Abstract
In several, but not all, previous studies, positive p21WAF1 expression has been suggested as an indicator of a good prognosis in patients with stage III/IV colorectal cancer. However, it is not known whether the same is true for stage B2 patients. The purpose of this study is to assess the influence of p21WAF1 expression in tumor cells on disease-free survival (DFS) and overall survival (OS) of Astler–Coller stage B2 and C patients with colorectal cancer who underwent 5-fluorouracil-based adjuvant chemotherapy. Nuclear p21WAF1 was detected by immunohistochemistry in tissue microarrays from 275 colorectal cancers. The expression of p21WAF1 was associated with DFS (p = 0.025) and OS (p = 0.008) in the subgroup of stage B2 patients that was treated with adjuvant chemotherapy. In multivariate analysis, it remained the only independent prognostic parameter in relation to DFS and OS (p = 0.035 and p = 0.02, respectively). In the subgroup of 72 stage B2 patients with positive p21WAF1 expression but not in the subgroup of 61 stage B2 patients with negative p21WAF1 expression, adjuvant chemotherapy was associated with better DFS (85% 5-year survival versus 65% without chemotherapy, p = 0.03) and OS (96% versus 82%, p = 0.014). In the combined stage B2 and C group of patients treated with adjuvant chemotherapy, positive p21WAF1 expression was also associated with better DFS and OS (p = 0.03, p = 0.002, respectively). Expression of p21WAF1 in colorectal tumor cells identifies a subgroup of Astler–Coller stage B2 patients who could benefit significantly from 5FU-based chemotherapy and may improve the selection of patients for adjuvant chemotherapy.
Keywords: Colorectal cancer, Survival, Chemotherapy, 5-fluorouracil, p21WAF1
Introduction
The efficacy of 5-fluorouracil (5FU)-based adjuvant chemotherapy and the associated survival benefit have been firmly established for patients with stage III colorectal cancer (CRC) [1]. However, the use of adjuvant therapy for stage II colon cancer patients remains controversial [2]. Typically, only high-risk stage II CRCs are treated. Hence, there is a need for predictive factors to support treatment decisions in this group of patients, the majority of whom will be cured by surgery alone [3]. This is especially important because a wider implementation of prophylactic programs in recent years has resulted in an increased number of patients with stage B2 tumors.
5-Fluorouracil has been used to treat colorectal cancer patients for a long time [4]. The target enzyme for 5FU is thymidylate synthase (TS). This enzyme catalyzes the conversion of deoxyuridine-5′monophosphate to deoxythymidine-5′-monophosphate and is therefore essential for DNA synthesis and repair. Resistance to 5FU-based treatment might depend both on the level of expression of TS and other enzymes involved in the metabolism of 5FU and on mechanisms involved in cell growth or apoptosis. p21WAF1 is a multifunctional cell cycle-related protein that inhibits cyclin-dependent kinases (CDKs), which results in cell cycle arrest in the G1 phase [5]. p21WAF1 expression is known to be inversely related to cell proliferation and directly related to terminal differentiation [6]. Recently, it has been shown that TS expression was upregulated in p21WAF1-/- human colorectal cancer HCT116 cells, and TS promoter activity was downregulated by ectopic p21 expression [7]. Furthermore, a CDK inhibitor reduced expression of TS in a dose-dependent manner, and the reduction resulted in enhancement of sensitivity to 5FU in cultured human colon cancer cells [8]. Taken together with other reports [9], this suggests a great importance of p21WAF1 for in vitro response to chemotherapeutic agents and that the CDK inhibitor p21WAF1 regulates thymineless stress-induced DNA damage. Hence, p21WAF1 expression might have a predictive significance in 5FU-treated CRC patients. In several [10–19] but not all [20–24] previous studies, positive p21WAF1 expression has been suggested as an indicator of good prognosis in patients with stage III/IV CRC. However, it is not known whether the same is true for stage B2 patients. Therefore, our purpose was to assess the influence of p21WAF1 expression on disease-free survival (DFS) and overall survival (OS) of Astler–Coller stage B2 patients with CRC who either were or were not treated with 5FU-based adjuvant chemotherapy, and of stage C patients treated with adjuvant chemotherapy.
Materials and methods
Patients
This retrospective study was based on tumor tissue from 275 unselected patients who underwent potentially curative colorectal resection for sporadic CRC (defined as an absence of relevant family history at the time of admission to the hospital). Distant metastases at the time of operation were excluded by preoperative liver ultrasonography, chest x-ray, and intraoperative exploration. Table 1 lists the clinico-pathological details of the 275 tumors and patients. Astler–Coller staging of CRC based on the extent of local invasion and the status of lymph nodes was used [25]. In this system, stage B2 denotes the tumor penetrating through the muscularis propria and uninvolved nodes, stage C—tumors extending into the muscularis propria or penetrating through it with lymph node metastases. The mean age of the patients was 61.1 ± 10.8 years (range, 33–83; median, 62). The splenic flexure was regarded as the border between the proximal and distal colon. The Research Ethics Committee of Pomeranian Medical University approved this study.
