Abstract
We characterized the reproductive cycle of Octodon degus to determine whether reproductive maturation is spontaneous in juveniles and if ovarian cyclicity and luteal function are spontaneous in adults. Laboratory-reared prepubertal and adult females were monitored for vaginal patency and increased wheel-running. Sexual receptivity was assessed by pairing adult females with a male 1) continuously, 2) at the time of vaginal patency, or 3) following estradiol treatment. Blood samples were assayed for estradiol and progesterone concentrations on Days 1, 4, 8, and 16 relative to vaginal opening. Ovarian tissues were collected 6 and 16 days after behavioral estrus and 6 days after copulation for histology. In juveniles, the onset of cyclic vaginal patency and increased wheel-running activity was spontaneous, occurred in the absence of proximal male cues, and appeared at regular intervals (17.5 ± 1.4 days). In adults, vaginal patency and increased wheel-running occurred cyclically (21.2 ± 0.6 days) in the absence of proximal male cues, and these traits predicted the time of sexual receptivity. Corpora lutea develop spontaneously and are maintained for 12–14 days. The ovaries had well-developed corpora lutea 6 days after mating and 6 days after estrus without mating. Progesterone concentrations were highest in the second half of the cycle when corpora lutea were present and estradiol concentrations peaked on the day of estrus. Thus, female degus appear to exhibit a spontaneous reproductive cycle consistent with other Hystricognathi rodents. Octodon degus is a novel model with which to examine the mechanisms underlying different reproductive cycles.
Keywords: circadian rhythm, corpus luteum, female mating behavior, ovary, ovulatory cycle
The hystricognath rodent Octodon degus has a spontaneous ovarian cycle that lasts 17–21 days and has a true luteal phase, thus, it is a unique animal model for studies of mechanisms of female reproductive cyclicity.
INTRODUCTION
The estrous cycle of female mammals is a cyclical change in hormone secretion and ovulation that can be divided into two phases based on ovarian structures and the hormones they produce. During the follicular phase, maturing follicles in the ovary release increasing concentrations of estrogen. When estradiol concentrations reach a threshold, a surge of gonadotropin-releasing hormone (GnRH) is released from cells in the hypothalamus. This surge in GnRH in turn triggers a surge of luteinizing hormone (LH) from the anterior pituitary, which then induces ovulation. The luteal phase occurs when the ruptured follicles develop into the corpora lutea and these structures primarily secrete progesterone. Maximal behavioral receptivity, or estrus, is typically associated with high blood concentrations of estradiol, although some species are able to display receptive behavior across the reproductive cycle [1].
Female reproductive cycles can be categorized with respect to whether ovulation, production of functional corpora lutea, and behavioral receptivity can occur spontaneously or be induced by external stimuli in a species-specific manner. Despite these known cycle variations, our knowledge of reproductive patterns is limited, in part because of the lack of knowledge about reproductive biology from a broad range of species. The analysis of hormonal secretion and sexual behavior patterns in additional species will aid in determining commonalities underlying female reproductive cycles as well as important species differences [2]. Generally speaking, however, species within a genus or order have reproductive cycles that share similar characteristics [3]. The most-studied animal models of female estrous cyclicity are myomorph rodents (i.e., rat, hamster, and mouse) [4]. These rodents exhibit spontaneous estrous cycles, follicular development, and ovulation. Corpora lutea form for 1–3 days, but they do not become functional in the absence of mating or vaginal cervical stimulation [5]. These species have a relatively short cycle length (4 or 5 days). The LH surge occurs 6–15 h prior to ovulation, and both events occur at precise circadian intervals [6–8]. For example, in hamsters housed in a light:dark cycle, the estrous cycle occurs every 96 h (four periods of 24 h). When animals are housed in constant light, this rhythm continues (range, 95.35–97.54 h), indicating an endogenous clock (rather than the environmental light:dark cycle) is regulating the timing of the LH surge during the estrous cycle [6]. Laboratory rodents have been extensively studied and have provided valuable information as to the mechanisms underlying estrous cyclicity; however, not all mammals share these same features [4, 9]. The study of additional species can provide novel additional information and breadth to our understanding of female reproductive cycles and behavior [2].
For example, many species with spontaneous ovulation exhibit functional, progesterone-secreting corpora lutea independent of mating stimulation (i.e., guinea pigs, coypu, sheep, and primates). In these species, the estrous cycle is much longer relative to laboratory rodents (14–35 days), and the luteal phase typically lasts for 12–13 days [5, 10, 11]. Most females in this group, including most Hystricognathi rodents, exhibit behavioral receptivity (estrus) only during the periovulatory period [12]. Although species with a long luteal phase exhibit spontaneous cycles of ovulation, it is unknown if the circadian system is involved in the timing or regulation of estrous-related events. The Hystricognathi infraorder contains South American rodent species, including tuco-tucos, guinea pigs, coypus, cuis, grasscutters, and chinchillas.
The Hystricognathi rodent Octodon degu is one of the most numerous and well-studied mammals of central Chile [13]. This precocious rodent species is small (∼200 g), social, and breeds readily in the laboratory [13]. Pups are born fully furred and mobile, and by age 2 wk they are eating solid food, although they continue to nurse. In both the field and laboratory, animals are weaned at age 4–5 wk [14, 15]. Females exhibit a vaginal opening as early as age 2 mo, and by age 3.5 mo, all females have demonstrated at least one occurrence of a vaginal opening [14]. Penile spikes, a testosterone-dependent structure, appear in male degus at age 2.5 mo, and all males have their full complement of spikes at 3.5 mo [14]. These signs of sexual development are not present in animals that have been gonadectomized. In the field, degus typically breed once a year (May–July), and litters of 3–10 pups are born in September–October [15]. Gestation is 90 days. In the field, it is not known how old females are at first conception, but presumably if the juveniles born in the fall breed the following summer, they are age 8–9 mo when they mate. In the lab, degus breed around age 6 mo, presumably because of the availability of ad libitum food and water [13].
