Abstract
Background/aims
Health-related quality of life (HRQoL) measures are used in healthcare to help inform clinical decision-making and policy-making decisions. A number of disease-specific or condition-specific measures have been developed and applied in ophthalmology; however, their use in the specific fields of amblyopia and strabismus are not as established. The purpose of this study is to identify and discuss specific HRQoL instruments that may be used in the investigation and management of patients with amblyopia and/or strabismus.
Methods
A systematic literature review was undertaken in November 2009. The electronic databases of AMED (Allied and Complementary Medicine: 1985 to November 2009), the British Nursing Index and Archive (1985 to October 2009), Ovid Medline In-Process and Other Non-Indexed Citations and Ovid Medline (1950 to present) and PsycINFO (1806 to November Week 1 2009) were searched. No language restrictions were applied to the search.
Results
Four instruments were identified: the Amblyopia and Strabismus Questionnaire (A&SQ), the Amblyopia Treatment Index (ATI), the Adult Strabismus Questionnaire (AS-20) and the Intermittent Exotropia Questionnaire (IXTQ).
Conclusion
The use of HRQoL measures in patients with amblyopia and/or strabismus is a developing area. Further research is necessary to determine the impact of issues such as diplopia and poor cosmesis upon patient groups, and to determine the influence of ethnicity and parental reporting in these patients.
INTRODUCTION
Health-related quality of life (HRQoL) measures are increasingly used in healthcare. Such measures help to inform clinical decision-making through evidence-based practice, and can also be used in economic evaluations to help to inform policy-making decisions. HRQoL is a concept that incorporates physical and functional status, emotional status and social functioning,1 and may be assessed using generic or disease or condition-specific instruments. When considering the application of HRQoL instruments within ophthalmology, there are concerns that generic measures are not sensitive to the recognised symptoms of vision loss or emotional aspects of a given ophthalmic condition, such as strabismus.
A number of validated disease-specific or condition-specific instruments have been developed and applied in ophthalmology, both in research and clinical practice. These include the Visual Function Index (VF-14), the National Eye Institute Visual Function Questionnaire2-4 (NEI-VFQ: 51- and 25-item questionnaires), the Impact of Visual Impairment5 (IVI) and the Activities of Daily Vision Scale6 (ADVS), to name a few. Such instruments have been used to describe the impact of a condition within a given population, as a measure to determine the success or suitability of treatment interventions, and to assess the impact of vision loss. The use of patient-reported outcome measures within the specific field of amblyopia and/or strabismus is not as well established. The purpose of this study is to identify and discuss HRQoL instruments that may be used in the investigation and management of patients with amblyopia and/or strabismus.
METHODS
A systematic literature review was undertaken during November 2009. A specific search strategy was employed to identify HRQoL instruments that might be used in the investigation and management of patients with amblyopia and/or strabismus. The electronic databases of AMED (Allied and Complementary Medicine: 1985 to November 2009), the British Nursing Index and Archive (1985 to October 2009), Ovid Medline In-Process and Other Non-Indexed Citations and Ovid Medline (1950 to present) and PsycINFO (1806 to November Week 1 2009) were searched. Details of the search strategy (ie, keywords used) are shown in box 1. No language restrictions were applied to the search.
Box 1. Search strategy employed.
Orthoptic
Strabismus
Amblyopia
Visual acuity
Vision
Loss
Impairment
5 and 6
5 and 7
1 or 2 or 3 or 7 or 8 or 9
Questionnaire
Quality of life
Cosme*
11 or 12 or 13
10 and 14
*denotes unlimited truncation retrieves all possible suffix variations of the root word indicated.
