Abstract
The purpose of this article is to review the recent developments of abnormal mitochondrial dynamics, mitochondrial fragmentation, and neuronal damage in neurodegenerative diseases, including Alzheimer’s, Parkinson’s, Huntington’s, and amyotrophic lateral sclerosis. The GTPase family of proteins, including fission proteins, dynamin related protein 1 (Drp1), mitochondrial fission 1 (Fis1), and fusion proteins (Mfn1, Mfn2 and Opa1) are essential to maintain mitochondrial fission and fusion balance, and to provide necessary adenosine triphosphate to neurons. Among these, Drp1 is involved in several important aspects of mitochondria, including shape, size, distribution, remodeling, and maintenance of X in mammalian cells. In addition, recent advancements in molecular, cellular, electron microscopy, and confocal imaging studies revealed that Drp1 is associated with several cellular functions, including mitochondrial and peroxisomal fragmentation, phosphorylation, SUMOylation, ubiquitination, and cell death. In the last two decades, tremendous progress has been made in researching mitochondrial dynamics, in yeast, worms, and mammalian cells; and this research has provided evidence linking Drp1 to neurodegenerative diseases. Researchers in the neurodegenerative disease field are beginning to recognize the possible involvement of Drp1 in causing mitochondrial fragmentation and abnormal mitochondrial dynamics in neurodegenerative diseases. This article summarizes research findings relating Drp1 to mitochondrial fission and fusion, in yeast, worms, and mammals. Based on findings from the Reddy laboratory and others’, we propose that mutant proteins of neurodegenerative diseases, including AD, PD, HD, and ALS, interact with Drp1, activate mitochondrial fission machinery, fragment mitochondria excessively, and impair mitochondrial transport and mitochondrial dynamics, ultimately causing mitochondrial dysfunction and neuronal damage.
1. Introduction
Increasing evidence suggests that structural and functional abnormalities in mitochondria are involved in aging and age-related neurodegenerative diseases, such as Alzheimer’s (AD), Parkinson’s (PD), Huntington’s (HD), and amyotrophic lateral sclerosis (ALS) (Beal, 2005; Reddy and Beal, 2005; Lin and Beal, 2006; Reddy, 2006a; 2006b; 2007a; 2008a; 2009; Reddy and Beal, 2008; Reddy and Reddy 2010). These structural abnormalities are caused by an imbalance in highly conserved, GTPase genes that are essential for mitochondrial division and mitochondrial fusion. Table 1 summarizes molecular features and biological functions involved in mitochondrial fission and fusion genes. GTPase genes – dynamin-related protein 1 (Drp1), fission 1 (Fis1), mitofusins 1 and 2 (Mfn1, Mfn2), and optic atrophy 1 (Opa1) – regulate, maintain, and remodel mammalian mitochondria (Chen and Chan, 2009).
Table 1.
Summary of molecular features and biological functions of mitochondrial structural genes
| Name of the gene | Chromosome location | Molecular structure | Functions |
|---|---|---|---|
| DRP1 | 12 | Drp1 encodes 736 aminoacids (aa) (20 exons) with a mass of 80.6 kDa. It has (exon 15 is deleted); isoform 3 699 aa (exons 15 and 16 are deleted); isoform 4 with 725 aa; isoform 5 with 710 aa and isoform 6 with 749 aa. | Drp1 establishes mitochondrial morphology through the distribution of mitochondrial tubules across the cytoplasm; Drp1 is involved in multiple functions, including mitochondrial fragmentation; it contains 6 spliced transcripts that encode various isoforms. |
| FIS1 | 7 | Fis1 encodes 152 aa (with a mass of 17 kDa). | Fis1 is a component of a mitochondrial complex that promotes mitochondrial fission. |
| MFN1 | 3 | Mfn1 encodes a 741 aa; it has 3 isoforms/variants. Isoform 2 encodes 370 aa and isoform 3 encodes 630 aa. | Mfn1 is a mediator of mitochondrial fusion; both mitofusion 1 and 2 facilitate mitochondrial targeting. |
| MFN2 | 1 | Mfn2 encodes 757 aa, and isoform 2 encodes 436 aa. | Mfn2 participates in mitochondrial fusion and contributes to the maintenance and operation of the mitochondrial network. Defects in Mfn2 are involved in causing Charcot-Marie-Tooth disease. It is involved in smooth muscle cell proliferation and may play a role in the pathophysiology of obesity. |
| OPA1 | 3 | Opa1 encodes 960 aa (contains 29 exons) and isoform 2 encodes 997 aa. | Opa1 is a nuclear-encoded mitochondrial protein; mutations in this gene lead to a loss of visual acuity; multiple transcript variants have been found in this gene. |
| TOMM40 | 19 | TOMM40 encodes a 361 aa and isoform 2 encodes 329 aa. | Tomm40 is a channel-forming subunit of the translocase of the mitochondrial outer membrane (TOMM). It is essential for protein transport into the mitochondria. Polymorphisms in Tomm40 has been found to occur more in late-onset AD patients. |
| CyPD (peptidylp rolyl isomease D) | 3 | Peptidylprolyl isomerase D encodes a 178 aa peptide that is found in the mitochondrion. | CypD is a part of mitochondrial permeability transition pore in the inner mitochondrial membrane; activation of this protein can induce apoptotic and necrotic cell death. |
Normal mammalian cells, including neurons, fission and fusion, via GTPase genes (Drp1, Fis1, Mfn1, Mfn2, and Opa1), balance equally and maintain mitochondrial dynamics and distribution (Chan, 2006; Chen and Chan, 2009). However, in aged neurons, in neurons exposed to toxins, and/or in neurons that express mutant proteins, an imbalance between fission and fusion leads to abnormalities in mitochondrial structure and function, inhibits adenosine triphosphate (ATP) production, and damages neurons (Lin and Beal, 2006; Reddy, 2008). Recent research has revealed abnormal functions of fission and fusion genes and their involvement in neurodegenerative diseases (Reddy, 2008). The purpose of this article is to provide an overview of mitochondrial GTPase genes, with a particular focus on Drp1.
2. Mitochondrial genes
Structurally, mitochondrion is comprised of 2 lipid membranes: the matrix that harbors beta-oxidation and tricarboxylic acid, and the highly porous outer membrane and the inner membrane that restricts ionic flow to the mitochondrial matrix. The electron transport chain is localized in the inner membrane and participates in the transport of electrons and in the production of essential ATP (energy) for the cell.
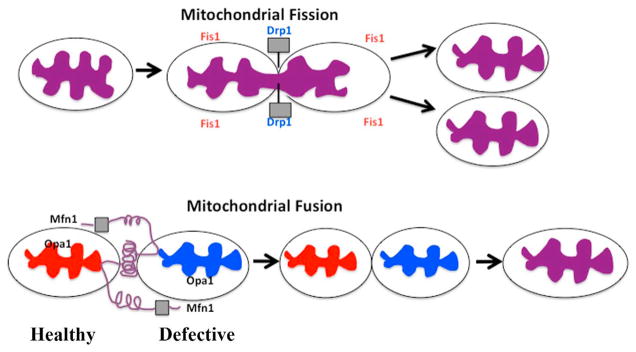
Mitochondria constantly divide and fuse, altering their size and shape while traveling through the neuron, from cell body to nerve terminals and synapses where energy is in high demand. Mitochondrial division is regulated and maintained by two GTPase genes: mitochondrial fission 1 (or Fis1) and Drp1. Fis1 is primarily localized on the outer mitochondrial membrane and participates in mitochondrial division (Chen and Chan, 2005). In diseased states, such as AD, the mitochondrially generated free radicals were found to activate Fis1, which corresponded to an increase in mitochondrial fragmentation (Reddy, 2008) (see Fig. 1). In knockout studies of Fis1 in cell culture, knockout Fis1 was found to stop mitochondrial fission. Further, in recent knockout mouse model studies of Drp1, in which Drp1 was not expressed, embryos died on day 11, indicating that Drp1 is critical for cell survival. Recent gene expression and biochemical studies using AD postmortem brains, AD transgenic mice, and amyloid beta precursor protein (AβPP) cell culture to determine amyloid beta (Aβ) toxicity revealed that Fis1 and Drp1 are upregulated in neurons affected by AD. These results suggest that increased Drp1 is important for mitochondrial fragmentation. They also indicate that Fis1 plays a key role in mitochondrial division (Wang et al., 2008, 2009; Manczak et al., 2010). Figure 1 shows mitochondrial fission and fusion.
Figure 1.
Summary of dynamin-related protein in different species. The GTPase domain is highly conserved in all species studied so far, including yeast, c. elegans, fruitflies, rats, mice, nonhuman primates, and humans.
In studies of the GTPase genes that control mitochondrial fusion (Mfn1, Mfn2, and Opa1), researchers used the knockout mouse model of Mfn1, Mfn2, and Opa1 to determine the normal function of Mfn1, Mfn2, and Opa1. Researchers found that the embryos died on days11 to 12. A lethal phenotype in embryos indicates that these fusion genes are essential for cell survival (Alavi et al., 2007; Chen et al., 2003; Davies et al., 2007). Mutations in the Opa1 gene caused an autosomal dominant, optic nerve atrophy in humans, which is a degeneration of retinal ganglial cells (Alexander et al., 2000; Delettre et al., 2000). Genetic mutations in Mfn2 caused Charcot-Marie-Tooth type 2A, a peripheral neuropathy affecting sensory and motor neurons of the distal extremities (Zuchner et al., 2007).
The C-terminal part of Mfn1 mediates oligomerization between Mfn molecules of adjacent mitochondria and facilitates mitochondrial fusion (Table 1 and Fig. 1). Mitochondrial fusion protects cells from the toxic effects of mitochondrial DNA by allowing functional complementation of mitochondrial DNA, proteins, and metabolites between two adjacent mitochondria.
Overall, the balance between fission and fusion is important to maintain normal mitochondrial size, shape, and distribution, and to supply energy to high-demand sites, such as nerve terminals.
3. Drp1 structure
Drp1 is critical for mitochondrial division, size, and shape, and for the distribution of mitochondria throughout the neuron, from cell body to axons, dendrites, and nerve terminals.