Octodon degus are an ideal model with which to examine the mechanisms underlying different reproductive cycles, and whether there are daily rhythms in the timing of reproductive events in a species with a true luteal phase. Despite their physical similarity to murid rodents, degus appear to have a reproductive cycle that lasts 17–21 days, similar to other hystricognathi rodents, such as guinea pigs, but also closely resembling large mammals, such as primates and sheep. Labyak and Lee [16] detected a vaginal opening for 3–5 days in degus, and this occurred at regular intervals. Despite this finding, earlier work reported that degus had a vaginal opening for 3–21 days, indicative of receptivity, but did not have regular estrous cycles [12]. In addition, corpora lutea were only detected in females that had mated; therefore, this species was described as an induced ovulator. In agreement with this hypothesis, Nowak and Paradiso [17] found that the female degu required the presence of a male to induce ovulation. It remains unclear whether the onset of reproductive maturity and the occurrence of regular estrous cycles depend on the presence of a male conspecific. There are several other examples of Hystricognathis that have variations in the characteristics of their reproductive cycles. The cuis (Galea musteloides) requires male chemosensory cues to induce a vaginal opening, but thereafter ovulation and corpora lutea function occur spontaneously [18, 19]. The grasscutter (Thryonomys swinderianus) is reported to have spontaneous and recurring vaginal opening; however, these cycles are irregular and range from 3 to 98 days [20]. Alternatively, degus might be similar to the majority of other Hystricognathis, including guinea pigs, that have both spontaneous ovulation and corpora lutea function independent of social contact [12].
The goals of these studies were to characterize the reproductive cycles of degus and determine 1) if the onset of cyclic patterns in vaginal patency and elevated wheel-running activity are induced or spontaneous in juvenile degus, 2) if ovulation and maintenance of corpora lutea are spontaneous in adult animals, 3) the temporal pattern of ovarian hormone secretion during the estrous cycle, 4) whether mating occurs at the time of increased activity and vaginal patency at estrus, and 5) whether there is a daily rhythm in the time of mating events. We determined if signs of estrous cyclicity occur spontaneously in peripubertal and adult female degus, the pattern of estradiol and progesterone secretion across the presumed estrous cycle, and the timing of mating in intact and steroid-primed degus.
MATERIALS AND METHODS
Animals and Housing
All procedures involving animals were approved by the University Committee for the Care and Use of Animals at the University of Michigan and were in accordance with the standards in the National Research Council Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize discomfort and the number of animals used in these experiments. Animals were housed individually in 47.5 × 26.25 × 20 cm cages in a 12L:12D cycle with temperature at 18°C ± 2°C. Purina Rodent Chow (5015) and water were available ad libitum. Males and females were housed in the same colony rooms, with air changes occurring 12–15 times per hour as required by the National Research Council Guide for the Care and Use of Laboratory Animals.
Degus were obtained from a laboratory colony maintained at the University of Michigan. This colony was established with animals obtained from three different national zoos and individuals from a laboratory colony that was founded with live trapped animals from Chile. In 2000, additional wild-caught animals were added to the colony as breeders (obtained from Dr. Peter Meserve). The colony is maintained as outbred as possible. Breeding pairs are never made from siblings, first cousins, or parent-sibling combinations. The earlier data from this colony [11] suggesting spontaneous, long estrous cycles were gathered from the first generation of animals in the laboratory, including offspring from pairings of wild and zoo-raised parents. The cycle durations have not changed in the subsequent years. Furthermore, we observe spontaneous increases in activity and vaginal opening in females housed in an environment isolated from male cues [21].
Females are housed in the same room as males; however, it is unlikely that circulating pheromonal cues are influencing the onset of puberty or ovulatory cyclicity. In species where the presence of males induces or accelerates the onset of estrous cyclicity, naive females are exposed to male urine or soiled bedding, or cohabitate with males for more than 24 h [22]. In the Hystricognathi cuis, females ovulate in response to the presence of a male, but do not continue to have cyclical occurrences of vaginal opening [19]. We observe cyclical changes in activity and vaginal patency in naive females that have never been housed with a male or exposed to male bedding and urine. Thus, we believe we are describing the natural cycles of animals that have ad libitum access to food and water, which is quite different than their natural habitat during parts of the year [23, 24].
Characterization of the Onset of Vaginal Patency in Juvenile Degus
Examinations of sexually naive juvenile degus (n = 14) began between Postnatal Days 25 and 58 (P25–P58) and continued for 4–6 mo (P135–P180). Females were monitored for the presence of vaginal opening three times weekly. Any changes in the color of the vaginal region and changes in the vaginal membrane were noted (closed, partial opening, or full opening). For analysis, total lack of vaginal opening was recorded as a “0,” full vaginal opening was recorded as a “2,” and all intermediate stages (dimpling, partial opening) were given a “1.” The date of the first full vaginal opening was defined as the onset of estrous cyclicity. Cycles were defined as the interval of time (days) between the appearances of full vaginal opening. All juvenile animals underwent a 10-min sham ovariectomy procedure as part of a larger study. We did not detect an effect on behavior, vaginal opening, or health in these animals.
The same juvenile degus were also housed in running wheels to determine whether they exhibited a regular increase in wheel running at the time of vaginal patency and/or a periodic increase in wheel running followed by a phase delay in activity the next day, both of which are characteristics of adult female estrous cycles. At weaning (P25), degus were housed in cages equipped with a running wheel. To prevent the detrimental effects of social isolation on development [25], degus were housed with an ovariectomized sibling on alternating weeks. During these times, the animals were still kept in the experimental environment with a running wheel, but activity data were not recorded. Activity data were collected in 10-min bins (Dataquest III hardware and Minimitter software). Activity records were examined by an experimenter blind to the status of the degu's vaginal opening. Records were examined for days that contained persistent or elevated activity (based on the daily number of wheel revolutions, an elongated active period [α] relative to other days, or increased activity occurring at times when degus are typically inactive, i.e., during the end of the night). These days were classified as “putative estrus.” The day immediately following this active period was then classified as “putative postestrus.” All other days of activity were used to calculate baseline activity. The percent change in activity levels on the days of putative estrus and postestrus were compared to baseline levels. The time of day when peak activity occurred on putative estrus and postestrus was determined using cosine analysis (Actiview). Two animals were not included in these analyses, because one died and the second exhibited two cycles of vaginal opening which then ceased for the remainder of the experiment.