Table 1.
Relations between p21WAF1 expression and clinico-pathological parameters
| Parameter | p21WAF1 Expression | ||
|---|---|---|---|
| n | n (%) | p | |
| Age (years) | |||
| <62 | 138 | 87 (63) | 0.39 |
| >62 | 137 | 79 (58) | |
| Sex | |||
| Females | 120 | 75 (63) | 0.54 |
| Males | 155 | 91 (59) | |
| Grade | |||
| G1 + G2 | 156 | 91 (58) | 0.46 |
| G3a | 119 | 75 (63) | |
| Astler–Coller | |||
| B2 | 133 | 72 (54) | 0.049 |
| C | 142 | 94 (66) | |
| Site | |||
| Proximal | 53 | 37 (70) | 0.16 |
| Distal | 222 | 129 (58) | |
| Site | |||
| Rectum | 138 | 81 (59) | 0.62 |
| Colon | 137 | 85 (62) | |
| Radiotherapy (rectal tumors) | |||
| Preoperative | 43 | 26 (61) | 0.85 |
| Postoperative RT and no RTb | 93 | 53 (57) | |
aIncluding mucinous carcinoma
bRadiotherapy status unknown in two cases
Tumors were resected between July 1997 and April 2004 in the Departments of Surgery of the teaching hospitals of Pomeranian Medical University in Szczecin and the Regional Oncological Center in Szczecin, Poland. The operations consisted of either a resection with lymphadenectomy or a total mesorectal excision for rectal carcinomas. A total of 203 (74%) patients (83 high-risk stage B2 and 120 stage C) were treated with adjuvant chemotherapy (six 5-day courses of bolus infusion of 5FU (425 mg/m2) every 4 weeks combined with leucovorin, 20 mg/m2). A total of 72 patients (22 stage C patients) did not receive adjuvant chemotherapy due to internist contraindications.
A total of 41 patients with rectal cancer received postoperative radiotherapy (50.4 Gy), and 43 patients, preoperative radiotherapy (5 × 5 Gy). Of the 133 Astler–Coller stage B2 tumors, there were 65 rectal tumors, and of these, 24 (18%) received preoperative and 11 (8.3%) received postoperative radiotherapy. Radiotherapy did not influence DFS (p = 0.61) nor OS (p = 0.83) of patients with rectal tumors. Since we did not find a statistically significant difference in p21WAF1 expression between the group of patients subjected to preoperative radiotherapy and the remaining 93 rectal tumors not treated with preoperative radiotherapy (Table 1), the former was included in the study.
Time from the surgery until the time of death due to cancer or to last known follow-up was regarded as OS, and the time until the first appearance of metastasis or local recurrence was regarded as DFS. The median follow-up was 54 months (mean, 57.3 ± 30.5 months; range, 5–143 months). During the follow-up, 66 of the 275 (24%) patients died of their disease, and 165 (60%) were alive without symptoms from the disease. Recurrences were found in 106 patients. Four patients died of non-cancer-related causes, and they were treated as censored observations.
Tissue microarray (TMA) construction
Tumor areas with the highest mitotic activity at the outer invasive zone of the cancer were chosen for tissue microarrays which were constructed as previously described [26].
Immunohistochemistry
Slides with tissue microarrays were deparaffinized, rehydrated, and had the endogenous peroxidase activity blocked. Slides were immersed in pH 9.0 buffer, and heat-induced antigen retrieval was performed in a pressure cooker (Pascal, Dako, Glostrup, Denmark). Monoclonal p21WAF1 antibody (Dako) was used (dilution, 1:25; incubation time, 30 min), and the slides were immunostained using a Dako EnVision+ kit according to the manufacturer’s instructions. We used the sensitive EnVision+ visualization system because the detection system used is regarded as a critically important variable in immunohistochemical analysis, and detection methods using signal amplification with HRP-labeled polymers have been shown to be more sensitive than methods without such a layer of amplification [27]. The reaction was developed with diaminobenzidine substrate–chromogen solution, and the slides were counterstained with hematoxylin. Positive controls included colorectal adenocarcinoma previously shown to have a high level of p21WAF1 expression. Negative controls omitted the primary antibody. The immunohistochemical procedure for all tissue microarrays from 275 tumors was performed at the same time in identical conditions because, instead of 275 histological slides of whole tissue sections, only four slides containing tissue cores from all 275 tumors were processed.