3.1. Drp1 structure and its variants
Drp1 has been found in the cytoplasm of plants, yeast, worms and, more recently, in several tissues from mammals (including humans), including the brain, heart, lung, kidney, spleen, testis, liver, hepatocytes, and fibroblasts. Figure 2 depicts the structure of Drp1 in different species. Drp1 has a highly conserved N-terminal GTPase domain, a helical domain in the middle, and a GED domain at the c-terminus.
Figure 2.
Mitochondrial dynamics in mammalian cells. Mitochondrial fission and fusion balance equally in healthy mammalian cells. However, cells exposed to toxins or cells that express mutant proteins impair mitochondrial dynamics, leading to abnormalities in mitochondrial structure and function.
Three groups of researchers have independently sequenced cDNA clones of mammalian Drp1 or dynamin-like proteins (Yoon et al., 1998; Shin et al., 1997; Imoto et al., 1998). As summarized in Table 1, human Drp1 has several splice variants: variant 1 consists of 736 amino acids with a calculated molecular mass of 81.6 kDa; in variant 2, exon 15 is spliced out and has a calculated molecular mass of 710 amino acids; in variant 3, exons 15 and 16 are spliced out and have a total of 699 amino acids; variant 4 has 725 amino acids; variant 5, 710 amino acids; and variant 6, 749 amino acids. The presence of a highly conserved GTPase domain in Drp1 indicates that Drp1 may be critically involved in essential cellular functions.
Similar to human Drp1, multiple variants of Drp1 have been found in the mouse. In the mouse, variant 1 consists of 712 amino acids, with a calculated molecular mass of 78.3 kDa; in variant 2, exon 3 is spliced out, and in variant 3, exons 15 and 16 are spliced out. It is possible that multiple splice variants are preset in mouse Drp1.
3.2. Drp1 in yeast
Otsuga and colleagues (1999) studied the relationship among Dnm1p (an orthologue to human Drp1), mitochondrial morphology, and the distribution of mitochondria in yeast. They found that Dnm1p controls the morphology and cortical distribution of mitochondrial membranes in yeast. Immunofluorescence analysis revealed that Dnm1p is distributed as punctate structures at the cell cortex and colocalizes with the mitochondrial compartment. These Dnm1p-containing structures are associated with spherical mitochondria found in an mdm10 mutant strain of yeast. In addition, a portion of Dnm1p cofractionates with mitochondrial membranes during differential sedimentation and sucrose gradient fractionation in wild-type cells. Otsuga showed that Dnm1p is required for the distribution of mitochondria in yeast, a novel function for a dynamin-related protein (Otsuga et al., 1999).
Disruption of the Dmn1gene caused mitochondrial membranes to collapse to one side of the cell but did not affect the morphology or distribution of other cytoplasmic organelles. Dnm1 proteins containing point mutations in the GTP-binding domain or completely lacking the GTP-binding domain failed to rescue mitochondrial morphology defects in a Dmn1 mutant, but they induced defects in wild-type cells.
3.3 Drp1 in worms
Using confocal and biochemical methods, Labrousse and colleagues (1999) studied the effects of Drp1 mutants in C. elegans and found that they disrupt mitochondria. Mutant Drp1 caused the mitochondrial matrix to retract into large blobs surrounded and connected by tubules from the outer mitochondrial membrane. Over-expressed wild-type Drp1 in C. elegans caused mitochondria to become excessively fragmented. Wild-type Drp1 fused to GFP was observed as spots on mitochondria where scission eventually occurs. Defective Drp1 increased mitochondrial fusion, possibly blocked mitochondrial transport from cell body to axons, dendrites, and nerve terminals, and damaged neurons. In contrast, increased Drp1 levels enhanced mitochondrial fragmentation, leading to the overproduction of defective mitochondria in mammalian cells, including neurons. Therefore, Drp1, at normal levels, has important roles in neurons.
4. Drp1 function
Recent basic research on Drp1 in yeast, worms, and mammalian cells revealed that Drp1 is involved in several functions, including: 1) mitochondrial division, 2) mitochondrial distribution, 3) peroxisomal fragmentation, 4) phosphorylation, 5) ubiquitination, and 6) SUMOylation.
4.1. Drp1 and mitochondrial division
Several recent studies suggest that Drp1 is involved in mitochondrial division (Labrousse et al., 1999; Sesaki and Jensen, 1999; Smirnova et al., 2001) and that a loss of Drp1 function increases mitochondrial fusion and mitochondrial connectivity (Labrousse et al., 1999; Sesaki and Jensen, 1999; Smirnova et al., 2001). These results were consistent with those from earlier studies of mitochondria and dynamin proteins using C. elegans. For example, Labrousse et al. (1999) reported that Drp1 expression is essential for mitochondrial division and that mitochondrial division is controlled and regulated by Drp1. It has also been reported that Drp1 is important for mitochondrial division in yeast (Sesaki and Jensen, 1999).
Using biochemical methods, and confocal and time-lapse photography of mitochondria, Smirnova et al. studied the involvement of Drp1 in mitochondrial division, in mammals (Smirnova et al., 2001). They demonstrated that endogenous Drp1 is localized to mitochondria and that it plays a key, but unknown, role in mitochondrial division. In time-lapse photography of transfected cells, green-fluorescent proteins that fused to Drp1 were found concentrated in spots, marking actual mitochondrial division events. They also found that purified human Drp1 self-assembled into multimeric, ring-like structures with dimensions similar to those of dynamin multimers. The structural and functional similarities between dynamin and Drp1 suggest that Drp1 wraps around the constriction points of dividing mitochondria, analogous to dynamin collars at the necks of budding vesicles (Smirnova et al., 2001).
Using cell culture, biochemical methods, and overexpressed Drp1 mutations, Smirnova et al. (2001) also studied the effects of Drp1 mutations on mitochondrial structure and function. Their extensive analysis of cells overexpressing mutant Drp1 revealed that Drp1 overexpression leads to perinuclear clusters of mitochondria and to increased inter-mitochondrial connectivity. These findings suggest that normal expression of Drp1 is critical for mitochondrial dynamics, and the overexpressions of Drp1 may cause abnormal mitochondrial dynamics in mammalian cells (Smirnova et al., 2001).
Using mitochondrially targeted EGFP and DsRed2 labeling, Uo et al. (2009) studied mitochondrial morphology. They observed a short, distinctly tubular mitochondrial morphology in postnatal cortical neurons in culture and in retinal ganglion cells in vivo. Differential expression patterns of fusion and fission genes in part appeared to distinguish these morphological differences when the neuronal cells expressed markedly high levels of Drp1 and Opa1 genes, compared to non-neuronal cells. Further, they found mouse cortical neurons expressed several splice variants of Drp1, including a neuron-specific isoform that incorporates exon 3. Knockdown or dominant-negative interference of endogenous Drp1 significantly increased mitochondrial length in both neurons and non-neuronal cells, but caused cell death in only cortical neurons. In contrast, the depletion of the fusion protein Mfn2, but not Mfn1, caused extensive mitochondrial fission and non-neuronal cell death. Thus, Drp1 and Mfn2 in normal cortical neurons appear not only to regulate mitochondrial morphology, but also to be required for cell survival. The present findings point to unique patterns of Drp1 expression and the selective vulnerability to reduced levels of Drp1 expression/activity in neurons, and the findings indicate that the regulation of mitochondrial dynamics is regulated in neurons.
In addition to the involvement of Drp1 in mitochondrial fragmentation, a neurotoxin oxidopamine or 6-hydroxydopamine (6-OHDA) was also found to be involved in mitochondrial fragmentation. Recently, using immunofluorescence and time-lapse fluorescence microscopy, Gomez-Lazaro et al. (2008) studied the cytotoxic effects of 6-OHDA on mitochondrial morphology, using SH-SY5Y neuroblastoma cells. They demonstrated that 6-OHDA induces profound mitochondrial fragmentation in SH-SY5Y cells and does not induce any changes in peroxisome morphology. Biochemical experiments revealed that 6-OHDA-induced mitochondrial fragmentation is an early event preceding the collapse of the mitochondrial membrane potential and the release of cytochrome c in SH-SY5Y cells. The silencing of Drp1, which is involved in mitochondrial and peroxisomal fission, prevented 6-OHDA-induced fragmentation of mitochondria. Further, in cells in which Drp1 was silenced, 6-OHDA-induced cell death was reduced, indicating that a block in mitochondrial fission may protect SH-SY5Y cells against 6-OHDA toxicity. Studies of mouse embryonic fibroblasts deficient in Bax or p53 revealed that both proteins are not essential for 6-OHDA-induced mitochondrial fragmentation. Overall, these findings suggest that Drp1-dependent mitochondrial fragmentation is involved in neuronal cell death.
Further, several recent reports suggest that overexpressed Drp1 fragments mitochondria in neurodegenerative diseases (Manczak et al., 2010; Shirendeb and Reddy, unpublished observations).
4.2. Drp1 and mitochondrial distribution
Several recent studies revealed thatDrp1 plays a role in mitochondrial distribution in mammalian cells (Pitts et al., 1999; Smirnova et al., 1998, Wang et al., 2010; Li et al., 2004). To determine the role of Drp1 in mitochondrial distribution, Smirnova et al. (1998) transfected the GTPase domain in wild-type cells with mutant Drp1 – a mutation that caused profound alterations in mitochondrial morphology. The tubular projections normally present in wild-type cells were retracted into large perinuclear aggregates in cells expressing mutant Drp1. Mutant Drp1 did not affect the morphology of other organelles, nor did it affect transport functions of the secretory and endocytic pathways. Mitochondrial aggregates were found in cells that were transfected with mutant Drp1; the aggregates appeared as clusters of tubules rather than as large masses of coalescing membranes. Based on these results, Smirnova et al. proposed that Drp1 is important for distributing mitochondrial tubules throughout the cell (Smirnova et al., 1998).
Pitts et al. (1999) studied the connection between Drp1 and mitochondria, and between Drp1 and the endoplasmic reticulum (ER) in mammalian cells. They transiently expressed both wild-type and two mutant Drp1 proteins and tagged them with green-fluorescent protein in cultured mammalian cells. Point mutations in the GTP-binding domain of Drp1 (K38A and D231N) dramatically changed the intracellular distribution from punctate vesicular structures into either an aggregated or a diffuse pattern. Strikingly, cells that expressed Drp1 mutants or that were microinjected with Drp1 antibodies showed a marked reduction in ER fluorescence, and a significant aggregation and tubulation of mitochondria. In addition, electron microscopy of Drp1 mutant cells revealed a striking and quantitative change in the distribution and morphology of mitochondria and the ER. These data support very recent findings implicating Drp1 in the maintenance of mitochondrial morphology in both yeast and mammalian cells.
