Abstract
Few species of true butterflies (Lepidoptera: Papilionoidea) have evolved a proboscis that greatly exceeds the length of the body. This study is the first to examine the morphology of an extremely long butterfly proboscis and to describe how it is used to obtain nectar from flowers with very deep corolla tubes. The proboscis of Eurybia lycisca (Riodinidae) is approximately twice as long as the body. It has a maximal length of 45.6 mm (mean length 36.5 mm ± 4.1 S.D., N = 20) and is extremely thin, measuring only about 0.26 mm at its maximum diameter. The proboscis has a unique arrangement of short sensilla at the tip, and its musculature arrangement is derived. The flower handling times on the preferred nectar plant, Calathea crotalifera (Marantaceae), were exceptionally long (mean 54.5 sec ± 28.5 S.D., N = 26). When feeding on the deep flowers remarkably few proboscis movements occur. The relationship between Eurybia lycisca and its preferred nectar plant and larval host plant, Calathea crotalifera, is not mutualistic since the butterfly exploits the flowers without contributing to their pollination. We hypothesize that the extraordinarily long proboscis of Eurybia lycisca is an adaptation for capitalizing on the pre-existing mutualistic interaction of the host plant with its pollinating long-tongued nectar feeding insects.
Keywords: Mouthparts, Insects, Evolution, Marantaceae, Flower-visiting behavior, Calathea crotalifera, Nectar feeding
Highlights
►The butterfly Eurybia lycisca has a proboscis measuring twice the body size. ►Flower-visiting and proboscis morphology is examined for the first time. ►Long flower handling times are balanced by high nectar rewards. ►Derived proboscis musculature and sensillum equipment were detected. ►The butterfly steals nectar without pollinating its preferred host plant.
1. Introduction
Elongate suctorial mouthparts have evolved convergently in various flower-visiting insects within the context of nectar feeding behavior (Krenn et al., 2005). Some insects have extremely long proboscides that greatly exceed body length. In a Neotropical hawk moth it reaches the remarkable length of 280 mm (Amsel, 1938). Darwin (1862) was the first to postulate that the development of greatly extended mouthparts takes place in a coevolutionary race that leads to a mutualistic specialization of nectar plants and their pollinating insects. Recent studies demonstrate that the long corollae of flowers exclude flower visiting insects that possess inappropriate mouthpart lengths, thus enhancing the chance of efficient pollination by insects with matching long proboscides (Anderson and Johnson, 2009; Anderson et al., 2008; Nilsson, 1988; Nilsson et al., 1985; Pauw et al., 2009). In return, these insects benefit from a greatly elongate proboscis by having more exclusive access to concealed nectar in deep corolla tubes (Haber and Frankie, 1989; Miller, 1997). Evolutionary scenarios postulate that the reciprocal relationships between flower depth and proboscis length in hawk moths (Lepidoptera: Sphingidae) result from mutualistic and coevolutionary adaptations (Alexandersson and Johnson, 2002; Johnson and Steiner, 1997; Nilsson, 1988; Nilsson et al., 1985). While previous studies largely focus on pollinator-mediated selection on flowers, specializations of the mouthparts have not been examined, except for proboscis length.
Compared with those of Hymenoptera and Diptera, the proboscis of Lepidoptera is a relatively simple organ. It is composed of two greatly elongate and coilable galeae, which together form the central food tube (reviewed by Kristensen, 2003). The morphology of the butterfly proboscis has been thoroughly studied along with its functional mechanisms and specializations to various food preferences (Eastham and Eassa, 1955; Knopp and Krenn, 2003; Krenn, 1990, 2010; Krenn and Mühlberger, 2002; Krenn et al., 2001).
In Papilionoidea, i.e. the true butterflies, the proboscis normally measures about two-thirds of the body length in species from Europe (Paulus and Krenn, 1996) and about 80% of body length in Neotropical butterflies (Kunte, 2007). Extremely long proboscides were recorded in the Neotropical genus Eurybia (Riodinidae), measuring up to 49.9 mm, equivalent to twice the body length (Kunte, 2007). While this is the greatest length recorded for a butterfly proboscis, neither the morphology nor the details of the flower-visiting behavior of any species of Eurybia have been examined to date. Species of Eurybia are known to frequently visit Calathea flowers to obtain nectar (DeVries, 1997). The aim of this study is to analyze the behavior of Eurybia lycisca (Westwood, 1851) while handling the flowers of Calathea crotalifera (Marantaceae), and to present the first morphological examination of the butterfly’s extraordinary proboscis. Furthermore, the question is addressed whether the adult butterflies act as pollinators on the larval host plants (DeVries, 1997) and therefore could be regarded as an example of coevolutionary and mutualistic pollinator–plant interaction, as reported for senita moths (Fleming and Holland, 1998; Holland and Fleming, 2002) and yucca moths (Pellmyr, 2003; Pellmyr and Krenn, 2002).
