Abstract
Eutopic endometrium in endometriosis has molecular evidence of resistance to progesterone (P4) and activation of the PKA pathway in the stromal compartment. To investigate global and temporal responses of eutopic endometrium to P4, we compared early (6-h), intermediate (48-h), and late (14-Day) transcriptomes, signaling pathways, and networks of human endometrial stromal fibroblasts (hESF) from women with endometriosis (hESFendo) with hESF from women without endometriosis (hESFnonendo). Endometrial biopsy samples were obtained from subjects with and without mild peritoneal endometriosis (n = 4 per group), and hESF were isolated and treated with P4 (1 μM) plus estradiol (E2) (10 nM), E2 alone (10 nM), or vehicle for up to 14 days. Total RNA was subjected to microarray analysis using a Gene 1.0 ST (Affymetrix) platform and analyzed by using bioinformatic algorithms, and data were validated by quantitative real-time PCR and ELISA. Results revealed unique kinetic expression of specific genes and unique pathways, distinct biological and molecular processes, and signaling pathways and networks during the early, intermediate, and late responses to P4 in both hESFnonendo and hESFendo, although a blunted response to P4 was observed in the latter. The normal response of hESF to P4 involves a tightly regulated kinetic cascade involving key components in the P4 receptor and MAPK signaling pathways that results in inhibition of E2-mediated proliferation and eventual differentiation to the decidual phenotype, but this was not established in the hESFendo early response to P4. The abnormal response of this cell type to P4 may contribute to compromised embryonic implantation and infertility in women with endometriosis.
Keywords: decidualization, endometrial fibroblast, endometriosis, eutopic endometrium, progesterone, transcriptome
Endometrial stromal cells from women with endometriosis demonstrate a blunted response to progesterone (P4), with limited expression of many P4-regulated genes and curtailed activation of canonical pathways and processes.
INTRODUCTION
Endometriosis, a common gynecologic disorder, is characterized by endometrium-like tissue on the pelvic peritoneum, ovary, and rectovaginal septum. It is present in up to 60% of women with chronic pelvic pain and dysmenorrhea and up to 50% of women with infertility [1, 2]. Retrograde transplantation of steroid hormone-sensitive endometrial tissue and cells into the pelvic cavity at the time of menses is believed to account for peritoneal disease and is accompanied by a local inflammatory response that contributes to the observed pain and infertility [3]. This shed eutopic endometrium of women with endometriosis has different innate properties that likely contribute to its attachment to, invasion of, and growth on peritoneal structures, giving rise to the disorder in genetically, environmentally, and immunologically predisposed individuals [4, 5]. Eutopic endometrium is composed of several cell types that respond to the circulating steroid hormones estradiol (E2) and progesterone (P4) in preparation for blastocyst nidation, placentation, and sustainability of an established pregnancy. The human endometrial stromal fibroblast (hESF), in response to P4 after E2 priming, undergoes distinct morphological differentiation, with changes in the cytoskeleton and a functional transition from fibroblast-like to epithelium-like cells, which is accompanied by a unique biosynthetic and secretory phenotype, and plays a critical role in successful embryonic implantation [6]. When pregnancy ensues, the endometrial stromal compartment becomes uniformly “decidualized” and constitutes the decidua, a morphologically and functionally distinct tissue that persists throughout gestation and represents the maternal aspect of the maternal-fetal interface, composed of decidualized stromal fibroblasts, vascular elements, epithelium, and immune cells [7]. Through their responses to P4, hESF communicate with the extracellular matrix, the invading trophoblast, and resident and peripheral leukocyte populations in the developing placental bed [8]. Thus, an abnormal hESF differentiation program can affect the success of implantation, early development, and pregnancy. In the setting of endometriosis, this differentiation program is compromised [4, 9].
hESF responses to P4 are complex and involve activation of PKA, the progesterone receptor (PGR) gene, and other pathways, with cross-talk between and among them [9–11], a consequence of P4 binding to its cognate nuclear receptors, and nongenomic mechanisms [12, 13]. Resistance to P4 actions in eutopic endometrium of women with endometriosis is believed to derive from lower levels of PGRA, PGRB [14, 15], and PGR coregulator expression [10], aberrant expression of specific transcription factors [16–18], and dysregulation of members and functions of specific pathways that drive hESF toward decidualization [4, 9, 19, 20]. We and others have demonstrated that hESF from women with endometriosis exhibit a blunted response to activation of the PKA pathway [9, 19]. However, it is unclear what the global response to P4 per se is in the impaired decidualization observed in hESF from women with endometriosis.
Thus, a comparative investigation of genes, gene families, and signaling and biological pathways involved in the hESF response to P4 in women with and without endometriosis is important to understand the mechanisms underlying normal and abnormal implantation and pregnancy maintenance. Herein, we compared early (6-h), intermediate (48-h), and late (14-Day) in vitro whole-genome responses of hESF from women with endometriosis (hESFendo) to hESF from women without endometriosis (hESFnonendo) treated with P4 plus E2 (E2P4), or E2 alone, or vehicle alone. Using this experimental paradigm, the data demonstrate unique phenotypes, gene expression processes, biochemical and signaling pathways, and networks suggestive of early, intermediate, and late responses of hESFnonendo and hESFendo to P4, giving insights into the complexity of events occurring normally in response to P4 and in the setting of endometriosis.
MATERIALS AND METHODS
Tissues and Cells
Endometrial tissue samples were obtained in accordance with the guidelines of the Declaration of Helsinki. Written, informed consent was obtained from all subjects. The study was approved by the Committee on Human Research of the University of California San Francisco (UCSF) and the Stanford University Committee on the Use of Human Subjects in Medical Research. Some samples were also obtained from the National Institutes of Health Specialized Cooperative Centers Program in Reproduction and Infertility Research (SCCPRR) Human Endometrial Tissue and DNA Bank at UCSF. Control subjects were premenopausal women (44.75 ± 1.7 years old; range 41–49 years old) undergoing hysterectomy for benign conditions (Table 1), with regular menstrual cycles (25–35 days), surgically confirmed absence of endometriosis, and no history of endometriosis. Endometriosis subjects were 39.5 ± 4.2-year-old (range, 31–49 years old; P = 0.35, vs. hESFnonendo subjects) women with regular menstrual cycles, in whom minimal to mild peritoneal endometriosis was diagnosed by visualization of pelvic lesions during laparoscopy and histologic evaluation (Table 1). Endometriosis was staged according to the revised American Fertility Society classification system [21]. All subjects were documented not to be pregnant and not to have had hormonal treatment for at least 3 months before surgery.
TABLE 1.
Subject characteristics for this study.
Endometrial tissue was digested with collagenase, and hESF were isolated and plated with Dulbecco modified Eagle medium (DMEM)/molecular cell developmental biology 105 (MCDB-105) medium with 10% charcoal-stripped fetal bovine serum (FBS), insulin (5 mg/ml), gentamicin, penicillin, and streptomycin, as described previously [22, 23]. All cells used were at Passage 2. Subsequently, hESF were plated in 6-cm plastic dishes, and, after they reached confluency, medium was changed to a low-serum medium (LSM; i.e., DMEM/MCDB-105 medium containing ascorbic acid, transferrin, and gentamicin with 2% FBS) for 24 h prior to treatment. The concentration of FBS (2%) in the culture medium was determined by culturing two preparations in triplicate for up to 16 days with 1μM P4 plus 10 nM E2 (E2P4) in 0%, 0.5%, 1%, 2%, 5%, and 10% FBS, and the classical decidual marker IGFBP1, in conditioned medium (CM) was measured by ELISA (Diagnostic Systems Labs, Webster, TX). Optimal IGFBP1 secretion was observed in the culture with 2% FBS (data not shown).