Characterization of the Estrous Cycle in Adult Animals
Intact adult (age >5 mo) female degus with and without sexual experience exhibit 1 day of increased activity every 18–22 days; this increased activity may be the day of estrus [16]. This cyclical increase in activity levels has been observed in all gonadal-intact females from our laboratory colony, including those that were wild caught (see above), first-generation animals, sexually naive females, and females housed in closed environmental chambers in the absence of male sensory cues. No regular changes in activity levels were observed in gonadectomized animals in earlier studies. Females were housed with running wheels for 2–3 mo to determine the interval between days of increased activity and to determine whether they occurred at regular periods indicative of estrous cyclicity (n = 20). Of these 20 animals, four females had a duration between peaks in wheel-running activity that was irregular; thus, these animals were not used in additional analyses. The day when there was a peak and phase advance in wheel-running activity was designated as Day 1 of the estrous cycle. This day was used for the subsequent determination of timing of blood collections, ovariectomies, and pairing of breeding couples. Degus were also checked daily for vaginal patency. We were unable to do daily vaginal cell inspections because degus have a closed vaginal membrane most of the time [26].
Mating Behavior of Intact and Estradiol-Primed Animals
We wanted to determine whether female receptivity (estrus) correlated with vaginal patency and the peak in wheel-running activity. Second, we wanted to determine whether there was a rhythm with respect to the time of day when mating occurred, as is the case in myomorph laboratory rodents. Mating pairs were videotaped using a 24-h time-lapse video camera (Panasonic video recorder with low-light lens); a low-intensity red light (<5 lux) was left on continuously. The time of day of mating events was scored. In the first group, intact females (n = 4) were observed every day for vaginal patency, or their wheel-running records were examined for an increase in activity. When they exhibited one of these signs, they were immediately housed with a male in a clear cage (53.3 × 53.3 cm) and videotaped until vaginal patency was absent. Vaginal patency typically lasted 1–2 days. After vaginal closure, the female was housed alone without a running wheel until ovariectomy (Day 6 after mating as determined from videotape observation). In the second group, females (n = 5) were monitored daily for vaginal patency for two to three putative estrous cycles before they were paired with a male. Females and males were paired 2–4 days prior to the next predicted putative estrus. We continued to monitor vaginal patency of females daily while they were living continuously with a male. In the third group, ovariectomized females were anesthetized with isoflurane, and a silastic capsule containing crystalline estradiol benzoate (EB; 10-mm effective length, inner diameter 1.98 mm and outer diameter 3.15 mm) was implanted subcutaneously at the nape of the neck. Capsules were soaked in saline overnight prior to implantation. Females received the capsule at either 2 h before lights-off (n = 7) or 2 h before lights-on (n = 4). Immediately after receiving the capsule, females were paired with a male in a glass aquarium and videotaped for 48 h. The number of copulatory events (male mount occurring with a female lordotic posture) was counted for each hour and converted to a percentage of the total number of events observed.
Blood Sampling Procedures
To measure estradiol and progesterone concentrations across the estrous cycle, blood was collected from adult females on Days 1, 4, 8, and 16 relative to the day of the increase in wheel-running and vaginal patency, which we refer to as putative estrus (n = 8–10 samples per time point). Degus were anesthetized with ketamine HCl (30 mg/kg body weight) or isoflurane inhalant. Blood (0.5–1 cc) was collected via infraorbital collection or cardiac heart puncture using a heparinized needle. Following blood sampling, degus were given yohimbine (2.5 mg/kg body weight, intraperitoneally) and lactated Ringer solutions (5 ml, subcutaneously). Blood samples were centrifuged for 15 min at 3000 rpm, and the plasma was collected and frozen at −20°C until the samples were assayed.
Hormone Assays
Estradiol and progesterone concentrations were analyzed in the laboratories of Dr. Padmanabhan and Dr. McConnell, University of Michigan Reproductive Sciences Program. Estradiol was measured by an automated chemiluminescent immunoassay developed by Chiron Corp. (Automated Chemiluminescence Analyzer) that has a lower limit of sensitivity of 0.5 pg/ml. The assay uses an antibody directed against an estradiol-6-bovine serum albumin conjugate (anti-E2-6) in duplicate 350-μl aliquots. All samples are reported as picograms per milliliter. Interassay and intraassay coefficients of variance for undiluted samples were 3%–6%, and were 8%–12% for the diluted samples. Samples were diluted 1:2 in MD-9 buffer prior to incubation. All of the reagents were provided in the ACS-180 E2-6 immunoassay. Chiron supplied a nine-point standard curve (0.5–125 pg/ml) prepared from the Estradiol-6 Master Curve material (Bayer Diagnostics). Specificity was very high, as demonstrated by minimal cross-reactivity with 19 other steroid compounds. Recovery of estradiol from serum with pools was 97% or better at three concentrations. Parallelism was demonstrated with two dilutions of pooled samples.
Progesterone concentrations were measured in duplicate in 50-μl aliquots diluted with 50 μl of diluent using a Coat-A-Count radioimmunoassay kit (Diagnostic Products Corp.). Values were expressed in nanograms per milliliter (1 ng/ml = 3.18 nmol/L), and the lower limit of sensitivity was 0.02 ng/ml. Interassay and intraassay coefficients of variance for undiluted samples were 3.9%–9.7%, and were 2.7%–8.8% for the diluted samples. Specificity was very high, as demonstrated by minimal reactivity to other steroids. Parallelism was demonstrated at three dilutions of sample.
Ovariectomies and Histology of Ovaries
Adult degus were ovariectomized following anesthesia with either ketamine HCl (30 mg/kg body weight) and xylazine (2.5 mg/kg body weight) or isoflurane gas anesthesia. Bilateral incisions were closed with sutures and wound clips, and were treated with topical antibiotic (Nolvasan; Fort Dodge Animal Health). Immediately after the ovariectomies, degus were given yohimbine (2.5 mg/kg body weight, intraperitoneally) and Lactated Ringer solution (5 ml, subcutaneously) to aid recovery.
For histological analysis, ovaries were collected from females on Day 1 (n = 1; relative to the onset of peak in wheel-running), Day 6 (n = 5), Day 16 (n = 5), or on Day 6 of presumed pregnancy (n = 4; see mating behavior below). Ovaries were stored in Bouin fixative until the time of histological processing. These ovariectomies were completed one putative estrous cycle after the collection of the activity data and the blood samples. All histological preparation was carried out by the University of Michigan Reproductive Sciences Program's Morphology Core. One half of each ovary was serially sectioned at 5 μm, and every eighth section was stained with hematoxylin and eosin. Follicular and luteal development was assessed in approximately 100 sections from each animal.