2. Material & methods
The field part of the study was conducted at Tropical Research Station La Gamba (Puntarenas, Costa Rica; 8°45′N, 83°10′W; 81 m above sea level) near the Esquinas rainforest of the Piedras Blancas National Park from August to September 2009. Flower-handling behavior of Eurybia lycisca was recorded with a Sony V50 digital video camera. Handling time was defined as the amount of time the butterfly spent on the flower beginning with the uncoiling of the proboscis and ending when the proboscis was recoiled following nectar uptake or when the butterfly flew from the flower without visibly recoiling the proboscis. Proboscis length was measured in cold-immobilized individuals whose proboscis was partly uncoiled.
To investigate the morphology of the proboscis light microscopy, serial semithin sectioning and scanning electron microscopy were employed.
External features of the proboscis were examined in specimens fixed in 70% alcohol and then soaked in lactic acid for 5–7 days. Afterwards, the galeae were rinsed in distilled water and 30% ethanol. The galeae were then separated, embedded in polyvinyl-lactophenol on microscope slides and covered with a coverslip. Light microscopy was used to record the shape, number and length of sensilla and to investigate the surface structure of the galeae. Scanning electron microscopy was also used for external examination. Specimens were dehydrated in ethanol, air dried after treatment with hexamethyldisilazane (Bock, 1987), coated with gold and examined using a Fei XL30 (ESEM) scanning electron microscope.
Serial semithin sectioning was used for the light microscopic examination of the internal anatomy of the proboscis (procedure see Blumer et al., 2002). Specimens were fixed in 70% ethanol. The proboscis was separated from the head prior to embedding and additionally cut at the middle of its length and near the tip to ensure efficient impregnation of Agar Low Viscosity Resin under vacuum impregnation (procedure see Pernstich et al., 2006).
Drawings of the embedded parts of the proboscis were made using a stereomicroscope with the aid of a drawing tube. The drawings were used to determine the most suitable cutting plane to section the proboscis at the following intervals: 10–20%, 30–35%, 50–70% and 85–90% of overall proboscis length. Serial sections were cut on a microtome (Leica EM UC6) with a diamond knife (Histo Jumbo Diatome) at a thickness of 1 μm. A series of cross sections for a span of 100 μm was taken from the various proboscis regions. Sections were stained with a mixture of 1% azure II and 1% methylene blue in an aqueous 1% borax solution (confer Romeis, 1989) at 60–70 °C. Micrographs were taken with an Olympus CX41 microscope equipped with an Olympus E330 digital camera.
The length and inner diameter of the corolla from 20 flowers of Calathea crotalifera (Marantaceae) were measured with a digital caliper from different plant individuals growing in the garden of the Tropical Research Station (Table 1). Nectar amounts of unreleased flowers, which were previously covered over night with plastic bags, were measured using 10 μl syringes at 08:00 and 16:00. The superinflorescence of Calathea crotalifera consists of several bracts, each containing one to four flowers. The asymmetrical androgynous flower is composed of three chorisepals and a synpetal corolla that consists of three petals. The androeceum is conjoined with the corolla and consists of one fertile petaloid stamen while other staminodia resemble petals. The superior gynoeceum consists of three conjoined carpels to form a single style (Kennedy, 1983). Pollinating euglossine bees (Apidae) release a non-reversible pollen transfer mechanism (Claßen-Bockhoff and Heller, 2008a). It can be readily determined by visual examination of the flower whether its pollen release mechanism has been activated after visitation of an insect. This permitted us to postulate whether the respective visitor acts as a pollinator or not.
Table 1.
Morphometric comparison of Eurybia lycisca and its nectar plant, Calathea crotalifera.
| [mm] |
Eurybia lycisca |
Calathea crotalifera |
|
|---|---|---|---|
| Mean ± S.D. Min – max N |
Mean ± S.D. Min – max N |
||
| Body length | 18.5 ± 1.9 15.8–23.1 20 |
Flower length | 39.5 ± 1.1 36.8–41.1 20 |
| Proboscis length | 36.5 ± 4.1 28.0–45.6 20 |
Corolla length | 26.9 ± 1.0 24.7 ± 29.0 20 |
| Proboscis diameter | – 0.24–0.26 – |
Corolla diameter | 0.7 ± 0.1 0.4–1.0 20 |
Statistical analyses were done using SPSS 16.0.