To determine the connection between mitochondrial fission proteins, including Drp1 and mitochondrial movement and distribution, Li and colleagues (2004) studied intraneuronal distribution of mitochondria, particularly at nerve terminals and synapses in neurons, using primary rat neurons. The extension or movement of mitochondria into dendritic protrusions correlated with the development and morphological plasticity of spines. Molecular manipulations of dynamin-like GTPases, Drp1, and Opa1, which were found to reduce dendritic mitochondrial content, led to a loss of synapses and dendritic spines. In contrast, increasing dendritic mitochondrial content or mitochondrial activity enhanced the number and the plasticity of spines and synapses. Thus, the movement or distribution of mitochondria into dendrites appears to be essential for the support of synapses, and reciprocally, synaptic activity appears to modulate the motility and fusion/fission balance of mitochondria and to control dendrites.
Recently, Verstreken and colleagues investigated the genetic basis for mitochondrial distribution using fruit flies that express mutant Drp1 (Verstreken et al., 2005). They found that mitochondria are absent in synapses in neurons from fruit flies that express mutant Drp1, indicating a genetic basis for mitochondria at synapses. Further, during intense stimulation, mutant flies failed to maintain normal neurotransmission. Synaptic vesicle labeling using FM1-43 indicated normal exo- and endocytosis, but a specific inability to mobilize reserve pool vesicles, which was partially rescued by exogenous ATP. Using a variety of drugs, they demonstrated that reserve pool recruitment depends on mitochondrial ATP production downstream of PKA signaling and that mitochondrial ATP limits myosin-propelled mobilization of reserve pool vesicles. They concluded a specific role for mitochondria in regulating synaptic strength.
Wang et al. reported that Drp1 is critical for mitochondrial distribution in cells treated with Aβ, and altered Drp1 expression impairs mitochondrial distribution in AD (Wang et al. 2010). However, further research is needed to confirm these results and also in other neurodegenerative diseases, including PD, HD, and ALS.
4.3. Drp1 and peroxisomal fragmentation
Several studies reported that Drp1 is involved in fragmenting peroxisomes in mammalian cells (Koch et al., 2003). To determine the effect of Drp1 on peroxisomal growth and division in mammalian cells, Koch and colleagues studied peroxisome proliferation by Pex11pbeta and the expression of dominant-negative mutants of Drp1 in a rat model (Koch et al., 2003). They localized Drp1 in spots on tubular peroxisomes in hepatocytes from rat liver and used immunoblot analysis, revealing the presence of Drp1 in highly purified peroxisomal fractions and an increase in Drp1 after the rats were treated with the peroxisome proliferator bezafibrate. The increase in Drp1 or the combination of Drp1 with Pex11pbeta caused the appearance of tubular peroxisomes, but Drp1 had no influence on the intracellular distribution of peroxisomes. In co-expressing cells, the increase in Drp1 promoted the formation of tubulo-reticular networks of peroxisomes and completely inhibited peroxisomal division. These findings were confirmed by silencing Drp1 with siRNA and observing decreased peroxisomal fragmentation. Based on this research, Koch et al. proposed a direct role for Drp1 in peroxisomal fission and in the maintenance of peroxisomal morphology in mammalian cells.
Koch et al. (2005) also studied whether human fission 1 (hFis1, a fission protein that is critically involved in mitochondrial fragmentation) is also involved in peroxisomal growth and division. Using biochemical and fluorescence methods, they demonstrated that hFis1 localizes to peroxisomes, in addition to mitochondria. Through differential tagging and deletion experiments, they found that the transmembrane domain and the short C-terminal tail of hFis1 are needed for targeting peroxisomes and mitochondria, and that the N-terminal region is required for organelle fission. hFis1 was found to promote peroxisome division upon ectopic expression, whereas the silencing of Fis1 with RNA inhibited fission and caused the tubulation of peroxisomes. These findings provide evidence of a role for Fis1 in peroxisomal fission and suggest that the fission machinery of mitochondria and peroxisomes shares common components (Koch et al., 2005).
Further research is needed to understand the role of Drp1 in peroxisomal fragmentation in normal and diseased states, in such neurodegenerative diseases as AD, PD, HD and ALS.
4.4. Drp1 phosphorylation, mitochondrial fragmentation, and cell death
Cribbs and Strack (2007) recently identified a phosphorylation site that is conserved in all metazoan Drp1 orthologues. Ser 656 is phosphorylated by the cyclic AMP-dependent protein kinase and is dephosphorylated by calcineurin, and its phosphorylation state is controlled by sympathetic tone, calcium levels, and cell viability. Pseudo-phosphorylation of Drp1 by the mutation of Ser 656 into aspartic acid leads to the elongation of mitochondria and confers resistance against various pro-apoptotic insults. Conversely, the constitutively dephosphorylated Ser656Ala mutant Drp1 promotes mitochondrial fragmentation and increases cell vulnerability. Thus, Drp1 phosphorylation at Ser 656 provides a mechanism for the integration of cAMP and calcium levels in the control of mitochondrial function, such as in terms of mitochondrial shape and apoptosis.
Chang and Blackstone (2007) have identified cAMP-dependent, protein kinase-dependent phosphorylation of Drp1 within the GED domain at Ser (637) as an inhibitor of Drp1 GTPase activity. Mechanistically, this effect likely derives from a decreased interaction of GTP-binding/middle domains with the GED domain since the phosphomimetic S637D mutation impairs intramolecular interactions but not Drp1-Drp1 intermolecular interactions. Using phosphomimetic S637D substitution, they demonstrated that mitochondrial fission is prominently inhibited in cells. Thus, protein phosphorylation at Ser 637 may results in the alteration of Drp1 function and mitochondrial morphology, both of which are likely involved in the dynamic regulation of mitochondrial division.
Frank and colleagues (2001) investigated the role of Drp1 localization from cytosol to mitochondria during apoptosis. Upon the induction of apoptosis, Drp1 translocates from the cytosol to mitochondria, where it preferentially localizes to potential sites of mitochondrial division. They also found that inhibition of Drp1 via overexpression of a dominant-negative mutant counteracts the conversion to a puncti form mitochondrial phenotype, prevents the loss of the mitochondrial membrane potential and the release of cytochrome c, and reveals a reproducible swelling of mitochondria. Remarkably, the inhibition of Drp1 blocks cell death, implicating mitochondrial fission as an important step in apoptosis (Frank et al., 2001). These findings suggest that Drp1 activation is essential for mitochondrial fragmentation and that the activation of Drp1 may initiate apoptotic cell death.
Further, to determine whether Bax-induced mitochondrial fragmentation is required for the release of cytochrome c, Sheridan and colleagues studied the roles of the Bcl xL family member, Bax and Bcl-2 family member, and Bak in cytochrome c release and in mitochondrial fragmentation (Sheridan et al., 2008). They found that Bcl xL and other members of the apoptosis inhibitory subset of the Bcl-2 family antagonized Bax and Bak-induced cytochrome c release, but failed to block mitochondrial fission that is associated with Bax/Bak activation. These findings suggest that Bax/Bak-initiated remodeling of mitochondrial networks and cytochrome c release are separate events and that the proteins in the Bcl-2 family influence mitochondrial fission-fusion dynamics, independent of apoptosis.
Recently, Wu and colleagues studied the role of Bax in apoptotic cell death (Wu et al., 2010). They found that, in human lung adenocarcinoma cells, Bax was activated after mitochondrial depolarization and the completion of cytochrome c release that was induced by photodynamic therapy with the photosensitizer photofrin. They also found that the knockdown of Bax expression by gene silencing had no effect on mitochondrial depolarization and cytochrome c release, indicating that Bax makes no contribution to mitochondrial outer membrane permeabilization. Further study revealed that Bax knockdown only slowed cell death that was induced by photodynamic therapy, indicating that Bax is not essential for photodynamic therapy-induced apoptosis. The fact that Bax knockdown totally inhibited the mitochondrial accumulation of Drp1 and of Drp1-knockdown attenuated cell apoptosis suggests that Bax can promote photodynamic therapy-induced apoptosis through the promotion of Drp1 activation. Taken together, these findings suggest that Bax activation is not essential for mitochondrial outer-membrane permeabilization but that it is essential for Drp1-mediated mitochondrial fission during apoptosis caused by Photofrin-PDT.
These finding suggest that Drp1 activation is critical for mitochondrial fragmentation and that Drp1 activation occurs as an early event in apoptotic cell death. Cytochrome c release is dependent of activities of Bax and Bak. Bax activation is not associated with mitochondrial outer-membrane permeabilization. However, further research is needed to understand the precise role of Drp1 and Bax/Bak in apoptotic cell death.
4.5. Drp1 and SUMOylation
Several recent studies reported that Drp1 interacts with several SUMOylation proteins and that this interaction may regulate mitochondrial fission (Harder et al., 2005; Zunino et al., 2007, 2009; Braschi et al., 2009; Wasiak et al., 2007).
Harder et al. (2005) identified 2 Drp1-interacting proteins (Ubc9 and Sumo1) and demonstrated that Drp1 is a Sumo1 substrate, which has implications for the interaction of Drp1 with Ubc9 and Sumo1. Using video microscopy, they found the yellow fluorescence protein (YEP):Sumo1 at the site of mitochondrial fission and at the tips of fragmented mitochondria. Consistent with these findings, fluorescence microscopy revealed a portion of total cytosolic YFP:Sumo1 colocalized with endogenous mitochondrial Drp1. Transient transfection of Sumo1 dramatically increased the level of mitochondrial fragmentation. Analysis of endogenous Drp1 levels indicated that the overexpression of Sumo1 specifically protected Drp1 from degradation, resulting in a more stable, active pool of Drp1. This overexpression may partially account for the excess in mitochondrial fragmentation. These data point to a function for Sumo1 in maintaining mitochondrial structure and suggest a novel role for Sumo1 in mitochondrial fission.
