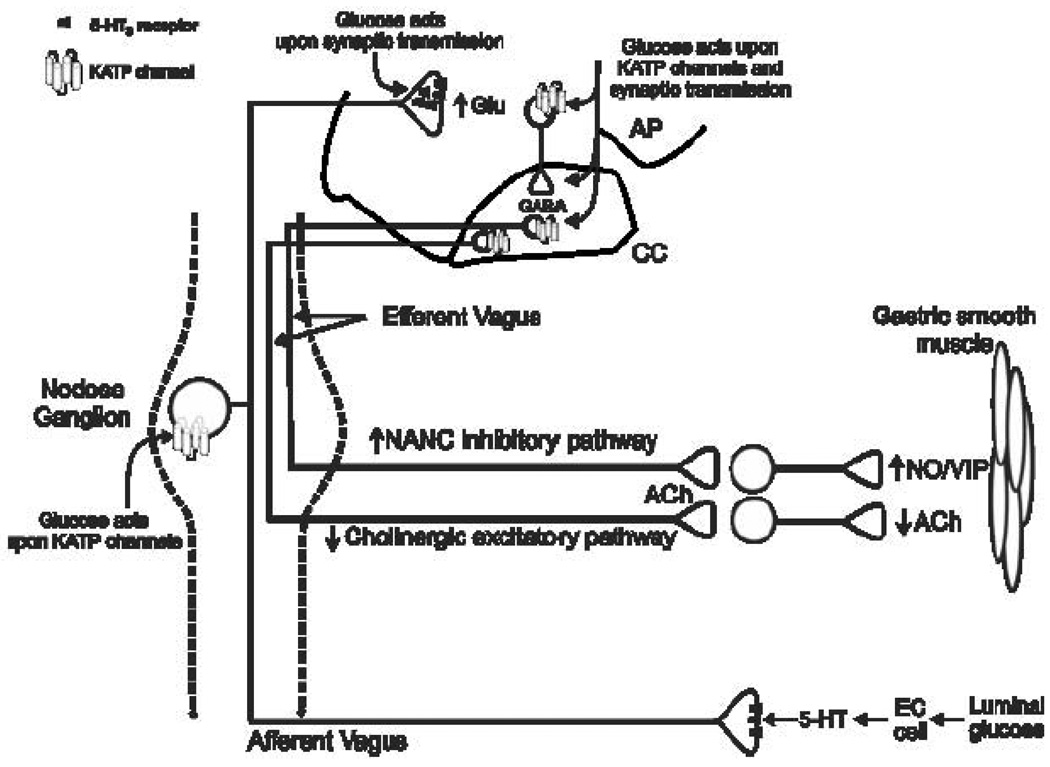
The precise mechanisms that underlie glucose-induced gastric inhibition remain controversial. As discussed above, an increase in the activity of the postganglionic parasympathetic NANC efferent pathway or an inhibition of the cholinergic efferent pathway could cause vagally-mediated inhibition of gastric function (Travagli et al., 2006) and published studies support the involvement of both pathways (Travagli et al., 2006) Part of the controversy stems from the different experimental methods used in these studies, but there are also problems with distinguishing the influence of peripheral versus central glucose effects on GI functions. In fact, it is possible that both cholinergic efferent and NANC efferent pathways are involved in glucose-dependent gastroinhibition. Glucose in the intestine, and its absorption into the blood, could first initiate vago-vagal reflexes through activation of NANC pathways. Subsequently, when glucose levels in the brainstem have risen, inhibition of cholinergic efferent pathways could cause a temporally distinct, prolonged direct gastroinhibition (Figure 6).
Figure 6. Schematic diagram illustrating the actions of glucose on vago-vagal reflexes controlling the stomach.