3. Results
3.1. Probing behavior on Calathea crotalifera flowers
The nectar-feeding behavior of Eurybia lycisca (Riodinidae) butterflies was observed on flowers of its preferred nectar plant, Calathea crotalifera (Marantaceae). The total handling time on single tubular flowers (mean corolla length 26.9 mm ± 1.0 S.D.; diameter 0.7 mm ± 0.1 S.D., N = 20) ranged between 23 sec and 151 sec (mean 54.5 sec ± 28.5 S.D., N = 26). Handling included proboscis uncoiling, searching for the corolla opening, uptake of nectar and proboscis withdrawal. After landing on a superinflorescence of Calathea crotalifera, the butterfly stepwise uncoils its proboscis. Full extension is achieved after 7–12 uncoiling steps which require a minimum of 2 sec and a maximum of 4 sec (N = 15). During uncoiling, the proboscis is turned steeply upward at the joint with the head. The butterfly stretches the legs to lift the body so that enough space is provided beneath to allow for the extension of the proboscis beyond the bend region in forwards and downwards direction (Fig. 1A). The uncoiled proboscis assumes a flexed position with the proximal region raised upward, a short bend region at about one third of the total length and the distal region in a vertical position. The butterfly uses the tip of the proboscis to locate the opening of the corolla before insertion. When the proboscis is inserted, the butterfly tilts forward enabling the tip of the proboscis to reach the bottom of the corolla tube. The inserted proboscis remains motionless for 9–130 sec (mean 35.1 sec ± 26.9 S.D., N = 30). During this time nectar is being taken up the proboscis. The mean nectar amount of unreleased flowers that can be taken up by butterflies ranges from 9.5 μl ± 2.9 S.D. (N = 20) in the morning to 20.6 μl ± 7.1 S.D. (N = 20) in the afternoon.
Fig. 1.
A. Eurybia lycisca probing with its extraordinary long proboscis for the corolla opening on a Calathea crotalifera flower. B. Untriggered flower in which the style (sty) holds back the hooded staminode (sta). C. Flower in which the pollen release mechanism has been triggered by an insect that is capable of pollinating the flower.
Eurybia lycisca can be ruled out as a pollinator of Calathea crotalifera, since examination of the flowers after visits revealed that they could not release the pollen trigger mechanism (Fig. 1B and C). In addition to Eurybia lycisca, only one species of hesperiid butterfly (Calpodes ethlius), two species of euglossine bee (Euglossa sp., Exaerete smaragdina) and the hummingbirds Glaucis aene and Threnetes ruckeri were observed to visit flowers of Calathea crotalifera. However, only euglossine bees were able to release the pollen trigger mechanism. Over a two day period, the number of flower visits to Calathea crotalifera was observed. 36.2–41.6% of flower visits were attributed to hummingbirds, 29.5–30.7% were Eurybia lycisca, 15.8–23.8% were euglossine bees and 10.5–11.9% were Calpodes ethlius.
3.2. Proboscis morphology
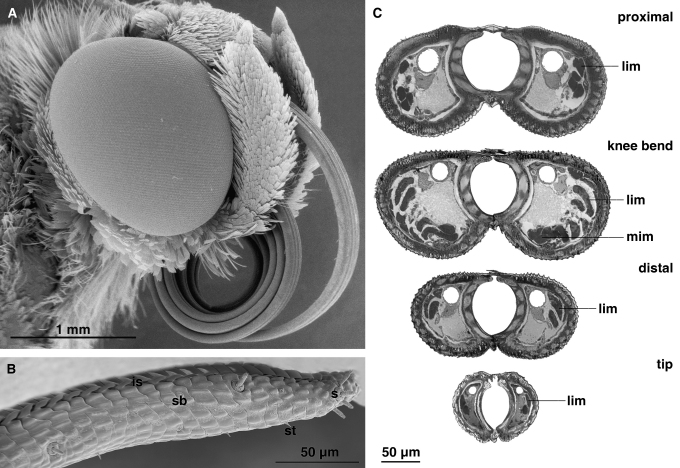
The length of the proboscis of Eurybia lycisca ranges between 28.0 mm and 45.6 mm (mean 36.5 mm ± 4.1 S.D., N = 20) (Table 1), which corresponds approximately to twice the body length. In resting position the proboscis forms at least 7 coils and is positioned between the labial palps beneath the head (Fig. 2A). In the uncoiled position, the region proximal to the bend region amounts to about 35% of the whole galeal length, while the region distal to the bend measures about 65% of the whole galeal length. The proboscis tapers continuously to the tip. Its diameter ranges from 0.26 mm in the bend region to 0.10 mm in the tip region in the male specimen and 0.24 mm–0.09 mm in the female specimen, respectively. The surface of the outer galeal wall bears continuous, vertical exocuticular ridges. The ridges have a corrugated texture that does not change from the proximal to the distal region of the proboscis. The tip region is characterized by slit openings between the otherwise tightly closed dorsal galeal linkage (Fig. 2B). The tip region is 0.71 mm long, which corresponds to approximately 2.0% of the proboscis length.