Hormonal Treatment, RNA Isolation, and Processing for Microarray Analysis
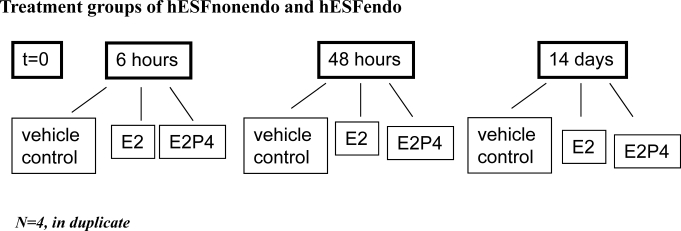
Figure 1 shows the experimental design of the present study. Duplicate cultures of hESF in LSM were treated with E2P4 or with 10 nM E2 alone (E2) or with vehicle (vehicle control), and cells and CM were collected at time (t) of 0 h, 6 h (early response), 48 h (intermediate response), and 14 days (late response), and CM was changed every 2 days. Total RNA was isolated from individual plates and purified using an RNeasy mini-kit (Qiagen, Valencia, CA) following the manufacturer's protocol. Samples were stored in RNase-free H2O, purity was analyzed by the A260:A280 nm ratio, and RNA quality and integrity were assessed by using a Bioanalyzer 2100 unit (Agilent Technologies, Santa Clara, CA). All samples had high-quality RNA (RNA integrity number [RIN] = 9.7–10).
FIG. 1.
Experimental design. Treatment groups were similar for hESFnonendo and hESFendo. For analysis of microarray and QRT-PCR data, all groups were normalized to t = 0, and then the normalization was conducted within each time-group: E2P4 was normalized to E2, which was normalized to the vehicle control, with the resulting data reflecting the pure P4 response.
Duplicate RNA preparations were pooled and prepared according to the manufacturer's microarray preparation protocol (Affymetrix, Inc., Santa Clara, CA) as described previously [9]. Briefly, for each sample, 100 ng of total RNA was reverse-transcribed to cDNA by using 500 ng of T7-(N6) primer and SuperScript II. A second strand of DNA was generated using DNA polymerase, followed by overnight in vitro transcription to generate cRNA. After samples were processed through cRNA clean-up spin columns (Affymetrix), 10 μg of cRNA was reverse-transcribed using random primers and SuperScript II. Mixtures were digested with RNase H, and cDNA was purified by cDNA clean-up spin columns (Affymetrix). Finally, 5.5 μg of sense cDNA was fragmented and labeled using a GeneChip WT-terminal labeling kit. The quality of the cDNA and the fragmented cDNA was assessed with an Agilent bioanalyzer. Individual samples were hybridized to Human Gene 1.0 ST arrays (Affymetrix) containing 19 492 genes. Data were scanned according to the protocol described in the WT sense target-labeling assay manual from Affymetrix (version 4; product code FS450_0007).
Microarray Gene Expression Data Analysis
The intensity values of different probe sets (genes) in the GeneChip operating software (Affymetrix) were imported into GeneSpring GX version 10.0 software (Agilent Technologies, Santa Clara, CA) and processed using the robust multiarray analysis algorithm for background adjustment, normalization, and log2 transformation of perfect-match values [9, 24]. The data at each time point were normalized to the corresponding sample at t = 0 h. Then, the normalization was conducted within each time-group: data from E2P4-treated samples were normalized to those from E2-treated samples, which were normalized to the vehicle control, with the resulting data thus reflecting the specific temporal response to P4. We performed three major comparisons of the response to P4: within the nonendometriosis group, within the endometriosis group, and between hESFendo and hESFnonendo. The resulting gene lists generated included only genes showing >1.5-fold change in expression and a P value of <0.05 by using a two-way ANOVA parametric test and Benjamini-Hochberg multiple testing correction for false discovery rate.
Principal Component Analysis and Hierarchical Clustering
Principal component analysis (PCA) and hierarchical clustering were performed as described previously [9, 24]. We applied the unbiased PCA algorithm in GeneSpring software to all samples, using all 19 492 genes on the Human Gene 1.0 ST array chip to look for similar expression patterns and underlying cluster structures. Hierarchical cluster analysis uses only differentially expressed genes from all samples and from among all experimental conditions and was conducted using the smooth-correlation-distance-measure algorithm (GeneSpring) to identify samples with similar patterns of gene expression.
Gene Ontology and Pathway Analyses
Gene ontology and functional annotations were carried out using Ingenuity Pathway analysis (IPA) (Ingenuity Systems, Redwood City, CA), into which gene symbols and fold changes of up- and downregulated genes in each pair-wise comparison were imported [9].
Microarray Validation by Real-Time PCR
Genes showing >1.5-fold up- or downregulation at each time point were randomly chosen for validation by quantitative real-time PCR (QRT-PCR). QRT-PCR assays were performed in duplicate using SYBR Green PCR Master Mix (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. The RPL19 housekeeping gene was used as the normalizer. Primer sequences (Table 2) were designed from public databases, and PCR assays were run using Mx4000 and Mx3005 QPCR systems (Stratagene, La Jolla, CA) under thermal cycling conditions as described previously [9, 22]. Pair-wise comparisons between treatments and control at each time point were performed. All validation experiments used four-subject samples in each group. Statistical analysis for the QRT-PCR assays was performed using the nonparametric Mann-Whitney U-test. Significance was determined at a P value of ≤0.05.
TABLE 2.
Primer sequences used in real-time RT-PCR experiments.
ELISA
ELISAs were carried out to quantify IGFBP1 (Alpha Diagnostic International, San Antonio, TX), PRL (Diagnostic Systems Labs, Webster, TX), and amphiregulin (AREG) and HBEGF (both, R&D Systems Inc., Minneapolis, MN) levels in CM from cultured hESF, performed according to manufacturers' instructions. All samples were assayed in duplicate, and values were plotted against a standard curve. Levels of IGFBP1, PRL, AREG, and HBEGF expression in CM for each sample were normalized to total RNA. The IGFBP1 ELISA kit had inter- and intra-assay coefficient of variation values (CV) of 5%–7.4% and 2.4%–3.4%, respectively, and the PRL ELISA inter- and intra-assay CVs were 6.7%–10.4% and 7.8%–8.2%, respectively. The sensitivity for the AREG ELISA was 15 pg/ml, with a linear range up to 1000 pg/ml. The sensitivity for the HBEGF ELISA was 30 pg/ml, with a linear range up to 2000 pg/ml. Statistical analysis of the ELISA data was performed using a two-tailed type 3 Student-t test.