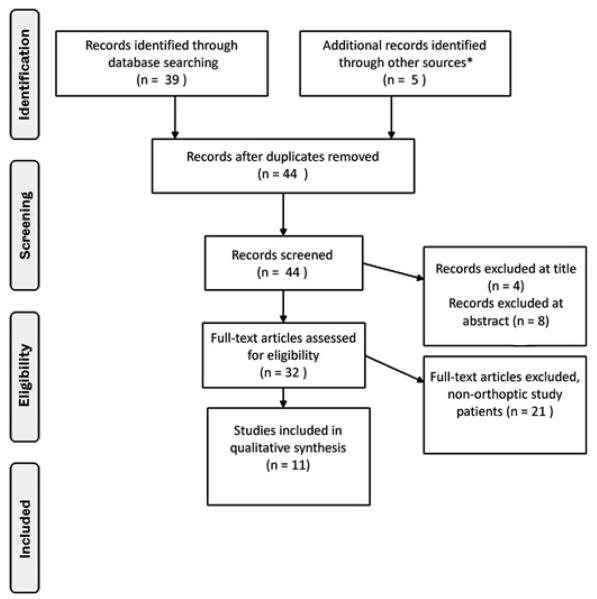
Following removal of duplicates, 39 articles were applicable for this review. Articles were rejected at title if they were not related to the subject area (n=4), rejected at abstract if a vision-specific or disease-specific instrument was not used in the study (n=8), and rejected at full paper if the study subjects could not be described as ‘orthoptic patients’—that is, did not include subjects with a diagnosis of amblyopia and/or strabismus (n=21). These included assessment of HRQoL in patients with sensory impairment, age-related macular degeneration, low vision, cataract, diabetic retinopathy, choroidal melanoma and glaucoma. This left 11 papers identified through the outlined search strategy subject to full review. These papers described the development or administration of four HRQoL measures: the Amblyopia and Strabismus Questionnaire (A&SQ)7-10, the Amblyopia Treatment Index (ATI)11-13, the Adult Strabismus Quality of Life Questionnaire (AS-20)14 15 and the Intermittent Exotropia Questionnaire (IXTQ).16 17 Additional literature was identified through a clinical expert (n=3) and through references of the papers identified through the electronic search (n=2). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)18 flow diagram of study identification is shown in figure 1. This additional searching was undertaken in order to identify any further papers that reported the psychometric properties of the instruments identified from the initial literature search. Instruments were assessed in terms of reliability, validity and responsiveness (see table 1). It is possible to have a reliable measure that is not valid; however, a valid measure must also be reliable. Explanations of reliability, validity and responsiveness are shown in table 1. For the purpose of this paper, construct validity will be determined if compared with objective clinical measures such as visual acuity or binocularity; concurrent validity will be a comparison to an existing vision-specific HRQoL measure.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of study identification. *Correspondence from clinical expert as articles were in press at time of search (n=3) and through references of identified literature (n=2).
Table 1.
Assessment of health-related quality of life (HRQoL) measures
| Definition | Other comments | |
|---|---|---|
| Reliability | ► Ability of a measure to reproduce the same value on two separate occasions when there has been no change in health'19 | ► Can be over time or between methods of administration19 may be considered in terms of internal consistency (the extent to which all items measure the same concept) or test–retest reliability (the extent to which the results of the instrument compare if the test is administered to the same subject on more than one occasion when there has been no demonstrable change of health status) |
| Validity | ► The extent to which a measure reflects the concept that it is intended to measure | ► May be considered in terms of content validity (‘degree to which the instrument is reflective of aspects important to the patients and disease of interest’); construct validity ‘how well a measure correlates with other indicators of similar or related constructs’); concurrent validity (‘the extent to which an instrument correlates to other measures of the same or similar construct’); and discriminant validity (‘the ability to discriminate between either cases versus controls or disease severity groups’)20 |
| ► Factor analysis is a method of determining the structure of an instrument in terms of domains or subscales. It can be used to identify redundant or duplicate items. It may also be used to determine domain structure. Some papers refer to this as a measure of internal validity | ||
| Responsiveness | ► The extent to which the instrument can detect in patients known to have a change in their physical condition |
RESULTS
The literature search identified four disease-specific instruments that can used in the investigation and management of orthoptic patients: the Amblyopia and Strabismus Questionnaire (A&SQ), the Amblyopia Treatment Index (ATI), the Adult Strabismus Questionnaire (AS-20) and the Intermittent Exotropia Questionnaire (IXTQ). Summary details of the instruments are shown in tables 2 and 3.
Table 2.