Statistical Analysis
Analysis of variance tests were used to determine whether estradiol and progesterone concentrations varied across the days of the putative estrous cycle. Based on the ovarian histological data, post hoc analyses were done with Student t-tests. A cosine analysis was used to determine the time of peak activity (wheel-running) in juvenile degus (Actiview). For juvenile data, we used a within-subject ANOVA to test whether the putative cycle stage was associated with significant variation in wheel-running activity levels and with the timing of peak activity. A chi-square analysis was used to determine whether the presence of vaginal opening corresponded with times when estrus-typical activity occurred in peripubertal animals. In adults, a chi-square analysis was done to compare the distribution of regular vs. irregular estrous cycles in adults. A chi-square analysis was also used to compare the number of mating events that occurred in the light and dark periods in animals given an EB capsule at two different times of day.
RESULTS
Characterization of the Onset of Vaginal Patency in Juvenile Degus
Young degus (n = 12) exhibited a cyclical presence of vaginal opening and elevated wheel-running activity. Of the remaining two juvenile animals, one died following her first vaginal opening, and the second showed two intermediate cycles (no full patency) and then ceased to show any indications of cyclicity. The median age of first vaginal opening was P74, and ranged from P33 to P89. In six animals, the first vaginal opening was preceded by the detection of intermediate vaginal stages one or two putative cycles earlier. In all animals, after the initial onset of vaginal opening was detected, it continued to be present in a cyclical manner. The interval between the detection of successive vaginal openings averaged 17.5 days (±1.38 days), with individual intervals varying from 9.7 to 21.7 days in length for juveniles. Because of our sampling frequency, it is difficult to accurately determine the number of days the vaginal opening was present during each cycle. However, using the shortest possible and longest possible intervals for each opening, we can estimate that degus showed full vaginal openings for an average interval between 2.4 days (±0.23 SEM) and 5 days (±0.30 SEM) during each cycle.
When all activity records were examined for juvenile females, there were days of elevated wheel running that occurred cyclically (putative estrus). To determine whether the increase in activity levels occurred on days with a vaginal opening (as previously described in adult females [12]), we examined days in which both of these variables were recorded. Elevated wheel-running (putative estrus) coincided with the beginning of full vaginal opening (83% of the days) or intermediate vaginal opening (12% of the days; Fig. 1). Using a chi-square test, we found a significant relationship between the presence of vaginal opening and estrus-typical wheel-running activity (χ2 = 31.790; P < 0.001). Week-long activity recordings that encompassed the time of full vaginal opening were likely to include estrus-typical wheel-running activity, whereas recordings that occurred during weeks when there was no vaginal opening were less likely to include estrus-typical wheel-running activity (Fig. 1). On the day of putative estrus, females had a phase advance in the time of peak of activity (Fig. 2A) and a relatively large increase in the amount of wheel-running activity (Fig. 2B) compared with baseline. On the day following putative estrus, animals had a delay in the time of peak of activity relative to baseline activity and a decrease in activity levels (Fig. 2). Using a within-subject ANOVA, we found that the variation in mean activity level related to putative cycle stage was significant (F1.126 = 23.929; P < 0.001), as was the variation in the daily timing of peak activity (F2 = 4.512; P = 0.023).
FIG. 1.
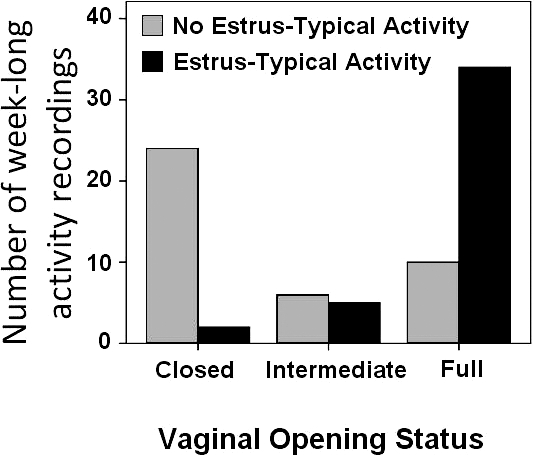
Estrus-typical wheel-running activity occurs during times of vaginal patency in juvenile degus. Depicted is the number of week-long activity recordings containing either 1) an event of estrus-typical wheel-running (black bars), characterized by a day of elevated, phase-advanced activity followed by a day of decreased, delayed activity (postestrus); or 2) no estrus-typical wheel-running (gray bars). Vaginal status of the animals is defined by whether a full vaginal opening was observed at some point during the week-long activity recording period. “Intermediate” means the presence of a partial opening or dimpling.
FIG. 2.
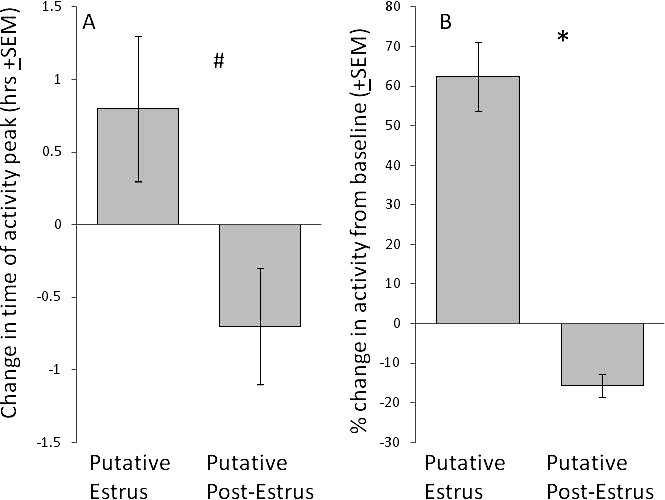
A) Change in the timing (phase) of the activity peak (hours ± SEM) in juveniles on the day of putative estrus and postestrus relative to baseline days. #P = 0.023. B) Activity levels in juvenile degus on the day of putative estrus and the day immediately following putative estrus (postestrus) relative to baseline days. *P < 0.001 Positive and negative values indicate phase advance and delay, respectively.
Characterization of the Estrous Cycle in Adult Animals
Adult female degus periodically exhibited increases in wheel-running activity that corresponded with vaginal patency. Similarly to juveniles, the increase in wheel-running activity generally occurred for 1 day, whereas patency lasted for several days. Of the 20 animals examined, 80% (n = 16) had regularly occurring periods of increased wheel-running and vaginal patency, and 20% had an irregular length of time between these events. Chi-square analysis confirmed that the vast majority of animals had regular cycles (χ2 =7.2; P < 0.0007). In adult females, the cycle length ranged from 17.8 to 25.4 days, and the mean length was 21.2 ± 0.56 days.
