Abstract
Under physiological conditions, cells receive fate-determining signals from their tissue surroundings, primarily in the form of polypeptide growth factors. Integration of these extracellular signals underlies tissue homeostasis. Although departure from homeostasis and tumor initiation are instigated by oncogenic mutations rather than by growth factors, the latter are the major regulators of all subsequent steps of tumor progression, namely clonal expansion, invasion across tissue barriers, angiogenesis, and colonization of distant niches. Here, we discuss the relevant growth factor families, their roles in tumor biology, as well as the respective downstream signaling pathways. Importantly, cancer-associated activating mutations that impinge on these pathways often relieve, in part, the reliance of tumors on growth factors. On the other hand, growth factors are frequently involved in evolvement of resistance to therapeutic regimens, which extends the roles for polypeptide factors to very late phases of tumor progression and offers opportunities for cancer therapy.
One of the first lines of evidence associating cancer with soluble growth factors (GFs) emerged from studies performed in the 1950s, in the laboratory of Victor Hamburger, by two fellows, Rita Levi-Montalcini and Stanley Cohen (23). When studying mechanisms enabling limb innervation in chick embryos, they grafted a lump of a mouse sarcoma onto an embryo and observed more extensive attraction of nerve fibers to the lump. They later found that snake venom and the murine submaxillary gland secrete a similarly active “nerve-stimulating factor,” which instigated the isolation of the first two GFs: nerve growth factor (NGF) and epidermal growth factor (EGF). Two decades later, Cohen and Todaro reported that cells infected by the feline sarcoma virus lost their ability to bind EGF (99). This observation led to the isolation from a murine sarcoma of two “transforming growth factors,” TGF-α and TGF-β (81). Subsequent studies revealed that not only virally transformed cells but also chemically transformed cells, as well as cells derived from human tumors, often secrete GFs, which are responsible for self-stimulation (autocrine) of growth. In support of autocrine theories, Waterfield and colleagues reported in 1983 that the transforming gene of the simian sarcoma virus is structurally related to the platelet-derived growth factor (PDGF) (103). In 1984 Waterfield provided yet another link between GFs and cancer: partial sequencing of the EGF-receptor (EGFR/ErbB-1) uncovered homology to another oncogene, the erbB gene of the avian erythroblastosis virus (27). Subsequent molecular cloning of EGFR by Ullrich and colleagues (100) boosted the understanding of the intracellular mechanisms of GF action: Like EGFR, the majority of GF receptors are single-pass transmembrane proteins harboring an intracellular tyrosine kinase domain (108) (a serine/threonine kinase in the case of TGF-β receptors). A similarly important development impacted our current understanding at the tissue level: similar to their roles in embryogenesis, GFs are the short-range mediators of the interplay between tumors and both the extracellular matrix (60) and stromal, non-cancer cells, such as myofibroblasts, macrophages, and endothelial cells. This cross talk underlies processes fundamental to tumor progression, such as sprouting of blood vessels (33) and local inflammatory responses. In this review, we highlight the multiplicity and importance of GFs for the stepwise progression of epithelial tumors, as well as discuss their burgeoning relevance to cancer therapy.
Cancer Initiation: Roles of Genetic Aberrations
The progressive transformation of normal cells into highly malignant derivatives entails accumulation of a number of genetic changes. Germ-line mutations, such as the loss of tumor suppressor functions and the induction of oncogene functions (30, 91), facilitate somatic mutations because they often fail DNA repair. The somatic mutations encompass single base mutations, inter- and intrachromosomal rearrangements, as well as copy number changes. Cancer cells may also acquire new DNA sequences from viruses (97), and somatic mutations in the mitochondrial genome have also been reported in tumors (17). Among somatic mutations, a fine line has to be drawn between “passenger mutations” and “driver mutations.” Passenger mutations are often found within cancer genomes but are not directly involved in oncogenesis and do not confer growth advantage (95). By contrast, a driver mutation confers considerable survival and growth advantage to the cancer cell and can drive clonal expansion. The number of driver mutations per a common adult epithelial cancer is estimated as five or more (7), but fewer events are required in hematological cancers.
In total, ~1.6% of the 22,000 functional genes in the human genome show recurrent somatic mutations in cancer (35). Yet, it is important to realize that there are many more genes than pathways, and driver mutations rarely impinge on more than one gene in a pathway. For example, some gene families, in particular protein kinase cascades placed downstream of GF receptors, are often mutated in tumors such as melanomas (B-RAF), pancreatic (RAS), breast (ErbB-2/HER2), and brain cancer (EGFR), but co-existence of such mutations is very rare. And although some tumors are characterized by enhanced secretion of GFs and chemotactic cytokines (e.g., EGF-like factors and IL-8 secreted by pancreatic tumors), driver mutations directly affecting GF genes are relatively rare. One example entails a PDGF-collagen fusion protein of dermatofibrosarcoma protuberans (39, 88).
Processes Enabling Growth Factors to Support Tumor Progression
Once initiated by driver mutations, premalignant epithelial cells may accumulate additional oncogenic mutations, but their expansion and progression to metastatic carcinomas depend on a multi-step process orchestrated primarily by GFs (FIGURE 1). GFs are compact polypeptides, which bind to transmembrane receptors harboring kinase activity, to stimulate specific combinations of intracellular signaling pathways, such as the mitogen-activated protein kinase (MAPK), the phosphatidylinositol 3-kinase (PI3K), phospholipase C-γ, and transcription factors like the signal transducers and activators of transcription (STATs) or SMAD proteins (FIGURE 2). These modules of cellular activation and the respective GFs are co-opted in several phases of tumor progression.
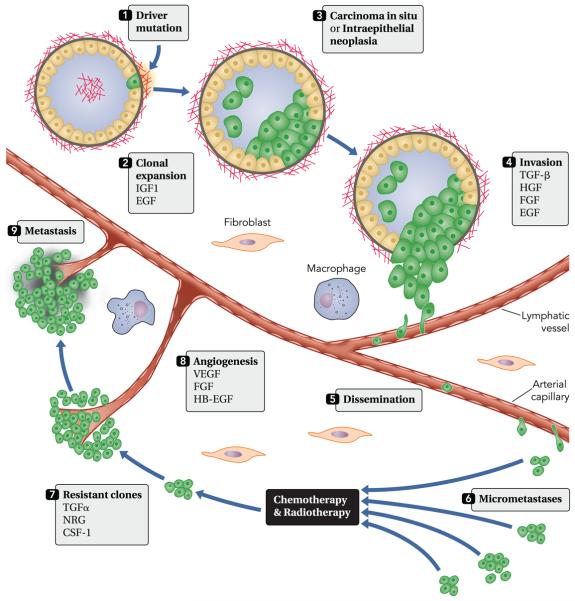
FIGURE 1. The stepwise progression of cancer and roles for growth factors.
The process is instigated by a somatic mutation, which confers considerable survival and growth advantages to the initiated cell (1). GFs like EGF and IGF1 support the consequent expansion of mutation-bearing clones (2), often leading to intraluminal lesions (3), such as carcinoma in situ or intraepithelial neoplasia, which are surrounded by the basal membrane. Invasion (4) refers to the migration and penetration by cancer cells into neighboring tissues. This process involves loss of epithelial polarity, acquisition of a motile, mesenchymal-like phenotype, and secretion of proteases. Both oncogenes and tumor suppressors, along with a large group of GFs, control this critical phase of tumor development. Cancer cells enter (extravasation) and exit (intravasation) lymphatic and blood vessels to disseminate (5) and metastasize to distant organs. Extra- and intravasation entail the supporting functions of macrophages, platelets, and endothelial cells. The resulting micrometastases (6) usually display sensitivity to chemotherapy and radiotherapy. However, the acquisition of new mutations and the ability of cancer cells to produce GFs (autocrine loops) propel the outgrowth of resistant clones (7). Angiogenesis (8) is essential for the establishment of secondary tumors larger than 1 ml. Both sprouting of existing vessels and recruitment of bone marrow-derived endothelial progenitor cells are stimulated by GFs secreted by tumor and stromal cells. In the final phase, relatively large metastases (9) populate a distinct set of target organs. Note that a latency period of several years may precede this final phase. CSF-1, colony stimulating factor 1; EGF, epidermal growth factor; FGF, fibroblasts growth factor; HB-EGF, heparin-binding EGF; NRG, neuregulin; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
FIGURE 2. Major signaling pathways stimulated by growth factors and their receptors.