Scoring
Immunohistochemical staining for each tumor core was independently assessed by two observers (PD and WD) who were blinded to the clinical and pathological data. In cases of disagreement, the result was reached by consensus. The percentage of tumor cell nuclei with unequivocal staining was recorded for each core. P21WAF1 expression in tumors was variable, and so, tumors were classified as negative (<1% of positive tumor cells) or positive (if ≥1% of tumor cells showed nuclear immunoreactivity).
Statistical analysis
Associations between the presence of p21WAF1 expression in tumors and other categorical variables were analyzed with the Fisher exact test. The Kaplan–Meier method was used for the univariate survival analysis, and the differences between compared groups were assessed by the log-rank test. A Cox proportional hazards model was used for univariate and multivariate analyses of factors associated with OS and DFS. The independent variables included in the model were: age, gender, tumor site, Astler–Coller stage, histological grade, 5FU-based adjuvant chemotherapy, and presence of p21WAF1 expression. A p < 0.05 was considered statistically significant. STATISTICA version 9.1 (StatSoft Inc., Tulsa, USA) was used for the statistical analysis.
Results
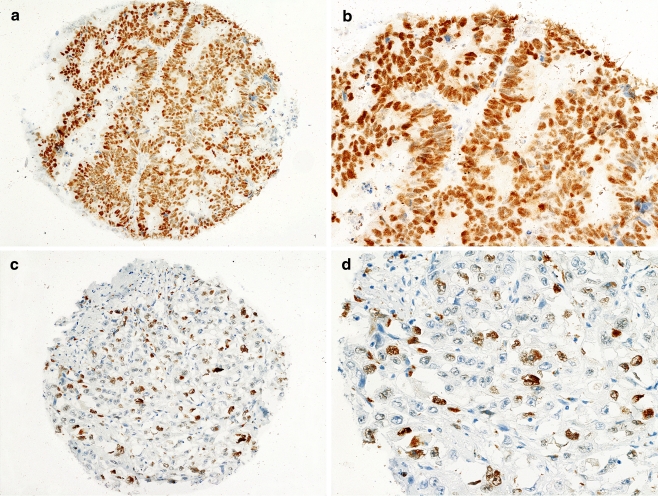
Expression of p21WAF1 (Fig. 1a–d) was found in the nuclei of 60.4% (166/275) of all cases, in 54.1% (72/133) of stage B2 cancers, and in 66.2% (94/142) of stage C tumors. Of all the parameters examined, only Astler–Coller stage was associated with p21WAF1 expression (Table 1).
Fig. 1.
Expression of p21WAF1in representative cores of CRC. a, b High expression of p21WAF1 in the nuclei of tumor cells. c, d Low expression of p21WAF1 in the nuclei of cancer cells. b, d Fragments of the cores from a and c, respectively, at high magnification
Group B2
No association was found between p21WAF1 expression and DFS or OS in the whole B2 group, neither in univariate or multivariate analysis (data not shown). However, 83 patients in this group were treated with 5FU-based adjuvant chemotherapy, and 50 patients were not. Further analysis of the association of p21WAF1 expression with DFS and OS in these subgroups showed that expression of p21WAF1 was associated with DFS (p = 0.025) and OS (p = 0.0079) only in the subgroup of stage B2 patients that were treated with adjuvant chemotherapy (Fig. 2a). Moreover, p21WAF1 expression remained in this subgroup as the only independent prognostic parameter in the multivariate analysis in relation to DFS and OS (p = 0.035 and p = 0.02, respectively; Table 2). In the stage B2 subgroup of patients not treated with chemotherapy, none of the parameters studied were associated with DFS or OS (data not shown).
Fig. 2.
DFS (black curves) and OS (red curves) of stage B2 patients treated with adjuvant chemotherapy categorized according to the p21 expression (n = 83) (a) and of stage B2 patients with p21-positive tumors categorized according to adjuvant chemotherapy (n = 72) (b). DFS (black curves) and OS (red curves) of stage C patients (n = 120) (c) and of stage B2 + C patients (n = 203) (d) treated with adjuvant chemotherapy categorized according to p21 expression
Table 2.
