Abstract
Seawater acclimation in killifish, Fundulus heteroclitus, is mediated in part by a rapid (1 hour) translocation of CFTR Cl- channels from an intracellular pool to the plasma membrane in gill and increased CFTR mediated Cl- secretion. This effect is mediated by serum and glucocorticoid-inducible kinase 1 (SGK1), which is stimulated by plasma hypertonicity rather than cortisol. Since arsenic exposure prevents acclimation to seawater by decreasing CFTR protein levels we tested the hypothesis that arsenic (as sodium arsenite) blocks acclimation to seawater by down regulating SGK1 expression. Freshwater adapted killifish were exposed to arsenic (48 hrs) and transferred to seawater containing arsenic, and SGK and CFTR expression were measured. Arsenic reduced the seawater induced increase in SGK1 mRNA and protein abundance, and reduced both the total amount of CFTR and the amount of CFTR in the plasma membrane. The decrease in membrane CFTR reduced Cl- secretion. Arsenic also increased the amount of ubiquitinated CFTR and its degradation by the lysosome. Thus, we propose a model whereby arsenic reduces the ability of killifish to acclimate to seawater by blocking the seawater induced increase in SGK1, which results in increased ubiquitination and degradation of CFTR.
Keywords: Acclimation, ubiquitination, lysosome, Abcc7, environmental toxicant
1. Introduction
Arsenic, a toxic metalloid, is prevalent in the environment, where it occurs both naturally and as result of pollution. Exposure to arsenic via occupational and environmental sources represents a major health concern worldwide according to the World Health Organization (WHO). Chronic exposure to arsenic contaminated water or air causes cancers of the skin, lung, bladder, prostate, liver and kidney (Karagas, et al., 1998; Abernathy, et al., 1999; Henke, 2009). Arsenic has also been linked to type 2 diabetes, vascular disease, cardiovascular disease, neuropathy and reproductive and developmental disorder (USEPA, 1980; Abernathy, et al., 1999; National Research Council, 1999). To protect against the adverse effects of chronic arsenic exposure, the U.S. EPA and the WHO have established a limit for safe drinking water of 10 ppb (USEPA, 1980). In addition, to protect humans consuming seafood from the risk of arsenic associated cancers, the U.S. EPA has established a maximum safe-level of total dissolved inorganic arsenic in seawater of 0.0175 ppb. However, these concentrations are often exceeded even in clean coastal waters (1 to 3 ppb), and arsenic can be as high as 1000 ppb in polluted seawater (Boyle and Jonasson, 1973; Neff, 1997).
Exposure to arsenic disrupts Cl- balance and blocks seawater acclimation in killifish, Fundulus heteroclitus (Stanton, et al., 2006; Shaw, et al., 2007b), but the underlying mechanism for this inhibition is unknown. When moving from freshwater to seawater, acclimation is accomplished in large part by increasing CFTR mediated Cl- secretion across the gill and opercula epithelium to balance Cl- (and Na+) intake in seawater. Acclimation to seawater involves both short-term (hours)(Sato, et al., 2007; Shaw, et al., 2008) and longer term (days) up-regulation of NaCl excretion (Wood and Laurent, 2003; Shaw, et al., 2007b). The short-term mechanism is mediated by the translocation of CFTR from an intracellular vesicular pool to the plasma membrane, an effect mediated by SGK1, which in killifish is increased by plasma hypertonicity (Shaw et al., 2008). The long-term increase in Cl- secretion is mediated by cortisol, which via activation of the glucocorticoid receptor (GR), enhances the mRNA and protein abundance of CFTR, Na+-K+-ATPase and NKCC1 (Singer, et al., 1998; Marshall, et al., 1999; Marshall, 2002; Marshall and Singer, 2002; Marshall, 2003; Scott, et al., 2004; Marshall, et al., 2005). Although arsenic has no effect on the cortisol-GR mediated increase in CFTR, Na+-K+-ATPase, and NKCC1 mRNA, or on the protein abundance of Na+-K+- ATPase and NKCC1 (Shaw, et al., 2007b) arsenic reduces CFTR protein in the gill and rapidly (hours) reduces CFTR Cl- currents (Stanton, et al., 2006). However, the mechanism for this effect of arsenic is unknown. Thus, the goal of this study is to test the hypothesis that arsenic reduces the seawater induced increase in CFTR abundance by inhibiting the seawater induced up-regulation of SGK1.