The major signaling pathways stimulated by receptor tyrosine kinases (RTKs) and TGF-β receptors are shown schematically. Note that TGF-β can stimulate both the canonical pathway, and non-canonical pathways (not shown), including some components which are listed here under RTK. Activation of the phospholipid PI3K pathway may be achieved by binding of the regulatory p85 subunit to an activated RTK or through the activation of the small GTP-binding protein RAS. On activation, PI3K induces formation of phosphatidylinositol(3,4,5)-phosphate (PIP3), which serves as a docking site for proteins containing phospholipid-binding domains (e.g., PH domains), including protein kinase B and AKT. Recruitment of AKT to the membrane enables its phosphorylation on two stimulatory residues; one of these phosphorylation events (threonine-308) is mediated by PDK, whereas the other (serine-473) is mediated by mTorC2. PTEN and INPP4B are phophatases that abolish AKT activation and act as tumor suppressors. AKT has a large number of substrates that regulate mainly survival and metabolism. One example is the proapoptotic protein BAD, which is inhibited following phosphorylation by AKT. BAD induces permeabilization of the mitochondrial membrane to enable release of cytochrome C. The latter is involved in the generation of a protein complex known as the apoptosome, which cleaves pro-caspases to generate the active form of caspase-9. The ERK pathway is a multiple layer kinase cascade, in which the most distal MAPK elements are activated on tyrosine/threonine phosphorylation. In the ERK1/2 kinase pathway, binding of a ligand to an RTK enables auto-phosphorylation and recruitment of adaptors like GRB2 and SHC. The adaptors mediate recruitment of a GTP/GDP exchange protein, SOS, which loads GTP onto RAS. Active, GTP-loaded RAS molecules stimulate RAF kinases, which instigate the cascade leading to MEK (MAPK kinase) and then to ERK activation. Active ERK molecules translocate to the nucleus to stimulate multiple transcription programs essential for cell cycle progression and cell migration. The phopholipase Cγ-protein kinase C (PKC) pathway is instigated on recruitment of PLCγ to ligand-bound receptors. The activated enzyme hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to form two second messengers, diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). Binding of IP3 to receptors on the membrane of the endoplasmic reticulum leads to Ca2+ release. Free cytosolic calcium ions, together with DAG, activate members of the protein kinase C (PKC) family, resulting in the phosphorylation of various effector proteins. In addition, cytosolic Ca2+ activates the calcium/calmodulin-dependent protein kinases and phosphatases. The SMAD signaling pathway is the canonical signaling pathway of TGF-β family members. TGF-β dimers bind to a type II receptor, which recruits and phosphorylates a type I receptor. The type I receptor then recruits and phosphorylates a receptor-regulated SMAD (R-SMAD). R-SMAD then binds to the common SMAD (SMAD4) to form a heterodimeric complex. This complex then translocates into the nucleus, where it acts as a transcription factor.
Autonomous cell growth and clonal expansion
GFs are essential for clonal expansion, which permits fixation of oncogenic mutations, as well as increases the pool of initiated cells susceptible to additional mutations. Unlike the paracrine (heterotypic) mode of action of GFs that dominates physiological processes like embryogenesis and wound healing, many cancer cells acquire the ability to synthesize GFs to which they are responsive (93). Along with such autocrine loops, several distinct mechanisms may lead to constitutive pathway activation in tumors. At the receptor level, overexpression may enable cancer cells to become hyper-responsive to GFs (e.g., EGFR in head and neck cancer, and ErbB-2/HER2 in breast cancer), whereas specific mutations or deletions can elicit ligand-independent signaling (e.g., brain tumor mutants of EGFR) (26, 45). Mutations affecting downstream mediators may similarly confer growth autonomy. For instance, RAS mutations characterize up to 25% of human cancers, and mutational inactivation of a TGF-β effector, SMAD4/DPC4, is abundant in pancreatic tumors.
Accelerated intraepithelial proliferation
Morphologically recognizable, putative precursor lesions to cancer have been described, for example in breast [ductal carcinoma in situ (DCIS)] and in prostate cancer [prostate intraepithelial neoplasia (PIN) (28)]. An important feature of intraepithelial lesions is the integrity of the surrounding basement membrane. Intraluminal cell proliferation, along with disruption of epithelial polarity and aneuploidy, commonly associates with molecular markers consistent with aberrant GF and hormone signaling. Thus ErbB-2/HER2, a close relative of ErbB-1/EGFR, is overexpressed in 40% of DCIS and up to 70% of the high-grade group (64). Likewise, ~20% of high-grade PIN lesions harbor a TMPRSS2-ERG fusion gene (15), which is a common abnormality detectable in up to 50% of prostate cancers. ERG belongs to the ETS family of oncogenic transcription factors, which undergo activation on stimulation of GF receptors and phosphorylation by several MAPKs.
GF-induced basement membrane breakdown and invasive growth
Tumor progression is licensed only when the basement membrane decorating secretory ducts undergoes dissolution. GFs play critical roles in basement membrane disruption, penetration by cancer cells into neighboring tissues, the vascular or lymphatic systems (intravasation), as well as their departure from the bloodstream (extravasation) and subsequent colonization of distant organs (109). The conversion of tightly packed polarized epithelial cells into motile individual cells, denoted epithelial-mesenchymal transition (EMT), heralds tumor spread (FIGURE 3). Autocrine GFs and genetic aberrations that mimic GF binding, in which case GF binding becomes needless, leading to constitutive signaling via the tyrosine kinase domain, may provide the second hits that propel EMT and eruption of intracellular lesions. For example, studies performed with clinical specimens and ErbB-2/HER2-overexpressing spheroids resembling mammary DCIS raised the possibility that TGF-β (85), or overexpression of the adaptor 14-3-3-zeta (69), underlay invasive growth. Based primarily on in vitro models, it appears that TGF-β and other growth factors implicated in such transitions (e.g., the hepatocyte and the fibroblast growth factors; HGF and FGFs) enhance the invasive potential of cancer cells by upregulating secreted proteases (e.g., the matrix metalloproteinases MMP-2 and MMP-9) and down-regulating protease inhibitors (94, 104). In parallel, GFs induce several molecular switches of adhesion, such as the loss of the epithelial E-cadherin and gain of the mesenchymal N-cadherin (71). Another molecular switch replaces tensin-3, linker of the actin cytoskeleton and the extracellular matrix, with tensin-4 (also called cten for c-terminal tensin-like protein), thereby disrupting the bridge and enhancing cell migration (54). In the majority of epithelial cancers, the dual growth arrest and adhesive functions of E-cadherin are lost through mutational inactivation, transcriptional repression, or proteolysis of the extracellular cadherin domain. A variety of GFs, including TGF-β and EGF, as well as cleaved Notch proteins, induce the expression of potent E-cadherin repressors (e.g., ZEB-2 and Twist). On the other hand, cleavage, ubiquitylation, and endocytosis of E-cadherin are among the inducible means that downregulate E-cadherin (34).
FIGURE 3. Molecules associated with the epithelial-mesenchymal transition (EMT).
EMT involves the functional transition of polarized epithelial cells into apolar and motile cells, which resemble fibroblasts and secrete extra-cellular matrix (ECM) components. Commonly used epithelial and mesenchymal cell markers are listed. Note that intermediate phenotype representing transition states occur in tissues. Cten, COOH-terminal tensin-like molecule; FOXC2, forkhead box C2; FSP-1, fibroblast-specific protein 1; LEF-1, lymphoid enhance factor 1; miR, microRNA; MUC1, mucin 1; ZEB-2, E-box zinc-binding protein; ZO-1, zona occludens 1.
Intravasation, extravasation, and dissemination
Paracrine interactions among cancer cells, macrophages, and endothelial cells critically facilitate intravasation of post-EMT cells. For example, mammary cancer cells secrete the colony-stimulating factor 1 (CSF-1), which attracts macrophages to tumors and increases local secretion of macrophage-derived GFs (37). Because macrophages are often found in close proximity to microvessels, this self-stimulatory loop enhances intravasation. The hypoxic and inflammatory conditions that occur during tumor progression increase secretion of TGF-β by macrophages. TGF-β-mediated induction of angiopoietin-like 4 (ANGPTL4) in breast cancer cells enables retention of tumor cells in the lungs, because ANGPTL4 enhances the permeability of lung capillaries and facilitates the trans-endothelial passage of tumor cells (76). Once in the circulation, tumor cells adhere to platelets, especially on stimulation by thrombin, thereby gaining mitogenic stimuli (e.g., PDGF and lysophosphatidic acid) and protection from natural killer cells (72). Another stromal source of pro-metastasis cytokines are cancer-associated fibroblasts (CAFs) (75). Animal studies demonstrated that CAFs co-injected with pancreatic tumor cells increased their metastasis (47), and mesenchymal stem cells co-injected with mammary cells similarly promoted metastasis via a paracrine loop involving the chemokine CCL5/RANTES (53). In line with clinical significance, a gene expression signature based on laser microdissection of the stroma reported prognostic value of angiogenic, immune, and hypoxic responses (31). In conclusion, paracrine loops involving stromal myeloid, endothelial, and mesenchymal cells complement the autocrine mechanisms of cancer cell stimulation.