Mating Behavior of Intact and Estradiol-Primed Animals
In the first group of intact females, paired after estrus had begun (noted by patency and/or activity), females mated between two and seven times (mean, 4.25 ± 1.1; median, 4). The majority of mating events (11 of 17 total) occurred in the dark (Fig. 3A). Three animals had their first mating bout 3–15 min after lights-off, during the period of high locomotor activity at light transition. In addition, each animal had a mating bout 3.5–5 h after dark onset. Three of the four animals mated at a phase of −1.25 h to +30 min relative to lights-on, whereas one animal had a mating bout 2.5 h before lights-on. Although it was less frequent, three animals also exhibited mating behavior during lights-on, during the day following the first observation of putative estrus. The duration over which females mated ranged from 7.4 to 34.5 h.
FIG. 3.
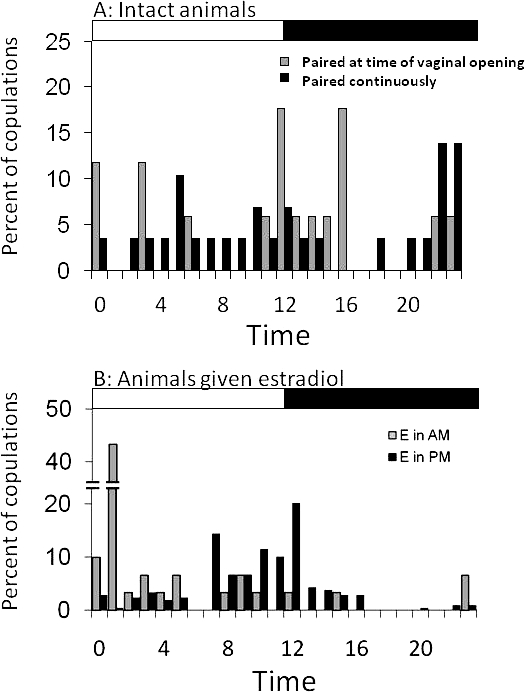
A) Percent of mating events over time (hours) in intact female degus that were continuously housed with males, or paired with a male at the time of vaginal opening. B) Percent of mating events over time in ovariectomized females given estradiol capsules at the time of lights-off or lights-on, then paired with a male. Light and dark bars represent lights-on and lights-off, respectively. E, estradiol capsules.
The second group of intact females (n = 5) was paired with an unfamiliar male and monitored continuously to determine their preferred time of mating (Fig. 3A). Animals were paired 2–4 days prior to the next predicted day of vaginal opening. Females mated on the day of predicted putative estrus, and we did not see advancement in the timing of vaginal patency in this group of animals. The onset of the first mating time varied from 7.0 h before lights-on to 9.5 h after lights-on (mean, 1.0 ± 2.5 h after lights-on). Four of five animals began mating before lights-on, during the time of peak running-wheel activity. Almost half of the mating bouts occurred during the day (48.3%). In both groups of intact female degus, there was an extended period of locomotor inactivity and lack of mating activity between 6 h after lights-out and 1.25 h before lights-on.
Ovariectomized females given an estradiol capsule at the time of lights-on began mating between 1 h 50 min and 4 h 40 min after the capsule was implanted. Mating events were observed throughout the lights-on portion of the photoperiod. The largest percent of mating events occurred at the time of lights-on, on the first day the animals received the hormone capsule (Fig. 4B); however, some mating bouts continued in the second day after pairing. Animals given estradiol at the time of lights-off also initiated mating relatively quickly, within 2 h of receiving the hormone capsule, and the majority of mating events occurred on the first day. They also mated throughout the lights-on portion of the following day. In this group, the largest percentage of mating events occurred at the time of lights-off. A chi-square analysis of the two groups of EB-treated females revealed a significant difference in the distribution of mating events across the day (χ2 = 6.4; P < 0.01). Specifically, regardless of when animals were treated with EB, more mating bouts occurred during the light compared with the dark (Fig. 3B). For both groups of females, relatively little or no mating activity occurred between 5 and 8 h after lights-off or between 6 and 7 h after lights-on. Thus, although mating occurred relatively soon after treatment with steroid hormones, time of day also appeared to influence when mating occurred.
FIG. 4.
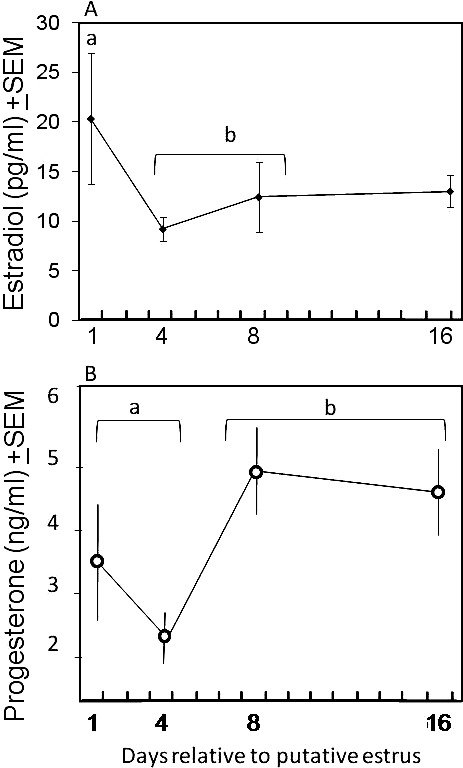
Estradiol (A) and progesterone (B) concentrations (±SEM) in degus. Day of estrous cycle is relative to the onset of estrus, as indicated by an increase in wheel-running and vaginal patency. Lowercase letters indicate data points that are significantly different from one another (P ≤ 0.02).
Hormonal Changes Across the Estrous Cycle
The estradiol secretion pattern of adults changed across the reproductive cycle and differed from that of progesterone (Fig. 4). On Day 1 of vaginal patency, concentrations were high, decreased midcycle (Day 4), and subsequently increased during early follicular development (Days 8 and 16; F = 3.655, P = 0.027; Fig. 4A). Based on the histological analysis, Day 4 and Day 8 samples have little follicular activity and would be expected to have much lower estradiol concentrations than Day 1 or Day 16 (when new follicle development is apparent). In accordance with this hypothesis, we found that Day 1 estradiol concentrations were significantly greater than the pooled Day 4 and Day 8 (P = 0.019) concentrations, but were not significantly different from those of Day 16.