Multivariate analysis of disease-free and overall survival of stage B2 and C patients treated with adjuvant chemotherapy
| Category | B2 (n = 83) | C (n = 120) | ||||||
|---|---|---|---|---|---|---|---|---|
| Disease-free survival | Overall survival | Disease-free survival | Overall survival | |||||
| Hazard ratio (95% Cl) | p | Hazard ratio (95% Cl) | p | Hazard ratio (95% Cl) | p | Hazard ratio (95% Cl) | p | |
| Sex | 0.97 (0.37–2.53) | 0.95 | 0.99 (0.24–4.04) | 0.99 | 1.21 (0.71–2.08) | 0.49 | 1.50 (0.73–3.05) | 0.27 |
| Age | 0.99 (0.95–1.03) | 0.63 | 1.00 (0.94–1.07) | 0.99 | 1.02 (0.99–1.05) | 0.12 | 1.02 (0.99–1.05) | 0.29 |
| Grade | 0.56 (0.20–1.57) | 0.27 | 0.39 (0.08–1.86) | 0.24 | 1.93 (1.12–3.33) | 0.018 | 2.87 (1.37–6.01) | 0.005 |
| Tumor site | 0.99 (0.29–3.47) | 0.10 | 2.55 (0.64–10.15) | 0.19 | 0.57 (0.27–1.17) | 0.13 | 0.35 (0.10–1.15) | 0.08 |
| P21WAF1 | 0.36 (0.14–0.93) | 0.035 | 0.16 (0.03–0.76) | 0.02 | 0.60 (0.35–1.04) | 0.07 | 0.48 (0.24–0.93) | 0.03 |
In the subgroup of 72 stage B2 patients with p21WAF1 expression, adjuvant chemotherapy was associated with better DFS (85% 5-year survival versus 65% without chemotherapy, p = 0.03; Fig. 2b) and OS (96% 5-year survival versus 82% without chemotherapy, p = 0.014; Fig. 2b). In the subgroup of 61 stage B2 patients with negative p21WAF1 expression, a trend for the association of chemotherapy with worse survival (both DFS and OS) was observed, but the differences were not statistically significant (data not shown).
Group C
The expression of p21WAF1 in tumors of patients treated with adjuvant chemotherapy was associated with DFS in a univariate analysis (HR = 0.58, 95% CI = 0.34–0.99, p = 0.047) and was on the verge of statistical significance in the multivariate analysis (p = 0.07) (Table 2). In terms of OS, there was a statistically significant association with p21WAF1 expression in both the univariate (HR = 0.41, 95% CI = 0.21–0.80, p = 0.008) and multivariate (p = 0.03) (Table 2) analyses. Kaplan–Meier survival curves of patients with p21WAF1 expression in the nuclei of cancer cells indicate better DFS (p = 0.055) and significantly better OS (72% versus 45% 5-year survival of patients with negative p21WAF1 expression, p = 0.0079; Fig. 2c).
Stage B2 + C patients treated with adjuvant chemotherapy
Expression of p21WAF1 was associated with DFS and OS (p = 0.03 and p = 0.002, respectively) in a univariate analysis. The Astler–Coller stage and p21WAF1 expression were found to be independent prognostic factors for DFS and OS (Table 3). In multivariate analysis, there was no statistically significant association between site of CRC (neither rectum versus colon nor proximal versus distal as defined by the splenic flexure) and DFS (p = 0.77, p = 0.28, respectively) or OS (p = 0.77, p = 0.28, respectively). Kaplan–Meier survival curves showed significantly better DFS and OS of patients with tumors showing p21WAF1 expression as compared to those negative for p21WAF1 (p = 0.03 and p = 0.0018, respectively; Fig. 2d).
Table 3.
Multivariate analysis of disease-free and overall survival of patients treated with adjuvant chemotherapy (group B2 + C, n = 203)
| Category | Disease-free survival | Overall survival | ||
|---|---|---|---|---|
| Hazard ratio (95% Cl) | p | Hazard ratio (95% Cl) | p | |
| Sex | 1.20 (0.75–1.90) | 0.44 | 1.42 (0.77–2.65) | 0.27 |
| Age | 1.01 (0.99–1.03) | 0.32 | 1.01 (0.98–1.04) | 0.58 |
| Astler–Coller | 3.16 (1.86–5.36) | <0.0001 | 3.60 (1.77–7.33) | 0.0004 |
| Grade | 1.48 (0.94–2.32) | 0.09 | 1.95 (1.07–3.56) | 0.03 |
| Tumor site | 0.64 (0.34–1.20) | 0.17 | 0.62 (0.26–1.49) | 0.28 |
| P21WAF1 | 0.52 (0.33–0.83) | 0.0062 | 0.36 (0.20–0.66) | 0.0009 |
Discussion
We found p21WAF1 nuclear expression in 60.4% CRCs which is comparable to the literature data (16–87%) [11, 13–15, 28, 29]. The majority of reports (but not all) concerning the prognostic role of p21WAF1 expression in CRCs indicate better OS and/or DFS for patients with tumors showing p21WAF1 expression (Table 4). In some of these reports, p21WAF1 expression was an independent prognostic factor for OS and/or DFS (Table 4). However, in those reports, patients with various stages of the disease who were subjected to different treatment protocols (surgery alone, surgery and adjuvant chemotherapy, radiochemotherapy) were often grouped together for the analysis, or no information on the mode of therapy is given. Thus, it is difficult to infer whether stage II patients with p21WAF1-positive tumors may receive any benefit from adjuvant chemotherapy.