GF-induced evasion from cytotoxic therapies
Some tumor types display 20-year or longer dormancy period (the time between primary tumor diagnosis and detectable metastatic outgrowth), thought to reflect adaptation of disseminated tumor cells to the new microenvironment. In the clinical setting of patients heavily treated with cytotoxic drugs, survival is a formidable challenge for micrometastases. Intrinsic resistance to anti-proliferative and death signals (e.g., TRAIL) is a well studied hallmark of cancer, which often involves the retinoblastoma protein (pRb) and E2Fs, transcription factors that control gene expression essential for cell proliferation (43). Resistance to apoptosis can also be acquired by cancer cells through the loss of a proapoptotic regulator, as is the case when the p53 tumor suppressor gene is mutated. In addition, the PI3K-AKT pathway, which transduces antiapoptotic signals, is often involved in weakening therapy-induced apoptosis in human tumors. This antiapoptotic survival pathway likely underlies the extensive involvement of GFs like IGF-1, as well as several EGF-like ligands, in evasion from apoptosis. As will be discussed below, blocking GF-mediated evasion from cell death bears clinical implications. Early studies indicated that anti-EGFR antibodies can sensitize tumors to chemotherapy, probably by blocking apoptosis evasion (1). This finding was later translated to combination therapies of colorectal and head and neck cancer patients (21).
GF-induced angiogenesis
The generation of new vessels is critical for tumor growth beyond few millimeters of size. New vessels are formed either by mature endothelial cells (angiogenesis) or by bone marrow-derived endothelial progenitor cells (EPCs), which are home to foci of angiogenesis (vasculogenesis) (4). Growth factors like the vascular endothelial growth factors (VEGFs), FGFs, and TGF-β play important roles in both vasculogenesis and angiogenesis, and VEGF antagonists limit angiogenesis in animals and in patients. EPCs regulate the angiogenic switch through paracrine secretion of proangiogenic GFs as well as by direct luminal incorporation into sprouting nascent vessels. Mobilization of circulating progenitors and other endothelial cells to tumors emerges as a critical step in tumor progression (62). For example, CAFs promote neoangiogenesis by recruiting EPCs into tumors, an effect mediated in part by their ability to secrete stromal cell-derived factor 1 (SDF-1) (74). Furthermore, Kerbel and associates reported that cancer therapy, including high-dose chemotherapy, could induce mobilization of EPCs to the viable rim of tumors (86).
Growth Factor Families Involved in Tumor Progression
Neuregulins and the EGF family
The family comprises eleven polypeptides sharing a conserved EGF domain (see Table 1). All EGF family members, like many other GFs, such as HGFs, are derived from membrane-bound precursor proteins. For instance, processing of the membrane-anchored Spitz, an EGF homolog in Drosophila, is essential for neurogenesis. Another example, HGF is inactive until activation is caused by a proteolytic cleavage in the single-chain HGF precursor, generating an active two-chain heterodimeric form. The activation of HGF in the extracellular milieu is a critical limiting step in HGF-induced signaling and controls bioavailability that is believed to have important roles in invasive growth of tumor cells and regeneration of injured tissues. All EGF family members bind to a group of four receptor tyrosine kinases, namely ErbB-1/EGFR through -4 (also called HER1–4). Like other tyrosine kinases, each ErbB molecule comprises an extracellular domain to allow ligand binding, a single transmembrane part, and an intracellular protein tyrosine kinase domain. It is important noting that not all ErbB receptors act autonomously: ErbB-2 (also called HER2 and Neu) binds no known EGF-like ligand (56), and ErbB-3 shows no tyrosine kinase activity (40). Hence, their activity is restricted to the formation of heterodimers with other ErbB receptors. Nevertheless, because heterodimers evade negative regulation, their signaling is stronger and longer than signals transmitted by the corresponding homodimers (107). Under normal conditions, the ErbB receptors are folded such that dimerization is prevented (14, 18). Only on ligand binding does the conformation of the extracellular domain change to expose a dimerization loop in a way that allows dimerization. What follows is the juxtaposition of the tyrosine kinase domain in a head-to-tail configuration that removes and transphosphorylates an inhibitory carboxyl terminal tail (50, 112). Phosphorylation of the tail leads to the recruitment of downstream signaling proteins to initiate dimer-specific signaling and transcription cascades (FIGURE 2). Translocation of receptor fragments (70), or full molecules (61), to the nucleus may directly regulate transcriptional programs.
Table 1.
Growth factors in normal physiology and cancer
| GF Family | GF Receptors |
Origins/ Targets |
Physiologcal Roles/Selected Examples of Gene Knockout (KO) Animals, Including Conditional KO |
|---|---|---|---|
| EGF | ErbB-1 (EGFR) ErbB-2 (HER2) ErbB-3 ErbB-4 |
Origins: Macrophages Monocytes Epithelial cells Neural cells Tumor cells Targets: Epithelial, Endothelial, Neural cells |
Cell proliferation, organ development, tissue repair ErbB-1 KO: defects in brain, cell proliferation, migration, differentiation of epithelial cells, e.g. skin, lung, intestine, placenta ErbB-2 KO: defects in muscle spindle, myoblast cell survival, cardiac phenotype (trabeculae formation) ErbB-3 KO: defects in ductal morphogenesis, mammary glands, early cardiac valve formation ErbB-4 KO: defects in neural development, mammary glands TGFα KO: defects in skin, coat, nails (waved phenotype), cardiovascular defects, defects in vision, eye, vibrissae, muscle, adiposis EGF KO: defects in digestive functions, skin, nails, vision, eye, reproductive, immune defects NRG1 KO: prenatal-perinatal lethality, defects in muscle, nervous system, cardiovascular defects NRG2 KO: postnatal lethality, defects in growth, size, reproductive defects |
| EGF | |||
| TGFα | |||
| NRG 1-4 | |||
| Amphiregulin | |||
| Betacellulin | |||
| Epiregulin | |||
| HB-EGF | |||
| Epigen | |||
| IGF | IGF1R IGF2R Insulin receptor |
Origins: Liver Tumor cells Targets: Many cell types/tissues Tumor cells |
IGF1: childhood, pubertal growth, produced throughout life time, anabolic effects Growth HormoneR KO and transgenics: defects in neuroendocrine, reproductive functions IGF1 KO: defects in growth, size, liver, biliary ducts, nervous system, homeostasis, inner ear maturation, cardiovascular defects, adipose IGF2 KO: prenatal-perinatal lethality, defects in embryogenesis, growth, size, skeleton, vision, eye, homeostasis, limbs, digits, tail, liver, biliary ducts, muscle, skeleton, cellular, cardiovascular, endocrine, exocrine, renal and urinary defects |
| IGF1 | |||
| IGF2 | |||
| TGF-β | TGF-βR1 TGF-βR2 |
Origins: Platelets Bone Several cell types Targets: Fibroblasts Endothelial cells Keratinocytes Lymphocytes Monocytes |
TGF-β: ECM formation, fibroblast activity, chemotaxis TGF-βR1 KO: embryonic lethal TGF-βR2 KO: embryonic lethal, defects in yolk sac hematopoiesis, vasculogenesis SMAD5 KO: embryonic lethal, defects in vascular, cardiac, craniofacial, heart development, ventral closure SMAD4 KO: embryonic lethal, heterozygous KO: duodenal polyps similar to human polyps TGF-β1 KO: immune, digestive, alimentary, renal, respiratory, endocrine, exocrine, hematopoietic, urinary defects, defects in growth, size, liver, biliary ducts, homeostasis, life span, aging TGF-β2 KO: prenatal-perinatal lethality, cardiovascular, endocrine, exocrine, immune, reproductive, respiratory hearing, vestibular, ear, renal, urinary, digestive, alimentary defects, defects in homeostasis, skeleton, vision, eye, nervous system, muscle, limbs, digits, tail, growth, size, craniofacial, embryogenesis |
| TGF-β1–3 | |||
| BMPs | |||
| Activins | |||
| VEGF | VEGFR1 (Flt-1) VEGFR2 (Flk-1, KDR) VEGFR3 (Flt-4) |
Origins: Endothelial cells Tumor cells Targets: Vascular endothelium Macrophages Monocytes |
Angiogenesis, blood flow, endothelial cell proliferation, enhanced chemotaxis/ homing of vascular precursor cells, recruitment of bone marrow progenitors VEGFA KO: prenatal-perinatal lethality, defects in life span, aging, growth, size, behavior, muscle, nervous system, skeleton, skin, coat, nails, cardiovascular and reproductive functions VEGFB KO: defects in vision, eye, homeostasis, cardiovascular function VEGFC KO: prenatal-perinatal lethality, defects in immune, cardiovascular functions |
| VEGFA (=VEGF) | |||
| VEGFB | |||
| VEGFC | |||
| VEGFD | |||
| PlGF | |||
| HGF | HGFR Met Ron |
Origins: Mesenchymal and tumor cells Targets: Endothelial Epithelial cells |