Fig. 2.
A. Head of Eurybia lycisca (SEM) with coiled proboscis. B. Proboscis tip with four sensilla of uncertain type (s) on the apex of each galea (SEM): Intake slits (is) for imbibing nectar are located on the dorsal side of the tip region. Three different kinds of sensilla are distributed on the proboscis: sensilla of uncertain type (s) are restricted to the tip region sensilla basiconica (sb) and sensilla trichodea (st) can be found along the whole proboscis. C. Semithin sections at various points along the proboscis. Lateral intrinsic muscles (lim) occur throughout the length of the proboscis, median intrinsic muscles (mim) are present only in the knee bend.
Bristle-shaped sensilla trichodea occur along the whole length of the galea with a mean length of 83 μm ± 6.4 S.D. (N = 10) in the basal region, 12.5 μm ± 7.4 S.D. (N = 10) in the proximal region and 6.1 μm ± 1.0 S.D. (N = 20) in the distal region. These sensilla become shorter from the basal to the distal region of the proboscis. Sensilla basiconica occur in the food canal and on the outside of the galea in a single row. Approximately 50 sensilla are present on the proboscis; with a mean density of 3.14 sensilla per mm. The apex of each galea is characterized by a cluster of 4–5 sensilla (Fig. 2B). The sensilla are composed of a short socket (mean length 3.4 μm ± 1.1 S. D., N = 12) and an elongated sensory cone (mean length 10.1 μm ± 1.0 S.D., N = 12).
The lumen of each galea contains a trachea and a nerve in the dorsal region of the cross-section, together with longitudinal musculature in the lateral and ventral regions (Fig. 2C). Lateral intrinsic galeal muscles occur throughout the whole length of each galea. The individual muscles follow a slightly oblique course; they originate on the dorso-lateral wall and extend obliquely to the ventro-lateral wall. Near the tip, the lateral intrinsic galeal muscles follow a nearly longitudinal course. A group of median intrinsic muscles is present only in the knee bend region. Their muscle strands run along the ventral galeal wall in a more or less longitudinal direction.
4. Discussion
Insect mouthparts that are considerably longer than the length of the body are known to occur in some specialized nectarivorous insects such as hawk moths (Sphingidae), species of euglossine bees (Euglossini) and South African flies of Nemestrinidae and Tabanidae (Brachycera) (Krenn et al., 2005). Such extraordinarily long proboscides are rare in true butterflies, even in tropical species, and seem to be restricted to few representatives of Riodinidae and Hesperiidae (Kunte, 2007). Here, we present the first study on the flower-handling behavior and the proboscis morphology of a riodinid butterfly from the genus Eurybia. Its proboscis is nearly twice the body length and is one of the longest among butterflies in terms of absolute length (Krenn, 2010).
We found Eurybia lycisca to have an exceptionally long handling time on the particularly deep and thin corolla tubes of Calathea, which were known to be among the preferred nectar sources (DeVries, 1997). Analysis of the proboscidial movements indicated that the phases of uncoiling, searching and insertion were slower compared to movements in butterflies with average-sized proboscides (Krenn, 1990, 2008). Kunte (2007) found that handling times were up to three times longer in butterflies with a relatively long proboscis. He concluded that they have a reduced foraging efficiency compared to butterflies with average proboscis lengths.
Eurybia lycisca butterflies remain on a single flower of Calathea for about 1 min during which time the proboscis is frequently motionless. Generally, butterflies perform a characteristic series of proboscis movements during flower probing, in which the distal region of the proboscis is repeatedly withdrawn and inserted into the flower (Krenn, 1990, 2008). These poking movements are nearly entirely absent in visits of Eurybia lycisca on Calathea crotalifera flowers. Probably, the poking movements, which serve to search for nectar inside tubular corollae (Krenn, 1998), are not necessary in the narrow corollae of Calathea crotalifera since these flowers are frequented by few visitors and provide liberal amounts of nectar.