SGK1, a 50 kDa, serine/threonine protein kinase, is transcriptionally regulated by a wide variety of environmental and cytotoxic stressors, including hypertonicity, as well as by steroids (including cortisol) and peptide hormones (Loffing et al., 2006). SGK1 regulates the location and abundance of many plasma membrane proteins including ion channels, receptors and peptide hormone receptors. For example, SGK1 increases K+ and Ca++ transport in epithelial cells by enhancing the plasma membrane expression of ROMK1 and TRPV5 channels, respectively (Lang, et al., 2006; Tessier and Woodgett, 2006). SGK1 also stimulates sodium reabsorption in the kidney by increasing the number of ENaC sodium channels in the plasma membrane (Lang, et al., 2003; Pearce, 2003; Thomas and Itani, 2004; Vallon, et al., 2005; Bhalla, et al., 2006; Lang, et al., 2006). Briefly, aldosterone and glucocorticoids, by binding to the mineralocorticoid (MR) and glucocorticoid receptor (GR), respectively, promote the transcription and subsequent phosphorylation of SGK1 (ppSGK). ppSGK1 phosphorylates, and thereby inhibits Nedd4-2, a E3 ubiquitin ligase, which decreases the Nedd4-2 induced ubiquitination of ENaC channels. Because ubiquitinated ENaC is removed from the membrane by endocytosis and is then degraded in the lysosome, reduced ubiquitination of ENaC leads to the accumulation of ENaC in the plasma membrane, which results in enhanced Na+ transport. SGK1 also enhances CFTR Cl- currents in Xenopus oocytes and in pancreatic cells in culture by increasing the abundance of CFTR in the plasma membrane (Wagner, et al., 2002; Sato, et al., 2007; Caohuy, et al., 2009). In recent studies in killifish we demonstrated that transfer from freshwater to seawater rapidly (hours) increased SGK1 mRNA and protein levels, and that the increase in SGK1 preceded the rise in the abundance of CFTR in the apical membrane of the opercula epithelium (which is similar in form and function to the gill (Shaw, et al., 2008)). In killifish, the increase in SGK1 is stimulated by plasma hypertonicity rather than steroid hormones (i.e., cortisol activation of the GR) (Shaw, et al., 2008).
Since arsenic blocks the acclimation to seawater in killifish by a mechanism that does not disrupt GR-mediated induction of CFTR gene expression, yet acutely decreases CFTR protein abundance (Shaw, et al., 2007b), studies in this manuscript were designed to test the hypothesis that arsenic interferes with the ability of killifish to acclimate to seawater by interfering with SGK1 regulated trafficking of CFTR to the apical plasma membrane. The data demonstrate for the first time that arsenic reduces SGK1 mRNA expression and protein abundance, increases the ubiquitination and lysosomal degradation of CFTR, and decreases the abundance of CFTR in the apical membrane of the opercula epithelium in the killifish. These findings demonstrate that environmentally relevant levels of arsenic (10 and 100 ppb), increase the ubiquitination and degradation of CFTR, which will reduce the ability of killifish to acclimate to increased salinity and to maintain NaCl homeostasis.
2. Materials and Methods
2.1 Animals
Studies were performed in compliance with Institutional animal care and use guidelines approved by MDIBL (#A3562-01) and Dartmouth Medical School (#A3259-01). Killifish, Fundulus heteroclitus, were collected from Northeast Creek (Bar Harbor, ME, USA) and held in glass aquaria containing running seawater (pH 8.1 ± 0.4; salinity 33 ± 0.5‰, 15°C) at the MDIBL for at least two weeks to ensure acclimation to seawater. For all tests fish were maintained outdoors under natural light cycles (photoperiod 15:9-h light:dark). Killifish were fed commercial flake food (48% protein, 9% fat; Tetracichlid, Tetra, Blacksburg, VA, USA) once a day that contained no detectable inorganic arsenic, or monomethyl- or dimethyl arsenic: only arsenobetaine (determined by ICP-MS in the Dartmouth Trace Elements Analysis Core).