Development, homeostasis, regeneration, cellular growth, motility, morphogenesis, organ development, regeneration, wound healing Matrix invasion in angiogenesis HGFR KO: embryonic lethal, defects in development of placenta, liver, skeletal muscle HGF KO: prenatal-perinatal lethality, defects in embryogenesis, growth, size, liver, biliary ducts, hematopoietic, cardiovascular defects |
| HGF | |||
| MSP | |||
| FGF | FGFR1–4 | Origins: Monocytes, Macrophages Endothelial cells Targets: Endothelium Fibroblasts Keratinocytes |
Proliferation of endothelial cells, keratinocytes, fibroblasts, chemotaxis, mesoderm induction, limb formation, brain development, angiogenesis, keratinocyte organization, wound healing FGF KO: embryonic/early postnatal lethality FGF signaling disorders in humans: hereditary diseases, cancer Epidermal FGFR2b KO: increased sensitivity to chemical carcinogenesis, induction of EMT FGF1 KO: nervous system, hematopoietic, homeostasis FGF2 KO: cardiovascular, hematopoietic, muscle, nervous system, behavior FGF3 KO: postnatal lethality, limbs/digits/tail, hearing, behavior FGF10 KO: prenatal-perinatal lethality, defects in embryogenesis, skeleton, digits, tail, vision, eye, respiratory, reproductive, cardiovascular, digestive, alimentary, renal and urinary defects FGF12 or 14 or 17 KO: behavior, nervous system FGF15 or 16 KO: prenatal-perinatal-postnatal lethality, cardiovascular defects |
| FGF1 (acidic) | |||
| FGF2 (basic) | |||
| (22 members in human) | |||
| PDGF | PDGFRα PDGFRβ |
Origins: Platelets Macrophages Neutrophils Smooth muscle Pericytes Fibroblasts Targets: Fibroblasts Smooth muscle |
Proliferation of smooth muscle cells, fibroblasts, blood vessel formation, smooth muscle cell recruitment, chemotaxis, role in connective tissues, endothelial cells, regulation of vessel growth, pericyte recruitment PDGFA KO: prenatal-perinatal-postnatal lethality, lung emphysema, defects in development of lung alveoli, alveolar smooth muscle cell progenitors, growth, size, cardiovascular defects PDGFB KO: prenatal-perinatal lethality, cardiovascular defects, defects in kidneys, development of smooth muscle cells of blood vessel, bleedings during birth PDGFC KO: prenatal-perinatal-postnatal lethality, perinatal death due to feeding defects, homeostasis, respiratory difficulties, skeleton, skin, coat, nails, digestive, alimentary defects PDGFRβ KO: pericyte deficient mice, vessel dilation, leakage, rupture |
| PDGFA/A | |||
| PDGFB/B | |||
| PDGFC/C | |||
| PDGFD/D | |||
| PDGFA/B | |||
| IL-8 | CXCR1, CXCR2 (Receptors are coupled to G-proteins) |
Origins: Macrophages Epithelial cells Endothelial cells Melanoma cells Targets: Neutrophils Granulocytes Endothelial cells |
Mediator of inflammatory responses, chemoattractant of neutrophils and granulocytes, angiogenic factor CXCR1/2 expression in malignant melanoma |
| (CXC chemokine) |
Along with autocrine loops involving an EGF-like ligand, mutation, amplification, or dysregulation of at least one of the ErbB family members has been identified in >20% of solid tumors. For example, ~50% of glial tumors harbor EGFR gene amplification (105), and a large fraction of these also present EGFRvIII, a mutant lacking a portion of the extra-cellular domain (49). Despite its inability to bind soluble ligands, EGFRvIII exhibits constitutive tyrosine phosphorylation and multiple downstream signaling pathways (46). Stimulatory EGFR mutations that concentrate at regulatory regions within the kinase domain are found in at least 10% of non-small cell lung cancer patients (63, 77). ErbB-2/HER2 rarely presents tumorigenic point mutations in tumors (87), but amplification of the corresponding portion of chromosome 17 is a feature of 20–25% of metastatic breast cancers, and it associates with worse prognosis (89). Overexpression of ErbB-2 was also observed in ovarian cancer, stomach cancer, and aggressive forms of uterine cancer. ErbB-3 is frequently expressed in human mammary tumors along with ErbB-2, and overexpression of neuregulins, the natural ligands for ErbB-3 and ErbB-4, leads to increased tumorigenicity (5, 58). Recent work demonstrated ErbB-4 mutations in 19% of individuals with melanoma (80). Seven missense mutations were identified, and they resulted in increased kinase activity and transformation ability.
Insulin-like growth factors
The insulin-like growth factor (IGF) axis consists of two cell surface receptors (IGF1R and IGF2R), two ligands (IGF1 and IGF2), a family of six high-affinity IGF binding proteins (IGFBP1–6), as well as associated IGFBP degrading enzymes. IGF1 is produced primarily in the liver under the control of the growth hormone. IGF1 plays an important role in childhood growth, and it exerts anabolic effects in adults. Almost all circulating IGF1 molecules are constitutively bound to an IGFBP, which attenuates the bioactivity of these GFs. The action of IGF1 is mediated by binding to two receptor tyrosine kinases, IGF1R and the insulin receptor (at low affinity), as well as their heterodimers. On the other hand, IGF2 can bind IGF1R, and it is the sole ligand for the IGF2R/mannose 6-phosphate receptor.
Early studies by Baserga and collaborators used fibroblast cell lines from mouse embryos homozygous for a targeted disruption of the IGF1R gene and demonstrated resistance to malignant transformation by several (not all) oncogenes (84). Based on this and other studies, it is currently assumed that, although the IGF1 axis may not generate strong oncogenic signals, its intactness is essential for survival of transformed cells. It is notable that the receptors of the IGF axis are expressed on most types of tumors, and by recruiting the PI3K-AKT pathway IGF1R generates extremely potent anti-apoptotic signals. Amplification of the IGF1R locus has been reported in a small number of breast and melanoma cases (2). In addition, a strong positive association was observed between plasma IGF1 levels and prostate cancer risk (16). In the same vein, mammo-graphic density studies have shown the importance of the IGF1 axis in the generation of these lesions, which represent a strong risk factor for breast cancer (98). Similar to IGF1, animal models support involvement of IGF2 in tumor development (19), and the corresponding imprinted gene has been linked to several neoplasias, including Wilms' tumors and colorectal cancer (111).
Transforming growth factor-β
TGF-β exists in three isoforms (TGF-β1, TGF-β2, and TGF-β3), but the extended superfamily includes more than 30 additional cytokines, classified into several subfamilies [e.g., bone morphogenetic proteins (BMPs) and activins]. Some cells secrete and respond to TGF-β in an autocrine manner. This cytokine induces a cytostatic effect on many epithelial cell types, and it is also able to control proliferation, differentiation, and programmed cell death in most other cell types because the receptors, heterotetrameric serine/threonine kinases, are widely expressed in derivatives of all three embryonic cell layers. Receptor signaling is regulated both positively, by metalloproteinases that cleave a latent form of the ligand, and negatively, by a cell surface antagonist called Bambi (73). The receptors play an important role in apoptosis by signaling through the SMAD pathway. In the SMAD pathway, TGF-β dimers bind to a type II receptor, which recruits and activates a type I receptor by phosphorylation. Consequently, the type I receptor recruits a receptor-regulated SMAD (R-SMAD). For instance, SMAD3, an R-SMAD, has been shown to induce apoptosis by binding to SMAD-4 (a common SMAD), translocating into the nucleus and acting as a transcription factor. TGF-β can also trigger apoptosis through death-domain-associated protein 6 (DAXX) (79).
Under normal physiological conditions, TGF-β prevents the ability of cells to progress through the cell cycle, and it stimulates apoptosis or differentiation. During tumorigenesis, however, genetic and epigenetic events can convert TGF-β into a tumor promoter. Accordingly, a survey of mammary tumors reported that TGF-β1 staining is positively associated with rate of disease progression (36). Similarly, high TGF-β-SMAD activity confers poor prognosis in patients with glioma (13). TGF-β activates epithelial-mesenchymal transition (EMT) through activation of both the canonical (i.e., SMAD2/3-dependent) and the noncanonical (i.e., SMAD2/3-independent) pathways (FIGURE 3). In line with this, SMAD4 deficiency abrogated mammary cell metastasis to the bone in response to TGF-β (25). On the other hand, several lines of evidence indicate that the noncanonical TGF-β signaling also plays an essential role in mediating TGF-β stimulation of EMT, invasion, and metastasis (66). This pathway includes RAS/MAPK, PI3K/AKT, RHO/ROCK, Jagged/Notch, WNT/β-catenin, and mTOR (59).