Glucose in the intestinal tract induces the release of serotonin from enterochromaffin (EC) cells(Freeman et al., 2006;Zhu et al., 2001). The released 5-HT acts upon 5-HT3 receptors present on the peripheral terminals of vagal afferent neurons to induce excitation (Savastano et al., 2005;Hillsley et al., 1998;Grundy, 2006); this peripheral signal is presumably relayed centrally via the afferent vagus nerve. Glucose, however, is also able to act directly upon vagal afferent neurons contained within the nodose ganglion via actions upon KATP channels to induce neuronal excitation (Grabauskas et al., 2009). Centrally, glucose acts to increase glutamate release from vagal afferent terminals (Wan & Browning, 2008a) by a mechanism that appears to involve 5-HT3 receptors(Wan & Browning, 2008b)). Glucose can also activate NTS neurons via actions upon KATP channels and increase synaptic transmission to gastric-projecting DMV neurons (Ferreira, Jr. et al., 2001;Shi et al., 2003). Glucose can also act directly upon DMV neurons to alter their excitability (Balfour & Trapp, 2007;Balfour et al., 2006;Trapp et al., 1994). The result of these peripheral and central actions is gastric relaxation and delayed gastric emptying (Raymer et al., 2000;Rayner et al., 2001;Schvarcz et al., 1997) which may involve both withdrawal of cholinergic excitation (Shi et al., 2003;Shi et al., 2005) as well as activation of non-adrenergic, non-cholinergic (NANC) efferent vagal pathways (Zhou et al., 2008).
Conclusions & Future Directions
Several laboratories have recently provided conclusive evidence that vagally-mediated GI reflexes do not rely on static neuronal circuits that produce stereotypical output responses to visceral signaling. Instead, these reflex pathways exhibit a high degree of modulation and plasticity. Within the brainstem circuits that regulate GI function, parasympathetic motor output can be modulated by visceral information, which can consists of either vagal afferent activity itself, GI-related neurohormones released in response to meal ingestion, macronutrients or a combination of these factors. GI responses are timed over either minutes or hours so that the response is appropriate to the homeostatic demands placed upon the gastrointestinal tract. These features of brainstem GI circuitry are clearly different to the circuitry mediating other autonomic reflexes. Hypertension, for example, causes modulation and plasticity in brainstem baroreflex circuits over much longer periods of time (1–5 weeks) (Tang & Dworkin, 2007b;Tang & Dworkin, 2007a;Hermes et al., 2008;Mei et al., 2003;Zhang et al., 2008). These differences support the long-standing idea that vagal pathways in the brainstem are organized into circuits that are anatomically and functionally specific.
Clearly, there is still much to be learned about the plasticity of vagal brainstem circuits. Serious long-term health consequences can result from malfunctioning GI vago-vagal reflexes but the precise mechanism(s) responsible for breakdowns in autonomic and homeostatic regulation remain to be discovered. Tonic vagal afferent input is obviously critical for setting output “tone” but the effects of long-term increases or decreases in vagal activity have not yet been investigated. Similarly, we know little about the effects of long-term alterations in the release of GI neurohormones, such as CCK and GLP-1, on vagally-mediated reflexes. The plasticity in vagal circuitry discussed here is readily reversible. Could more permanent re-modeling of brainstem vagal circuitry underlie chronic GI disturbances, such as functional dyspepsia or persistent reflux; and what circumstances would evoke more persistent remodeling? Is the “gain” of plasticity set during development and what processes might disrupt this gain-setting? Are all second messenger systems within vago-vagal brainstem circuits subject to plasticity? Clearly, these questions and many others must be answered before we can understand the role of vagal reflexes and plasticity in autonomic control of homeostasis.