RESULTS
PCA and Hierarchical Clustering Analysis
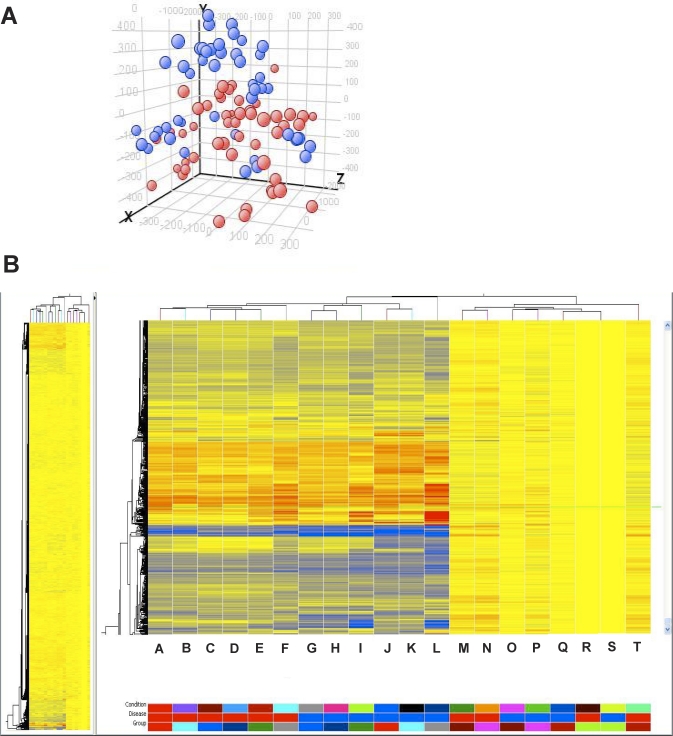
PCA distributes samples into a three-dimensional space based on variations in gene expression, with samples that have similar gene expression profile trends clustering close together. When we used all genes on the Affymetrix array and a completely unbiased approach, the samples roughly clustered into two major groups, in a comparison of disease versus no disease (Fig. 2A).
FIG. 2.
Clustering and cluster trees. A) Principal component analysis of hESFnonendo and hESFendo at t = 0, 6, and 48 h and at 14 days, treated with or without E2 and P4. PCA was applied to all samples that were characterized by the gene expression of all probes on a Gene 1.0 ST platform. Blue, no endometriosis samples; red, endometriosis samples. B) Hierarchical clustering analysis of hESFnonendo and hESFendo at t = 0, 6, and 48 h and at 14 days, treated with or without (control [c]) estrogen (E2), and progesterone (P4), using only the profiles of significantly regulated genes. The heat map represents relative expression levels of genes in the hESF samples: each horizontal line represents a single gene, and each column represents a single sample. The relative expression of each gene is color coded as high (red) or low (blue) or no change (yellow). Groups: A, E2 14-Day endo; B, c 14-Day endo; C, E2 48-h endo; D, c 48-h endo; E, E2P4 48-h endo; F, E2P4 14-Day endo; G, E2 48-h nonendo; H, c 48-h nonendo; I, E2P4 48-h nonendo; J, E2 14-Day nonendo; K, c 14-Day nonendo; L, E2P4 14-h nonendo; M, E2 6-h endo; N, E2P4 6-h endo; O, E2 6-h nonendo; P, E2P4 6-h nonendo; Q, c 6-h nonendo; R, t = 0 endo; S, t = 0 nonendo; T, c 6-h endo.
Unsupervised hierarchical clustering analyses based on the combined gene list derived from pair-wise comparisons as described above (Fig. 1) at each time point resulted in a dendrogram of sample clustering and a heat map of gene expression (Fig. 2B) in which all control samples (t = 0 h and vehicle control) and all treated samples fell into two main branches. Short-term treatment (6-h) groups (vehicle control, E2-treated, and E2P4-treated) clustered together in one sub-branch with t = 0. Intermediate (48-h) and long-term (14-Day) treatment groups clustered into other main sub-branches; the latter group clustered further into two sub-branches, based on the disease state, followed by further branching according to the treatment time (Fig. 2B). This clustering demonstrates that cultured, treated hESF cluster according to the duration of hormonal exposure, similar to in vivo hormonal exposure of whole-tissue samples, which cluster based primarily on cycle phase [24, 25] and thereafter based on disease state [25].
Differences in Gene Expression Between hESFendo and hESFnonendo at t = 0
Comparisons between gene expression in hESFendo and hESFnonendo at t = 0 (untreated uncultured control) revealed differences between the two (Table 3). In particular, we observed upregulation of members of the EGF and TGFB families (HBEGF, AREG, TGFB2), the SLC16A6 solute carrier, and the neural precursor cell expressed developmentally downregulated 4-like (NEDD4L) gene in hESFendo compared with the hESFnonendo samples. Also, there was significant downregulation of several genes at t = 0 in hESFendo versus hESFnonendo, including the FKBP5, IL1R1, sulfatase 2, prolactin receptor, methyltransferase-like 7A, and plexin C1 genes; the cytoskeletal MYLIP and ACTA2 genes; and the progesterone receptor (PGR [1.75-fold]) gene (Table 3). IPA analysis of the comparison of these groups showed that the HBEGF and AREG genes were allocated to several different signaling pathways, including neuregulin signaling, ERBB2 (HER-2) signaling in breast cancer, pancreatic adenocarcinoma signaling, and others. Analysis of hESF transcriptomes at t = 0 revealed no gene profile was unique to one menstrual cycle phase versus another (data not shown), consistent with previous reports [22].
TABLE 3.
List of up- and downregulated genes (BH corrected P value < 0.05) in comparison of hESFendo at t = 0 (hESFendo t = 0) versus hESFnonendo at t = 0, expressed as fold change (FC).
Early, Intermediate, and Late Responses to P4
We investigated the temporal responses presumably to P4 (by comparison of E2P4 vs. E2 response) of hESFnonendo and hESFendo and then compared the two responses to each other. These comparisons enabled us to define the “normal” P4-regulated program from early response genes to the full decidual phenotype, as well as the hESFendo response.
Early Response
hESFnonendo.
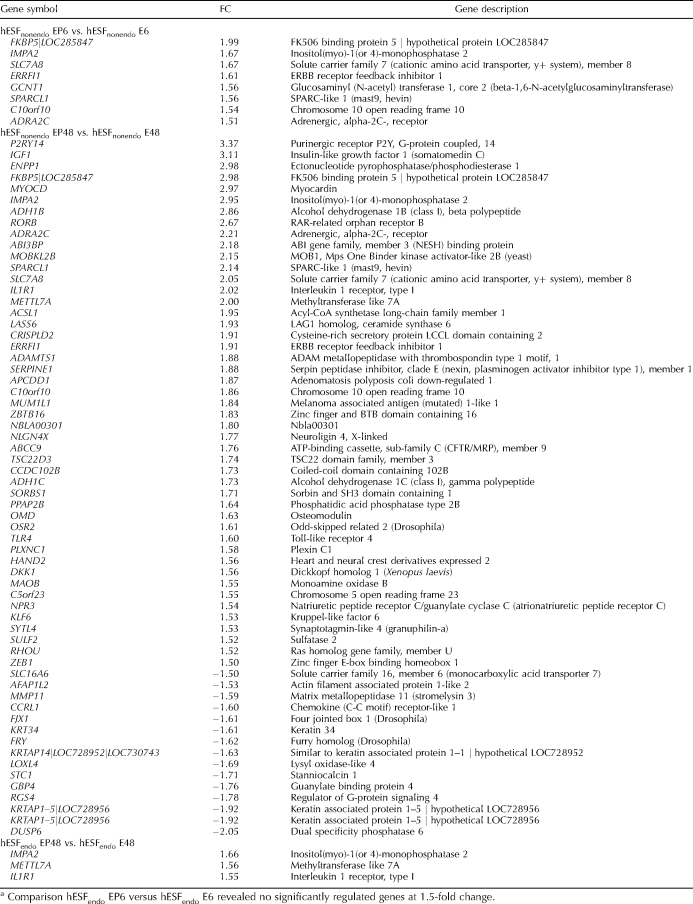
In a comparison of hESFnonendo treated with E2P4 versus E2 alone, upregulated genes included the SLC7A8 solute carrier, the PGR FKBP5 chaperone, the SPARCL1 gene, the ERBB receptor feedback inhibitor 1 (ERRFI1, MIG6), IMPA2, and GCNT1 genes, the adrenergic alpha-2C receptor, and chromosome 10 open reading frame 10 (C10orf10), among others (Table 4). There were no significantly downregulated genes at the 1.5-fold change cutoff.