The oncogenic activities of TGF-β are mediated by dysregulated autocrine and paracrine signaling networks involving epithelial, fibroblast, endothelial, and immune cells that subtly promote tumor angiogenesis and metastasis as well as inhibit host immunosurveillance. The pro-angiogenic functions of TGF-β have been linked to its ability to regulate the expression and/or activities of other angiogenic factors, such as FGF and VEGF (38). Likewise, stromal fibroblasts may determine whether TGF-β suppresses or promotes tumor formation. For example, conditional inactivation of the TGF-β type II receptor gene in mouse fibroblasts resulted in epithelial lesions of the prostate and the forestomach, presumably by activation of paracrine HGF signaling (10).
VEGFs
VEGFs regulate both vasculogenesis and angiogenesis. The family consists of five glycoproteins, VEGFA (=VEGF), VEGFB, VEGFC, VEGFD, and PlGF (placenta growth factor). In addition, alternative exon splicing generates four VEGF isoforms. Multiplicity also characterizes the respective surface receptors, co-receptors like neuropilins (NPs) and proteogly-cans, as well as the downstream signaling pathways (57). The VEGF family members bind to at least one of the three known VEGFRs, namely VEGFR-1 (FLT-1), VEGFR-2 (FLK-1 or KDR), and VEGFR-3 (FLT-4). VEGFA binds to VEGFR-1 and VEGFR-2, whereas VEGFB and PlGF bind exclusively to VEGFR-1. VEGFR-2 seems to mediate most known cellular responses to VEGF and has much higher intracellular signaling intermediates than VEGFR-1 (102). Unlike VEGFR-3, which is largely restricted to lymphatic endothelial cells, both VEGFR-1 and VEGFR-2 are expressed in vascular endothelial cells, as well as monocytes, macrophages (VEGFR-1), and hematopoietic stem cells (VEGFR-2). Importantly, expression of VEGFR-1 and VEGFR-2, as well as the co-receptors NP1 and NP2, has been detected on subsets of solid tumor cells, and according to a recent study activation of VEGFR-1 in breast cancer cells supports their growth and survival (106).
VEGFRs control angiogenesis by simultaneous signaling through several intermediates: cell proliferation and vasopermeability are stimulated by the protein kinase C pathway, cell survival and proliferation by the AKT and MAPK pathway, and cell migration results from signaling through SRC and Paxillin. Other than the proangiogenic effects, VEGF exerts effects independent of vascular processes, such as autocrine effects on tumor cell function (i.e., survival, migration, invasion), immune suppression, and recruitment of bone marrow progenitors. The latter may dictate organ-specific tumor spread by homing to tumor-specific premetastatic sites and forming clusters that provide a permissive niche for incoming tumor cells (51).
Cancer Therapeutics Targeting Growth Factor Signaling
The understanding gained over the last 25 years on the roles played by GFs in progression of solid tumors has transformed the discipline of clinical oncology in multiple ways, including disease stratification, prognosis, and ultimately cancer therapy. A new class of treatment, called molecular targeted therapy, has joined over the last decade the classical pillars of cancer treatment, namely chemotherapy and radiotherapy. Like its predecessor, hormonal therapy, targeted therapy intercepts signaling pathways by making use of highly specific drugs, such as tyrosine kinase inhibitors (TKIs) and monoclonal antibodies (mAbs). These drugs are characterized by relatively mild adverse effects and optimum, rather than maximum, tolerable doses (9). Although their targets differ, responses to targeted drugs are often low/moderate, but they can be enhanced by combining the drugs with chemo- or radiotherapy (6). Another general lesson learned while applying the new drugs in oncology wards relates to patient's resistance, which may be primary or acquired, namely evolving in a few months or years (48). Mechanisms underlying resistance remain poorly understood, but in a few cases they have been attributed to compensatory circuits, such as enhancement of IGF1R signaling in breast cancer patients treated with an anti-ErbB-2/HER2 antibody (68), or to preemptive downstream mutations, such as K-RAS mutations conferring primary resistance to anti-EGFR antibodies (82). What follows is a concise description of targeted therapies aimed at intercepting some major GF pathways involved in epithelial tumor progression (see FIGURE 4).
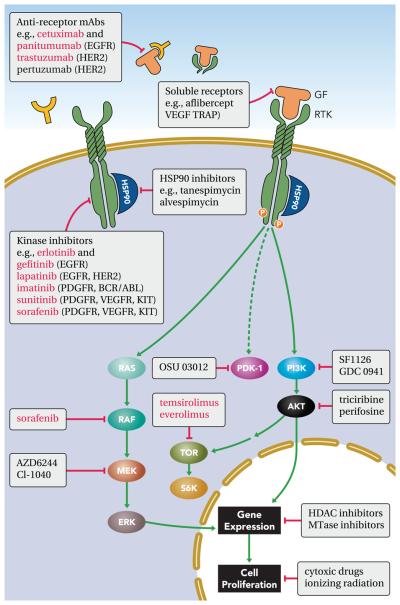
FIGURE 4. Therapeutic targeting of growth factor signaling pathway in solid tumors.
A scheme showing the major therapeutic strategies aimed at blocking growth factor signaling in cancer. The strategies include monoclonal antibodies (mAbs) against the GFs and their receptors, soluble receptors (TRAPs): small molecule tyrosine kinase inhibitors (TKIs), blockers of heat shock proteins (HSPs), antagonists of specific signaling pathway proteins, as well as inhibitors of histone deacetylases (HDACs) and methyl-transferase (MTase). Also represented are cytotoxic treatments. Examples of drugs are shown in yellow boxes; therapeutic agents that were approved for clinical use are highlighted in red, and targets are shown in parentheses. See details of signaling pathways in FIGURE 2.
Clinical interceptors of the EGFR/HER2 network
The mAb cetuximab is a human-mouse anti-ErbB-1 chimeric IgG1 antibody, which has shown efficacy in colorectal cancer (24) and in head and neck cancer (11, 101). Cetuximab binds to the ligand-binding domain of ErbB-1 and prevents dimerization and subsequent activation by auto-phophorylation. Panitumumab is a fully human IgG2 antibody specific to ErbB-1, which is effective and well tolerated in colorectal cancer. Nimotuzumab is yet another humanized mAb that inhibits EGF binding and shows effectiveness in nasopharyngeal cancer and in glioma. One striking feature of Nimotuzumab is the absence of severe adverse effects, such as skin rash, which commonly associate with similar mAbs to EGFR (78). Low toxicity of nimotuzumab might be due to intermediate affinity and incomplete abrogation of the active conformation (96). Trastuzumab is an ErbB-2/HER2-specific mAb, which was approved in 1998 for the treatment of metastasizing breast cancer, only if tumors overexpress ErbB-2/HER2 and secrete no soluble version of this protein (90). By suppressing ErbB-2/HER2 signaling, trastuzumab interferes with cell cycle control, angio-genesis, and the PI3K pathway. Another mechanism of action of trastuzumab involves the induction of antibody-dependent cell-mediated cytotoxicity (ADCC) (22, 67). Yet another potential mechanism entails antibody-induced degradation of ErbB-2/HER2, a process enhanced on combining two antibodies directed at distinct sites of the oncoprotein (8). Compared with mAbs, TKIs are low molecular weight mimics of ATP, whose mechanism of action is clear but target specificity quite broader. Along with mono-specific inhibitors like the EGFR inhibitor erlotinib (approved for treatment of lung and pancreatic cancer), pan-ErbB, or dual-specificity TKIs, like lapatinib, show encouraging clinical efficacies. Moreover, lapatinib holds promise for treatment of trastuzumab-resistant patients (92). Another class of experimental therapeutics comprises inhibitors of heat shock proteins (HSPs), chaperones involved in the folding and conformational maturation of signaling proteins, including ErbB-2. Disruption of HSP90 results in ubiquitylation and proteasomal degradation of ErbB-2, leading to abrogation of the PI3K/AKT and cyclin D pathways (20).
Experimental interceptors of the IGF1 axis
Although no drug targeting the IGF1 axis has entered clinical use, a large number of candidate TKIs and mAbs are being tested in clinical trials (12). For example, BMS-554417 is a dual TKI of both IGF1R and insulin receptor, which can induce cell cycle arrest, prevent nuclear accumulation of cyclin D1, and reduce tumor xenograft size (41). Currently, extensive efforts focus on mAbs to IGF1R, such as CP-751,871 (42). Due to the metabolic effects of the IGF1 axis and cross talk with the insulin receptor, adverse effects are considered a major concern. For example, treatment with CP-751,871 increased serum insulin and human growth hormone levels, with modest increases in serum glucose levels.