Acknowledgements
The authors would like to thank the NIH (DK 55530) and NSF (IBN-08-18736) for their support. We also thank Cesare M. & Zoraide Travagli and W. Nairn Browning for support and encouragement.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Andresen MC, Kunze DL. Nucleus tractus solitarius--gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259:H1307–H1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol. 2002;2:650–656. doi: 10.1016/s1471-4892(02)00227-8. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS) J Neurophysiol. 2008;99:1712–1722. doi: 10.1152/jn.00038.2008. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci. 2006;26:11893–11902. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J Physiol. 2006;570:469–484. doi: 10.1113/jphysiol.2005.098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour RH, Trapp S. Ionic currents underlying the response of rat dorsal vagal neurones to hypoglycaemia and chemical anoxia. J Physiol. 2007 doi: 10.1113/jphysiol.2006.126094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H-R, Sutton GM, Townsend RL, Patterson LM, Zheng H. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol Behav. 2006;89:517–524. doi: 10.1016/j.physbeh.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Bonham AC, Chen C-Y. Synaptic transmission in the nucleus tractus solitarius (NTS) In: Undem BJ, Weinreich D, editors. Advances in Vagal Afferent Neurobiology. Boca Raton, FL: CRC Press; 2005. pp. 193–208. [Google Scholar]
- Brismar H, Asghar M, Carey RM, Greengard P, Aperia A. Dopamine-induced recruitment of dopamine D1 receptors to the plasma membrane. Proc Natl Acad Sci U S A. 1998;95:5573–5578. doi: 10.1073/pnas.95.10.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Holmes GM, Tong-Chamberlain M, Travagli RA. Plasticity in rat vago-vagal circuits may underlie meal-related exacerbation of functional dyspepsia symptoms. Neurogastroenterol.Motil. 2009;21 Suppl 1:141–142. Ref Type: Abstract. [Google Scholar]
- Browning KN, Kalyuzhny AE, Travagli RA. Opioid peptides inhibit excitatory but not inhibitory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Neurosci. 2002;22:2998–3004. doi: 10.1523/JNEUROSCI.22-08-02998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Kalyuzhny AE, Travagli RA. Mu-opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci. 2004;24:7344–7352. doi: 10.1523/JNEUROSCI.1676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. TRH uncovers the presence of presynaptic 5-HT1A receptors via activation of a second messenger pathway in the rat dorsal vagal complex. J Physiol. 2001;532:425–435. doi: 10.1111/j.1469-7793.2001.0425i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Short-term receptor trafficking in the dorsal vagal complex: an overview. Auton Neurosci. 2006;126–127:2–8. doi: 10.1016/j.autneu.2006.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Functional organization of presynaptic metabotropic glutamate receptors in vagal brainstem circuits. J Neurosci. 2007;27:8979–8988. doi: 10.1523/JNEUROSCI.1105-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Modulation of inhibitory neurotransmission in brainstem vagal circuits by NPY and PYY is controlled by cAMP levels. Neurogastroenterol Motil. 2009;21:1309–1318. doi: 10.1111/j.1365-2982.2009.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Zheng Z, Gettys TW, Travagli RA. Vagal afferent control of opioidergic effects in rat brainstem circuits. J Physiol. 2006;575:761–776. doi: 10.1113/jphysiol.2006.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Stevens CF. Increased transmitter release at excitatory synapses produced by direct activation of adenylate cyclase in rat hippocampal slices. J Neurosci. 1994;14:310–317. doi: 10.1523/JNEUROSCI.14-01-00310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P, Mollard P, Bockaert J, Manzoni O. Visualization of cyclic AMP-regulated presynaptic activity at cerebellar granule cells. Neuron. 1998;20:773–781. doi: 10.1016/s0896-6273(00)81015-6. [DOI] [PubMed] [Google Scholar]