TABLE 4.
List of regulated genes (BH corrected P value < 0.05) in comparison of hESFnonendo or hESFendo treated with E2P4 for 6-h (EP6) or 48-h (EP48) versus hESFnonendo or hESFendo treated with E2 for 6-h (E6) or 48-h (E48), expressed as fold change (FC).a
hESFendo.
Analysis of microarray data of gene expression after 6 h of treatment in hESFendo revealed no genes were up- or downregulated at the >1.5-fold cutoff (P < 0.05) (Table 4). Interestingly, in the E2-treated groups, the E2 target PGR gene was upregulated in hESFnonendo at 6 h, demonstrating early responsiveness to E2; however, in hESFendo, there was delayed PGR upregulation, first noted at 48 h of E2 treatment (data not shown). This time shift in E2 regulation of the PGR gene may account for the relative resistance to P4 observed in hESFendo at the 6-h time point of cells treated with E2P4. Importantly, the early response to P4 (E2P4 vs. E2) signaling involving the PGR chaperone and the MAPK pathway was not established in hESFendo.
Intermediate Response
hESFnonendo.
In a comparison of E2P4- and E2-treated hESFnonendo at 48 h, in addition to the sustained or enhanced upregulation of the early response genes already described above at 6 h, the first upregulation was observed for the IGF1, DKK1, KLF6, MAOB, IL1R1, ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) (regulator of extracellular inorganic pyrophosphate [PPi]), sulfatase 2, plexin C1, and osteomodulin genes and others (Table 4). Among the downregulated were the SLC16A6 soluble carrier, the MMP11, and the dual-specificity phosphatase 6 genes and others (Table 4).
hESFendo.
In hESFendo, there were only three significantly upregulated genes: the IMPA2 (upregulated in hESFnonendo at 6 h), methyltransferase-like 7A, and IL1R1 genes (Table 4).
Late Response
hESFnonendo.
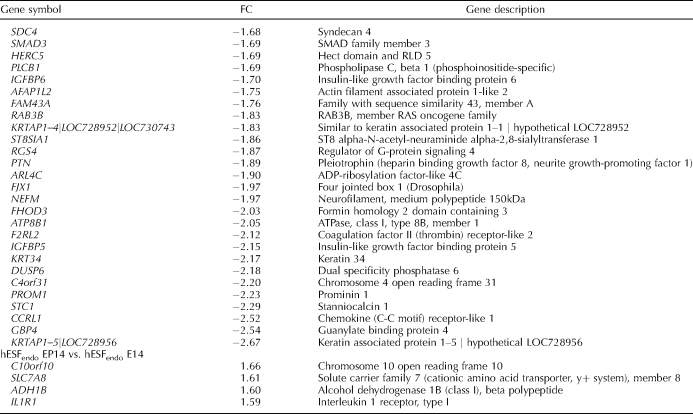
After hESFnonendo was treated for 14 days, in addition to the continued or enhanced expression of the genes mentioned above, the uniquely upregulated (>1.5-fold, P < 0.05) group included the classical P4-regulated PRL, IGFBP1, GPX3, MAOA, tenascin family, prolactin receptor, parathyroid hormone-like hormone, adenylate cyclase 1, hydroxysteroid (17-beta) dehydrogenase type 11 (HSD17B11), and FOXO1A genes and others (Table 5). Among those newly observed and significantly downregulated were the IGFBP5, cyclin D1, neurofilament medium polypeptide (NEFM), and pleiotrophin (PTN, heparin-binding growth factor 8, neurite growth-promoting factor 1) genes and others (Table 5).
TABLE 5.
List of regulated genes (BH corrected P value < 0.05) in comparison of hESFnonendo or hESFendo treated with E2P4 for 14 days (EP14) versus hESFnonendo or hESFendo treated with E2 for 14 days (E14), expressed as fold change (FC).
hESFendo.
In contrast, in hESFendo, only a few P4-dependent genes were (up)regulated, including the C10orf10, SLC7A8 (both upregulated at 6 h in hESFnonendo), ADH1B (upregulated at 48 h in hESFnonendo), and IL1R1 genes (Table 5).
Validation of Microarray Data by RT-PCR
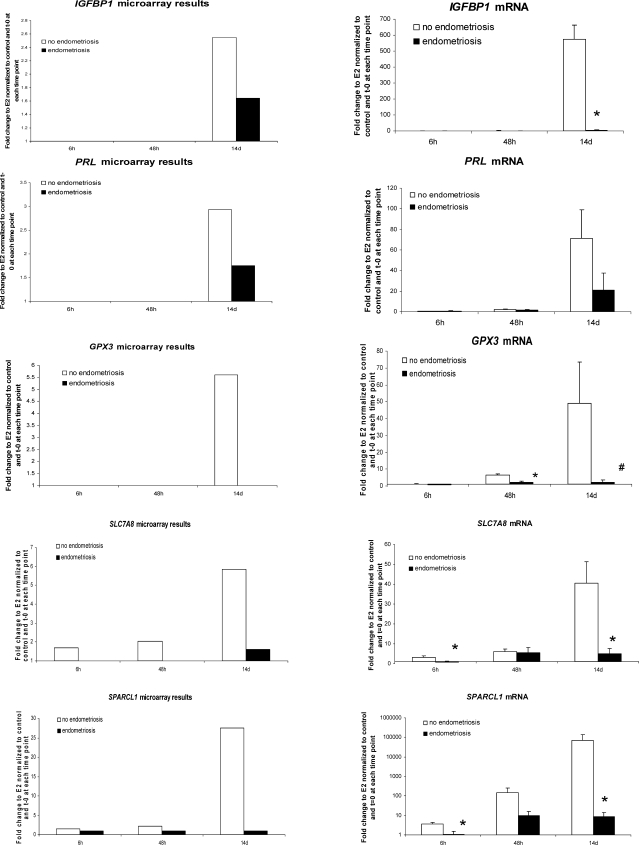
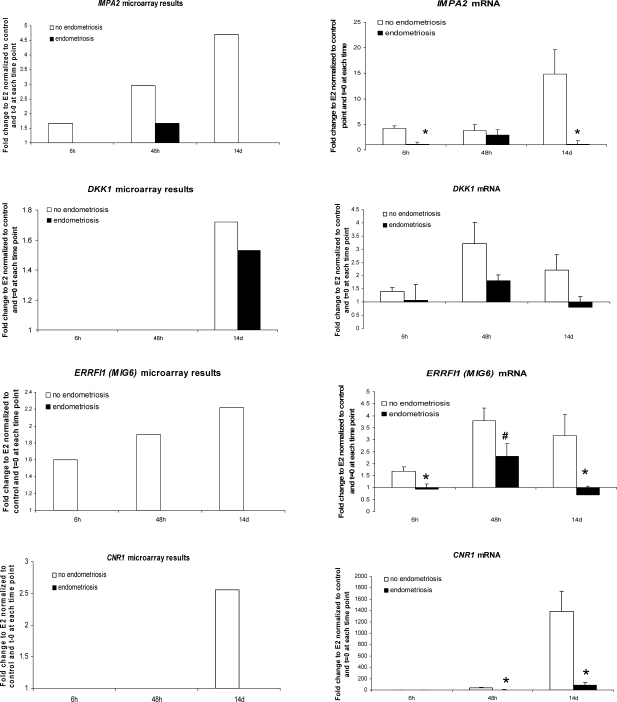
To validate microarray data by QRT-PCR, we randomly selected nine genes from the microarray data analysis: IGFBP1, PRL, IMPA2, SLC7A8, DKK1, GPX3, ERRFI1 (MIG6), SPARCL1, and CNR1 (Fig. 3). There was very high concordance of gene regulation observed in the microarray analysis and the validation studies. We confirmed significant dysregulation of P4-regulated endometrial genes, such as IGFBP1, IMPA2, SLC7A8, GPX3, ERRFI1, SPARCL1, and CNR1, in hESFendo compared to those in hESFnonendo in response to E2P4 versus E2 treatment, even at earlier time points (Fig. 3).