Targeting TGF-β signaling
The functional duality of TGF-β in tumor progression requires inhibition in advanced metastatic cancer, while retaining the growth inhibitory abilities exhibited in early stages of tumorigenesis. One approach employs the antisense oligodeoxynucleotide AP 12009, a synthetic 18-mer phosphorothioate oligodeoxynucleotide, which is complementary to the sequence of the TGF-β2 mRNA (44). Promising results were obtained in phase I clinical trials of AP 12009 performed in recurrent or refractory glioma patients. In laboratory studies, administration of a TGF-β neutralizing antibody has been demonstrated to restore natural killer cell activity and to reduce metastasis (3). Potentially, CAT-192 (Metelimumab), a human antibody that neutralizes TGF-β is a candidate agent. Likewise, preclinical studies employing a dominant-negative mutant approach identified TGF-βR2 as a target. Hence, it is likely that future attempts to inhibit the pathway will involve, in addition to antisense oligonucleotides and antibodies, also kinase inhibitors specific to TGF-βR2 (83).
Targeting of VEGF signaling pathways
Two early lines of preclinical evidence identified VEGF and the respective receptors as effective targets for cancer therapy: a neutralizing anti-VEGF antibody suppressed both sarcomas and a glioblastoma, as well as angiogenesis in mouse xenograft models (55), and a dominant-negative form of VEGFR2 similarly inhibited a glioblastoma xenograft in animals (65). The original murine antibody was later humanized (bevacizumab) and approved for treatment of colorectal and other tumors. Other anti-angiogenesis compounds under development are a soluble receptor able to sequester three different ligands, an antisense oligonucleotide able to inhibit several VEGFs, anti-VEGFR2 antibodies, an aptamer to the most abundant iso-form of VEGF, and an anti-PlGF antibody that showed promising results in animal models (32). In addition, several TKIs with selectivity to VEGFRs have been approved: The TKI sorafenib was approved for patients with hepatocellular carcinoma and renal cell carcinoma (RCC) (29), and sunitinib was also approved for patients with RCC. As is the case for all kinase inhibitors, the TKIs target several VEGFRs, as well as several related tyrosine kinases (52). The mechanisms of action of VEGF-targeted therapies variably include inhibition of new vessel growth, induction of endothelial cell apoptosis, blockade of the incorporation of hematopoietic and endothelial progenitor cells, and direct effects on target tumor cells. Patients treated with VEGF inhibitors may experience longer survival, but they eventually succumb to their disease. Acquired resistance may be attributed to VEGF-unrelated angiogenic factors allowing disease progression (e.g., PDGF-B and angiopoietin-1), switching of the tumor vasculature into drug-resistent mature vessels, or emergence of hypoxia-resistent tumor cell variants carrying mutations in p53 or in other genes (110).
Concluding Remarks
Some remarkable leaps into cancer progression have been accomplished, since the early discovery of GFs by Rita Levi-Montalcini and Stanley Cohen. Beyond the isolation of many other GFs and the elucidation of the respective autocrine loops in tumors, we now understand that paracrine loops involving myeloid, mesenchymal, and endothelial cells fulfill equally important functions in cancer progression. Consequently, the emerging high content picture of tumor progression is bound to rival the complexity of GFs' contributions to embryonic development (see Table 1), and similarly to embryos it portrays tumors as extensions of host tissues. And although a continuum of cancer genome, tumor biology, and signaling networks remain beyond reach, it is already clear that the integrated outcome will change the way cancer is being diagnosed and treated. Indeed, pharmaceutical interceptors of GF signaling first hit oncology wards some 12 years ago, but although their number rises steadily, the promises attached to the new drugs come with great challenges: optimal scheduling and combination with cytotoxic regimens lack conceptual grounds, as do the understanding of patient resistance to therapy and the dynamic impact of GFs on tumor progression. Nevertheless, recent glimpses into the vibrant future of translational GF research bear optimism. This may be exemplified by the application of anti-EGFR antibodies in colorectal cancer: whereas the antibodies are only weakly active in patients, it was their combination with chemotherapy that permitted approval and clinical application in 2004. In 2007, we learned that K-RAS mutations confer resistance to the antibody, which enabled elimination of a large fraction of non-responders. On the other hand, reports of the last 1–2 years propose that best responses are achieved in patients whose tumors present autocrine loops of EGFR. Similar knowledge-based spirals of refined patient selection and identification of resistance mechanisms will likely result in powerful drugs, which are active on biologically well defined but relatively small cohorts of patients. ■
| Roles in Tumors | Therapeutic Targeting (Including Experimental) |
|---|---|
| ErbB-1 overexpression: lung cancer, head and neck, brain tumors ErbB-1 internal deletions (EGFRvIII): in glioblastoma multiforme ErbB1/2 kinase mutations: NSCLC ErbB-2 amplification/overexpression: breast, ovarian, lung, uterine, stomach cancer ErbB-4 mutations: melanoma TGFα overexpression: gastric, head and neck cancer, more |
mAbs to ErbB-1 (e.g., Cetuximab, Panitumumab): colorectal, head and neck cancer mAbs to ErbB-2 (e.g., Trastuzumab): breast cancer ErbB1-TKIs (e.g., Erlotinib, Gefitinib): lung, pancreatic cancer ErbB1/2-TKIs (e.g., Lapatinib): breast cancer Pan ErbB-TKI (e.g., Canertinib) |
| IGF1R: Activates pro-survival pathways High IGF1R: in poorly differentiated tumors Low IGF1R: in highly differentiated tumors IGF1 expression: in tumor cells (hypoxia) IGF1R amplification: breast cancer, melanoma IGF1R overexpression: in pediatric cancers IGF2 overexpression: in colorectal cancer Under/overexpression of IGFBPs |
No drug approved so far. Several mAbs to IGF1R (e.g., CP-751871) IGF1R- and InsulinR-TKIs (e.g., BMS-554417) IGF1R-TKIs (e.g., NVP-AEW541-ADW742) |
| Tumor suppressor activity: preventing cell cycle progression, stimulating apoptosis, differentiation, antiproliferative activity in epithelial cells TGF-β pathways mutations: oncogenic activities in epithelial, fibroblast, endothelial, immune cells, angiogenesis, invasion, metastasis TGF-β overexpression in tumor cells, induction of EMT SMAD-4, SMAD-2 gene mutations in pancreas, lung, colon cancer |
TGFβ1, TGFβ2 antisense oligonucleotides mAb to TGFβ1 Kinase inhibitors to TGF-βR2 |
| Survival, migration, invasion, regulation of vessel permeability Autocrine effects on tumor cells (survival, migration, invasion) Immune suppression, vessel dilation, regulation of blood flow Increased VEGF mRNA levels in endothelial cells during hypoxia |
mAb to VEGF (bevacizumab) in colon, renal, lung cancer VEGF Trap (Fc of IgG fused to VEGFR1/2, aflibercept) VEGFR-TKIs (e.g., vatalanib) Multi target TKIs (VEGFR, PDGFR) (e.g., sorafenib) in renal cell cancer |
| Matrix invasion, EMT Paracrine/Autocrine activation via HGF MET overexpression: adenocarcinomas, large cell carcinomas, squamous cell carcinomas, SCLCs (25%) Associated with resistance to EGFR TKI in lung cancer |
MET targeted through HSP90 inhibition (geldanamycin, anisomycin) mAb to HGF (e.g., AMG102) HGF competitor NK4 MET-TKIs (e.g., K252a, SU11274, PHA665752) |
| Tumor cells under hypoxia secrete FGF Polymorphisms within FGFR2 FGFR2 missense mutations/copy number gains: breast cancer, gastric cancer Aberrant FGFR2 signaling: proliferation, tumor cell survival Class switch from FGFR2b to FGFR2c in prostate, bladder cancer |
Small-molecule FGFR inhibitors, e.g., PD173074, SU5402, AZD2171, and Ki23057 Human antibody, peptide mimetic, RNA aptamer, siRNA, synthetic microRNA to FGFR2 |
| Direct tumor growth promoting effects, angiogenic activity, angiogenesis. Tumor cells under hypoxia secrete PDGF PDGFB translocation: in dermatofibrosarcoma protuberans PDGFRα mutations: in gastrointestinal stromal tumors PDGFRα amplification: in glioblastoma PDGFRα translocation: in chronic myelomonocytic leukemia |
PDGFR-TKIs (e.g. imatinib) in chronic myeloid leukemia, gastrointestinal stromal tumors mAbs to PDGFRs (e.g., 1B3, IMC-2C5) |
| Promotes tumor cell survival in prostate cancer Increases expression of AKT in androgen-independent pathways Autocrine/Paracrine growth, invasive, and angiogenic effects |
Neutralizing mAb to CXCR1/2 in mouse models CXCR1-small molecule inhiitors (e.g., repertaxin) CXCR1-small molecule inhibitors (e.g., SCH-479833, SCH-527123) |
Acknowledgments
We thank members of our groups for insightful comments.