Summary of health-related quality of life (HRQoL) orthoptic-specific instruments
| Instrument | Item pool development |
Number of questions | Likert-type scale used | Domains or subscales (items) |
Mode of administration | Psychometrics |
|---|---|---|---|---|---|---|
| A&SQ | CB | 26 | 5-point | Fear of losing better eye (2) Distance estimation (10) Visual disorientation (3) Diplopia (4) Problems with social contact and cosmetic problems (4) |
Self | IC, DV, CV, CCV |
| ATI | CB, LB | 18 (atropine) 19 (patching) | 5-point 5-point | Adverse effects (8) Compliance (5) Social stigma (3) |
Parent | IC, CV |
| AS-20 | PB | 20 | 5-point | Psychosocial (10) Function (10) |
Self | IC, DV, CCV, |
| IXTQ | PB | 12 (proxy) 17 (parent) 12 (child aged 5–7 years) 12 (child aged 8–17 years) |
5-point 5-point 3-point 5-point |
In parent only Function (8) Psychosocial (7) Surgery (2) |
Parent, proxy or child | DV, IC, |
A&SQ, Amblyopia and Strabismus Questionnaire; AS-20, Adult Strabismus Questionnaire; ATI, Amblyopia Treatment Index; CB, clinician-based; CV, construct validity; CCV, concurrent validity; DV, discriminant validity; IC, internal consistency; IXTQ; Intermittent Exotropia Questionnaire; LB, literature-based; PB, patient-based; R, responsiveness; TRR, test–retest reliability.
The instruments identified all used factor analysis in their development. Factor analysis is a technique that can reduce the number of items (ie, questions) within an instrument, by identifying redundant items. It may also be used to assess the structure of a domain (ie, subgroup or theme, such as ‘cosmesis’ or ‘ability’) by grouping items together that are related to each other. Factor analysis can be thought of as a confirmatory tool to assess the structure of a developed instrument or measure.
Each instrument was shown to be reliable (in that internal consistency data was reported; see table 1 for details); however, no test–retest data was reported for any instrument. Test–retest data would add to the evidence of reliability for the instrument. Each instrument was also deemed to be valid; however, only the A&SQ and AS-20 were tested against another recognised HRQoL measure (the NEI-VFQ-25).
The A&SQ
The A&SQ was designed to assess the ‘decrease in quality of life’ of patients with amblyopia and strabismus.7 The instrument was designed by clinicians who listed problems that amblyopic and strabismic patients experience. These were categorised into five domains. All items in the questionnaire are measured on Likert-type rating scales, which are assumed to be linear and run from 0 to 100 (0 corresponding to the least favourable score and 100 corresponding to the most favourable score). The total A&SQ score is defined as the mean of all item scores.
The reliability of the A&SQ has been reported in terms of internal consistency.7 Validity has been reported in terms of discriminatory validity,7 concurrent validity7 and construct validity9 10 (see table 1). An English-language version has also been developed and has shown to be both reliable and valid.8
The ATI
The ATI was developed following consultation with specialists and parents of children with amblyopia. A list of 20 items was created and the initial questionnaire was issued to parents of children participating in the Amblyopia Treatment Study (a multi-centre randomised controlled clinical trial designed to compare atropine and conventional patching treatment). Slight modifications/rewording of some of the questions were made depending upon whether the child was undertaking conventional patching treatment or atropine therapy for the management of their amblyopia. The ATI now consists of 18-items, scored on a 5-part Likert-type scale. The overall ATI score is the mean of all the item scores. The reliability of the ATI has been reported in terms of internal consistency.11-13 The validity of the ATI has been reported in terms of construct validity12 and the structure of the instrument has also been investigated.11-13
The AS-20
The AS-20 is a patient-derived instrument for use in patients with a diagnosis of strabismus. Subjects with strabismus were interviewed and statements or phrases were used to generate a 181-item questionnaire. A number of items were subsequently removed as they were found to be not applicable. Of the remaining items, two subscales were identified that were applicable to the strabismic population (psychosocial and function), with each subscale containing 10 items. A 5-point Likert type scale is applied to each question. The overall AS-20 is given from 0 (worst HRQoL) to 100 (best HRQoL). The reliability of the AS-20, in terms of internal consistency, and validity, in terms of discriminative validity, has been reported.14 The instrument has also been validated in terms of concurrent validity against the VFQ-25, and was found to be more sensitive than the NEI-VFQ-25 in detecting reduced HRQoL in adult subjects with strabismus.15
The IXTQ
The IXTQ is a patient- and parent-derived instrument developed to measure the impact of intermittent exotropia upon HRQoL. Children and parents of children with intermittent exotropia were interviewed, and the results analysed to identify items and themes associated with the diagnosis.16 Three types of questionnaire were developed: child (addressing the child's QoL), proxy (parallel to the child questionnaire addressing parent's perception of the child's HRQoL) and parent (addressing the parent's own HRQoL). The child questionnaire was developed in two formats: 5–7 year olds, with a 3-point Likert-type scale; and 8–17 years, with a five-point Likert-type scale. The final child (both versions) and proxy questionnaires contain 12 items. The parent questionnaire consists of three subscales: function (eight items), psychosocial (seven items) and surgery (two items). The parent instrument is also measured on a 5-point Likert-type scale. The overall IXTQ score is calculated as the mean of all the item scores. The IXTQ has undergone initial validation, in terms of internal consistency reliability,17 with further validity testing planned.