The deep corolla tube of Calathea crotalifera (mean length = 26.9 mm) restricts access of general flower visiting butterflies having a mean proboscis length of about 15.9 mm, calculated from data of 89 Neotropical butterfly species (Kunte, 2007). In comparison to Lantana flowers, which are visited by a great number of various butterfly species (Kunte, 2007), the flowers of Calathea species have a small spectrum of visiting butterflies, euglossine bees, hummingbirds and sugar birds (Claßen-Bockhoff and Heller, 2008b and present study). Only two species of butterflies, both with extraordinarily long mouthparts, were recorded during our study. It can be argued that the functional costs and the reduced foraging efficiency due to the extended flower handling time (Kunte, 2007) is balanced by a rich amount of nectar, which is available only to these few insects possessing a proboscis of matching length.
In contrast to that of most butterflies, the proboscis of Eurybia lycisca is equipped with only a small number of short sensilla. The sensory function of the various proboscis sensilla was described for the nymphalid butterfly, Vanessa cardui (Krenn, 1998). The bristle-shaped sensilla on the proboscis are interpreted to be mechanoreceptors which are responsible for detecting the width of the flower’s opening. The short blunt-tipped sensilla basiconica function as chemoreceptors (Inoue et al., 2009). The sensilla styloconica are interpreted to be contact chemo-mechanoreceptors, and are characterized by pores on the sensory cone and the presence of a tubular body (Altner and Altner, 1986; Krenn and Penz, 1998; Nagnan-Le Meillour et al., 2000). They are crucial for the detection of nectar and for assessing the position of the proboscis tip once inside a flower (Krenn, 1998). Two kinds of sensilla are present at the proboscis tip in Eurybia lycisca, i.e. very short bristle-shaped sensilla and short basiconica-like sensilla. Future work should concentrate on TEM examination of the tip sensilla to provide information on their ultrastructural composition and on their likely functional role. Meanwhile, the question remains open as to whether the tip sensilla are particularly short sensilla styloconica or sensilla basiconica. In the latter case, the sensilla styloconica would be entirely absent. Short or reduced sensilla styloconica were reported on the proboscides of some Papilionidae, Danainae and Ithomiinae (Paulus and Krenn, 1996; Petr and Stewart, 2004). The short size of sensilla on the proboscis of Eurybia lycisca can be interpreted to be an adaptation to the small diameter of the corolla of the preferred nectar plant, e.g. Calathea crotalifera. Presumably, long sensilla at the tip would hinder entrance into small lumina of a corolla, as observed in Morpho peleides butterflies where the long sensilla styloconica of the tip prevent access to flowers (Knopp and Krenn, 2003). Because flower morphology restricts most competing nectar feeding insects with a medium sized proboscis, there is a high probability that those with exceptionally long mouthparts will find greater amounts of nectar and the common sensillum equipment of the tip would be superfluous for nectar detection in simple and tubular shaped flowers. The morphology of the proboscis of Eurybia lycisca is seen as a modification of the proboscis morphology of the nectar feeding butterfly guild that is characterized by a rather short tip region with sparsely arranged short sensilla, in comparison to fruit, sap or dung-feeding butterflies which have a relatively long tip region with numerous and long sensilla styloconica (Krenn et al., 2001).
In principle, the proboscis anatomy of Eurybia lycisca resembles that of other butterflies. However, the proboscis contains remarkably few intrinsic galeal muscles. Nearly all species of butterflies possess two series of intrinsic galeal muscles in the proboscis lumen (Krenn and Mühlberger, 2002) which are responsible for coiling the proboscis (Krenn, 1990, 2000; Wannenmacher and Wasserthal, 2003). In Eurybia lycisca the two series of intrinsic muscles are found only in the short bend region at about one third of the proboscis length. Such a muscle arrangement was erroneously described for Pieris brassicae (Eastham and Eassa, 1955) and could not be verified in any Lepidoptera species in additional studies (Krenn and Kristensen, 2004; Krenn and Mühlberger, 2002) until now. The specific muscle arrangement is not common to other representatives of Riodinidae, as far as known, since the European species Hamearis lucina has a rather short proboscis which is equipped with lateral and medial intrinsic muscles throughout the proboscis. A Neotropical representative of genus Nymphidium has only lateral intrinsic musculature throughout the whole proboscis (Krenn and Mühlberger, 2002).