Y. Yarden is the incumbent of the Harold and Zelda Goldenberg Professorial Chair. Our work is supported by research grants from the Goldhirsh Foundation, the U.S. National Cancer Institute (CA072981), the Seventh Framework Program (FP7) of the European Commission, the Israel Science Foundation, the German Research Foundation (DFG), Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Marc Rich Foundation for Education, Culture and Welfare, and the M. D. Moross Institute for Cancer Research.
References
- 1.Aboud-Pirak E, Hurwitz E, Pirak ME, Bellot F, Schlessinger J, Sela M. Efficacy of antibodies to epidermal growth factor receptor against KB carcinoma in vitro and in nude mice. J Natl Cancer Inst. 1988;80:1605–1611. doi: 10.1093/jnci/80.20.1605. [DOI] [PubMed] [Google Scholar]
- 2.Almeida A, Muleris M, Dutrillaux B, Malfoy B. The insulin-like growth factor I receptor gene is the target for the 15q26 amplicon in breast cancer. Genes Chromosomes Cancer. 1994;11:63–65. doi: 10.1002/gcc.2870110110. [DOI] [PubMed] [Google Scholar]
- 3.Arteaga CL, Hurd SD, Winnier AR, Johnson MD, Fendly BM, Forbes JT. Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J Clin Invest. 1993;92:2569–2576. doi: 10.1172/JCI116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 5.Atlas E, Cardillo M, Mehmi I, Zahedkargaran H, Tang C, Lupu R. Heregulin is sufficient for the promotion of tumorigenicity and metastasis of breast cancer cells in vivo. Mol Cancer Res. 2003;1:165–175. [PubMed] [Google Scholar]
- 6.Baselga J. Targeting tyrosine kinases in cancer: the second wave. Science. 2006;312:1175–1178. doi: 10.1126/science.1125951. [DOI] [PubMed] [Google Scholar]
- 7.Beerenwinkel N, Antal T, Dingli D, Traulsen A, Kinzler KW, Velculescu VE, Vogelstein B, Nowak MA. Genetic progression and the waiting time to cancer. PLoS Comput Biol. 2007;3:e225. doi: 10.1371/journal.pcbi.0030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Kasus T, Schechter B, Lavi S, Yarden Y, Sela M. Persistent elimination of ErbB-2/HER2-overexpressing tumors using combinations of monoclonal antibodies: relevance of receptor endocytosis. Proc Natl Acad Sci USA. 2009;106:3294–3299. doi: 10.1073/pnas.0812059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Kasus T, Schechter B, Sela M, Yarden Y. Cancer therapeutic antibodies come of age: targeting minimal residual disease. Mol Oncol. 2007;1:42–54. doi: 10.1016/j.molonc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bierie B, Stover DG, Abel TW, Chytil A, Gorska AE, Forrester E, Yang L, Moses HL. Transforming growth factor-beta regulates mammary carcinoma cell survival and interaction with the adjacent microenvironment. Cancer Res. 2008;68:1809–1819. doi: 10.1158/0008-5472.CAN-07-5597. [DOI] [PubMed] [Google Scholar]
- 11.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 12.Bruchim I, Attias Z, Werner H. Targeting the IGF1 axis in cancer proliferation. Expert Opin Ther Targets. 2009;13:1179–1192. doi: 10.1517/14728220903201702. [DOI] [PubMed] [Google Scholar]
- 13.Bruna A, Darken RS, Rojo F, Ocana A, Penuelas S, Arias A, Paris R, Tortosa A, Mora J, Baselga J, Seoane J. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11:147–160. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, Lemmon MA, Sliwkowski MX, Ward CW, Yokoyama S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 15.Cerveira N, Ribeiro FR, Peixoto A, Costa V, Henrique R, Jeronimo C, Teixeira MR. TMPRSS2-ERG gene fusion causing ERG overexpression precedes chromosome copy number changes in prostate carcinomas and paired HGPIN lesions. Neoplasia. 2006;8:826–832. doi: 10.1593/neo.06427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee A, Mambo E, Sidransky D. Mitochondrial DNA mutations in human cancer. Oncogene. 2006;25:4663–4674. doi: 10.1038/sj.onc.1209604. [DOI] [PubMed] [Google Scholar]
- 18.Cho HS, Leahy DJ. Structure of the extracellular region of HER3 reveals an interdomain tether. Science. 2002;297:1330–1333. doi: 10.1126/science.1074611. [DOI] [PubMed] [Google Scholar]
- 19.Christofori G, Naik P, Hanahan D. Deregulation of both imprinted and expressed alleles of the insulin-like growth factor 2 gene during beta-cell tumorigenesis. Nat Genet. 1995;10:196–201. doi: 10.1038/ng0695-196. [DOI] [PubMed] [Google Scholar]
- 20.Citri A, Kochupurakkal BS, Yarden Y. The achilles heel of ErbB-2/HER2: regulation by the Hsp90 chaperone machine and potential for pharmacological intervention. Cell Cycle. 2004;3:51–60. [PubMed] [Google Scholar]
- 21.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 22.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S, Levi-Montalcini R, Hamburger V. A nerve growth-stimulating factor isolated from sarcoma AS 37 and 180. Proc Natl Acad Sci USA. 1954;40:1014–1018. doi: 10.1073/pnas.40.10.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 25.Deckers M, van Dinther M, Buijs J, Que I, Lowik C, van der Pluijm G, ten Dijke P. The tumor suppressor Smad4 is required for transforming growth factor beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res. 2006;66:2202–2209. doi: 10.1158/0008-5472.CAN-05-3560. [DOI] [PubMed] [Google Scholar]
- 26.Di Fiore PP, Pierce JH, Kraus MH, Segatto O, King CR, Aaronson SA. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987;237:178–182. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- 27.Downward J, Yarden Y, Mayes E, Scrace G, Totty N, Stockwell P, Ullrich A, Schlessinger J, Water-field MD. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984;307:521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- 28.Epstein JI. Precursor lesions to prostatic adenocarcinoma. Virchows Arch. 2009;454:1–16. doi: 10.1007/s00428-008-0707-5. [DOI] [PubMed] [Google Scholar]
- 29.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 30.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 31.Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 32.Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, Autiero M, Wyns S, Plaisance S, Moons L, van Rooijen N, Giacca M, Stassen JM, Dewerchin M, Collen D, Carmeliet P. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 33.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 34.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 35.Futreal PA, Coin L, Marshall M, Down T, Hubbard T, Wooster R, Rahman N, Stratton MR. A census of human cancer genes. Nat Rev Cancer. 2004;4:177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorsch SM, Memoli VA, Stukel TA, Gold LI, Arrick BA. Immunohistochemical staining for transforming growth factor beta 1 associates with disease progression in human breast cancer. Cancer Res. 1992;52:6949–6952. [PubMed] [Google Scholar]
- 37.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 38.Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell. 2003;12:817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 39.Greco A, Fusetti L, Villa R, Sozzi G, Minoletti F, Mauri P, Pierotti MA. Transforming activity of the chimeric sequence formed by the fusion of collagen gene COL1A1 and the platelet derived growth factor b-chain gene in dermatofibrosarcoma protuberans. Oncogene. 1998;17:1313–1319. doi: 10.1038/sj.onc.1202051. [DOI] [PubMed] [Google Scholar]
- 40.Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL., 3rd Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci USA. 1994;91:8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haluska P, Carboni JM, Loegering DA, Lee FY, Wittman M, Saulnier MG, Frennesson DB, Kalli KR, Conover CA, Attar RM, Kaufmann SH, Gottardis M, Erlichman C. In vitro and in vivo antitumor effects of the dual insulin-like growth factor-I/insulin receptor inhibitor, BMS-554417. Cancer Res. 2006;66:362–371. doi: 10.1158/0008-5472.CAN-05-1107. [DOI] [PubMed] [Google Scholar]
- 42.Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, Yap TA, Roberts ML, Sharma A, Gualberto A, Adjei AA, de Bono JS. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5834–5840. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 44.Hau P, Jachimczak P, Schlingensiepen R, Schulmeyer F, Jauch T, Steinbrecher A, Brawanski A, Proescholdt M, Schlaier J, Buchroithner J, Pichler J, Wurm G, Mehdorn M, Strege R, Schuierer G, Villarrubia V, Fellner F, Jansen O, Straube T, Nohria V, Goldbrunner M, Kunst M, Schmaus S, Stauder G, Bogdahn U, Schlingensiepen KH. Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides. 2007;17:201–212. doi: 10.1089/oli.2006.0053. [DOI] [PubMed] [Google Scholar]
- 45.Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD, Huang CM, Gill GN, Wiley HS, Cavenee WK. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;272:2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- 46.Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma. Sci Signal. 2009;2:re6. doi: 10.1126/scisignal.287re6. [DOI] [PubMed] [Google Scholar]