Strabismus and amblyopia—should these issues be considered together in the same measure?
The presence of strabismus can be associated with three main HRQoL issues: poor cosmetic appearance, diplopia and loss of stereopsis. The psychosocial implications of strabismus have been well documented.21-24 The impact of amblyopia upon a person's HRQoL has not been as adequately explored. It is possible that the impact upon HRQoL may be related more to the treatment of amblyopia, rather than the condition itself. To investigate the impact of amblyopia upon HRQoL, it would be appropriate to use the ATI; this instrument was specifically designed to quantify the impact of amblyopia on the child and the family. On a similar note, to investigate the impact of strabismus alone on HRQoL, the AS-20 could be used. An argument against the A&SQ as a measure is that if decreased HRQoL was found, could the detriment to HRQoL be attributed to amblyopia or strabismus, or indeed both. While it may not be necessary to make such a differentiation, this disadvantage of the A&SQ as a HRQoL measure should be acknowledged.
Practical issues
All of the measures described are short instruments and as such can be easily administered, and can be considered as a low burden for the patient/parent (see table 2). Each instrument involves a 5-point Likert-type scale, with a 3-point scale used for the child-respondent version aged 5–7 years in the IXTQ measure. Only the IXTQ contains a child-respondent version. It has been recognised that proxy reporting of HRQoL can differ from self-reported HRQoL, particularly paediatric HRQoL.25 Parents have been shown to be more able to judge physical HRQoL components compared with emotional or social components.26 It is possible that the HRQoL implications of strabismus and amblyopia are different depending upon whether the child or adult perspective is taken. Ethnic differences in the impact of strabismus and/or amblyopia upon HRQoL have not been examined. The identified measures did not disclose their study populations in terms of ethnicity; the country of origin for the study is shown in table 3. While the items included in any of the developed measures may not have differed should a racially heterogeneous group have been used, the impact of strabismus and/or amblyopia upon different groups has not been fully investigated. Socio-economically deprived groups or ethnic minority groups may report different HRQoL implications of strabismus and/or amblyopia.
Table 3.
Summary of research results of published studies using orthoptic-specific health-related quality of life (HRQoL) instruments
| Reference | Sample | Description of study | Results |
|---|---|---|---|
| A&SQ | |||
| Van de Graaf et al (the Netherlands)7 | n=68 current amblyopic and strabismic patients n=53 healthy controls n=174 patients from historical cohort |
Clinical experts identified problems patients with strabismus and amblyopia experience Complaints categorised into themes (loss of depth perception; diplopia and visual disorientation; appearance) From the themes, five domains were developed (distance estimation; visual disorientation; fear of losing better eye; diplopia; appearance) Developed a 26-item questionnaire to be completed by the patient Tested on three groups (current amblyopic and strabismic patients; healthy controls; historical cohort) Also administered 12-item SF-12 and the VFQ-25 |
Average scores for SF-12, VFQ-25 and A&SQ were: 47.16, 93.66, 95.76 for controls; 47.13, 92.40, 83.31 for historical cohort; and 45.77, 79.16, 67.52 for patients, respectively. Cronbach's α=fear of losing better eye (0.93); distance estimation (0.76); visual disorientation (0.87); diplopia (0.78); social contact and cosmetic problems (0.83) |
| Van de Graaf et al (the Netherlands)9 | n=174 patients from historical cohort | Assessed clinical validity of A&SQ by correlating domains to orthoptic parameters of amblyopic patients treated 30–35 years previously | Current DRT of amblyopic eye correlated to four domains (p<0.01) (fear of losing better eye; distance estimation; diplopia; social contact and cosmetic problems) Current degree of binocular vision correlated to two domains (p<0.01) (distance estimation; social contact and cosmetic problems) |