Mean progesterone concentrations were higher in the second half of the cycle. Using an ANOVA across four collection times, the differences across the cycle were not significant (F = 2.162; P = 0.055; Fig. 4B). However, we observed histologically functional corpora lutea from ovaries collected on Days 8 and 16 (see below), whereas we saw no functional corpora lutea from ovaries collected on Days 1 and 4 of the cycle. When the samples from animals with functional corpora lutea were pooled (Days 8 and 16) and compared to those from the remaining animals (Days 1 and 4), progesterone was significantly higher in the Day 8/Day 16 group samples (t = −2.12, P = 0.02).
Histology of Ovarian Samples
Corpora lutea were identified as groups of large, round structures on the perimeter of the ovary. The corpora lutea differed from the other round structures on the edge of the ovary, the follicles, because they lacked an oocyte. In addition, the corpora lutea were composed of hypertrophied granulosa cells. The granulosa cells of the corpus luteum were larger than the granulosa cells observed in the developing primary and secondary follicles. Hypertrophied thecal cells surrounded the granulosa cells.
Follicles at various stages of development were also observed. Primary follicles were identified as small follicles with oocytes surrounded by granulosa cells. Secondary follicles appeared larger and were characterized as having a follicular antrum and an oocyte that had not completed first meiosis. Graafian follicles displayed a large follicular antrum and a zona granulosa forming an even layer of cells around the periphery of the follicle [27]. Corpora albicans appeared as fibrous masses. Atretic follicles were identified by degenerated oocytes and disorganized granulosa cells. In addition, both atretic and functional corpora lutea were apparent in many ovaries.
One animal's ovaries were collected on the putative day of estrus. This animal displayed Graafian follicles (Fig. 5, A and B). Secondary follicles and corpora albicans were also observed. All ovaries (n = 5 samples) collected from animals on Day 6 relative to the daily rise in wheel-running had well-developed corpora lutea (Fig. 5, C and D). Primary follicles were seen in four of these animals. None of the degus in this group exhibited secondary follicles. In contrast, ovaries collected from animals on Day 16 (n = 5) showed a great deal of follicular development. Three of the five animals had both primary and secondary follicles; one of these animals had only primary follicles; and one animal had only secondary follicles. Fewer corpora lutea were seen in these animals compared with the Day 6 group, and they were becoming atretic (Fig. 5, E and F [primary follicles], and G and H [secondary follicles]).
FIG. 5.
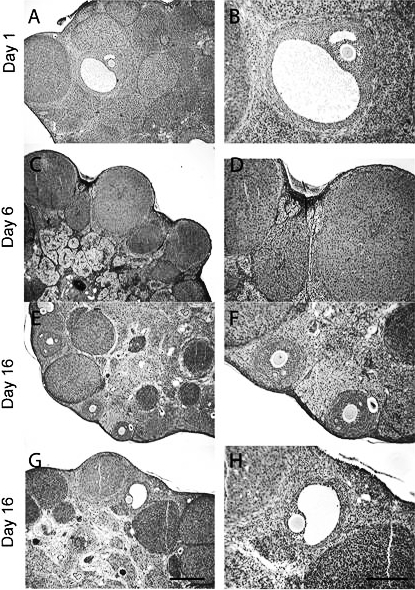
Representative ovary from early on the first day of estrus. A) Graafian follicle with a large follicular antrum. B) Graafian follicle with granulosa and thecal cells surrounding the follicular antrum. C) Ovary from a female collected on Day 6 containing well-developed corpora lutea lining the ovary. D) Higher magnification of corpora lutea to show hypertrophied granulosa and thecal cells. The lighter area (cytoplasm) illustrates how the granulosa cells have enlarged in relation to the darker nuclei. Thecal cells are darker and surround the larger granulosa cells. E–H) Representative ovaries collected from animals on Day 16 relative to estrus. E) Example of primary follicles, which were located close to the edge of the ovary and were developing concurrently. F) The same follicle shown in E at higher magnification illustrates granulosa cells surrounding an oocyte in the center. A thin layer of thecal cells surrounds the granulosa cells. Secondary follicles are seen in G and at a higher magnification in H. Disorganized and lighter areas in the interior of the follicle are atretic cells. Bar in G = 0.4 mm for A, C, and E (original magnification ×40). Bar in H = 0.2 mm for B, D, and F (original magnification ×100).
We also collected ovaries from animals 6 days after we observed mating. Similarly to the Day 6 unmated animals, 100% (n = 4) of these animals had well-developed corpora lutea. Three animals had very small primary follicles, and one animal did not exhibit any follicles in the primary or secondary stages of development.
DISCUSSION
In this experiment, the reproductive cycle of the Hystricognathi rodent, Octodon degu, was examined. The ovarian, hormonal, activity, and mating patterns were examined in intact and ovariectomized, steroid-primed animals. We determined that 1) the onset of reproductive maturity is not dependent on the presence of proximal male cues during adolescence; 2) reproductive cycles occur spontaneously and cyclically in juveniles and adults; 3) degus have both spontaneous ovulation and corpora lutea formation; 4) mating activity occurs on the day of increased activity, coinciding with vaginal patency and peak estradiol concentrations; 5) in intact and estradiol-primed animals, mating behavior preferentially occurs at times of increased activity, specifically just before or after light-to-dark transitions and during the light phase of the L:D cycle; and 6) the total time over which mating bouts occur varies greatly, ranging from 7 to 34 h.
We found that corpora lutea appeared functional for approximately 12–14 days. Progesterone concentrations were highest on Days 8 and 16 relative to vaginal patency (Fig. 4). Histological data from intact adults indicated that all animals showed well-developed corpora lutea on Day 6 following vaginal patency (Fig. 5). The corpora lutea structures likely formed and became active between Days 4 and 8, and deteriorated by Day 16, when follicular structures are dominant. The average cycle length of young degus was moderately shorter than that of adults (17.5 vs. 21.2 days), and was more variable. Irregular or non-standard-length reproductive cycles are a typical occurrence in peripubertal animals, and early cycles are often anovulatory and infertile because the hypothalamic pituitary gonadal axis is still developing [28, 29]. Similar to our previous results [16], adult cycles averaged 21 days in length. Because cycle length is determined by the lifetime of the functional corpora lutea [30], it is probable that the degus' 21-day cycle is composed of a 12- to 14-day luteal phase. This is relatively long compared with Myomorpha rodents (for example, rats and mice with 1–3 days) but is similar to other Hystricognathi rodents, such as guinea pigs and cavys [12, 20]. Thus, degus have spontaneous and regularly occurring estrous cycles with functional corpora lutea.