- 47.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 49.Jeuken J, Sijben A, Alenda C, Rijntjes J, Dekkers M, Boots-Sprenger S, McLendon R, Wesseling P. Robust detection of EGFR copy number changes and EGFR variant III: technical aspects and relevance for glioma diagnostics. Brain Pathol. 2009;19:661–671. doi: 10.1111/j.1750-3639.2009.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jura N, Endres NF, Engel K, Deindl S, Das R, Lamers MH, Wemmer DE, Zhang X, Kuriyan J. Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment. Cell. 2009;137:1293–1307. doi: 10.1016/j.cell.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, Faraoni R, Floyd M, Hunt JP, Lockhart DJ, Milanov ZV, Morrison MJ, Pallares G, Patel HK, Pritchard S, Wodicka LM, Zarrinkar PP. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 53.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 54.Katz M, Amit I, Citri A, Shay T, Carvalho S, Lavi S, Milanezi F, Lyass L, Amariglio N, Jacob-Hirsch J, Ben-Chetrit N, Tarcic G, Lindzen M, Avraham R, Liao YC, Trusk P, Lyass A, Rechavi G, Spector NL, Lo SH, Schmitt F, Bacus SS, Yarden Y. A reciprocal tensin-3-cten switch mediates EGF-driven mammary cell migration. Nat Cell Biol. 2007;9:961–969. doi: 10.1038/ncb1622. [DOI] [PubMed] [Google Scholar]
- 55.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 56.Klapper LN, Glathe S, Vaisman N, Hynes NE, Andrews GC, Sela M, Yarden Y. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci USA. 1999;96:4995–5000. doi: 10.1073/pnas.96.9.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- 58.Krane IM, Leder P. NDF/heregulin induces persistence of terminal end buds and adenocarcinomas in the mammary glands of transgenic mice. Oncogene. 1996;12:1781–1788. [PubMed] [Google Scholar]
- 59.Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98:146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 62.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 63.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 64.Meijnen P, Peterse JL, Antonini N, Rutgers EJ, van de Vijver MJ. Immunohistochemical categorisation of ductal carcinoma in situ of the breast. Br J Cancer. 2008;98:137–142. doi: 10.1038/sj.bjc.6604112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367:576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- 66.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 67.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, Neri TM, Ardizzoni A. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 68.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 69.Neal CL, Yao J, Yang W, Zhou X, Nguyen NT, Lu J, Danes CG, Guo H, Lan KH, Ensor J, Hittelman W, Hung MC, Yu D. 14-3-3zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res. 2009;69:3425–3432. doi: 10.1158/0008-5472.CAN-08-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ni CY, Murphy MP, Golde TE, Carpenter G. Gamma-secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 71.Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–644. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10:355–362. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massague J, Niehrs C. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature. 1999;401:480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- 74.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 75.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth–bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 76.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massague J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 78.Perez-Soler R, Delord JP, Halpern A, Kelly K, Krueger J, Sureda BM, von Pawel J, Temel J, Siena S, Soulieres D, Saltz L, Leyden J. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. Oncologist. 2005;10:345–356. doi: 10.1634/theoncologist.10-5-345. [DOI] [PubMed] [Google Scholar]
- 79.Perlman R, Schiemann WP, Brooks MW, Lodish HF, Weinberg RA. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat Cell Biol. 2001;3:708–714. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- 80.Prickett TD, Agrawal NS, Wei X, Yates KE, Lin JC, Wunderlich JR, Cronin JC, Cruz P, Rosenberg SA, Samuels Y. Analysis of the tyrosine kinome in melanoma reveals recurrent mutations in ERBB4. Nat Genet. 2009;41:1127–1132. doi: 10.1038/ng.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roberts AB, Anzano MA, Lamb LC, Smith JM, Frolik CA, Marquardt H, Todaro GJ, Sporn MB. Isolation from murine sarcoma cells of novel transforming growth factors potentiated by EGF. Nature. 1982;295:417–419. doi: 10.1038/295417a0. [DOI] [PubMed] [Google Scholar]
- 82.Sartore-Bianchi A, Di Nicolantonio F, Nichelatti M, Molinari F, De Dosso S, Saletti P, Martini M, Cipani T, Marrapese G, Mazzucchelli L, Lamba S, Veronese S, Frattini M, Bardelli A, Siena S. Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer. PLoS One. 2009;4:e7287. doi: 10.1371/journal.pone.0007287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saunier EF, Akhurst RJ. TGF beta inhibition for cancer therapy. Curr Cancer Drug Targets. 2006;6:565–578. doi: 10.2174/156800906778742460. [DOI] [PubMed] [Google Scholar]
- 84.Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14:3604–3612. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seton-Rogers SE, Brugge JS. ErbB2 and TGF-beta: a cooperative role in mammary tumor progression? Cell Cycle. 2004;3:597–600. [PubMed] [Google Scholar]
- 86.Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, Hicklin DJ, Chaplin D, Foster FS, Benezra R, Kerbel RS. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 87.Shigematsu H, Takahashi T, Nomura M, Majmudar K, Suzuki M, Lee H, Wistuba II, Fong KM, Toyooka S, Shimizu N, Fujisawa T, Minna JD, Gazdar AF. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005;65:1642–1646. doi: 10.1158/0008-5472.CAN-04-4235. [DOI] [PubMed] [Google Scholar]
- 88.Shimizu A, O'Brien KP, Sjoblom T, Pietras K, Buchdunger E, Collins VP, Heldin CH, Dumanski JP, Ostman A. The dermatofibrosarcoma protuberans-associated collagen type Ialpha1/platelet-derived growth factor (PDGF) B-chain fusion gene generates a transforming protein that is processed to functional PDGF-BB. Cancer Res. 1999;59:3719–3723. [PubMed] [Google Scholar]
- 89.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 90.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 91.Solomon E, Borrow J, Goddard AD. Chromosome aberrations and cancer. Science. 1991;254:1153–1160. doi: 10.1126/science.1957167. [DOI] [PubMed] [Google Scholar]
- 92.Spector N, Xia W, El-Hariry I, Yarden Y, Bacus S. HER2 therapy. Small molecule HER-2 tyrosine kinase inhibitors. Breast Cancer Res. 2007;9:205. [Google Scholar]
- 93.Sporn MB, Todaro GJ. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980;303:878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- 94.Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest. 1999;103:1237–1241. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Talavera A, Friemann R, Gomez-Puerta S, Martinez-Fleites C, Garrido G, Rabasa A, Lopez-Requena A, Pupo A, Johansen RF, Sanchez O, Krengel U, Moreno E. Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation. Cancer Res. 2009;69:5851–5859. doi: 10.1158/0008-5472.CAN-08-4518. [DOI] [PubMed] [Google Scholar]
- 97.Talbot SJ, Crawford DH. Viruses and tumours–an update. Eur J Cancer. 2004;40:1998–2005. doi: 10.1016/j.ejca.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 98.Tamimi RM, Cox DG, Kraft P, Pollak MN, Haiman CA, Cheng I, Freedman ML, Hankinson SE, Hunter DJ, Colditz GA. Common genetic variation in IGF1, IGFBP-1, and IGFBP-3 in relation to mammographic density: a cross-sectional study. Breast Cancer Res. 2007;9:R18. doi: 10.1186/bcr1655. R 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Todaro GJ, De Larco JE, Cohen S. Transformation by murine and feline sarcoma viruses specifically blocks binding of epidermal growth factor to cells. Nature. 1976;264:26–31. doi: 10.1038/264026a0. [DOI] [PubMed] [Google Scholar]
- 100.Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, Lee J, Yarden Y, Libermann TA, Schlessinger J, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- 101.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, Knecht R, Amellal N, Schueler A, Baselga J. Open-label, uncontrolled, multi-center phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 102.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 103.Waterfield MD, Scrace GT, Whittle N, Stroobant P, Johnsson A, Wasteson A, Westermark B, Heldin CH, Huang JS, Deuel TF. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983;304:35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- 104.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 105.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA. 1987;84:6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu Y, Hooper AT, Zhong Z, Witte L, Bohlen P, Rafii S, Hicklin DJ. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer. 2006;119:1519–1529. doi: 10.1002/ijc.21865. [DOI] [PubMed] [Google Scholar]
- 107.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 108.Yarden Y, Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- 109.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 110.Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science. 2002;295:1526–1528. doi: 10.1126/science.1068327. [DOI] [PubMed] [Google Scholar]
- 111.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 112.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]