In the garden of the Tropical Research Station La Gamba, the most abundant flower visitors of Calathea crotalifera were hummingbirds and Eurybia lycisca. Furthermore, several euglossine bees regularly utilize this plant as a nectar source. Pollination is possible in all members of Marantaceae when the insect touches a trigger-like appendage of the hooded staminode which holds the style under tension. The style springs forward and scrapes off the foreign pollen, if present on the pollinator, and places its own pollen onto the pollinator’s mouthparts (Pischtschan and Claßen-Bockhoff, 2008). The flower can be inspected after visitation to determine whether the trigger mechanism was released and thus if the visitor served as a potential pollinator. Euglossine bees were found to be the pollinators of Calathea crotalifera since they were able to release the trigger mechanism of the staminode–style complex, corroborating previous results (Claßen-Bockhoff and Heller, 2008a). All other flower visitors failed to trigger the pollen transfer mechanism including Eurybia lycisca. Future work should confirm our hypothesis that releasing the trigger is unlikely due to the thinness of the butterfly’s proboscis and the lack of energetic body movements of the butterfly when feeding, in contrast to the vigorous behavior of the euglossine bees. We therefore regard Eurybia lycisca as a nectar thief and not as a potential pollinator of Calathea crotalifera. Furthermore, Eurybia lycisca larvae predominantly feed on Calathea floral tissue (DeVries, 1997). Therefore we conclude that a two-fold antagonistic relationship exists between Eurybia lycisca and Calathea crotalifera that can be regarded as a parasitic interaction.
In flower-visiting insects with extraordinarily long mouthparts (e.g. hawk moths) the fit between nectar spur length and pollinator proboscis length has been regarded as a result of mutualistic coevolution, i.e., the matching traits evolved due to their functional interaction. The underlying evolutionary pathways were recently discussed (Whittall and Hodges, 2007). Either the nectar spurs and the proboscis length of a pollinator evolved in a one-to-one coevolutionary interaction, as proposed for species of Sphingidae and Nemestrinidae (Alexandersson and Johnson, 2002; Anderson and Johnson, 2009; Nilsson, 1987, 1988; Pauw et al., 2009) or the shift hypothesis applies, i.e. a one-sided selection favored the evolution of a particular long nectar spur to match a rather fixed proboscis length of pollinating insects (Whittall and Hodges, 2007). Neither of those evolutionary scenarios applies to the relationship between Eurybia lycisca and Calathea crotalifera. Since Eurybia lycisca is not the pollinator of its preferred nectar plant, Calathea crotalifera, which is the main larval host plant, the antagonistic nature of the animal–plant relationship is in no doubt. We argue that the enormous length of the proboscis in Eurybia lycisca evolved as an adaptation for stealing nectar from long and thin flowers, which are subject to selection from the pollinator’s proboscis morphology and, therefore, probably have a fixed corolla length. In this case, the proboscis length of the nectar thief is subject to selection by the flower length. We conclude that the extraordinary proboscis length of Eurybia lycisca is an adaptation for gaining access to the nectar resources of flowers which are pollinated by other long-tongued visiting insects. Obviously, the extra costs of an extremely long proboscis and extended handling times are balanced by the high amounts of nectar in the flowers, which limit access to few flower visitors.
Acknowledgements
We are grateful to the Department of Ultrastructure at the University of Vienna for providing the SEM facilities and to the Tropical Research Station La Gamba, Costa Rica, for making available their laboratory facilities and tropical garden. Thanks are due to John Plant for correcting the English manuscript. The Costa Rican Ministerio del Ambients y Energia kindly granted research permits. The study was funded by FWF (P 22248 B17).
References
- Alexandersson R., Johnson S.D. Pollinator–mediated selection on flower-tube length in a hawkmoth–pollinated Gladiolus (Iridaceae) Proceedings of the Royal Society of London B. 2002;269:631–636. doi: 10.1098/rspb.2001.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altner H., Altner I. Sensilla with both terminal pore and wall pores on the proboscis of the moth Rhodogastria bubo Walker (Lepidoptera: arctiidae) Zoologischer Anzeiger - A Journal of Comparative Zoology. 1986;216:129–150. [Google Scholar]