FIG. 3.
Validation of microarray gene expression profiling by QRT-PCR. Right column indicates fold change expression of genes in the microarray data set in the present study. Left column indicates QRT-PCR validation of microarray data of gene expression in hESFnonendo and hESFendo treated with or without E2 and P4 for 6 h, 48 h, and 14 days (n = 4 in each group). Y axis displays the fold change of expression in hESF treated withP4 and E2, relative to E2 and normalized to the vehicle control and t = 0 at each time point. *, Significance accepted at P ≤ 0.05. #, Significance accepted at P = 0.05. Error bars represent means ± SEM.
Validation of Microarray Data by ELISA
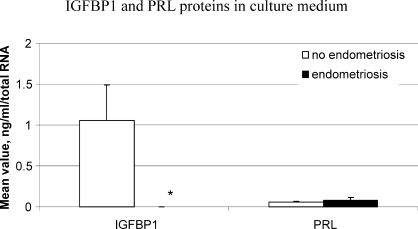
IGFBP1 and PRL.
Secretion of the IGFBP1 decidualization marker, but not PRL, in response to E2P4 versus E2 treatment differed between hESFendo and hESFnonendo (Fig. 4), confirming the mRNA validation data (Fig. 3).
FIG. 4.
Validation of microarray gene expression profiling by ELISA. IGFBP1 and PRL protein secretion in CM from hESFnonendo and hESFendo treated with or without E2 and P4 for 14 days (n = 4 in each group), normalized to the total RNA level. *, Significance accepted at P ≤ 0.05. Error bars represent means ± SEM.
Amphiregulin and heparin-binding EGF-like growth factor secretion by hESF.
Previous publications have implicated the EGF signaling network in the pathogenesis of endometriosis [25, 26]. Because amphiregulin (AREG) and heparin-binding EGF-like growth factor (HBEGF) transcripts were upregulated in hESFendo versus hESFnonendo at t = 0 and at 6 h (Table 3, Supplemental Table S1 [available online at www.biolreprod.org]), we measured AREG and HBEGF protein in CM of hESF. No detectable AREG protein was found in CM from hESFnonendo at any time point or from any treatment group. However, all hESFendo secreted AREG (data not shown). There was a tendency toward increased AREG expression with time; however, differences from one time point to another were not significant, and there was no apparent effect of hormonal treatment on AREG levels in CM (data not shown), suggesting constitutive expression of this growth factor in hESFendo. There was no HBEGF protein detected in CM from hormonally treated and control hESFnonendo at different time points, and hESFendo secretion levels of HBEGF in CM were at the lower limit of detection of the assay (data not shown).
Early and Late Responses in hESFendo Versus hESFnonendo: Canonical Pathways and Networks
The degree of regulation of signaling pathways and network formations in different comparison groups depends on the total number of genes regulated in each group (see gene lists in Tables 3–5 and Supplemental Tables S1–S3). Significantly regulated canonical pathways and network formations in response to E2P4 versus that of E2 treatment in hESFnonendo and hESFendo and in hESFendo versus hESFnonendo are shown in Supplemental Tables S4 and S5.
Canonical pathways.
Canonical pathways regulated at early and intermediate time points in the hESFnonendo response to E2P4 versus E2 treatment were related to lipid and carbohydrate metabolism, O-glycan biosynthesis, neuregulin, and cAMP signaling. At 14 days, there was activation of the IGF1 signaling and integrin-linked kinase (ILK) pathways and activation by the VDR/RXR and hepatic fibrosis pathways. In contrast, the hESFendo response to E2P4 versus E2 treatment was blunted, with fewer genes being regulated and subsequently being involved in canonical pathways. Moreover, pathways activated in hESFendo differed from those activated in hESFnonendo in response to E2P4 versus E2 treatment. Differences were observed in interleukin (IL)10 and IL6 signaling pathways, PPAR signaling, LXR/RXR activation, and bile acid and glucose biosynthesis and metabolism pathways in early and late responses to E2P4 versus E2 treatment (Supplemental Table S4).
Networks.
Network analysis of genes upregulated in the early (6-h) response to E2P4 versus E2 treatment in hESFnonendo revealed enrichment of biological processes involving carbohydrate, lipid, and amino acid metabolism and tissue morphology. In contrast, in the (48-h) intermediate response, disease and development-associated networks prevailed, including cancer, cardiovascular, and gastrointestinal disease, tissue development, cell morphology, and embryonic development. The late response of hESFnonendo at 14 days showed involvement of networks including DNA replication, recombination, and repair, nucleic acid metabolism, skeletal and muscular system development and function, endocrine system disorders, and gene expression. IPA analysis of the hESFendo early response involved tissue developmental processes (nervous system, skeletal, muscular, connective tissue, and hematologic) and cell–cell signaling and cell cycle. The intermediate response revealed the formation of cancer, cell death, neurological disease, posttranslational modification, cell cycle, cellular development, and cell signaling networks, which differs from the hESFnonendo response at this time point. Differences between hESFendo and hESFnonendo at 14 days of treatment revealed networks involving cancer, cell growth and proliferation, cell–cell signaling, cell movement, nucleic acids, drugs, and lipid metabolism (Supplemental Table S5).