- Chen CY, Bonham AC. Glutamate suppresses GABA release via presynaptic metabotropic glutamate receptors at baroreceptor neurones in rats. J Physiol. 2005;562:535–551. doi: 10.1113/jphysiol.2004.076885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Ling Eh EH, Horowitz JM, Bonham AC. Synaptic transmission in nucleus tractus solitarius is depressed by Group II and III but not Group I presynaptic metabotropic glutamate receptors in rats. J Physiol. 2002;538:773–786. doi: 10.1113/jphysiol.2001.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GT, Ferguson AV. Sensory circumventricular organs: central roles in integrated autonomic regulation. Regul Pept. 2004;117:11–23. doi: 10.1016/j.regpep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86:805–847. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Sanders NM, Compton D, Becker TC, Eiki J, Zhang BB, Levin BE. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J Neurosci. 2009;29:7015–7022. doi: 10.1523/JNEUROSCI.0334-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris PL, Hofbauer RD, Daughters R, Vandenlangenberg E, Iversen L, Goodale RL, Maxwell R, Eckert ED, Hartman BK. De-stabilization of the positive vago-vagal reflex in bulimia nervosa. Physiol Behav. 2008;94:136–153. doi: 10.1016/j.physbeh.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M, Jr, Browning KN, Sahibzada N, Verbalis JG, Gillis RA, Travagli RA. Glucose effects on gastric motility and tone evoked from the rat dorsal vagal complex. J Physiol. 2001;536:141–152. doi: 10.1111/j.1469-7793.2001.t01-1-00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel R, Zhang X, Renehan WE. Relationships between the morphology and function of gastric and intestinal distention-sensitive neurons in the dorsal motor nucleus of the vagus. J Comp Neurol. 1996;364:78–91. doi: 10.1002/(SICI)1096-9861(19960101)364:1<78::AID-CNE7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Freeman SL, Bohan D, Darcel N, Raybould HE. Luminal glucose sensing in the rat intestine has characteristics of a sodium-glucose cotransporter. Am J Physiol Gastrointest Liver Physiol. 2006;291:G439–G445. doi: 10.1152/ajpgi.00079.2006. [DOI] [PubMed] [Google Scholar]
- Gentilcore D, Little TJ, Feinle-Bisset C, Samsom M, Smout AJ, Horowitz M, Jones KL. Role of 5-hydroxytryptamine mechanisms in mediating the effects of small intestinal glucose on blood pressure and antropyloroduodenal motility in older subjects. Am J Physiol Gastrointest Liver Physiol. 2007;293:G692–G698. doi: 10.1152/ajpgi.00199.2007. [DOI] [PubMed] [Google Scholar]
- Ghia JE, Blennerhassett P, Collins SM. Vagus nerve integrity and experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G560–G567. doi: 10.1152/ajpgi.00098.2007. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ. Metabotropic glutamate receptors mediate excitatory transmission in the nucleus of the solitary tract. J Neurosci. 1992;12:2251–2258. doi: 10.1523/JNEUROSCI.12-06-02251.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ. Metabotropic glutamate receptors depress afferent excitatory transmission in the rat nucleus tractus solitarii. J Neurophysiol. 1993;70:2669–2672. doi: 10.1152/jn.1993.70.6.2669. [DOI] [PubMed] [Google Scholar]
- Grabauskas G, Song I, Zhou SY, Owyang C. Electrophysiological identifications of glucose-sensing neurons in the rat nodose ganglia. J Physiol. 2009 doi: 10.1113/jphysiol.2009.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabauskas G, Zhou SY, Song I, Owyang C. Nodose ganglia neurons demonstrate gluco-responsiveness mediated via voltage-dependent potassium channels. Gastroenterology DDW 2006. 2006;507(A74) Ref Type: Abstract. [Google Scholar]
- Greydanus MP, Vassallo M, Camilleri M, Nelson DK, Hanson RB, Thomforde GM. Neurohormonal factors in functional dyspepsia: insights on pathophysiological mechanisms. Gastroenterology. 1991;100:1311–1318. [PubMed] [Google Scholar]
- Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol. 1990;259:R1131–R1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- Grundy D. Serotonin and sensory signalling from the gastrointestinal lumen. J Physiol. 2006;575:1–2. doi: 10.1113/jphysiol.2006.113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay M, Lindsley KA. Metabotropic glutamate receptor inhibition of visceral afferent potassium currents. Brain Res. 1995;698:169–174. doi: 10.1016/0006-8993(95)00889-x. [DOI] [PubMed] [Google Scholar]
- Hay M, McKenzie H, Lindsley K, Dietz N, Bradley SR, Conn PJ, Hasser EM. Heterogeneity of metabotropic glutamate receptors in autonomic cell groups of the medulla oblongata of the rat. J Comp Neurol. 1999;403:486–501. [PubMed] [Google Scholar]
- Hermes SM, Mitchell JL, Silverman MB, Lynch PJ, McKee BL, Bailey TW, Andresen MC, Aicher SA. Sustained hypertension increases the density of AMPA receptor subunit, GluR1, in baroreceptive regions of the nucleus tractus solitarii of the rat. Brain Res. 2008;1187:125–136. doi: 10.1016/j.brainres.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillsley K, Kirkup AJ, Grundy D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J Physiol. 1998;506(Pt 2):551–561. doi: 10.1111/j.1469-7793.1998.551bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang CJ, Hay M. Expression of metabotropic glutamate receptors in nodose ganglia and the nucleus of the solitary tract. Am J Physiol Heart Circ Physiol. 2001;281:H457–H462. doi: 10.1152/ajpheart.2001.281.1.H457. [DOI] [PubMed] [Google Scholar]
- Holtmann G, Goebell H, Jockenhoevel F, Talley NJ. Altered vagal and intestinal mechanosensory function in chronic unexplained dyspepsia. Gut. 1998;42:501–506. doi: 10.1136/gut.42.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Afferent signalling of gastric acid challenge. J Physiol Pharmacol. 2003;54 Suppl 4:43–53. [PubMed] [Google Scholar]
- Holzer P. Efferent-like roles of afferent neurons in the gut: Blood flow regulation and tissue protection. Auton Neurosci. 2006;125:70–75. doi: 10.1016/j.autneu.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, Livingston EH, Saria A, Guth PH. Sensory neurons mediate protective vasodilatation in rat gastric mucosa. Am J Physiol. 1991;260:G363–G370. doi: 10.1152/ajpgi.1991.260.3.G363. [DOI] [PubMed] [Google Scholar]
- Holzer P, Painsipp E, Jocic M, Heinemann A. Acid challenge delays gastric pressure adaptation, blocks gastric emptying and stimulates gastric fluid secretion in the rat. Neurogastroenterol Motil. 2003;15:45–55. doi: 10.1046/j.1365-2982.2003.00382.x. [DOI] [PubMed] [Google Scholar]
- Hornby PJ. Receptors and transmission in the brain-gut axis II. Excitatory amino acids receptors in the brain-gut axis. Am J Physiol. 2001;280:G1055–G1060. doi: 10.1152/ajpgi.2001.280.6.G1055. [DOI] [PubMed] [Google Scholar]
- Hornby PJ, Abrahams TP. Central control of lower esophageal sphincter relaxation. Am J Med. 2000;108 Suppl 4a:90S–98S. doi: 10.1016/s0002-9343(99)00345-9. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ. Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci. 1998;18:10269–10276. doi: 10.1523/JNEUROSCI.18-24-10269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Jin YH, Bailey TW, Andresen MC. Cranial afferent glutamate heterosynaptically modulates GABA release onto second-order neurons via distinctly segregated metabotropic glutamate receptors. J Neurosci. 2004a;24:9332–9340. doi: 10.1523/JNEUROSCI.1991-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci. 2004b;24:4709–4717. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders AG, Sheng ZH. Modulation of neurotransmitter release by the second messenger-activated protein kinases: implications for presynaptic plasticity. Pharmacol Ther. 2005;105:69–84. doi: 10.1016/j.pharmthera.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunding JA, Nordstrom LM, Haukelid AO, Gilja OH, Berstad A, Hausken T. Vagal activation by sham feeding improves gastric motility in functional dyspepsia. Neurogastroenterol Motil. 2008;20:618–624. doi: 10.1111/j.1365-2982.2007.01076.x. [DOI] [PubMed] [Google Scholar]