Table 4.
Major reports on p21WAF1 expression and survival of patients with CRCs
| Source | Study group | Stage of CRC and type of treatment | Results |
|---|---|---|---|
| Cheng J.D., et al. [10] | n = 39 | Metastatic CRC 5FU CHTH | 5FU responders had greater p21 expression |
| Ropponen K.M, et al. [11] | n = 162 | 0–D incl. 62–B majority: surgery only; 22–CHTH | Better OS and RFS for patients with p21+ tumors; p21: independent prognostic parameter for OS and RFS (MA) |
| Viale G., et al. [12] | n = 191 | I–IV CHTH? | ↓p21 → poor OS and DFS (UA) |
| Bukholm I.K., et al. [13] | n = 61 | B–D CHTH? | Low p21 → increased risk of metastases and death |
| Zirbes T.K., et al. [14] | n = 294 | I–IV incl. 90–II. surgery CHTH? | Better OS for patients with p21+ tumors; p21 independent prognostic parameter (MA) |
| Holland T.A., et al. [15] | n = 126 | A–D RCHTH? | Better OS for patients with high p21 expression |
| Pasz-Walczak G., et al. [16] | n = 122 | I–IV CHTH? | Better OS for patients with p21+ tumors (UA) |
| Watanabe T., et al. [20] | n = 460 | II (B)–105 III (C)–355, 5FU CHTH | No association of p21 expression with survival in all and in stage B tumors |
| Hoss A., et al. [21] | n = 100 | T2–3, N0 rectum, surgery only | No association of p21 expression with survival |
| Schwandner O., et al. [17] | n = 160 | I–III rectum, surgery, n = 69 surgery + CHTH + RT, n = 91 | Better RFS but not OS for patients with p21+ tumors; p21 independent prognostic parameter for RFS but not OS (MA) |
| Rau B., et al. [22] | n = 66 | T3–4, N0–2, M0–1 rectum, RCHTH | No prognostic significance of pretreatment p21 expression. Post-treatment increase of p21: shorter DFS |
| Prall F., et al. [18] | n = 184 | I–IV incl. 55-III, III: n = 32 -5FU, n = 23-5FU + RT | Better OS for patients with p21 + tumors (MA) |
| Mitomi H., et al. [19] | n = 211 | B–D incl. 83-B CHTH for C–D | Better OS for patients with high p21 expression; p21 independent prognostic parameter (MA) |
| Ioachim E., et al. [23] | n = 97 | Dukes B, C, surgery, CHTH? | No association of p21 expression with OS and DFS |
| Noske A., et al. [24] | n = 116 | II–III RCHTH | Better RFS and OS for patients with p21- tumors |
↓ down regulation, UA univariate analysis, MA multivariate analysis, OS overall survival, DFS disease-free survival, RFS relapse-free survival, RT radiotherapy, CHTH chemotherapy, RCHTH radiochemotherapy, ? not known
The benefit of adjuvant 5FU-based chemotherapy has been firmly established for patients with stage III CRC. However, in the stage B2 group, it is not known whether the survival benefit from chemotherapy is sufficient to outweigh the toxicity and cost of the treatment [2]. Only one study [20] has addressed the issue of the influence of 5FU-based adjuvant chemotherapy on the survival of patients with stage B2 CRC in relation to p21WAF1 expression. In that study, pretreatment levels of p21WAF1 were not related to survival of patients with stage II (and III) CRC treated with adjuvant chemotherapy. However, increased levels of p21WAF1 were associated with the sensitivity of metastatic CRC to 5FU-based chemotherapy [10].