- Amsel H.G. Amphimoea walkeri Bsd., der Schwärmer mit dem längsten Rüssel! Entomologische Rundschau. 1938;55:165–167. [Google Scholar]
- Anderson B., Johnson S.D. Geographical covariation and local convergence of flower depth in a guild of fly-pollinated plants. New Phytologist. 2009;182:533–540. doi: 10.1111/j.1469-8137.2009.02764.x. [DOI] [PubMed] [Google Scholar]
- Anderson B., Johnson S.D., Kohn J. The geographical mosaic of coevolution in a plant-pollinator mutualism. Evolution. 2008;62:220–225. doi: 10.1111/j.1558-5646.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Blumer M.J.F., Gahleitner P., Narzt T., Handl C., Ruthensteiner B. Ribbons of semithin sections: an advanced method with a new type of diamond knife. Journal of Neuroscience Methods. 2002;120:11–16. doi: 10.1016/s0165-0270(02)00166-8. [DOI] [PubMed] [Google Scholar]
- Bock C. A quick and simple method for preparing soft insect tissues for scanning electron microscopy using Carnoy and Hexamethyldisilazane. Beiträge zur elektronenmikroskopischen Direktabbildung und Analyse von Oberflächen. 1987;20:209–214. [Google Scholar]
- Claßen-Bockhoff R., Heller A. Floral synorganization and secondary pollen presentation in four Marantaceae from Costa Rica. International Journal of Plant Science. 2008;169:745–760. [Google Scholar]
- Claßen-Bockhoff R., Heller A. Style release experiments in four species of Marantaceae from the Golfo Dulce area, Costa Rica. Stapfia, zugleich Kataloge der oberösterreichischen Landesmuseen Neue Serie. 2008;88:557–571. [Google Scholar]
- Darwin C. first ed. John Murray; London: 1862. On the Various Contrivances by Which British and Foreign Orchids Are Fertilised by Insects and on the Good Effects of Intercrossing. [PMC free article] [PubMed] [Google Scholar]
- DeVries P.J. Princeton University Press; Chichester: 1997. The Butterflies of Costa Rica and Their Natural History Volume II: Riodinidae. [Google Scholar]
- Eastham L.E.S., Eassa Y.E.E. The feeding mechanism of the butterfly Pieris brassicae L. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 1955;239:1–43. [Google Scholar]
- Fleming T.H., Holland J.N. The evolution of obligate pollination mutualisms: senita cactus and senita moth. Oecologia. 1998;114:368–375. doi: 10.1007/s004420050459. [DOI] [PubMed] [Google Scholar]
- Haber W.A., Frankie G.W. A tropical hawkmoth community: Costa Rican dry forest Sphingidae. Biotropica. 1989;21:155–172. [Google Scholar]
- Holland J.N., Fleming T.H. Co-pollinators and specialization in the pollinating seed-consumer mutualism between senita cacti and senita moths. Oecologia. 2002;133:534–540. doi: 10.1007/s00442-002-1061-y. [DOI] [PubMed] [Google Scholar]
- Inoue T., Asaoka K., Seta K., Imaeda D., Ozaki M. Sugar receptor response of the food-canal taste sensilla in a nectar-feeding swallowtail butterfly, Papilio xuthus. Naturwissenschaften. 2009;96:355–363. doi: 10.1007/s00114-008-0483-8. [DOI] [PubMed] [Google Scholar]
- Johnson S.D., Steiner K.E. Long-Tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae) Evolution. 1997;51:45–53. doi: 10.1111/j.1558-5646.1997.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Kennedy H. Plants: species accounts: Calathea insignis (Marantaceae) In: Janzen D.H., editor. Costa Rican Natural History. University of Chicago Press; Chicago, Illinois, USA: 1983. [Google Scholar]
- Knopp M.C.N., Krenn H.W. Efficiency of fruit juice feeding in Morpho peleides (Nymphalidae, Lepidoptera) Journal of Insect Behavior. 2003;16:67–77. [Google Scholar]
- Krenn H.W. Functional morphology and movements of the proboscis of Lepidoptera (Insecta) Zoomorphology. 1990;110:105–114. [Google Scholar]
- Krenn H.W. Proboscis sensilla in Vanessa cardui (Nymphalidae, Lepidoptera): functional morphology and significance in flower-probing. Zoomorphology. 1998;118:23–30. [Google Scholar]
- Krenn H.W. Proboscis musculature in the butterfly Vanessa cardui (Nymphalidae, Lepidoptera): settling the proboscis recoiling controversy. Acta Zoologica (Stockholm) 2000;81:259–266. [Google Scholar]
- Krenn H.W. Feeding behaviours of neotropical butterflies (Lepidoptera, Papilionoidea) Denisia, zugleich Kataloge der oberösterreichischen Landesmuseen Neue Serie. 2008;88:295–304. [Google Scholar]