Figueroa-Romero et al. (2009) studied Drp1 regulation in conjunction with SUMOylation of small ubiquitin-like modifier (SUMO) proteins. They found that Drp1 interacts with the SUMO-conjugating enzyme Ubc9 via multiple regions and demonstrated that Drp1 is a direct target of SUMO modification by SUMO isoforms. While Drp1 does not harbor consensus SUMOylation sequences, 2 clusters of lysine residues were found within the B domain and served as noncanonical conjugation sites. Although initial analysis indicates that mitochondrial recruitment of ectopically expressed Drp1 in response to staurosporine is unaffected by the loss of SUMOylation, Drp1 SUMOylation is enhanced in the context of a K38A mutation. This dominant-negative mutant, which is deficient in GTP binding and hydrolysis, does not associate with mitochondria and prevents normal mitochondrial fission. These findings suggest that SUMOylation of Drp1 is linked to the activity cycle and is influenced by Drp1 localization.
Zunino et al. (2009) investigated Drp1 regulators of SUMOylation proteins. They identified a SUMO protease, SenP5, that desumoylates a number of mitochondrial targets, including Drp1. In interphase, SenP5 resides primarily within the nucleoli, in addition to within a cytosolic pool. This study also involved the relocalization of SenP5 from the nucleoli to the mitochondrial surface at G2/M transition prior to the breakdown of the nuclear envelope. The recruitment of SenP5 resulted in a significant loss in mitochondrial SUMOylation and a concomitant increase in the pool of Drp1 that drives mitochondrial fragmentation. Importantly, the silencing of SenP5 led to an arrest in the cell cycle precisely at the time when the protease was translocated to the mitochondria. These data indicate that transition of SenP5 to mitochondria may play an important role in mitochondrial fragmentation during mitosis. The altered intracellular localization of SenP5 represented the first example of the mitochondrial recruitment of a SUMO protease and provided new insights into the mechanisms of interorganellar communication during the cell cycle.
Braschi et al. (2009) studied Drp1 regulator proteins in mammalian cells. They found that SUMOylation of Drp1 stimulates mitochondrial fission, suggesting that SUMOylation has an important function in mitochondrial dynamics. The conjugation of SUMO to its substrates requires a regulatory SUMO E3 ligase; however, so far, none has been functionally associated with the mitochondria. Using biochemical assays, overexpression, and RNA interference experiments, Braschi et al. characterized the mitochondrial-anchored protein ligase (MAPL) as the first mitochondrial-anchored SUMO E3 ligase. They also demonstrated that Drp1 is a substrate for MAPL, thus providing a direct link between MAPL and fission machinery. However, a large number of mitochondrial SUMO targets remain to be identified. Braschi and colleagues’ findings suggest a global role for SUMOylation in mitochondrial function, with MAPL a crucial component in the regulation of multiple conjugation events.
Further research is needed to understand the role of Drp1 in SUMOylation in normal and diseased states in neurodegenerative diseases.
4.6. Drp1 and ubiquitination
Drp1 has been reported to ubiquitinate with mitochondrial E3 ubiquitin ligase MARCH5 and to regulate mitochondrial fission. Using immunofluorescence and biochemicalmethods, Karbowski et al. (2007) studied Drp1 in association with the MARCH5 protein. They found that the interference of the mutant proteins MARCH5 and MARCH5 RNA induces an abnormal elongation and interconnection of mitochondria, suggesting that MARCH5 mutations interfere with mitochondrial division. These aberrant mitochondrial phenotypes were reversed by the ectopic expression of Drp1, but not by Fis1. Moreover, as indicated by the abnormal clustering and the accumulation of Drp1 in mitochondria, as well as decreased cellular mobility of YFP-Drp1 in cells expressing MARCH5 RING mutants, MARCH5 activity regulated the subcellular trafficking of Drp1, likely by impacting the correct assembly at scission sites or by disassembling fission complexes. The loss of MARCH5 may account for the observed defects in mitochondrial division. Finally, MARCH5 RING mutants and endogenous Drp1, but not wild-type MARCH5 or Fis1, co-assembled into abnormally enlarged clusters in a Drp1 GTPase-dependent manner, suggesting molecular interactions among these proteins. Collectively, these data suggest a model, in which mitochondrial division is regulated by a MARCH5 ubiquitin-dependent switch.
Using RNA silencing and biochemical methods, Park et al. (2010) studied the connection between decreased MARCH5 protein and mitochondrial connectivity. They found that shRNA-mediated MARCH5 knockdown promotes the accumulation of highly interconnected and elongated mitochondria. Cells transfected with MARCH5 shRNA or a MARCH5 RING domain mutant displayed cellular enlargement and flattening that was accompanied by increased senescence-associated beta-galactosidase (SA-beta-Gal) activity, indicating that these cells had undergone cellular senescence. Notably, a significant increase in the level of Mfn1 – but not in the level of Mfn2, Drp1, or hFis1 levels – was observed in MARCH5-depleted cells, indicating that Mfn1 may be a ubiquitylation substrate. Introduction of Mfn1 (T109A), a GTPase-deficient mutant form of Mfn1, into MARCH5-RNAi cells not only disrupted mitochondrial elongation, but also abolished any increase in SA-beta-Gal activity. Moreover, the aberrant mitochondrial phenotypes in MARCH5-RNAi cells were reversed by ectopic expression of Drp1, but not by hFis1, and reversion of mitochondria morphology in MARCH5-depleted cells was accompanied by a reduction in SA-beta-Gal activity. Park et al. concluded that a lack of MARCH5 results in mitochondrial elongation, which promotes cellular senescence by blocking Drp1 activity and/or promoting an accumulation of Mfn1 in mitochondria.
4.7. Research on Drp1 function using knockout mice
To understand the normal functioning of Drp1, two groups independently generated knockout mouse models for Drp1 (Wakabayashi et al., 2009; Ishihara et al., 2009). The first group, led by Ishihara et al. (2009), generated a knockout mouse model for Drp1 and studied biochemistry and molecular biology of both heterozygote and homozygote knockout mice. They also extensively studied primary neurons from knockout mice to assess synapse formation, the number of synapses generated, and the neuronal structure.
Ishihara et al. (2009) found that mice lacking Drp1 have developmental abnormalities, particularly in the forebrain, and die on average shortly after embryonic day 12.5. Neural cell-specific (NS) Drp1 (−/−) mice died shortly after birth as a result of brain hypoplasia with apoptosis. Primary culture of NS-Drp1 (−/−) mouse forebrain showed a decrease in the number of neurites and also defective synapse formation, the latter which they thought was due to aggregated mitochondria that failed to distribute properly within cell processes. These researchers noted that these defects highlight the importance of Drp1-dependent mitochondrial fission within highly polarized cells, such as neurons. Moreover, Drp1 (−/−) murine embryonic fibroblasts and embryonic stem cells revealed that Drp1 is required for a normal rate of cytochrome c release and caspase activation during apoptosis.
The second research group, led by Sesaki (Wakabayashi et al., 2009), developed complete and tissue-specific mouse knockouts of Drp1. Homozygote knockout Drp1 mice died by embryonic day 11.5, but this embryonic lethality was not likely caused by ATP deprivation, as Drp1-null cells showed normal intracellular ATP levels. These researchers found that mitochondria formed extensive networks, and peroxisomes were elongated in Drp1-null embryonic fibroblasts. Brain-specific Drp1 depletion caused developmental defects of the cerebellum: Purkinje cells contained few giant mitochondria instead of the many short, tubular mitochondria observed in control cells. In addition, Drp1-homozygote embryos failed to undergo developmentally regulated apoptosis during neural tube formation in vivo. However, homozygote embryonic fibroblasts exhibited normal responses to apoptotic stimuli in vitro, suggesting that the apoptotic function of Drp1 may depend on physiological cues.
Overall, these knockout studies revealed that Drp1 is essential for cell survival and critical for development of the brain, mitochondrial division, and distribution of mitochondria in neurons.
5. Drp1 and neurodegenerative diseases
Abnormal mitochondrial dynamics has been studied in yeast, worms, and mammalian cells. The involvement of Drp1 in mitochondria, and its role in maintaining the shape, size, and distribution of mitochondria, have been determined to be crucial for normal cell functioning. However, the role of Drp1 in maintaining the shape, size, and distribution of mitochondria has been investigated in only a few studies in AD, PD, and HD.
5.1. Drp1, mitochondrial fragmentation, and Alzheimer’s disease
Several recent studies reported that impaired mitochondrial dynamics involve the abnormal expression of Drp1 in postmortem brains from AD patients, AD mouse models, and APP cell lines (Barsoum et al., 2006; Wang et al., 2008, 2009, 2009; Cho et al., 2009; Manczak et al., 2010).
Using in situ hybridization to mtDNA, immunocytochemistry, and morphometry of electron micrographs of biopsy specimens from AD patients and control subjects, Hirai et al. studied mitochondrial abnormalities in AD (Hirai et al., 2001). They found that the same neurons showing increased oxidative damage in AD have a striking and significant increase in mtDNA and cytochrome oxidase. Surprisingly, much of the mtDNA and cytochrome oxidase was found in the neuronal cytoplasm, and in the case of mtDNA, the mtDNA and cytochrome oxidase was found in vacuoles associated with lipofuscin, suggesting the presence of mitochondrial abnormalities and autophagosomes in neurons from AD patients
Recently, the Reddy laboratory extensively studied abnormal mitochondrial dynamics in primary neurons from AβPP transgenic mice (brain tissues from transgenic mice (Tg2576 mice), brain tissues from postmortem brains of AD patients, and neuroblastoma cells that were treated with the Aβ peptide (Manczak et al., 2010).
Using electron and confocal microscopy, gene expression analysis, and biochemical methods, we sought to determine mitochondrial structure (the expression of Drp1, Fis1, Mfn1, Mfn2, and Opa1) and function, and neurite outgrowth in neurons treated with Aβ (Manczak et al., 2010). In the neurons treated with only Aβ, we found increased expressions of Drp1 and Fis1 (fission genes), and decreased expressions of Mfn1, Mfn2, and Opa1 (fusion genes), indicating abnormal mitochondrial dynamics in AD neurons. Our immunohistochemistry of N2a cells treated with Aβ revealed increased immunoreactivity of Drp1 and Fis1, suggesting that Aβ elevates fission genes and fragments mitochondria. Electron microscopy of the N2a cells incubated with Aβ revealed a significantly increased number of mitochondria, indicating that Aβ fragments mitochondria.