Our results indicate for the first time that p21WAF1 expression in CRC tumor cells is an independent factor that is associated with favorable DFS and OS in patients with stage B2 tumors treated with 5FU-based adjuvant chemotherapy. Striking survival benefits were seen for stage B2 patients who received adjuvant chemotherapy compared with those who did not. Conversely, chemotherapy did not significantly influence DFS or OS of stage B2 patients with p21WAF1-negative tumors. Rather, a statistically non-significant trend towards worse survival was observed for stage B2 patients with p21WAF1-negative tumors treated with chemotherapy. The differences between our results and those of Watanabe et al. [20] may be attributed to the different scoring systems and different cutoff points used for the interpretation of immunohistochemical staining, as well as to differences in immunohistochemical methods. The major advantage of tissue microarrays is that tens of cases can be processed in identical laboratory conditions which greatly improves the reproducibility of the immunohistochemical method. Prall et al. [18] used tissue microarrays (n = 184) and found better OS for combined group of stage I–IV patients with p21WAF1-positive tumors. Our results (from the whole group) are well in accord with this report and give further support for the association of p21WAF1 expression and longer survival of patients with stage C/III CRCs treated with adjuvant 5FU-based chemotherapy as has been reported previously (Table 4). One limitation of TMA technology is that “punched” cores from donor tissues may not always be representative of the entire tumor. In this report, we applied one core from carefully identified, histologically relatively homogenous area with the highest mitotic activity in the outer invasive zone of each CRC. Using this approach, we found 60.4% of p21WAF1-positive CRCs which is within the range reported in the literature. Hoos et al. [30] reported that correlations between phenotypes and clinical outcome were not significantly different between full sections and triplicate 0.6-mm core tissue microarrays. However, on the other hand, they were not significantly different when only one 0.6-mm core tissue microarray was used [31]. The authors of the latter report conclude that tissue microarray “with a single core per specimen ensures full biological representativeness to identify the associations between biomarkers and clinico-pathological parameters, with no significant associated sampling bias.” So, careful sampling of the representative region of the tumor is regarded as the key step in the construction of tissue microarrays.
TS, the target enzyme for 5FU, is essential for DNA synthesis [32], and it may function as an oncogene [33]. Inhibition of TS induces apoptosis and cytotoxicity in human colorectal cancer cells [7]. The importance of p21WAF1 in the response of CRC to chemotherapeutic agents is supported by in vitro studies [7–9]. It has been reported that p21WAF1 (a CDK inhibitor) regulates thymineless stress-induced cytotoxicity of human colon carcinoma cell lines [7]. Also, TS expression is mediated through the inhibition of CDK: TS expression was upregulated by the knockout of the p21WAF1 gene in a CRC cell line [8]. In addition, reduction of TS expression results in enhancement of the sensitivity to 5FU in human CRC cell lines [8]. Our results are in line with other reports that show that p21WAF1 is a critical mediator of the cytotoxic action of TS inhibitors in cultured human colorectal cancer cells [7] and that CDK inhibitor enhances the sensitivity to 5FU in colorectal cancer cell lines [8]. In fact, p21WAF1 is required for maximal cytotoxicity induced by thymineless stress in colorectal cancer cells in culture [7]. Poor survival of patients with p21WAF1-negative tumors treated with adjuvant 5FU-based chemotherapy may perhaps be attributed to inherent resistance to 5FU. It has been shown that development of resistance to 5FU by colon cancer cell lines is associated with downregulation of the CDKN1A gene, along with other genes engaged in DNA damage response/repair pathway [34].
In summary, we found that p21WAF1 expression in CRC tumor cells identifies a subgroup of Astler–Coller stage B patients who would benefit significantly from 5FU-based chemotherapy and may therefore allow for better selection of patients for adjuvant chemotherapy. We believe that, in order to maximize the benefit of 5FU-based adjuvant therapy and to spare patients from unnecessary toxicity, stage B2 patients should be stratified according to p21WAF1 status. However, because this is a retrospective study, our results should be confirmed by further prospective randomized investigations.
Acknowledgments
This work was supported by the grant KBN 2P05B 174 28 from the Committee for Scientific Research.