| Felius et al (USA)8 | n=150 patients | Administered ASQE and compared with clinical outcomes of VA, diplopia and asthenopia; and disability questionnaire of six items on specific health, daily functioning, social interaction, concerns about the future, self-image, and job-related difficulties | ASQE correlated with disability questionnaire r=−0.76, p<0.001 Cronbach's α=fear of losing better eye (0.80); distance estimation (0.90); visual disorientation (0.82); diplopia (0.82); social contact and cosmetic problems (0.92) Spearman rank correlation coefficient of ASQE total score and level of unilateral acuity loss (−0.23, p<0.01); diplopia assessment (−0.29, p<0.001); and asthenopia assessment (−0.45, p<0.001) |
| Van de Graaf et al (the Netherlands)10 | n=72 current amblyopic and strabismic patients n=53 healthy controls n=173 patients from historical cohort |
Used factor analysis to compare correlations among 26-item responses | Determined that the hypothesised five domains of the A&SQ are valid. Report that the domain of distance estimation ought to be separated into two domains (near distance estimation and far distance estimation) resulting in an instrument that now consists of six domains |
| ATI | |||
| Cole et al (USA)11 | n=64 parents of patients participating in Amblyopia Treatment Study | 20-item questionnaire developed from clinical experience and literature evidence issued 4 weeks after treatment started | Used factor analysis to identify three treatment-related factors: adverse effects (five items), compliance (four items) and social stigma (two items) Internal consistency Cronbach's α=0.82 for adverse effects; 0.81 for compliance; 0.84 for social stigma |
| Pediatric Eye Disease Investigator Group (PEDIG) (USA)12 |
n=364 parents of patients participating in Amblyopia Treatment Study | 18-item questionnaire issued 5 weeks after treatment started | Overall internal consistency Cronbach's α=0.89 Used factor analysis to identify three treatment-related factors: adverse effects (eight items), compliance (five items) and social stigma (three items) Internal consistency Cronbach's α=0.86 for adverse effects; 0.86 for compliance; 0.75 for social stigma |
| Holmes et al (USA)13 | n=794 parents of patients participating in Amblyopia Treatment Study | 18-item questionnaire issued 5 weeks after treatment started | Overall internal consistency Cronbach's α=0.88 Used factor analysis to identify three treatment-related factors: adverse effects (eight items), compliance (five items) and social stigma (three items) Internal consistency Cronbach's α=0.85 for adverse effects; 0.85 for compliance; 0.71 for social stigma |
| AS-20 | |||
| Hatt et al (USA)14 | First phase n=29 strabismic patients Second phase n=32 strabismic patients, n=13 controls, n=18 other eye conditions |
First phase 181-item questionnaire issued. Subsequent removal of 132 items leaving 49 items for factor analysis Final questionnaire (20-items) issued in second phase, containing two domains (psychosocial and function) |
Overall Cronbach's α=0.94 (psychosocial 0.95, function 0.94) Significantly lower scores for strabismus patients (56) compared with controls (98; p<0.001) and patients with other eye conditions (88, p<0.001) |
| Hatt et al (USA)15 | n=84 strabismic patients | Completed AS-20 and VFQ-25 Compared results with normal VFQ-25 data and normal AS-20 data |
Strabismic patients scored below normal on AS-20 than VFQ-25 (90% cf 29%, p<0.0001). AS-20 detected subnormal HRQoL more than the VFQ-25 in subjects both with and without diplopia |
| IXTQ | |||
| Hatt et al (USA)17 | n=33 patients n=49 controls | Two child versions developed (5–7 yrs and 8–17 yrs); proxy version and parent version Questionnaires issued to patient and control groups |
Child questionnaire Cronbach's α=0.93 Proxy questionnaire Cronbach's α=0.97 Parent questionnaire overall Cronbach's α=0.92 (psychosocial=0.94, function=0.94, surgery=0.91) |
A&SQ, Amblyopia and Strabismus Questionnaire; AS-20, Adult Strabismus Questionnaire; ASQE, English version of the Amblyopia and Strabismus Questionnaire; ATI, Amblyopia Treatment Index; DRT, Dutch reading test; IXTQ, Intermittent Exotropia Questionnaire; SF-12, 12-item Short Form; VA, visual acuity; VFQ-25, 25-item Visual Function Questionnaire.