Biochemical analysis revealed Aβ in association with defective mitochondria. Neurite outgrowth was significantly decreased in N2a cells that were incubated with Aβ, indicating that Aβ affects neurite outgrowth. Increased neurite outgrowth was found in N2a cells treated with the mitochondria-targeted antioxidants MitoQ and SS31. Further, in N2a cells treated with MitoQ and SS31, and then incubated with Aβ, abnormal expressions of Drp1, Fis1, Mfn1, Mfn2, and Opa1 were prevented and mitochondrial function was normal; intact mitochondria were present and neurite outgrowth was significantly increased (Manczak et al., 2010).
The Reddy laboratory also investigated primary neurons from AβPP transgenic mice to determine the expression of mitochondrial fission and fusion genes, and mitochondrial dynamics. Our immunocytochemistry of Drp1 revealed an increase in Drp1 immunoreactivity, in primary neurons. Further, our electron microscopy analyses of primary neurons from the AβPP mice revealed a significantly increased number of mitochondria and, of them, a notable increase in structurally damaged mitochondria. These results indicate that mutant AβPP and/or Aβ may activate Drp1 and Fis1, consequently damaging mitochondrial structure and impairing mitochondrial function (Calkins and Reddy, unpublished observations).
In primary neurons from the AβPP mice treated with mitochondria-targeted antioxidant SS31, neurite outgrowth was significantly increased and the expression of cyclophilin D (mitochondrial matrix protein) was significantly decreased. These findings suggest that SS31 prevents Aβ toxicity, which may protect mitochondria from AD neurons, thus warranting the study of SS31 as a potential drug to treat patients with AD. Mechanistically, the SS31 peptide targets the inner mitochondrial membrane, due to the electrostatic attraction between these cationic peptides (positive charge) and the highly anionic cardiolipin molecules (negative charge) of the inner mitochondrial membrane (Szeto, 2006a; 2006b). Further, SS31 has a dimethyltyrosine residue, allowing SS31 to scavenge oxyradicals and inhibit linoleic acid and low density lipoprotein oxidation. By reducing mitochondrial ROS, SS31 was able to prevent the opening of the MPT pore, prevent mitochondrial swelling, and reduce cytochrome c release in response to a high Ca2+ overload.
In a study of postmortem brain specimens (frontal cortices) from patients at all stages of AD development (Braak stage I/II [early AD], III/IV [definite AD], and V/VI [severe AD]) and from control subjects, the Reddy laboratory found increased expressions of Drp1 and Fis1, and decreased expression of Mfn1, Mfn2, and Opa1 I (Manczak and Reddy, unpublished observations), indicating mitochondrial fragmentation and abnormal mitochondrial dynamics are involved in neurons from AD patients. Our immunohistochemistry of Drp1 revealed an increased immunoreactivity of Drp1 in AD patients. Interestingly, we also found an increase in the mitochondrial matrix protein, CypD (for mRNA and protein), and an increase in CypD in postmortem brain specimens from patients at all stages of AD development, suggesting the possibility of mitochondrial permeability transition pore opening, and suggesting alterations in mitochondrial structure and function the neurons of AD patients. These findings suggest that Aβ fragments mitochondria and causes abnormal mitochondrial dynamics, leading to mitochondrial dysfunction.
As described above, CypD is activated in affected neurons from AβPP mice, neurons expressing Aβ, and neurons from AD patients, and CypD initiates the opening of the mitochondrial permeability transition pore. The increased entry of AβPP and Aβ into mitochondria, and an increase in Ca2+ levels in the matrix of mitochondria may cause the inner mitochondrial pore to open (Starkov and Beal, 2008; Reddy, 2009). In general, the inner mitochondrial membrane provides a highly efficient barrier to ionic flow and protects mitochondria from toxic insults. However, in neurons from patients with AD, an age-dependent accumulation of AβPP and Aβ1–42 oligomers and -secretase complex proteins, PS1, APH, and nicastrin may induce a massive entry of Ca2+ into neurons and may promote mitochondrial Ca2+ overload. Excess mitochondrial Ca2+ may in turn promote the opening of the permeability transition pore in mitochondria and destroy the neuron via apoptotic cell death (Reddy, 2009).
5.2. Drp1, mitochondrial fission, and Huntington’s disease
Mitochondrial dysfunction has been described in HD, but the role of drp1 in HD mitochondrial dysfunction has not (Panov et al., 2002, 2005; Lin and Beal, 2006; Reddy et al., 2009; Knott et al., 2008; Wang et al., 2009; Pandey et al., 2010; Chen and Chan, 2009; Su et al., 2010; Liot et al., 2009; Oliveira, 2010; Li and Li, 2010; Ferreira et al., 2010; Quintanilla and, Johnson, 2009; Truishna et al., 2004; Kim et al., 2010). Recently, however, using quantitative fluorescence time-lapse microscopy and cortical neurons from HD mice, Liot et al. (2009) studied temporal and spatial relationships among energy decline, impairment of mitochondrial dynamics, and neuronal cell death in response to 3-NP. 3-NP caused an immediate drop in ATP, which corresponded to a mild rise in ROS, but mitochondrial morphology remained unaltered. Unexpectedly, several hours after this immediate drop in ATP, ROS dramatically increased – an increase that occurred simultaneously with profound mitochondrial fission that was characterized by the conversion of filamentous to punctate mitochondria and neuronal cell death. Glutamate receptor antagonist AP5 abolished the second peak in ROS, resulting in mitochondrial fission and cell death. Thus, secondary excitotoxicity, mediated by glutamate receptor activation of the NMDA subtype, and consequent oxidative and nitrosative stress, may have likely caused mitochondrial fission, rather than energy deficits per se. These results improve our understanding of the cellular mechanisms underlying HD pathogenesis.
Using several mitochondrial markers of COX2, SOD2, and cytochrome c, Kim et al. studied mitochondria in preferentially vulnerable striatal calbindin-positive neurons in moderate-to-severe grade HD patients (Kim et al. 2010). Combined calbindin and mitochondrial marker immunofluorescence revealed a significant and progressive grade-dependent reduction in the number of mitochondria, in spiny striatal neurons, and the mitochondria were markedly altered in size. Kim and colleagues found a reduction in COX2 protein levels that was consistent with mitochondrial loss and that corresponded with disease severity. In addition, both mitochondrial transcription factor A – a regulator of mtDNA, and PGC1α, a key transcriptional regulator of energy metabolism – and mitochondrial biogenesis were also significantly reduced as disease severity increased. Abnormalities in mitochondrial dynamics were observed: Drp1 significantly increased and Mfn1 decreased. Further, mitochondrial PCR array profiling in caudate nucleus specimens showed increased mRNA expression of proteins involved in mitochondrial localization and in membrane translocation, polarization, and transport that paralleled mitochondrial derangement. These findings revealed both mitochondrial loss and altered mitochondrial morphogenesis as mitochondrial fission increased and as fusion decreased in neurons from HD patients. These findings provide further evidence that mitochondrial dysfunction plays a critical role in the pathogenesis of HD.
To determine the effect of mutant Htt in abnormal mitochondrial dynamics in HD, using real-time RT-PCR and immunoblotting analysis, the Reddy laboratory quantified mRNA levels of mitochondrial structural genes; Drp1 and Fis1; Mfn1, Mfn2, Opa1; Tomm40; and the mitochondrial matrix gene CypD in brain specimens from the frontal cortex (affected in HD) of several HD3 and HD4 subjects and of age-matched control subjects (Shirendeb and Reddy, unpublished observations). Using immunohistochemistry, we also examined the localization of proteins related to mitochondrial structure and mutant Htt in different brain regions of subjects with HD3 and HD4, and of age-matched control subjects. We found increased expression of Drp1 and Fis1, and decreased expression of Mfn1, Mfn2, Opa1, and Tomm40 in the brain specimens from HD patients. Interestingly, we found CypD upregulated in the HD3 and HD4 specimens. These findings indicated the presence of abnormal mitochondrial dynamics in the progression of HD.
Taken together, these findings suggest that an increase in fission genes and a decrease in fusion genes may be responsible for abnormal mitochondrial dynamics in the striatum and cortex of HD patients, and that these changes may contribute to neuronal damage.
Further, in HD, as in AD, CypD expression increases particularly in the affected regions (striatum cortex and hippocampus) of brains from AD patients (Shirendeb and Reddy unpublished observations), an increase that may initiate the opening of the mitochondrial permeability transition pore. In addition, the increase in intracellular Ca2+ levels in the matrix of mitochondria may cause the inner mitochondrial pore to open. An excess of mitochondrial Ca2+ may promote the opening of the mitochondrial permeability transition pore and may destroy the neuron by apoptotic cell death.
5.3. Drp1, mitochondrial fission, and Parkinson’s disease
Mitochondrial dysfunction and oxidative stress are well-documented in PD; in particular, mitochondria in complex I have been found to be defective in both familial and sporadic PD (Lin and Beal, 2006; Matsuda et al., 2010; Martin, 2006, 2010; Chu and Zhe, 2010; Cookson, 2010; Morais et al., 2009; Lin et al. 2009). With the focus on abnormal mitochondrial dynamics, little research attention has been paid to elucidating the involvement of Drp1 and mitochondrial fission and fusion imbalance in PTEN induced putative kinase 1 (PINK1) and parkin mutants of fruitflies and mouse models. Inconsistent reports have been published regarding PINK1/Parkin and Drp1 involvement in PD and mitochondrial dysfunction. Earlier studies, including Poole et al. (2008) and Deng et al. (2008), reported that PINK1 and Parkin pathways induce mitochondrial fragmentation in fly models. Other recent studies, including Dagda et al. (2009), Sandebring et al. (2010), and Lutz et al. (2010), suggested that a PINK1 deficiency causes mitochondrial fragmentation through Drp1 overexpression.–
Deng et al. (2008) explored interactions between PINK1/Parkin and the mitochondrial fusion/fission machinery in order to determine the role of pink1 and park in mitochondrial dynamics and pathogenesis of PD. Muscle-specific knockdown of the fly homologue of Mfn (Marf) or Opa1, or the overexpression of Drp1, resulted in significant mitochondrial fragmentation. Mfn-knockdown flies also displayed altered cristae morphology. Interestingly, knockdown of Mfn or Opa1, or the overexpression of Drp1 rescued the phenotypes of muscle degeneration, cell death, and mitochondrial abnormalities in PINK1 or Parkin mutants. In the male germline, genetic interactions were observed between PINK1 and the testes-specific mfn homologue fuzzy onion, and between PINK1 and Drp1. These data suggest that the PINK1/Parkin pathway promotes mitochondrial fission and/or inhibits fusion by negatively regulating Mfn and Opa1 function, and/or positively regulating Drp1. However, PINK1 and Parkin mutant flies showed distinct mitochondrial phenotypes from Drp1 mutant flies, and flies carrying a heterozygous mutation in Drp1 were found to enhance the pink1-null phenotype, resulting in lethality. These results suggest that PINK1 and Parkin are likely not core components of the Drp1-mediated mitochondrial fission machinery. Modification of fusion and fission may represent a novel therapeutic strategy for PD.