Conflict of interest
We declare that we have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Wolpin BM, Meyerhardt JA, Mamon HJ, Mayer RJ. Adjuvant treatment of colorectal cancer. CA Cancer J Clin. 2007;57:168–185. doi: 10.3322/canjclin.57.3.168. [DOI] [PubMed] [Google Scholar]
- 2.Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology. 2008;134:1296–1310. doi: 10.1053/j.gastro.2008.02.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segal NH, Saltz LB. Is adjuvant therapy for stage II colon cancer worthwhile, and for whom? Nat Clin Pract Gastroenterol Hepatol. 2008;5:422–423. doi: 10.1038/ncpgasthep1181. [DOI] [PubMed] [Google Scholar]
- 4.Pinedo HM, Peters GF. Fluorouracil: biochemistry and pharmacology. J Clin Oncol. 1988;6:1653–1664. doi: 10.1200/JCO.1988.6.10.1653. [DOI] [PubMed] [Google Scholar]
- 5.Dotto GP. p21(WAF1/Cip1): more than a break to the cell cycle? Biochim Biophys Acta. 2000;1471:M43–56. doi: 10.1016/s0304-419x(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 6.Doglioni C, Pelosio P, Laurino L, Macri E, Meggiolaro E, Favretti F, Barbareschi M. p21/WAF1/CIP1 expression in normal mucosa and in adenomas and adenocarcinomas of the colon: its relationship with differentiation. J Pathol. 1996;179:248–253. doi: 10.1002/(SICI)1096-9896(199607)179:3<248::AID-PATH571>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Geller JI, Szekely-Szucs K, Petak I, Doyle B, Houghton JA. P21Cip1 is a critical mediator of the cytotoxic action of thymidylate synthase inhibitors in colorectal carcinoma cells. Cancer Res. 2004;64:6296–6303. doi: 10.1158/0008-5472.CAN-04-0863. [DOI] [PubMed] [Google Scholar]
- 8.Takagi K, Sowa Y, Cevik OM, Nakanishi R, Sakai T. CDK inhibitor enhances the sensitivity to 5-fluorouracil in colorectal cancer cells. Int J Oncol. 2008;32:1105–1110. [PubMed] [Google Scholar]
- 9.O’Connor PM, Jackman J, Bae I, Myers TG, Fan S, Mutoh M, Scudiero DA, Monks A, Sausville EA, Weinstein JN, Friend S, Fornace AJ, Jr, Kohn KW. Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997;57:4285–4300. [PubMed] [Google Scholar]
- 10.Cheng JD, Werness BA, Babb JS, Meropol NJ. Paradoxical correlations of cyclin-dependent kinase inhibitors p21waf1/cip1 and p27kip1 in metastatic colorectal carcinoma. Clin Cancer Res. 1999;5:1057–1062. [PubMed] [Google Scholar]
- 11.Ropponen KM, Kellokoski JK, Lipponen PK, Pietilainen T, Eskelinen MJ, Alhava EM, Kosma VM. p22/WAF1 expression in human colorectal carcinoma: association with p53, transcription factor AP-2 and prognosis. Br J Cancer. 1999;81:133–140. doi: 10.1038/sj.bjc.6690662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viale G, Pellegrini C, Mazzarol G, Maisonneuve P, Silverman ML, Bosari S. p21WAF1/CIP1 expression in colorectal carcinoma correlates with advanced disease stage and p53 mutations. J Pathol. 1999;187:302–307. doi: 10.1002/(SICI)1096-9896(199902)187:3<302::AID-PATH243>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 13.Bukholm IK, Nesland JM. Protein expression of p53, p21 (WAF1/CIP1), bcl-2, Bax, cyclin D1 and pRb in human colon carcinomas. Virchows Arch. 2000;436:224–228. doi: 10.1007/s004280050034. [DOI] [PubMed] [Google Scholar]
- 14.Zirbes TK, Baldus SE, Moenig SP, Nolden S, Kunze D, Shafizadeh ST, Schneider PM, Thiele J, Hoelscher AH, Dienes HP. Prognostic impact of p21/waf1/cip1 in colorectal cancer. Int J Cancer. 2000;89:14–18. doi: 10.1002/(SICI)1097-0215(20000120)89:1<14::AID-IJC3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Holland TA, Elder J, McCloud JM, Hall C, Deakin M, Fryer AA, Elder JB, Hoban PR. Subcellular localisation of cyclin D1 protein in colorectal tumours is associated with p21(WAF1/CIP1) expression and correlates with patient survival. Int J Cancer. 2001;95:302–306. doi: 10.1002/1097-0215(20010920)95:5<302::AID-IJC1052>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Pasz-Walczak G, Kordek R, Faflik M. P21 (WAF1) expression in colorectal cancer: correlation with P53 and cyclin D1 expression, clinicopathological parameters and prognosis. Pathol Res Pract. 2001;197:683–689. doi: 10.1078/0344-0338-00146. [DOI] [PubMed] [Google Scholar]
- 17.Schwandner O, Bruch HP, Broll R. p21, p27, cyclin D1, and p53 in rectal cancer: immunohistology with prognostic significance? Int J Colorectal Dis. 2002;17:11–19. doi: 10.1007/s003840100333. [DOI] [PubMed] [Google Scholar]