- Krenn H.W. Feeding mechanisms of adult Lepidoptera: structure, function, and evolution of the mouthparts. Annual Review of Entomology. 2010;55:307–327. doi: 10.1146/annurev-ento-112408-085338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenn H.W., Kristensen N.P. Evolution of proboscis musculature in Lepidoptera. European Journal of Entomology. 2004;101:565–575. [Google Scholar]
- Krenn H.W., Mühlberger N. Groundplan anatomy of the proboscis of butterflies (Papilionoidea, Lepidoptera) Zoologischer Anzeiger - A Journal of Comparative Zoology. 2002;241:369–380. [Google Scholar]
- Krenn H.W., Penz C.M. Mouthparts of Heliconius butterflies (Lepidoptera: Nymphalidae): a search for anatomical adaptations to pollen-feeding behavior. International Journal of Insect Morphology and Embryology. 1998;27:301–309. [Google Scholar]
- Krenn H.W., Plant J.D., Szucsich N.U. Mouthparts of flower-visiting insects. Arthropod Structure & Development. 2005;34:1–40. [Google Scholar]
- Krenn H.W., Zulka K.P., Gatschnegg T. Proboscis morphology and food preferences in nymphalid butterflies (Lepidoptera: Nymphalidae) Journal of Zoology. 2001;254:17–26. [Google Scholar]
- Kristensen N.P. DeGruyter Walter; 2003. Handbook of Zoology. [Google Scholar]
- Kunte K. Allometry and functional constraints on proboscis lengths in butterflies. Functional Ecology. 2007;21:982–987. [Google Scholar]
- Miller W.E. Diversity and evolution of tongue length in hawkmoths (Sphingidae) Journal of the Lepidopterists’ Society. 1997;51:9–31. [Google Scholar]
- Nagnan-Le Meillour P., Cain A.H., Jacquin-Joly E., Francois M.C., Ramachandran S., Maida R., Steinbrecht R.A. Chemosensory proteins from the proboscis of Mamestra brassicae. Chemical Senses. 2000;25:541–553. doi: 10.1093/chemse/25.5.541. [DOI] [PubMed] [Google Scholar]
- Nilsson L.A. Angraecoid orchids and hawkmoths in central Madagascar: specialized pollination systems and generalist foragers. Biotropica. 1987;19:310–318. [Google Scholar]
- Nilsson L.A. The evolution of flowers with deep corolla tubes. Nature. 1988;334:147–149. [Google Scholar]
- Nilsson L.A., Jonsson L., Rason L., Randrianjohany E. Monophily and pollination mechanisms in Angraecum arachnites Schltr.(Orchidaceae) in a guild of long-tongued hawk-moths (Sphingidae) in Madagascar. Biological Journal of the Linnean Society. 1985;26:1–19. [Google Scholar]
- Paulus H.F., Krenn H.W. Vergleichende Morphologie des Schmetterlingsrüssels und seiner Sensillen - Ein Beitrag zur phylogenetischen Systematik der Papilionoidea (Insecta, Lepidoptera) Journal of Zoological Systematics and Evolutionary Research. 1996;34:203–216. [Google Scholar]
- Pauw A., Stofberg J., Waterman R.J. Flies and flowers in Darwin’s race. Evolution. 2009;63:268–279. doi: 10.1111/j.1558-5646.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- Pellmyr O. Yuccas, yucca moths, and coevolution: a Review. Annals of the Missouri Botanical Garden. 2003;90:35–55. [Google Scholar]
- Pellmyr O., Krenn H.W. Origin of a complex key innovation in an obligate insect-plant mutualism. Proceedings of the National Academy of Sciences. 2002;99:5498–5502. doi: 10.1073/pnas.072588699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernstich A., Krenn H.W., Pass G. Preparation of serial sections of arthropods using 2,2-dimethoxypropane dehydration and epoxy resin embedding under vacuum. Biotechnic and Histochemistry. 2006;78:5–9. doi: 10.1080/10520290312120002. [DOI] [PubMed] [Google Scholar]
- Petr D., Stewart K.W. Comparative morphology of sensilla styloconica on the proboscis of North American Nymphalidae and other Selected Taxa (Lepidoptera): systematic and ecological considerations. Transactions of the American Entomological Society. 2004;130:293–409. [Google Scholar]
- Pischtschan E., Claßen-Bockhoff R. Setting-up tension in the style of Marantaceae. Plant Biology. 2008;10:441–450. doi: 10.1111/j.1438-8677.2008.00051.x. [DOI] [PubMed] [Google Scholar]
- Romeis B. Urban Schwarzenberg; Wien: 1989. Mikroskopische Technik. [Google Scholar]
- Wannenmacher G., Wasserthal L.T. Contribution of the maxillary muscles to proboscis movement in hawkmoths (Lepidoptera: Sphingidae) - an electrophysiological study. Journal of Insect Physiology. 2003;49:765–776. doi: 10.1016/s0022-1910(03)00113-6. [DOI] [PubMed] [Google Scholar]
- Whittall J.B., Hodges S.A. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature. 2007;447:706–710. doi: 10.1038/nature05857. [DOI] [PubMed] [Google Scholar]