In another study, Poole et al. (2008) studied genetic alterations affecting abnormal mitochondrial dynamics on the PINK1 and parkin mutant phenotypes in fruit flies. They found that heterozygous loss-of-function mutations of Drp1 are lethal in a PINK1 or parkin mutant background. Conversely, flight-muscle degeneration and mitochondrial morphological alterations that result from mutations in PINK1 and parkin are strongly suppressed by increased Drp1 expression and by heterozygous loss-of-function mutations affecting the mitochondrial fusion-promoting factors Opa1 and Mfn2. They also found that an eye phenotype associated with increased PINK1/Parkin pathway activity is suppressed by perturbations that reduce mitochondrial fission. The Poole study suggests that the PINK1/Parkin pathway promotes mitochondrial fission and that a loss of mitochondrial and tissue integrity in PINK1 and parkin mutants [derives]? may derive? from reduced mitochondrial fission.
Dagda et al. (2009) sought to determine the relationship between PINK1 knocking down and mitochondrial dysfunction in PD patients. Mutations in PINK1 are associated with familial PD. Although overexpressed PINK1 is neuroprotective, less is known about neuronal responses to the loss of PINK1 function, which has been found to occur in PD. Dagda et al. (2009) found a stable knockdown of PINK1 induced mitochondrial fragmentation and autophagy in SH-SY5Y cells, which was reversed by the reintroduction of an RNA interference (RNAi)-resistant plasmid for PINK1. Moreover, stable or transient overexpression of wild-type PINK1 increased mitochondrial interconnectivity and suppressed toxin-induced autophagy/mitophagy. The production of a mitochondrial oxidant played an essential role in triggering mitochondrial fragmentation and autophagy in PINK1 shRNA lines. Autophagy/mitophagy served a protective role in limiting cell death, and overexpression of Parkin further enhanced this protective mitophagic response. The dominant negative Drp1 mutant inhibited both fission and mitophagy in PINK1-deficient cells. Interestingly, RNAi knockdown of autophagy proteins Atg7 and LC3/Atg8 also decreased mitochondrial fragmentation without affecting oxidative stress, suggesting active involvement of autophagy in morphologic remodeling of mitochondria for clearance. To summarize, the loss of PINK1 function elicited oxidative stress and mitochondrial turnover that was coordinated by autophagic and fission/fusion machineries. Furthermore, PINK1 and Parkin may cooperate through different mechanisms to maintain mitochondrial homeostasis.
Sandebring et al. studied the effects of PINK1 deficiency in mitochondrial function and morphology, in fruit flies (Sandberg et al. 2010). They generated cell lines that stably transduced human dopaminergic M17 cells with lentiviral constructs, which increased or knocked down PINK1. They found that wild-type PINK1, but not recessive mutant or kinase dead versions, protects against rotenone-induced mitochondrial fragmentation, but PINK1-deficient cells showed lower mitochondrial connectivity.
Drp1 overexpression exaggerated PINK1 deficiency phenotypes and Drp1 RNAi rescued them. Sandebring and colleagues also showed that Drp1 is dephosphorylated in PINK1-deficient cells due to the activation of the calcium-dependent phosphatase calcineurin. Accordingly, the calcineurin inhibitor FK506 blocked both the dephosphorylation of Drp1 and the loss of mitochondrial integrity in PINK1-deficient cells, but did not fully rescue the mitochondrial membrane potential. These findings suggest that Drp1 overexpression causes PINK1 deficiency and decreases mitochondrial connectivity. Lutz et al. studied the connection between the PINK1 deficiency and mitochondrial pathology in human SH-SY5Y cells (Lutz et al., 2010). They found that an acute down-regulation of parkin in human SH-SY5Y cells severely affects mitochondrial morphology and function – a phenotype comparable with that induced by a deficiency in PINK1. Alterations in both mitochondrial morphology and ATP production caused by either parkin or PINK1 loss of function could be rescued by Mfn2 and OPA1, or by a dominant, negative mutant of Drp1. Both parkin and PINK1 were able to suppress mitochondrial fragmentation induced by Drp1. Moreover, in Drp1-deficient cells, the parkin/PINK1 knockdown phenotype did not occur, indicating that mitochondrial alterations observed in parkin- or PINK1-deficient cells are associated with an increase in mitochondrial fission. Notably, mitochondrial fragmentation is an early phenomenon that is linked to PINK1/parkin silencing and that occurs in primary mouse neurons and Drosophila S2 cells.
Using aged human endothelial cells (HUVECs) in vitro, Mai et al. studied the connection between extensive elongation of mitochondria and Drp1 expression (Mai et al., 2010). Young HUVECs had tubular mitochondria, whereas senescent cells were characterized by long, interconnected mitochondria. The change in mitochondrial morphology was caused by the downregulation of Fis1 and Drp1 expressions. Targeted photodamage of mitochondria induced the formation of ROS, which triggered mitochondrial fragmentation and a loss of membrane potential in young cells, whereas senescent cells proved to be resistant. Alterations of the Fis1 and Drp1 expression levels also influenced the expression of PINK1. Downregulation of PINK1 or overexpression of a PINK1 mutant (G309D) increased the sensitivity against ROS in young cells. These results indicated that there is a Drp1- and Fis1-induced, and a PINK1-mediated protection mechanism in senescent cells, which, when compromised, could contribute to the age-related progression of disease seen in PD and arteriosclerosis.
The discrepancies found in these studies may be due to the species studied (fly, human cells, mouse models) and also to the timing of mitochondrial characterization since Drp1 activity and expression are highly dynamic and change with disease progression. Further research using time-course analysis of PINK1/Parkin and Drp1 expressions from different species is needed to resolve these inconsistent reports.
5.4. Drp1, mitochondrial fission, and ALS
In ALS, mitochondrial abnormalities have been extensively described in both familial and sporadic ALS patients and also in transgenic mouse models generated with mutant SOD1 (Magrane and Manfredi 2009 2010; Zhou et al. 2009, Kawamata et al. 2008, Cassina et al. 2008, Ilieva et al. 2007, Martin 2006, 2009, 2010, Martin et al. 2009, Raimondi et al. 2006, Krasnianski et al. 2005, Kirkinezos et al. 2005, Zhu et al. 2002, Dhaliwal and Grewal 2000). Histopathological research revealed that mitochondria may be targets of toxicity in ALS since vacuolated and dilated mitochondria with disorganized cristae and membranes in motor neurons have been observed in patients with ALS (Reddy and Reddy, 2010), and mitochondrial defects (e.g., impaired respiration and increased uncoupling proteins) have been reported in the spinal cords and muscle biopsies of patients with ALS.
The direct involvement of Drp1 in mitochondrial fragmentation of tissues from ALS patients and mouse models of mutant SOD1 has not yet been studied. However, recently, Magrané et al. (2009) have generated motor neuronal cell lines expressing wild-type or mutant SOD1 containing a cleavable intermembrane space targeting signal for directly investigating the pathogenic role of mutant SOD1 in mitochondria. Using biochemical and immunofluorescence techniques, they studied mitochondrial dynamics in cells expressing mutant SOD1 localized to the intermembrane space of mitochondria. They demonstrated that mitochondria-targeted SOD1 localizes to the intermembrane space, where it is enzymatically active. They found neurite mitochondrial fragmentation and impaired mitochondrial dynamics in motor neurons expressing intermembrane space mutant SOD1. These defects are associated with impaired maintenance of neuritic processes.
Overall, these findings demonstrate that mutant SOD1 localized in the intermembrane space is sufficient to induce mitochondrial abnormalities and neuronal toxicity, and contributes to ALS pathogenesis.
6. Drp1 expression, mitochondrial fragmentation and impaired mitochondrial transport in neurodegenerative diseases
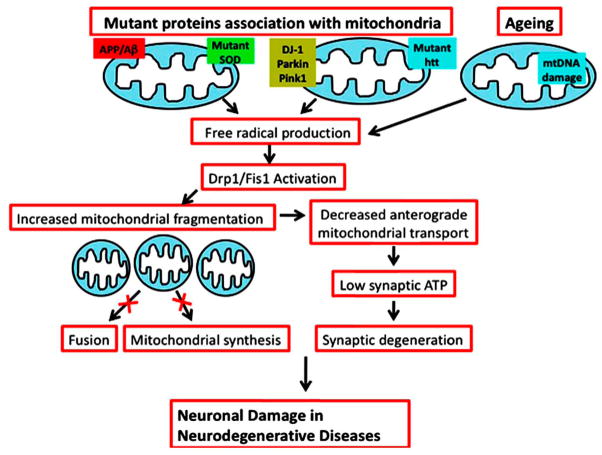
Based on findings from the Reddy laboratory (Reddy et al., 2004; Reddy, 2008; Manczak et al., 2004; 2005; 2006; 2010; Shirendeb and Reddy unpublished observations), and others (Barsoum et al., 2006; Dagda et al., 2009; Lutz et al., 2009; Sandebring et al., 2009; Magrane et al., 2009), we propose that an age-dependent, increased production and subsequent accumulation of mutant proteins in mitochondria (such as Aβ in AD, mutant Htt in HD, mutant PINK/Parkin in PD, and mutant SOD in ALS) induce free radical production and activate the expression of Drp1 and Fis1 (Fig. 3). In turn, the activated Drp1 and Fis1 lead to the excessive fragmentation of mitochondria, producing small and defective mitochondria that may not transport actively to synapses and may not supply the necessary ATP (energy) at nerve terminals. Further, these defective mitochondria may not be able to participate in mitochondrial fusion, may not be able to synthesize healthy mitochondria, and may ultimately, prematurely die. The continuous production of excessive numbers of defective mitochondria in neurons may ultimately damage synapses and cause synaptic neurodegeneration. Such a hypothesized explanation for neurodegenerative diseases needs further research. In pursuit of a better understanding of mitochondria, we need clarification and further research on: 1) mitochondrial transport in healthy and disease states, in neurons for every neurodegenerative disease, for example, in terms of mitochondrial mass (or total number), static/defective mitochondria, and motile/active mitochondria; 2) factors that control/regulate mitochondrial transport and role of aging in mitochondrial transport and abnormal mitochondrial dynamics, if any. Addressing these issues may take us closer to the development of therapeutic strategies capable of treating neurodegenerative diseases.