- 18.Prall F, Ostwald C, Nizze H, Barten M. Expression profiling of colorectal carcinomas using tissue microarrays: cell cycle regulatory proteins p21, p27, and p53 as immunohistochemical prognostic markers in univariate and multivariate analysis. Appl Immunohistochem Mol Morphol. 2004;12:111–121. doi: 10.1097/00129039-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Mitomi H, Ohkura Y, Fukui N, Kanazawa H, Kishimoto I, Nakamura T, Yokoyama K, Sada M, Kobayashi K, Tanabe S, Saigenji K. P21WAF1/CIP1 expression in colorectal carcinomas is related to Kras mutations and prognosis. Eur J Gastroenterol Hepatol. 2007;19:883–889. doi: 10.1097/MEG.0b013e3282e1c5f3. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, Benson AB, 3rd, Hamilton SR. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–1206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoos A, Nissan A, Stojadinovic A, Shia J, Hedvat CV, Leung DH, Paty PB, Klimstra D, Cordon-Cardo C, Wong WD. Tissue microarray molecular profiling of early, node-negative adenocarcinoma of the rectum: a comprehensive analysis. Clin Cancer Res. 2002;8:3841–3849. [PubMed] [Google Scholar]
- 22.Rau B, Sturm I, Lage H, Berger S, Schneider U, Hauptmann S, Wust P, Riess H, Schlag PM, Dorken B, Daniel PT. Dynamic expression profile of p21WAF1/CIP1 and Ki-67 predicts survival in rectal carcinoma treated with preoperative radiochemotherapy. J Clin Oncol. 2003;21:3391–3401. doi: 10.1200/JCO.2003.07.077. [DOI] [PubMed] [Google Scholar]
- 23.Ioachim E. Expression patterns of cyclins D1, E and cyclin-dependent kinase inhibitors p21waf1/cip1, p27kip1 in colorectal carcinoma: correlation with other cell cycle regulators (pRb, p53 and Ki-67 and PCNA) and clinicopathological features. Int J Clin Pract. 2008;62:1736–1743. doi: 10.1111/j.1742-1241.2006.01105.x. [DOI] [PubMed] [Google Scholar]
- 24.Noske A, Lipka S, Budczies J, Muller K, Loddenkemper C, Buhr HJ, Kruschewski M. Combination of p53 expression and p21 loss has an independent prognostic impact on sporadic colorectal cancer. Oncol Rep. 2009;22:3–9. doi: 10.3892/or_00000398. [DOI] [PubMed] [Google Scholar]
- 25.Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954;139:846–852. doi: 10.1097/00000658-195406000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sulzyc-Bielicka V, Domagala P, Majdanik E, Chosia M, Bielicki D, Kladny J, Kaczmarczyk M, Safranow K, Domagala W. Nuclear thymidylate synthase expression in sporadic colorectal cancer depends on the site of the tumor. Virchows Arch. 2009;454:695–702. doi: 10.1007/s00428-009-0787-x. [DOI] [PubMed] [Google Scholar]
- 27.Chung GG, Kielhorn EP, Rimm DL. Subjective differences in outcome are seen as a function of the immunohistochemical method used on a colorectal cancer tissue microarray. Clin Colorectal Cancer. 2002;1:237–242. doi: 10.3816/CCC.2002.n.005. [DOI] [PubMed] [Google Scholar]
- 28.Sinicrope FA, Roddey G, Lemoine M, Ruan S, Stephens LC, Frazier ML, Shen Y, Zhang W. Loss of p21WAF1/Cip1 protein expression accompanies progression of sporadic colorectal neoplasms but not hereditary nonpolyposis colorectal cancers. Clin Cancer Res. 1998;4:1251–1261. [PubMed] [Google Scholar]
- 29.Valassiadou KE, Stefanaki K, Tzardi M, Datseris G, Georgoulias V, Melissas J, Tsiftsis DD, Delides G, Kanavaros P. Immunohistochemical expression of p53, bcl-2, mdm2 and waf1/p21 proteins in colorectal adenocarcinomas. Anticancer Res. 1997;17:2571–2576. [PubMed] [Google Scholar]
- 30.Hoos A, Urist MJ, Stojadinovic A, Mastorides S, Dudas ME, Leung DH, Kuo D, Brennan MF, Lewis JJ, Cordon-Cardo C. Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol. 2001;158:1245–1251. doi: 10.1016/S0002-9440(10)64075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang D, Salto-Tellez M, Putti TC, Do E, Koay ES. Reliability of tissue microarrays in detecting protein expression and gene amplification in breast cancer. Mod Pathol. 2003;16:79–84. doi: 10.1097/01.MP.0000047307.96344.93. [DOI] [PubMed] [Google Scholar]
- 32.Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 33.Rahman L, Voeller D, Rahman M, Lipkowitz S, Allegra C, Barrett JC, Kaye FJ, Zajac-Kaye M. Thymidylate synthase as an oncogene: a novel role for an essential DNA synthesis enzyme. Cancer Cell. 2004;5:341–351. doi: 10.1016/S1535-6108(04)00080-7. [DOI] [PubMed] [Google Scholar]
- 34.De Angelis PM, Svendsrud DH, Kravik KL, Stokke T. Cellular response to 5-fluorouracil (5-FU) in 5-FU-resistant colon cancer cell lines during treatment and recovery. Mol Cancer. 2006;5:20. doi: 10.1186/1476-4598-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]