DISCUSSION
Within the field of treating patients with a diagnosis of amblyopia and/or strabismus, the application of HRQoL measures is a relatively new area. However, the need for such instruments has become recognised, with the development of four new measures over recent years. These instruments are important in that they address the specific HRQoL issues that patients with amblyopia and/or strabismus face, issues that would not be detectable using generic HRQoL instruments, such as the Health Utilities Index27 (HUI) or the Euroqol Quality of Life questionnaire28 (EQ-5D). HRQoL instruments in orthoptics (the speciality of investigating and managing patients with binocular disorders) are likely to determine the impact of amblyopia and/or strabismus, such as the A&SQ,7-10 ATI11-13 and AS-20.14 15 The IXTQ16 17 also addresses specific issues that patients with intermittent exotropia experience. It is possible that other strabismic conditions have their own specific issues that impact upon HRQoL, and to that end other condition-specific measures may be required to be developed in the future. Further research on subgroups of patients is necessary to determine whether this is the case.
The appropriateness of condition- or disease-specific measures in determining HRQoL is a contentious issue. While it is acknowledged that generic measures are unlikely to be sensitive enough to detect the implications of vision-loss, poor ocular alignment or diplopia, the question as to which of the condition-specific measures should be taken as the ‘gold standard’ has yet to be answered. Further studies are required to compare the appropriateness of the AS-20, A&SQ, ATI and (if appropriate) the IXTQ against each other and a well-validated comparator (such as the VFQ-25). Further research is also required to investigate any differences between self-reported HRQoL and proxy-reported HRQoL in orthoptic patients, specifically the differences between parental and self-reported HRQoL components. It is possible that the HRQoL implications of strabismus and/or amblyopia are not the same for both adult and child patients. Should this be the case then amendments to existing measures or development of new measures are necessary in order to quantify the HRQoL implications of strabismus and/or amblyopia for children. Exploration of the impact of ethnicity upon HRQoL in orthoptic patients is also required.
The use of HRQoL instruments need not be confined to clinical trials or research. Their application within everyday clinical practice should also be encouraged. The information provided from an HRQoL measure may assist in the clinical decision-making process of which treatment to prescribe and when. All of the questionnaires described can be used without licence, and therefore do not add any significant financial burden to the patient's investigation, aside from the extra time taken to administer the instrument. Their inclusion in patient investigations can be considered justified as another tool to facilitate the implementation of evidence-based practice.
Acknowledgments
Funding
This work was produced by JC under the terms of Personal Development Award research training fellowship issued by the NIHR. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, The National Institute for Health Research or the Department of Health.
Footnotes
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Schumacher M, Olschewski M, Schulgen G. Assessment of quality of life in clinical trials. Stat Med. 1991;10:1915–30. doi: 10.1002/sim.4780101207. [DOI] [PubMed] [Google Scholar]
- 2.Mangione CM, Berry S, Spritzer K, et al. Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 1998;116:227–33. doi: 10.1001/archopht.116.2.227. [DOI] [PubMed] [Google Scholar]
- 3.Mangione CM, Lee PP, Pitts J, et al. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ) Arch Ophthalmol. 1998;116:1496–504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 4.Mangione CM, Lee PP, Gutierrez P, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 5.Keeffe JE, McCarty CA, Hassell BA, et al. Description and measurement of handicap caused by vision impairment. Aust N Z J Ophthalmol. 1999;27:184–6. doi: 10.1046/j.1440-1606.1999.00179.x. [DOI] [PubMed] [Google Scholar]
- 6.Mangione CM, Phillips RS, Seddon JM, et al. Development of the activities of daily vision scale. Med Care. 1992;30:1111–26. doi: 10.1097/00005650-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 7.van de Graaf ES, van der Sterre GW, Polling JR, et al. Amblyopia and strabismus questionnaire: design and initial validation. Strabismus. 2004;12:181–93. doi: 10.1080/09273970490491196. [DOI] [PubMed] [Google Scholar]