- Mei L, Zhang J, Mifflin S. Hypertension alters GABA receptor-mediated inhibition of neurons in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1276–R1286. doi: 10.1152/ajpregu.00255.2003. [DOI] [PubMed] [Google Scholar]
- Mimidis K, Tack J. Pathogenesis of dyspepsia. Dig Dis. 2008;26:194–202. doi: 10.1159/000121346. [DOI] [PubMed] [Google Scholar]
- Page AJ, Young RL, Martin CM, Umaerus M, O'donnell TA, Cooper NJ, Coldwell JR, Hulander M, Mattsson JP, Lehmann A, Blackshaw LA. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology. 2005;128:402–410. doi: 10.1053/j.gastro.2004.11.062. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Rosa P, Sala C, Clementi F, Sher E. N-type Ca2+ channels are present in secretory granules and are transiently translocated to the plasma membrane during regulated exocytosis. J Biol Chem. 1996;271:30096–30104. doi: 10.1074/jbc.271.47.30096. [DOI] [PubMed] [Google Scholar]
- Quick MW, Corey JL, Davidson N, Lester HA. Second messengers, trafficking-related proteins, and amino acid residues that contribute to the functional regulation of the rat brain GABA transporter GAT1. J Neurosci. 1997;17:2967–2979. doi: 10.1523/JNEUROSCI.17-09-02967.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, Sternini C. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2003;284:G367–G372. doi: 10.1152/ajpgi.00292.2001. [DOI] [PubMed] [Google Scholar]
- Raybould HE, Zittel TT. Inhibition of gastric motility induced by intestinal glucose in awake rats: role of Na(+)-glucose co-transporter. Neurogastroenterol Motil. 1995;7:9–14. doi: 10.1111/j.1365-2982.1995.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Raymer CK, Park HS, Wishart JM, Kong M-F, Doran SM, Horowitz M. Effects of intraduodenal glucose and fructose on antropyloric motility and appetite in healthy humans. Am J Physiol. 2000;278:R360–R366. doi: 10.1152/ajpregu.2000.278.2.R360. [DOI] [PubMed] [Google Scholar]
- Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24:371–381. doi: 10.2337/diacare.24.2.371. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol. 2003;285:R479–R489. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh VH. Glucose-sensing neurons: are they physiologically relevant? Physiol Behav. 2002;76:403–413. doi: 10.1016/s0031-9384(02)00761-8. [DOI] [PubMed] [Google Scholar]
- Saito Y, Kawashima Y, Kondo A, Chikumaru Y, Matsui A, Nagata I, Ohno K. Dysphagia-gastroesophageal reflux complex: complications due to dysfunction of solitary tract nucleus-mediated vago-vagal reflex. Neuropediatrics. 2006;37:115–120. doi: 10.1055/s-2006-924428. [DOI] [PubMed] [Google Scholar]
- Savastano DM, Carelle M, Covasa M. Serotonin-type 3 receptors mediate intestinal Polycose- and glucose-induced suppression of intake. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1499–R1508. doi: 10.1152/ajpregu.00745.2004. [DOI] [PubMed] [Google Scholar]
- Schvarcz E, Palmer M, Aman J, Horowitz M, Stridsberg M, Berne C. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology. 1997;113:60–66. doi: 10.1016/s0016-5085(97)70080-5. [DOI] [PubMed] [Google Scholar]
- Shi M, Jones AR, Ferreira M, Jr, Sahibzada N, Gillis RA, Verbalis JG. Glucose does not activate nonadrenergic, noncholinergic inhibitory neurons in the rat stomach. Am J Physiol Regul Integr Comp Physiol. 2005;288:R742–R750. doi: 10.1152/ajpregu.00561.2004. [DOI] [PubMed] [Google Scholar]
- Shi M, Jones AR, Niedringhaus MS, Pearson RJ, Biehl AM, Ferreira M, Jr, Sahibzada N, Verbalis JG, Gillis RA. Glucose acts in the CNS to regulate gastric motility during hypoglycemia. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1192–R1202. doi: 10.1152/ajpregu.00179.2003. [DOI] [PubMed] [Google Scholar]
- Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. Stimulus-dependent translocation of kappa opioid receptors to the plasma membrane. J Neurosci. 1999;19:2658–2664. doi: 10.1523/JNEUROSCI.19-07-02658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterology and Motility. 1998;10:305–313. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- Tack J, Lee KJ. Pathophysiology and treatment of functional dyspepsia. J Clin Gastroenterol. 2005;39:S211–S216. doi: 10.1097/01.mcg.0000156109.97999.d1. [DOI] [PubMed] [Google Scholar]
- Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Tang X, Dworkin BR. Baroreflexes of the rat. IV. ADN-evoked responses at the NTS. Am J Physiol Regul Integr Comp Physiol. 2007a;293:R2243–R2253. doi: 10.1152/ajpregu.00142.2007. [DOI] [PubMed] [Google Scholar]
- Tang X, Dworkin BR. Baroreflexes of the rat. V. Tetanus-induced potentiation of ADN A-fiber responses at the NTS. Am J Physiol Regul Integr Comp Physiol. 2007b;293:R2254–R2259. doi: 10.1152/ajpregu.00143.2007. [DOI] [PubMed] [Google Scholar]
- Thumshirn M. Pathophysiology of functional dyspepsia. Gut. 2002;51 Suppl 1:I63–I66. doi: 10.1136/gut.51.suppl_1.i63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Ballanyi K, Richter DW. Spontaneous activation of KATP current in rat dorsal vagal neurones. Neuroreport. 1994;5:1285–1288. doi: 10.1097/00001756-199406020-00033. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol. 1991;260:G531–G536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Browning KN. D-Glucose modulates synaptic transmission from the central terminals of vagal afferent fibers. Am J Physiol Gastrointest Liver Physiol. 2008a;294:G757–G763. doi: 10.1152/ajpgi.00576.2007. [DOI] [PubMed] [Google Scholar]
- Wan S, Browning KN. Glucose increases synaptic transmission from vagal afferent central nerve terminals via modulation of 5HT3 receptors. Am J Physiol Gastrointest Liver Physiol. 2008b doi: 10.1152/ajpgi.90288.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, Berthoud HR, Travagli RA. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci. 2008;28:4957–4966. doi: 10.1523/JNEUROSCI.5398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano M, Kamato T, Nagakura Y, Miyata K. Effects of gastroprokinetic agents on gastroparesis in streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:145–150. doi: 10.1007/pl00005022. [DOI] [PubMed] [Google Scholar]
- Young RL, Cooper NJ, Blackshaw LA. Anatomy and function of group III metabotropic glutamate receptors in gastric vagal pathways. Neuropharmacology. 2008;54:965–975. doi: 10.1016/j.neuropharm.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Young RL, Page AJ, O'donnell TA, Cooper NJ, Blackshaw LA. Peripheral versus central modulation of gastric vagal pathways by metabotropic glutamate receptor 5. Am J Physiol Gastrointest Liver Physiol. 2007a;292:G501–G511. doi: 10.1152/ajpgi.00353.2006. [DOI] [PubMed] [Google Scholar]
- Young RL, Page AJ, O'donnell TA, Cooper NJ, Blackshaw LA. Peripheral versus central modulation of gastric vagal pathways by metabotropic glutamate receptor 5. Am J Physiol Gastrointest Liver Physiol. 2007b;292:G501–G511. doi: 10.1152/ajpgi.00353.2006. [DOI] [PubMed] [Google Scholar]
- Zhang W, Carreno FR, Cunningham JT, Mifflin SW. Chronic sustained and intermittent hypoxia reduce function of ATP-sensitive potassium channels in nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1555–R1562. doi: 10.1152/ajpregu.90390.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Fogel R, Renehan WE. Physiology and morphology of neurons in the dorsal motor nucleus of the vagus and the nucleus of the solitary tract that are sensitive to distension of the small intestine. J Comp Neurol. 1992;323:432–448. doi: 10.1002/cne.903230310. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Lu YX, Owyang C. Gastric relaxation induced by hyperglycemia is mediated by vagal afferent pathways in the rat. Am J Physiol Gastrointest Liver Physiol. 2008 doi: 10.1152/ajpgi.00067.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JX, Wu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]