At consistent intervals, we observed cycles of vaginal patency and heightened locomotor activity typical of estrus in most rodents [20, 31, 32]. Additionally, the onset of estrous cycles in juvenile degus occurred spontaneously, without being induced by the presence of proximal male cues. Degus have been described as being induced ovulators without regular estrous cycles, although it was noted that some animals may ovulate spontaneously [12, 26]. The results of the current study may differ from earlier reports because those animals had a different diet, may have been handled less and experienced stress when examined, may have experienced “jet-lag” disruptions as the colony had been shipped from one hemisphere to another, or were housed with inadequate amounts of available light [26]. It is unlikely that the colony of degus at the University of Michigan has recently acquired a spontaneous ovulatory phenotype, because founding females, including those with a wild-caught parent, also demonstrated a cyclical increase in wheel-running [16].
We predicted that females would mate on the day of estrus at the time of highest wheel-running activity. Animals paired continuously with males throughout their estrous cycle began mating before lights-on. The onset of increased wheel running was concurrent with vaginal patency and also occurred several hours before lights-on. In contrast, a majority of females paired at the time that we determined vaginal opening (estrus) had occurred (after lights-on) mated immediately after lights-off. The observation that mating occurred immediately after lights-out may be the result of females being introduced to the males for the first time early in the day and socialization being required, or that most mating occurs during periods of peak activity, which occur during the hours just before and after the lights go on or off. We tested these possibilities with hormone-primed females paired with males at lights-on or lights-off. Copulatory events occurred across much of the light phase and early dark phase and were not strongly dependent on when females received hormones. This suggests that time of day does not tightly constrain time of mating in this species, as is also reported for primates [33], but is unlike data from laboratory rodents [34]. Future work will determine whether time of day or seasonal changes in light regulate the occurrence of reproductive events in this species.
In mammals, there are a broad range of reproductive strategies, including spontaneous or socially induced estrus, ovulation, and maintenance of corpora lutea. However, research has focused on the type of reproductive cycle found in hamsters, rats, and mice, despite the fact that this variety of reproductive cycle is found in a relatively small number of mammalian species [4]. Hystricognathi rodents, such as Octodon degus, present a unique and interesting animal model for investigating the relationship between life history traits and type of reproductive cycle. This group of animals appears to closely resemble many laboratory myomorphs in body size and ecology as well as in requiring circulating steroids to induce sexual receptivity. In contrast, however, they possess an estrous cycle more similar to guinea pigs or large mammals, such as sheep or primates. This unique suite of physical, social, and reproductive characteristics makes this species suitable for both proximate questions regarding the hormonal regulation of mating and ovulation, and ultimate questions regarding how social behavior and reproductive pattern influence reproductive success. Furthermore, degus breed readily in lab [13], yet represent a wild animal, providing an opportunity for us to manipulate hormonal status and social environment and assess the individual outcomes easily.
These data raise interesting questions about the evolution of reproductive patterns among rodents. It is unclear why all mammals with spontaneous estrous cycles do not either rely on mating to induce corpora lutea survival or have a spontaneous luteal phase. Indeed, it is still a mystery why evolution resulted in some species having spontaneous rather than induced ovulation [3]. It is clear, however, that circadian hormone release is critical to fertility in myomorph rodents with either induced or spontaneous ovulation followed by a very short luteal phase. Additionally, in some species with a long luteal phase, such as humans and guinea pigs, luteinizing hormone release appears to be linked to certain times of day, but these species tend to have much longer periods during the day in which mating can result in pregnancy [35–37]. Even if estrus behavior does not appear closely linked to time of day in these species, ovulation and successful conception may still be dependent on timing information.
Octodon degus are a unique animal model for investigating how rodents have evolved different types of reproductive cycles under similar ecological conditions. These data may help improve assisted reproductive technologies used to improve pregnancy rate in farm animals, endangered species (in zoos), and humans. If this research establishes that estrous-related events are typically circadian in nature in a species with a long luteal phase, then this would enable us to determine whether successful pregnancies result from matings at a specific time of day, even if the luteinizing hormone surge can be induced at other times of day.
Acknowledgments
We would like to thank Dr. Warren Holmes for comments on an earlier draft, and the Department of Psychology and the Honors Program (BVR) at the University of Michigan. Additional thanks go to Kay Brabec, Dr. Daniel McConnell, and Dr. Vasantha Padmanabhan of the Reproductive Sciences Program for assistance and preparations, and Jennifer Hee Young Ku for help collecting data and taking care of the animals.
Footnotes
Supported by the National Institutes of Health-National Institute of Child Health and Human Development Center for the Study of Reproduction (P30 HD18258).