DISCUSSION
General Comments
This study reveals for the first time the kinetic expressions of specific genes, pathways, and networks during early, intermediate, and late responses to P4 (E2P4 vs. E2) in hESF from women with and without endometriosis. In this in vitro model, there are likely a multiplicity of pathways and processes that are initiated and perhaps synergistically affected by E2P4 treatment and separately by E2 treatment, although the response to E2 was significantly limited to PGR upregulation at all time points, supporting the data obtained herein as likely to be P4-mediated events. Nonetheless, there may be other responses that are not strictly P4-mediated in our model; however, identifying the classically P4-regulated genes was consistent with the effectiveness of a functional P4 pathway in hESFnonendo and a dysfunctional pathway in hESFendo.
In hESFnonendo, distinct biological and molecular processes and signaling pathways were observed; whereas, signaling pathways in the hESFendo responses to E2P4 versus E2 treatment were not active compared to those in hESFnonendo, due to the limited number of genes regulated. A blunted hESFendo response to P4 was observed, with limited expression of many P4-regulated genes and curtailed activation of distinct canonical pathways and processes. While the PGRB/PGRA ratio was reported to be lower in endometrial tissues from women with endometriosis than in tissues from healthy volunteers [15], our earlier studies of eutopic endometrium did not confirm significant differences in PGR isoforms in either tissue biopsies or hESF from women with endometriosis versus those without endometriosis [10]. In the present study, we hypothesized and have found that the P4 resistance in eutopic endometrium from women with endometriosis results not from the PGR aberrant expression but rather from its delayed upregulation in the early response to E2. Moreover, in the current study, the PGR FKBP5 (immunophilin) chaperone expression level, which was upregulated in hESFnonendo in early, intermediate, and late responses to P4, was not regulated in hESFendo and was significantly lower in hESFendo than in hESFnonendo at t = 0. Interestingly, diminished FKBP4 expression has been observed in eutopic and ectopic endometrium in humans and baboons with endometriosis [27, 28]. Furthermore, Fkbp4-null mice are prone to develop endometriotic lesions upon inoculation with endometrial tissue [28]. Overall, PGR chaperones (and coregulators [10]) appear to play a role in the blunted response of hESF to P4 in women with endometriosis.
We propose that the normal response of hESF to P4 (E2P4 vs. E2) involves a tightly regulated kinetic cascade involving key components in the PGR and MAPK signaling pathways that results in inhibition of E2-mediated proliferation, establishment of key networks, and eventual differentiation to the decidual phenotype. Furthermore, we propose that these early events, which are likely temporal and spatial in nature, are not established in the early hESFendo response to P4, contributing to abnormal P4 responsiveness of this cell type in eutopic endometrium of women with endometriosis. A similar mechanism may be ongoing in ectopic foci.
Our earlier studies using eutopic endometrial tissue samples from women with and without endometriosis [25, 26] revealed dysregulation of a number of P4-responsive genes and signaling molecules. In those earlier studies we specifically validated the dysregulation of the primarily stromal fibroblast (hESF) products ERRFI1, FOXO1A, CDC2, IGFBP1, PRL, GPX3, and DKK1 in endometrial tissue from women with disease [25, 26]. Many of these gene products were found to be similarly dysregulated in our current in vitro study of hESF, and thus, essentially their in vivo expression has been validated by our previous work.
hESFnonendo Response to Progesterone
Early and intermediate responses.
The first observable response to P4 in vivo occurs in early secretory endometrium and includes P4 inhibition of E2-induced cellular proliferation and an increase in cholesterol, fatty acid, prostaglandin, glycogen, and transporter biosynthesis, mostly in epithelium, with little known about the early hESF response [8]. In mid-secretory endometrium, hESF begin to make a transition from a fibroblast-like spindle to a rounder morphology and a secretory phenotype, typical of epithelium, producing classical “decidual markers” (e.g., prolactin and IGFBP1). In vitro, hESF respond to P4 (plus E2), P4 plus cAMP, or only cAMP, with characteristic morphologic and gene expression and protein changes, as in vivo. Treating cells with cAMP or P4 plus cAMP results in upregulation of decidual markers within 48–72 h, compared to 8–14 days (as in vivo [12]), with P4 treatment alone. Direct transcriptional control by P4 has been questioned due to the time course in vivo [12], and P4 has been proposed to maintain the decidual response initiated by a yet-to-be identified ligand stimulating the PKA pathway [12]. The data herein support an early response to P4 that includes unique early response genes and intracellular signaling pathways and subsequent expression of decidual markers involving stimulation of the PKA pathway.
What is striking in the early hESFnonendo response to P4 is EGFR-mediated and MAPK signaling. The ERBB receptor feedback inhibitor 1 (ERRFI1, or mitogen-inducible gene 6 [MIG6]) is a negative regulator of EGFR-mediated mitogenic signaling [29] and is one of the earliest transcription factors upregulated in P4-treated hESF. The protein regulates the duration of MAPK activation via attenuation of EGFR autophosphorylation in a mouse knockout model [29]. Our data support P4 regulation of this signaling pathway early in the P4 response in hESFnonendo.
Other early and intermediate response genes upregulated in the hESFnonendo response to P4 (E2P4 vs. E2) are the DKK1 gene of the Wnt family, the solute carrier family SLC7A8 and IMPA2 proteins, and, interestingly, the adrenergic receptor alpha-2C (ADRA2C) gene; this last gene is one of several neuronal receptors found in endometrium [24, 30]. Expression of the ADRA2C transcript in P4-treated hESFnonendo suggests that normal endometrium possesses the machinery for regulating neurotransmission [31]. Marked IMPA2 upregulation underscores the importance of the phosphatidylinositol signaling pathway [32] in the response of hESF to P4.
The SPARCL1 protein (a SPARC-like 1 [hevin], or mast9, or high endothelial venule protein), an extracellular matrix-associated protein member of the SPARC family, is upregulated during the implantation window and is associated with the E2P4 versus the E2 response of cultured explants from late proliferative phase human endometrium [33]. It is expressed in many tissues and is associated with collagen fibrils [34]. SPARCL1 expression is downregulated in several tumors and negatively regulates cell cycle progression, cell proliferation, and migration [35, 36]. In the present study, SPARCL1 expression was highly upregulated in response to P4 in hESFnonendo at 6 and 48 h and 14 days, suggesting a major role for the protein product in the P4 response of hESF, perhaps participating in or directly inhibiting cell cycle progression and enabling differentiation in response to P4.
Late response.
At 14 days, genes corresponding to secretory and cell adhesion proteins, including classical decidual markers, as well as interleukins, signaling components, enzymes, and receptors, are upregulated (Table 5), with the SPARCL1 gene mentioned above being the most highly upregulated gene. Also of interest is the CNR1 cannabinoid receptor, shown recently to be expressed in human endometrium throughout the cycle, without significant cyclic variations [37]. Although P4 has been shown to activate the endocannabinoid-degrading enzyme fatty acid amide hydrolase in human lymphocytes [38], this is the first study of P4 regulation of the endocannabinoid system in human endometrium. The significance of CNR1 expression in hESF warrants further investigation.
One of the most highly upregulated genes, ENPP1, regulates extracellular pyrophosphate, a major inhibitor of extracellular matrix (ECM) calcification [39]. Also, by Day 14, many known P4-regulated genes are increased, and several genes associated with actin filaments and bundle formation are up- or downregulated (Table 5), consistent with changes in cell shape in the late response to P4. Thus, the gene expression profile at Day 14 represents classically decidualizing hESF, based on the cytoskeletal and stress fiber changes, the secretory phenotype, and a predominance of genes involved in the ECM, cell matrix, and cell–cell communication.