Acclimation to freshwater was achieved as described previously (Shaw, et al., 2007b) by gradually reducing the aquaria salinity to 10% and maintaining killifish in 10% seawater for two weeks. Subsequently, the water was changed gradually to very “soft” freshwater ([Ca++] = 50μM, [Cl-] = 20μM, [Mg++] = 50μM, [Na+] = 100μM; American Society for Testing and Materials, 1985) and the fish were maintained in “soft” freshwater for at least two weeks (i.e., freshwater adapted). Studies investigating seawater acclimation involved direct transfer of freshwater acclimated fish to 100% seawater (15°C) as previously described (Shaw, et al., 2007a; Shaw, et al., 2007b; Shaw, et al., 2008). For arsenic exposure, fish were exposed to 10 ppb, 100 ppb, or 12,000 ppb sodium arsenite in freshwater for 48 hours and then transferred to seawater that also contained the same arsenic concentration for up to 8 hrs. The 12,000 ppb concentration was selected to match our previous studies investigating the effects of arsenic on chronic seawater acclimation (Shaw, et al., 2007b). 12,000 ppb arsenic over the short time-course employed in these studies is non-toxic, does not cause cellular stress (e.g., no effect on actin or HSP70 expression), and results in environmentally relevant arsenic concentrations in killifish tissue (4.27 μg/g) (Shaw, et al., 2007b). The 10 ppb and 100 ppb concentrations were selected to represent concentrations of arsenic commonly observed in the environment (U.S. EPA, 1980) and range from the current U.S. EPA drinking water standard (10 ppb) to the criterion values for the protection of aquatic life (http://www.epa.gov/waterscience/criteria/wqctable/). Control fish were maintained in freshwater or seawater without the addition of arsenic. Measured background concentrations of arsenic in freshwater was below detection limit (1 ppb) and was between 1.5 and 3 ppb in seawater. Water to which arsenic had been added was tested by ICP-MS to ensure that the arsenic concentrations were as expected. Swim water was aerated and replaced daily, resulting in no appreciable differences in general water quality parameters (total ammonia, pH, salinity, temperature) over the test period.
2.2 Ussing chamber analysis of CFTR-mediated Cl- currents
To examine the effect of arsenite on CFTR Cl- secretion, opercular membranes were isolated from fish anesthetized in tricane (0.2 g/l) and then euthanized by double pithing. Opercular membranes were mounted in Ussing chambers, and the isoproterenol-stimulated CFTR mediated Cl- current was measured using a Physiological Instruments Voltage Clamp (Model VCC MC6, San Diego, CA), as described in detail (Karnaky and Kinter, 1977; Ernst, et al., 1980; Stanton, et al., 2006; Shaw, et al., 2008). Data collection and analysis were conducted with Labscribe software (iWORX).
2.3 Cell surface biotinylation and Western blot analysis
To examine the effect of arsenite on the abundance of CFTR in gill and the operculum, fish were anesthetized and euthanized, as described above, and gill and opercular membranes attached to the bony operculum were rapidly isolated (5 fish/observation). The biochemical determination of apical membrane CFTR in the opercular membrane was performed by cell surface biotinylation using EZ-Link™ Sulfo-NHS-LC-Biotin at 4°C, as described previously in detail (Moyer, et al., 1998; Swiatecka-Urban, et al., 2002). Previous attempts to measure plasma membrane CFTR in gill was unsuccessful, most likely because the thick mucus layer prevented the penetration of EZ-Link™ Sulfo-NHS-LC-Biotin, despite our attempts to remove this mucus. Killifish CFTR was detected in biotinylated samples and in cell lysates of gill and opercular membranes using an anti-CFTR antibody (clone 24-1; R&D Systems) as described in detail previously (Swiatecka-Urban, et al., 2002). The R&D antibody recognizes human and killifish CFTR as described by Singer and colleagues (Singer, et al., 1998) SGK1 was detected in gill using an anti-rabbit antibody from Sigma (anti-SGK, #S5188). To determine if the SGK antibody recognized killifish SGK (kfSGK), HEK 293T cells were transfected with increasing amounts of kfSGK1 cDNA (pcDNA3.1) using Effectene (Qiagen, Valencia, CA) according to the manufacturer's instructions, and SGK1 abundance was determined by Western blot analysis. Results, presented in Figure 5A, demonstrate that the SGK antibody recognized kfSGK. Actin, a loading control, was detected using an anti-actin antibody as described previously (Shaw, et al., 2007b). Because SGK1 expression is low in all tissues studied, even after stimulation with glucocorticoids, it was not possible to detect SGK1 in opercular membranes since there is very little opercular tissue. Thus, all Western blot and PCR (see below) studies on SGK1 were performed on gill, which is functionally equivalent to the operculum (Wood and Laurent, 2003). The amount of CFTR and SGK1 in Western blots was measured using NIH Image J. In each blot the entire signal from each lane shown in the Figures was measured.
Figure 5.