REFERENCES
- Wallen K. Desire and ability: hormones and the regulation of female sexual behavior. Neurosci Biobehav Rev 1990; 14: 233 241 [DOI] [PubMed] [Google Scholar]
- Smale L, Heideman PD, French JA. Behavioral neuroendocrinology in nontraditional species of mammals: things the ‘knockout’ mouse CAN'T tell us. Horm Behav 2005; 48: 474 483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Baum MJ. Neuroendocrine regulation of GnRH release in induced ovulators. Front Neuroendocrinol 2000; 21: 220 262 [DOI] [PubMed] [Google Scholar]
- Conaway CH. Ecological adaptation and mammalian reproduction. Biol Reprod 1971; 4: 239 247 [DOI] [PubMed] [Google Scholar]
- Schwartz NB. Neuroendocrine regulation of reproductive cyclicity. Conn PM, Freeman ME. (Eds.), Neuroendocrinology in Physiology and Medicine. Totowa, NJ: Humana Press; 2000: 135 145 [Google Scholar]
- Sridaran R, Rodriguez-Sierra JF, Blake CA. Ovarian involvement in the timing mechanism that controls ovulation in rats. Biol Reprod 1979; 21: 505 509 [DOI] [PubMed] [Google Scholar]
- Alleva JJ, Waleski MV, Alleva FR. A biological clock controlling the estrous cycle of the hamster. Endocrinology 1971; 88: 1368 1379 [DOI] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci U S A 2005; 102: 15682 15687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ. An Introduction to Behavioral Endocrinology. Sunderland, MA: Sinauer Associates; 2000. [Google Scholar]
- Felipe A, Cabodevila J, Callejas S. Characterization of the estrous cycle of the Myocastor coypus (coypu) by means of exfoliative colpocytology. J Neotrop Mammal 2001; 82: 129 137 [Google Scholar]
- Hilliard J. Corpus luteum function in guinea pigs, hamsters, rats, mice and rabbits. Biol Reprod 1975; 8: 203 221 [Google Scholar]
- Weir BJ. Reproductive characteristics of hystricomorph rodents. Symp Zool Soc Lond 1974; 34: 265 301 [Google Scholar]
- Lee TM. Octodon degus: a diurnal, social, and long-lived rodent. ILAR J 2004; 45: 14 24 [DOI] [PubMed] [Google Scholar]
- Hummer DL, Jechura TJ, Mahoney MM, Lee TM. Gonadal hormone effects on entrained and free-running circadian activity rhythms in the developing diurnal rodent Octodon degus. Am J Physiol Regul Integr Comp Physiol 2007; 292: R586 R597 [DOI] [PubMed] [Google Scholar]
- Reynolds TJ, Wright JW. Early postnatal physical and behavioural development of degus (Octodon degus). Lab Anim 1979; 13: 93 99 [DOI] [PubMed] [Google Scholar]
- Labyak SE, Lee TM. Estrus- and steroid-induced changes in circadian rhythms in a diurnal rodent, Octodon degus. Physiol Behav 1995; 58: 573 585 [DOI] [PubMed] [Google Scholar]
- Nowak RM, Paradiso JL. Walker's Mammals of the World. Baltimore: The Johns Hopkins University Press; 1983. [Google Scholar]
- Weir BJ. The evocation of oestrus in the cuis, Galea musteloides. J Reprod Fertil 1971; 26: 405 408 [DOI] [PubMed] [Google Scholar]
- Touma C, Palme R, Sachser N. Different types of oestrous cycle in two closely related South American rodents (Cavia aperea and Galea musteloides) with different social and mating systems. Reproduction 2001; 121: 791 801 [DOI] [PubMed] [Google Scholar]
- Addo PG, Awumbila B, Awotwi E, Ankrah NA. Comparative characterization of the oestrous cycles of the grasscutter (Thryonomys swinderianus) and the guinea pig (Cavia porcellus) by the hystricomorph vaginal membrane perforation phenomenon. Livestock Research for Rural Development 2007; 19: e63. [Google Scholar]
- Jechura TJ, Lee TM. Ovarian hormones influence olfactory cue effects on reentrainment in the diurnal rodent, Octodon degus. Horm Behav 2004; 46: 349 355 [DOI] [PubMed] [Google Scholar]
- Bronson FH, Maruniak JA. Male-induced puberty in female mice: evidence for a synergistic action of social cues. Biol Reprod 1975; 13: 94 98 [DOI] [PubMed] [Google Scholar]
- Previtali MA, Lima M, Meserve PL, Kelt DA, Gutierrez JR. Population dynamics of two sympatric rodents in a variable environment: rainfall, resource availability, and predation. Ecology 2009; 90: 1996 2006 [DOI] [PubMed] [Google Scholar]
- Previtali MA, Meserve PL, Kelt DA, Milstead WB, Gutierrez JR. Effects of more frequent and prolonged El Nino events on life-history parameters of the degu, a long-lived and slow-reproducing rodent. Conserv Biol 2010; 24: 18 28 [DOI] [PubMed] [Google Scholar]
- Poeggel G, Nowicki L, Braun K. Early social deprivation alters monoaminergic afferents in the orbital prefrontal cortex of Octodon degus. Neuroscience 2003; 116: 617 620 [DOI] [PubMed] [Google Scholar]
- Weir BJ. The management and breeding of some more hystricomorph rodents. Lab Anim 1970; 4: 83 97 [DOI] [PubMed] [Google Scholar]
- Wheater PR, Burkitt HG, Daniels VG. Functional Histology. New York: Churchill Livingston; 1987. [Google Scholar]
- Adams Hillard PJ. Menstruation in young girls: a clinical perspective. Obstet Gynecol 2002; 99: 655 662 [DOI] [PubMed] [Google Scholar]
- Diaz A, Laufer MR, Breech LL. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Pediatrics 2006; 118: 2245 2250 [DOI] [PubMed] [Google Scholar]
- Baird DT, Baker TG, McNatty KP, Neal P. Relationship between the secretion of the corpus luteum and the length of the follicular phase of the ovarian cycle. J Reprod Fertil 1975; 45: 611 619 [DOI] [PubMed] [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science 1977; 196: 305 307 [DOI] [PubMed] [Google Scholar]
- Refinetti R, Menaker M. Evidence for separate control of estrous and circadian periodicity in the golden hamster. Behav Neural Biol 1992; 58: 27 36 [DOI] [PubMed] [Google Scholar]
- Wallen K. Sex and context: hormones and primate sexual motivation. Horm Behav 2001; 40: 339 357 [DOI] [PubMed] [Google Scholar]
- Hansen S, Sodersten P, Eneroth P, Srebro B, Hole K. A sexually dimorphic rhythm in oestradiol-activated lordosis behaviour in the rat. J Endocrinol 1979; 83: 267 274 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Rodriguez JS, Bridson WE, Wiegand SJ. Factors influencing the positive feedback action of estrogen upon the luteinizing hormone surge in the ovariectomized guinea pig. Endocrinology 1979; 104: 680 686 [DOI] [PubMed] [Google Scholar]
- Kerdelhue B, Brown S, Lenoir V, Queenan JT, Jr, Jones GS, Scholler R, Jones HW., Jr Timing of initiation of the preovulatory luteinizing hormone surge and its relationship with the circadian cortisol rhythm in the human. Neuroendocrinology 2002; 75: 158 163 [DOI] [PubMed] [Google Scholar]
- Cahill DJ, Wardle PG, Harlow CR, Hull MG. Onset of the preovulatory luteinizing hormone surge: diurnal timing and critical follicular prerequisites. Fertil Steril 1998; 70: 56 59 [DOI] [PubMed] [Google Scholar]