Several groups have investigated the transcriptome of decidualized human hESF (summarized in Supplemental Table S6). The current study compared hESF treated with E2P4 versus E2 and showed a time course of this comparison, focusing on early, intermediate, and late responses to P4, per se, of hESF (both hESFnonendo and hESFendo). Okada and colleagues [40] analyzed genes expressed in hESFnonendo after 3 days of P4 treatment (without E2) using a 1000-gene cDNA platform and found 6 genes were upregulated and 27 genes were downregulated by P4, compared to controls (vehicle alone). Among those downregulated was the IGFBP5 gene and pregnancy-specific glycoproteins, and among those upregulated was the IL1RI gene, as also found herein. The differences observed between the two studies were probably due to different treatment protocols (e.g., no priming with E2), duration of hormonal treatments, and use of different array platforms.
hESFendo Response to Progesterone
Early, intermediate, and late responses.
There were significantly lower numbers of genes regulated by P4 (E2P4 vs. E2) in hESFendo versus hESFnonendo (Tables 4, 5). In women with endometriosis, in contrast to those without disease, ERRFI1 is not upregulated in secretory phase tissue [25, 41], further supporting its regulation by P4 and dysregulation in this P4-resistant disorder, confirmed herein on the cellular level. We have previously shown that increased activation of MAPK1/3 in hESFendo contributes to persistent proliferative changes in secretory-phase endometrium in women with endometriosis [41]. Constitutive expression of the AREG and HBEGF EGFR ligands in hESFendo that can activate MAPK1/3 [42–44], and were observed herein, provides a potential mechanism for the observed constitutive MAPK1/3 phosphorylation and the proliferative phenotype of hESFendo. This may play an important role in the pathophysiology of the disease and design of targeted therapies for endometriosis and is under study in our laboratory.
We previously analyzed transcriptomes of hESF (both hESFnonendo and hESFendo) in response to 8-Br-cAMP for 96 h (full decidualization phenotype) [9]. A comparison of lists of genes of decidualized hESFnonendo in response to P4 (14 days) versus those of 8-Br-cAMP (96 h) revealed 30 common genes, among them PRL, IGFBP1, ADRA2C, CCND1, IL1R1, and stanniocalcin (Supplemental Table S7), reflecting the decidualization signature of hESFnonendo. Of note, no common genes were found when similar comparisons were performed with hESFendo (Supplemental Table S7).
Herein, the (CCND1) cyclin D1 transcript was increased in hESFendo versus hESFnonendo treated with E2P4 for 14 days, while it was decreased in hESFnonendo in response to P4 (E2P4 vs. E2). This confirms our previous results showing an increased proliferative potential with increased CCND1 expression in decidualized hESFendo versus hESFnonendo and a blunted response of hESFendo to cAMP (decreased PKA activation in hESFendo here) [9, 41].
In contrast to the upregulation of the SPARCL1 gene in response to P4 in hESFnonendo, SPARCL1 expression was not regulated by P4 in hESFendo. Importantly, it was the most downregulated gene in hESFendo versus hESFnonendo, underscoring the decreased responsiveness of hESFendo to P4 treatment. As SPARCL1 expression is an inhibitor of cell proliferation, its upregulation in decidualized hESFnonendo and downregulation in hESFendo suggest a role for it in the proliferative phenotype of hESFendo.
Interestingly, cancer-associated signaling pathways, such as those in colorectal cancer metastasis and bladder cancer signaling, were regulated in hESFendo versus hESFnonendo (Supplemental Table S4). Regulation of these canonical pathways in hESFendo is consistent with the invasive phenotype of endometriosis that may be shared by other invasive cell types and tissues.
Among several signaling pathways that were significantly dysregulated in the hESFendo response to P4, versus that of hESFnonendo (Supplemental Table S4), are those involving axonal guidance and neuropathic pain signaling (P = 0.0048 and P = 0.01, respectively) (Supplemental Table S4). Adrenergic and sensory nerve fibers have recently been described in eutopic endometrium of women with endometriosis (but not in women without endometriosis) [45], which may contribute to pain associated with the disorder. Thus, it is of particular interest that in the hESFnonendo response to P4 (E2P4 vs. E2), there is sustained ADRA2C upregulation and downregulation of neurofilament medium polypeptide (NEFM) and pleiotrophin (heparin-binding growth factor 8, neurite growth-promoting factor 1). Pleiotrophin, associated with angiogenesis and neurite growth, is expressed in normal endometrium throughout the menstrual cycle and is significantly upregulated in endometrium from women with advanced-stage endometriosis [46]. The molecular mechanisms underlying the presence of nerve fibers within eutopic endometrium of women with endometriosis are not well understood; however, the data herein support a role for hESF in promoting neurite growth and axonal guidance within the endometrium of women with endometriosis and, potentially, hormonal regulation of nerve fibers and nerve bundle density in this tissue from women with disease [47]. We are currently investigating this in our laboratory.
The current study has given unique insight into the genome-wide transcriptome of the human endometrial stromal fibroblast at early, intermediate, and late responses to P4, in an experimental paradigm that reveals kinetic changes in gene expression, biological processes, and signaling pathways. Some of these changes are novel and some are consistent with known hESF responses to P4 in vivo and resistance to P4 actions and dysregulation in disorders such as endometriosis. The signaling pathways described herein may serve as unique targets for drugs to treat endometrial disorders.
Supplementary Material
Footnotes
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health cooperative agreement 1U54HD055764-04, as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, to L.C.G.
3These authors contributed equally to this work.