Figure 3.
Schematic of proposed model for mitochondrial fragmentation, impaired mitochondrial transport, and abnormal mitochondrial dynamics caused by Drp1, in neurodegenerative diseases.
7. Conclusions and Future Directions
The science of mitochondrial dynamics (or fission fusion balance) is relatively new and has been studied in AD and PD. Currently, several investigators are studying abnormal mitochondrial dynamics in HD and ALS, and also in aging. Increasing evidence suggests that balanced mitochondrial fission and fusion are critically important in the maintenance of mitochondrial size, shape, distribution, and normal neuronal function. Impairments in mitochondrial fission and fusion cause irregular mitochondrial shapes and abnormal mitochondrial distributions in neurons from brain tissues of persons with AD, PD, AD, and ALS. Recent mitochondrial studies of yeast, worms, rodents and humans have identified several GTPase proteins, including Drp1, Fis1, Mfn1, Mfn2, and Opa1. Among these, Drp1 is evolutionarily conserved in lower to higher organisms and may have some structural and functional importance in maintaining cell survival and cell death. Recent knockout mice studies of Drp1 suggest that Drp1 is essential for cell survival and mitochondrial division.
Recent advancements in molecular, cellular, electron microscopy, and confocal and imaging studies revealed that Drp1 is associated with several cellular functions, including mitochondrial and peroxisomal fragmentation, phosphorylation, SUMOylation, ubiquitination, and cell death. Researchers of neurodegenerative diseases are beginning to recognize the possible involvement of a mitochondrial fission and fusion proteins, particularly Drp1, in causing mitochondrial fragmentation and the involvement of fission and fusion proteins in abnormal mitochondrial dynamics in neurodegenerative diseases HD. However, the importance of Drp1 and other mitochondrial structural proteins in neurodegenerative diseases are not well understood. Further, the significance of splice variants of Drp1 and other mitochondrial structural proteins is unclear, and the importance of Drp1 in different cell types in the brain, in diseased and non-diseased states, is still unclear. Research is needed to answer these important questions.
Acknowledgments
This research presented was supported by NIH grants AG028072, AG026051, and RR00163, Alzheimer Association grant IIRG-09-92429, and Medivation, Inc.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- AβPP
amyloid beta precursor protein
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- ATP
adenosine triphosphate
- Drp1
dynamin related protein 1
- Fis1
mitochondrial fission 1
- HD
Huntington’s disease
- Mfn1
mitochondrial fusin 1
- Opa1
optic atrophy 1
- PD
Parkinson’s disease
- PINK1
PTEN-induced kinase1
- ROS
reactive oxygen species
- TOMM40
translocase of outer mitochondria membrane 40
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alavi MV, Bette S, Schimpf S, Schuettauf F, Schraermeyer U, Wehrl HF, Ruttiger L, Beck SC, Tonagel F, Pichler BJ, Knipper M, Peters T, Laufs J, Wissinger B. A splice site mutation in the murine Opa1 gene features pathology of autosomal dominant optic atrophy. Brain. 2007;130(Pt 4):1029–1042. doi: 10.1093/brain/awm005. [DOI] [PubMed] [Google Scholar]
- Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26(2):211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Gräber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25(16):3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58(4):495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Braschi E, Zunino R, McBride HM. MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep. 2009;10(7):748–754. doi: 10.1038/embor.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassina P, Cassina A, Pehar M, Castellanos R, Gandelman M, de León A, Robinson KM, Mason RP, Beckman JS, Barbeito L, Radi R. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants. J Neurosci. 2008;28(16):4115–4122. doi: 10.1523/JNEUROSCI.5308-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Chang CR, Blackstone C. Drp1 phosphorylation and mitochondrial regulation. EMBO Rep. 2007;8(12):1088–9. doi: 10.1038/sj.embor.7401118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 2005;14(Spec No 2):R283–R289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC. Mitochondrial dynamics - fusion, fission, movement, and mitophagy - in neurodegenerative diseases. Hum Mol Genet. 2009;18(R2):R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160(2):189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324(5923):102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HY, Zhen X. Hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels in the regulation of midbrain dopamine systems. Acta Pharmacol Sin. 2010 doi: 10.1038/aps.2010.105. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR. DJ-1, PINK1, and their effects on mitochondrial pathways. Mov Disord. 2010;25(Suppl 1):S44–S48. doi: 10.1002/mds.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8(10):939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Cherra SJ, 3rd, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrialfission. J Biol Chem. 2009;284(20):13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies VJ, Hollins AJ, Piechota MJ, Yip W, Davies JR, White KE, Nicols PP, Boulton ME, Votruba M. Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum Mol Genet. 2007;16(11):1307–1318. doi: 10.1093/hmg/ddm079. [DOI] [PubMed] [Google Scholar]
- Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26(2):207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105(38):14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal GK, Grewal RP. Mitochondrial DNA deletion mutation levels are elevated in ALS brains. Neuroreport. 2000;11(11):2507–2509. doi: 10.1097/00001756-200008030-00032. [DOI] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1(4):515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Ferreira IL, Nascimento MV, Ribeiro M, Almeida S, Cardoso SM, Grazina M, Pratas J, Santos MJ, Januário C, Oliveira CR, Rego AC. Mitochondrial-dependent apoptosis in Huntington’s disease human cybrids. Exp Neurol. 2010;222(2):243–255. doi: 10.1016/j.expneurol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Figueroa-Romero C, Iñiguez-Lluhí JA, Stadler J, Chang CR, Arnoult D, Keller PJ, Hong Y, Blackstone C, Feldman EL. SUMOylation of the mitochondrial fission protein Drp1 occurs at multiple nonconsensus sites within the B domain and is linked to its activity cycle. FASEB J. 2009;23(11):3917–3927. doi: 10.1096/fj.09-136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lazaro M, Bonekamp NA, Galindo MF, Jordán J, Schrader M. 6-Hydroxydopamine (6-OHDA) induces Drp1-dependent mitochondrial fragmentation in SH-SY5Y cells. Free Radic Biol Med. 2008;44(11):1960–1969. doi: 10.1016/j.freeradbiomed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol. 2004 Feb 17;14(4):340–345. doi: 10.1016/j.cub.2004.02.004. 2009. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21(9):3017–23. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto M, Tachibana I, Urrutia R. Identification and functional characterization of a novel human protein highly related to the yeast dynamin-like GTPase Vps1p. J Cell Sci. 1998;111 ( Pt 10):1341–1349. doi: 10.1242/jcs.111.10.1341. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11(8):958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- Ju WK, Liu Q, Kim KY, Crowston JG, Lindsey JD, Agarwal N, Ellisman MH, Perkins GA, Weinreb RN. Elevated hydrostatic pressure triggers mitochondrial fission and decreases cellular ATP in differentiated RGC-5 cells. Invest Ophthalmol Vis Sci. 2007;48(5):2145–2151. doi: 10.1167/iovs.06-0573. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Neutzner A, Youle RJ. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol. 2007;178(1):71–84. doi: 10.1083/jcb.200611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Moody JP, Edgerly CK, Bordiuk OL, Cormier K, Smith K, Beal MF, Ferrante RJ. Mitochondrial loss, dysfunction and altered dynamics in Huntington’s disease. Hum Mol Genet. 2010 Aug 4; doi: 10.1093/hmg/ddq306. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkinezos IG, Bacman SR, Hernandez D, Oca-Cossio J, Arias LJ, Perez-Pinzon MA, Bradley WG, Moraes CT. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J Neurosci. 25(1):164–172. doi: 10.1523/JNEUROSCI.3829-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9(7):505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A, Yoon Y, Bonekamp NA, McNiven MA, Schrader M. A role for Fis1 in both mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2005;16(11):5077–5086. doi: 10.1091/mbc.E05-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KV, Suelmann R, Fischer R. Deletion of mdmB impairs mitochondrial distribution and morphology in Aspergillus nidulans. Cell Motil Cytoskeleton. 2003;55(2):114–124. doi: 10.1002/cm.10117. [DOI] [PubMed] [Google Scholar]
- Krasnianski A, Deschauer M, Neudecker S, Gellerich FN, Müller T, Schoser BG, Krasnianski M, Zierz S. Mitochondrial changes in skeletal muscle in amyotrophic lateral sclerosis and other neurogenic atrophies. Brain. 2005;128(Pt 8):1870–1876. doi: 10.1093/brain/awh540. [DOI] [PubMed] [Google Scholar]
- Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4(5):815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- Li XJ, Orr AL, Li S. Impaired mitochondrial trafficking in Huntington’s disease. Biochim Biophys Acta. 2010;1802(1):62–65. doi: 10.1016/j.bbadis.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119(6):873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lin TK, Liou CW, Chen SD, Chuang YC, Tiao MM, Wang PW, Chen JB, Chuang JH. Mitochondrial dysfunction and biogenesis in the pathogenesis of Parkinson’s disease. Chang Gung Med J. 2009;32(6):589–599. [PubMed] [Google Scholar]
- Liot G, Bossy B, Lubitz S, Kushnareva Y, Sejbuk N, Bossy-Wetzel E. Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ. 2009;16(6):899–909. doi: 10.1038/cdd.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lämmermann K, Brunner B, Kurz-Drexler A, Vogel F, Reichert AS, Bouman L, Vogt-Weisenhorn D, Wurst W, Tatzelt J, Haass C, Winklhofer KF. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem. 2010;284(34):22938–51. doi: 10.1074/jbc.M109.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrané J, Hervias I, Henning MS, Damiano M, Kawamata H, Manfredi G. Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities. Hum Mol Genet. 2009;18(23):4552–4564. doi: 10.1093/hmg/ddp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrané J, Manfredi G. Mitochondrial function, morphology, and axonal transport in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11(7):1615–16126. doi: 10.1089/ars.2009.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai S, Klinkenberg M, Auburger G, Bereiter-Hahn J, Jendrach M. Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J Cell Sci. 2010;123(Pt 6):917–26. doi: 10.1242/jcs.059246. [DOI] [PubMed] [Google Scholar]
- Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5(2):147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- Manczak M, Jung Y, Park BS, Partovi D, Reddy PH. Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. J Neurochem. 2005;92(3):494–504. doi: 10.1111/j.1471-4159.2004.02884.x. [DOI] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15(9):1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis. 2010;20(Suppl 2):S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Gertz B, Pan Y, Price AC, Molkentin JD, Chang Q. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Exp Neurol. 2009;218(2):333–346. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ. Mitochondrial pathobiology in Parkinson’s disease and amyotrophic lateral sclerosis. J Alzheimers Dis. 2010;20(Suppl 2):S335–S356. doi: 10.3233/JAD-2010-100348. [DOI] [PubMed] [Google Scholar]
- Martin LJ. Mitochondriopathy in Parkinson disease and amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2006;65(12):1103–1110. doi: 10.1097/01.jnen.0000248541.05552.c4. [DOI] [PubMed] [Google Scholar]
- Martin LJ. The mitochondrial permeability transition pore: a molecular target for amyotrophic lateral sclerosis therapy. Biochim Biophys Acta. 2010;1802(1):186–197. doi: 10.1016/j.bbadis.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Tanaka K. Does impairment of the ubiquitin-proteasome system or the autophagy-lysosome pathway predispose individuals to neurodegenerative disorders such as Parkinson’s disease? J Alzheimers Dis. 2010;19(1):1–9. doi: 10.3233/JAD-2010-1231. [DOI] [PubMed] [Google Scholar]
- Morais VA, Verstreken P, Roethig A, Smet J, Snellinx A, Vanbrabant M, Haddad D, Frezza C, Mandemakers W, Vogt-Weisenhorn D, Van Coster R, Wurst W, Scorrano L, De Strooper B. Parkinson’s disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol Med. 2009;1(2):99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JM. Nature and cause of mitochondrial dysfunction in Huntington’s disease: focusing on huntingtin and the striatum. J Neurochem. 2010;114(1):1–12. doi: 10.1111/j.1471-4159.2010.06741.x. [DOI] [PubMed] [Google Scholar]
- Otsuga D, Keegan BR, Brisch E, Thatcher JW, Hermann GJ, Bleazard W, Shaw JM. The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J Cell Biol. 1998;143(2):333–349. doi: 10.1083/jcb.143.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey M, Varghese M, Sindhu KM, Sreetama S, Navneet AK, Mohanakumar KP, Usha R. Mitochondrial NAD+-linked State 3 respiration and complex-I activity are compromised in the cerebral cortex of 3-nitropropionic acid-induced rat model of Huntington’s disease. J Neurochem. 2008;104:420–34. doi: 10.1111/j.1471-4159.2007.04996.x. [DOI] [PubMed] [Google Scholar]
- Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines, Nat. Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- Panov AV, Lund S, Greenamyre JT. Ca2+-induced permeability transition in human lymphoblastoid cell mitochondria from normal and Huntington’s disease individuals. Mol Cell Biochem. 2005;269:143–152. doi: 10.1007/s11010-005-3454-9. [DOI] [PubMed] [Google Scholar]
- Park YY, Lee S, Karbowski M, Neutzner A, Youle RJ, Cho H. Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J Cell Sci. 2010;123(Pt 4):619–626. doi: 10.1242/jcs.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts KR, Yoon Y, Krueger EW, McNiven MA. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol Biol Cell. 1999;10(12):4403–4417. doi: 10.1091/mbc.10.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105(5):1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla RA, Johnson GV. Role of mitochondrial dysfunction in the pathogenesis of Huntington’s disease. Brain Res Bull. 2009;80(4–5):242–247. doi: 10.1016/j.brainresbull.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi A, Mangolini A, Rizzardini M, Tartari S, Massari S, Bendotti C, Francolini M, Borgese N, Cantoni L, Pietrini G. Cell culture models to investigate the selective vulnerability of motoneuronal mitochondria to familial ALS-linked G93ASOD1. Eur J Neurosci. 2006;24(2):387–399. doi: 10.1111/j.1460-9568.2006.04922.x. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Manczak M, Mao P, Calkins MJ, Reddy AP, Shirendeb U. Amyloid-beta and mitochondria in aging and Alzheimer’s disease: implications for synaptic damage and cognitive decline. J Alzheimers Dis. 2010;20(Suppl 2):S499–S512. doi: 10.3233/JAD-2010-100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH. Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease. J Neurochem. 2006;96(1):1–13. doi: 10.1111/j.1471-4159.2005.03530.x. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial oxidative damage in aging and Alzheimer’s disease: implications for mitochondrially targeted antioxidant therapeutics. J Biomed Biotechnol. 2006;2006(3):31372. doi: 10.1155/JBB/2006/31372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: strategies to protect neurons. Antioxid Redox Signal. 2007;9(10):1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008;10(4):291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Res Brain Res Rev. 2005;49(3):618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14(2):45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Mao P, Manczak M. Mitochondrial structural and functional dynamics in Huntington’s disease. Brain Res Rev. 2009;61(1):33–48. doi: 10.1016/j.brainresrev.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer’s disease. Hum Mol Genet. 2004;13(12):1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Reddy TP. Mitochondria as a Therapeutic Target for Aging and Neurodegenerative Diseases. Current Alz Dis. 2010 doi: 10.2174/156720511795745401. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandebring A, Thomas KJ, Beilina A, van der Brug M, Cleland MM, Ahmad R, Miller DW, Zambrano I, Cowburn RF, Behbahani H, Cedazo-Mínguez A, Cookson MR. Mitochondrial alterations in PINK1 deficient cells are influenced by calcineurin-dependent dephosphorylation of dynamin-related protein 1. PLoS One. 2009;4(5):e5701. doi: 10.1371/journal.pone.0005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesaki H, Jensen RE. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J Cell Biol. 1999;147(4):699–706. doi: 10.1083/jcb.147.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C, Delivani P, Cullen SP, Martin SJ. Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome C release. Mol Cell. 2008;31(4):570–585. doi: 10.1016/j.molcel.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Shin HW, Shinotsuka C, Torii S, Murakami K, Nakayama K. Identification and subcellular localization of a novel mammalian dynamin-related protein homologous to yeast Vps1p and Dnm1p. J Biochem. 1997;122(3):525–530. doi: 10.1093/oxfordjournals.jbchem.a021784. [DOI] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12(8):2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol. 1998;143(2):351–358. doi: 10.1083/jcb.143.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA, Beal FM. Portal to Alzheimer’s disease. Nat Med. 2008;14(10):1020–1021. doi: 10.1038/nm1008-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Wang X, Zheng L, Perry G, Smith MA, Zhu X. Abnormal mitochondrial dynamics and neurodegenerative diseases. Biochim Biophys Acta. 2010;1802(1):135–142. doi: 10.1016/j.bbadis.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH. Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J. 2006a;8(3):E521–531. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J. 2006b;8(2):E277–283. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushina E, Dyer RB, Badger JD, Ure D, Eide L, Tran DD, Vrieze BT, Legendre-Guillemin V, McPherson PS, Mandavilli BS, Van Houten B, Zeitlin S, McNiven M, Aebersold R, Hayden M, Parisi JE, Seeberg E, Dragatsis I, Doyle K, Bender A, Chacko C, McMurray CT. Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol Cell Biol. 2004;24:8195–8209. doi: 10.1128/MCB.24.18.8195-8209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uo T, Dworzak J, Kinoshita C, Inman DM, Kinoshita Y, Horner PJ, Morrison RS. Drp1 levels constitutively regulate mitochondrial dynamics and cell survival in cortical neurons. Exp Neurol. 2009;218(2):274–85. doi: 10.1016/j.expneurol.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47(3):365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186(6):805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lim PJ, Karbowski M, Monteiro MJ. Effects of overexpression of huntingtin proteins on mitochondrial integrity. Hum Mol Genet. 2009;18:737–752. doi: 10.1093/hmg/ddn404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Perry G, Smith MA, Zhu X. Amyloid-beta-derived diffusible ligands cause impaired axonal transport of mitochondria in neurons. Neurodegener Dis. 2010;7(1–3):56–59. doi: 10.1159/000283484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009;29(28):9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105(49):19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177(3):439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Zhou F, Zhang Z, Xing D. Bax is essential for Drp1-mediated mitochondrial fission but not for mitochondrial outer membrane permeabilization caused by photodynamic therapy. J Cell Physiol. 2010;226:530–541. doi: 10.1002/jcp.22362. [DOI] [PubMed] [Google Scholar]
- Yoon Y, Pitts KR, Dahan S, McNiven MA. A novel dynamin-like protein associates with cytoplasmic vesicles and tubules of the endoplasmic reticulum in mammalian cells. J Cell Biol. 1998;140(4):779–93. doi: 10.1083/jcb.140.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417(6884):74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- Züchner S, De Jonghe P, Jordanova A, Claeys KG, Guergueltcheva V, Cherninkova S, Hamilton SR, Van Stavern G, Krajewski KM, Stajich J, Tournev I, Verhoeven K, Langerhorst CT, de Visser M, Baas F, Bird T, Timmerman V, Shy M, Vance JM. Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann Neurol. 2006;59(2):276–281. doi: 10.1002/ana.20797. [DOI] [PubMed] [Google Scholar]
- Zunino R, Braschi E, Xu L, McBride HM. Translocation of SenP5 from the nucleoli to the mitochondria modulates DRP1-dependent fission during mitosis. J Biol Chem. 2009;284(26):17783–17795. doi: 10.1074/jbc.M901902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, McBride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci. 2007;120(Pt 7):1178–1188. doi: 10.1242/jcs.03418. [DOI] [PubMed] [Google Scholar]