- 8.Felius J, Beauchamp GR, Stager DR, Sr, et al. The amblyopia and strabismus questionnaire: English translation, validation, and subscales. Am J Ophthalmol. 2007;143:305–10. doi: 10.1016/j.ajo.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 9.van de Graaf ES, van der Sterre GW, van Kempen-du SH, et al. Amblyopia and strabismus questionnaire (A&SQ): clinical validation in a historic cohort. Graefes Arch Clin Exp Ophthalmol. 2007;245:1589–95. doi: 10.1007/s00417-007-0594-5. [DOI] [PubMed] [Google Scholar]
- 10.van de Graaf ES, Felius J, van Kempen-du SH, et al. Construct validation of the amblyopia and strabismus questionnaire (A&SQ) by factor analysis. Graefes Arch Clin Exp Ophthalmol. 2009;247:1263–8. doi: 10.1007/s00417-009-1112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole SR, Beck RW, Moke PS, et al. The amblyopia treatment index. J AAPOS. 2001;5:250–4. doi: 10.1067/mpa.2001.117097. [DOI] [PubMed] [Google Scholar]
- 12.Pediatric Eye Disease Investigator Group Impact of patching and atropine treatment on the child and family in the amblyopia treatment study. Arch Ophthalmol. 2003;121:1625–32. doi: 10.1001/archopht.121.11.1625. [DOI] [PubMed] [Google Scholar]
- 13.Holmes JM, Strauber S, Quinn GE, et al. Further validation of the amblyopia treatment index parental questionnaire. J AAPOS. 2008;12:581–4. doi: 10.1016/j.jaapos.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatt SR, Leske DA, Bradley EA, et al. Development of a quality-of-life questionnaire for adults with strabismus. Ophthalmology. 2009;116:139–44. doi: 10.1016/j.ophtha.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatt SR, Leske DA, Bradley EA, et al. Comparison of quality-of-life instruments in adults with strabismus. Am J Ophthalmol. 2009;148:558–62. doi: 10.1016/j.ajo.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatt SR, Leske DA, Adams WE, et al. Quality of life in intermittent exotropia: child and parent concerns. Arch Ophthalmol. 2008;126:1525–9. doi: 10.1001/archopht.126.11.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatt SR, Leske DA, Yamada T, et al. Development and initial validation of quality-of-life questionnaires for intermittent exotropia. Ophthalmology. 2010;117:163–8. doi: 10.1016/j.ophtha.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. doi:10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brazier JE, Ratcliffe J, Salomon JA, et al. Methods for obtaining health state values: generic preference-based measures of health and the alternatives. Measuring and valuing health benefits for economic evaluation. 1st edn Oxford University Press; Oxford: 2007. pp. p175–256. [Google Scholar]
- 20.Margolis MK, Coyne K, Kennedy-Martin T, et al. Vision-specific instruments for the assessment of health-related quality of life and visual functioning. Pharmacoeconomics. 2002;20:791–812. doi: 10.2165/00019053-200220120-00001. [DOI] [PubMed] [Google Scholar]
- 21.Satterfield D, Keltner JL, Morrison TL. Psychosocial aspects of strabismus study. Arch Ophthalmol. 1993;111:1100–5. doi: 10.1001/archopht.1993.01090080096024. [DOI] [PubMed] [Google Scholar]
- 22.Menon V, Saha J, Tanson R, et al. Study of the psychosocial aspects of strabismus. J Pediatr Ophthalmol Strabismus. 2002;39:203–8. doi: 10.3928/0191-3913-20020701-07. [DOI] [PubMed] [Google Scholar]
- 23.Olitsky SE, Sudesh S, Graziano A, et al. The negative psychosocial impact of strabismus in adults. J AAPOS. 1999;3:209–11. doi: 10.1016/s1091-8531(99)70004-2. [DOI] [PubMed] [Google Scholar]
- 24.Archer SM, Musch DC, Wren PA, et al. Social and emotional impact of strabismus surgery on quality of life in children. J AAPOS. 2005;9:148–51. doi: 10.1016/j.jaapos.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Matza LS, Swensen AR, Flood EM, et al. Assessment of health-related quality of life in children: a review of conceptual, methodological, and regulatory issues. Value Health. 2004;7:79–92. doi: 10.1111/j.1524-4733.2004.71273.x. [DOI] [PubMed] [Google Scholar]
- 26.Eiser C, Morse R. Can parents rate their child's health-related quality of life? Results of a systematic review. Qual Life Res. 2001;10:347–57. doi: 10.1023/a:1012253723272. [DOI] [PubMed] [Google Scholar]
- 27.Horsman J, Furlong W, Feeny D, et al. The health utilities index (HUI®): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. doi: 10.1186/1477-7525-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Euroqol Group Euroqol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;1:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]