REFERENCES
- Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann N Y Acad Sci 2002; 955: 11 22 [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin NorthAm 1997; 24: 235 258 [DOI] [PubMed] [Google Scholar]
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med 2010; 362: 2389 2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Velarde MC, Giudice LC. Altered gene expression profiling in endometrium: evidence for progesterone resistance. Semin Reprod Med 2010; 28: 51 58 [DOI] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. N Engl J Med 2009; 360: 268 279 [DOI] [PubMed] [Google Scholar]
- Giudice LC. Endometrium in PCOS: Implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab 2006; 20: 235 244 [DOI] [PubMed] [Google Scholar]
- Irwin JC, Giudice LC. The decidua. Knobil E, Neill JD. (Eds.), Encyclopedia of Reproduction. San Diego: Aademic Press; 1998: 823 835 [Google Scholar]
- Giudice LC. Application of functional genomics to primate endometrium: insights into biological processes. Reprod Biol Endocrinol 2006; 4 (suppl 1): S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Horcajadas JA, Weeks JL, Esteban FJ, Nezhat CN, Conti M, Giudice LC. The protein kinase A pathway-regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis. Endocrinology 2010; 151: 1341 1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Velarde MC, Giudice LC. The progesterone receptor coactivator Hic-5 is involved in the pathophysiology of endometriosis. Endocrinology 2009; 150: 3863 3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar AK, Frank GR, Kessler CA, Cedars MI, Handwerger S. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine 1997; 6: 301 307 [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol 2003; 178: 357 372 [DOI] [PubMed] [Google Scholar]
- Jones MC, Fusi L, Higham JH, Abdel-Hafiz H, Horwitz KB, Lam EW, Brosens JJ. Regulation of the SUMO pathway sensitizes differentiating human endometrial stromal cells to progesterone. Proc Natl Acad Sci U S A 2006; 103: 16272 16277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab 2000; 85: 2897 2902 [DOI] [PubMed] [Google Scholar]
- Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, Osteen KG. Reduced expression of progesterone receptor-B in the endometrium of women withendometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil Steril 2005; 84: 67 74 [DOI] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod 2007; 77: 681 687 [DOI] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab 2007; 92: 3261 3267 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Utsunomiya H, Lin Z, Yin P, Cheng YH, Pavone ME, Tokunaga H, Trukhacheva E, Attar E, Gurates B, Milad MP, Confino E, et al. Steroidogenic factor-1 and endometriosis. Mol Cell Endocrinol 2009; 300: 104 108 [DOI] [PubMed] [Google Scholar]
- Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women withendometriosis have reduced decidualization capacity. Fertil Steril 2006; 85: 564 572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, Wu Y, Guo SW, Fazleabas AT. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod 2007; 13: 323 332 [DOI] [PubMed] [Google Scholar]
- Revised American Fertility Society classification of endometriosis. Fertil Steril 1985; 43: 351 352 [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women withversus without endometriosis. Biol Reprod 2009; 80: 105 114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC. Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab 2006; 91: 1453 1461 [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006; 147: 1097 1121 [DOI] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women withendometriosis. Endocrinology 2007; 148: 3814 3826 [DOI] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women withendometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 2003; 144: 2870 2881 [DOI] [PubMed] [Google Scholar]
- Jackson KS, Brudney A, Hastings JM, Mavrogianis PA, Kim JJ, Fazleabas AT. The altered distribution of the steroid hormone receptors and the chaperone immunophilin FKBP52 in a baboon model of endometriosis is associated withprogesterone resistance during the window of uterine receptivity. Reprod Sci 2007; 14: 137 150 [DOI] [PubMed] [Google Scholar]
- Hirota Y, Tranguch S, Daikoku T, Hasegawa A, Osuga Y, Taketani Y, Dey SK. Deficiency of immunophilin FKBP52 promotes endometriosis. Am J Pathol 2008; 173: 1747 1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferby I, Reschke M, Kudlacek O, Knyazev P, Pante G, Amann K, Sommergruber W, Kraut N, Ullrich A, Fassler R, Klein R. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat Med 2006; 12: 568 573 [DOI] [PubMed] [Google Scholar]
- Popovici RM, Kao LC, Giudice LC. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology 2000; 141: 3510 3513 [DOI] [PubMed] [Google Scholar]
- Kohli U, Muszkat M, Sofowora GG, Harris PA, Friedman EA, Dupont WD, Scheinin M, Wood AJ, Stein CM, Kurnik D. Effects of variation in the human alpha2A- and alpha2C-adrenoceptor genes on cognitive tasks and pain perception. Eur J Pain 2010; 14: 154 159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Turner G, Esterling LE, Sanders AR, Detera-Wadleigh SD. A novel human myo-inositol monophosphatase gene, IMP.18p, maps to a susceptibility region for bipolar disorder. Mol Psychiatry 1997; 2: 393 397 [DOI] [PubMed] [Google Scholar]
- Dassen H, Punyadeera C, Kamps R, Klomp J, Dunselman G, Dijcks F, de Goeij A, Ederveen A, Groothuis P. Progesterone regulation of implantation-related genes: new insights into the role of oestrogen. Cell Mol Life Sci 2007; 64: 1009 1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambrock HO, Nitsche DP, Hansen U, Bruckner P, Paulsson M, Maurer P, Hartmann U. SC1/hevin. An extracellular calcium-modulated protein that binds collagen I. J Biol Chem 2003; 278: 11351 11358 [DOI] [PubMed] [Google Scholar]
- Sullivan MM, Puolakkainen PA, Barker TH, Funk SE, Sage EH. Altered tissue repair in hevin-null mice: inhibition of fibroblast migration by a matricellular SPARC homolog. Wound Repair Regen 2008; 16: 310 319 [DOI] [PubMed] [Google Scholar]
- Claeskens A, Ongenae N, Neefs JM, Cheyns P, Kaijen P, Cools M, Kutoh E. Hevin is downregulated in many cancers and is a negative regulator of cell growthand proliferation. Br J Cancer 2000; 82: 1123 1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AH, Abbas MS, Habiba MA, Konje JC. Histomorphometric evaluation of cannabinoid receptor and anandamide modulating enzyme expression in the human endometrium throughthe menstrual cycle. Histochem Cell Biol 2010; 133: 557 565 [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Bari M, Di Rienzo M, Finazzi-Agro A, Rossi A. Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes throughthe transcription factor Ikaros. Evidence for a synergistic effect of leptin. J Biol Chem 2003; 278: 32726 32732 [DOI] [PubMed] [Google Scholar]
- Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, Manor E, Buriakovsky S, Hadad Y, Goding J, Parvari R. Autosomal-recessive hypophosphatemic rickets is associated withan inactivation mutation in the ENPP1 gene. Am J Hum Genet 2010; 86: 273 278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Nakajima T, Yoshimura T, Yasuda K, Kanzaki H. Microarray analysis of genes controlled by progesterone in human endometrial stromal cells in vitro. Gynecol Endocrinol 2003; 17: 271 280 [DOI] [PubMed] [Google Scholar]
- Velarde MC, Aghajanova L, Nezhat CR, Giudice LC. Increased mitogen-activated protein kinase kinase/extracellularly regulated kinase activity in human endometrial stromal fibroblasts of women withendometriosis reduces 3′,5′-cyclic adenosine 5′-monophosphate inhibition of cyclin D1. Endocrinology 2009; 150: 4701 4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooz M, Gooz P, Luttrell LM, Raymond JR. 5-HT2A receptor induces ERK phosphorylation and proliferation throughADAM-17 tumor necrosis factor-alpha-converting enzyme (TACE) activation and heparin-bound epidermal growthfactor-like growthfactor (HB-EGF) shedding in mesangial cells. J Biol Chem 2006; 281: 21004 21012 [DOI] [PubMed] [Google Scholar]
- Jessmon P, Kilburn BA, Romero R, Leach RE, Armant DR. Function-specific intracellular signaling pathways downstream of heparin-binding EGF-like growthfactor utilized by human trophoblasts. Biol Reprod 2010; 82: 921 929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HS, Lee HJ, Nishida M, Lee MS, Tamura R, Yamashita S, Matsuzawa Y, Lee IK, Koh GY. Betacellulin and amphiregulin induce upregulation of cyclin D1 and DNA synthesis activity throughdifferential signaling pathways in vascular smoothmuscle cells. Circ Res 2003; 93: 302 310 [DOI] [PubMed] [Google Scholar]
- Tokushige N, Markham R, Russell P, Fraser IS. Different types of small nerve fibers in eutopic endometrium and myometrium in women withendometriosis. Fertil Steril 2007; 88: 795 803 [DOI] [PubMed] [Google Scholar]
- Chung J, Uchida E, Grammer TC, Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol 1997; 17: 6508 6516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina MG, Lebovic DI. Endometriosis-associated nerve fibers and pain. Acta Obstet Gynecol Scand 2009; 88: 968 975 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.