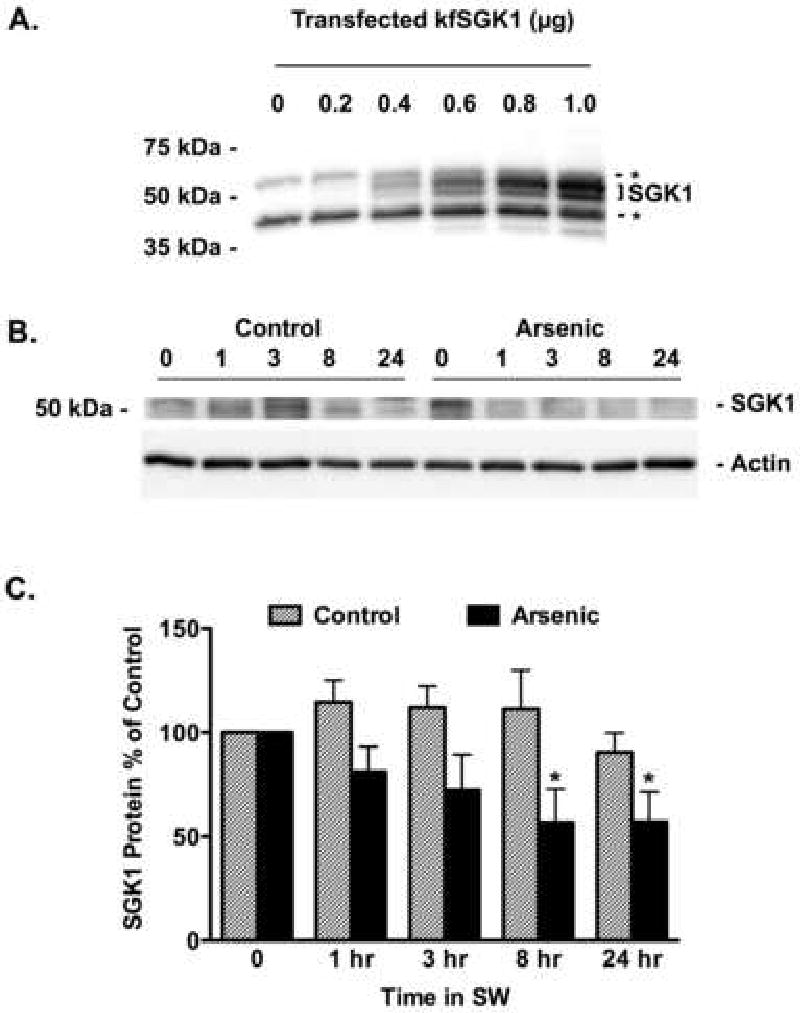
Arsenic reduced the abundance of SGK1 protein in the gill of fish transferred from freshwater (FW) to seawater (SW). A) HEK cells were transfected with kfSGK1 (0, 0.2, 0.4, 0.6, 0.8 or 1 μg cDNA) and SGK1 abundance was measured by Western blot. The nonspecific protein bands, indicated by the dashed lines and asterisks, are most evident in the lanes marked 0 and 0.2 μg cDNA. As shown previously, SGK1 is a double ∼50 kDa (Shaw et al., 2008), and its abundance increases as a function of the cDNA concentration. By contrast, the nonspecific bands do not change. This experiment demonstrates that the SGK1 antibody from Sigma recognizes kfSGK1. Fish were adapted to freshwater (FW) for two weeks, then exposed to arsenic (12,000 ppb) or vehicle for 48 hours and then placed in seawater (SW) containing arsenic (12,000 ppb) or vehicle for 1 hr, 3 hr, 8 hr and 24 hrs. B) Representative Western blots of kfSGK1. C) After the indicated times, gills were isolated and SGK1 protein in control (hatched bars) and arsenic treated fish (solid bars) were measured as described in methods. Data presented as percent of time 0. N=7/group. *P<0.05 versus control.
2.4 Detection of Ubiquitinated (Ub)-CFTR in Killifish
Killifish were adapted to freshwater for 14 days with or without 10, 100 or 12,000 ppb AsNaO2 for the last 48 hours. Fish were then moved into saltwater containing 50 μM chloroquine, a lysosomal inhibitor to allow Ub-CFTR to accumulate, with or without 10, 100 or 12,000 ppb AsNaO2. After one or three hour exposure times, fish were sacrificed and gills removed. Gill tissue was homogenized in boiling lysis buffer containing 1 mM EDTA, 50 mM NaF, 2% SDS, and Complete Protease Inhibitor Mixture (Roche Diagnostics) and heated for 10 min at 100°C. Samples were then cooled to room temperature and 4 volumes of immunoprecipitation dilution buffer was added (12.5 mM Tris pH 7.5, 187.5 mM NaCl, 2.5% Triton X-100, and Complete protease inhibitor Mixture (Roche Diagnostics)). After centrifugation at 14,000 × g for 10 min to pellet insoluble material, the soluble lysates were precleared by incubation with protein G conjugated to Sepharose beads (Pierce) at 4 °C. The precleared lysates (1000 μl) were added to the antibody-protein G-Sepharose bead (wet volume of 120 μl) complexes. CFTR was immunoprecipitated by incubation with the antibody clone 24-1 CFTR-protein G-Sepharose complex. After washing the protein G-Sepharose complex with immunoprecipitation dilution buffer four times, immunoprecipitated proteins were eluted by incubation at 100°C for 3 min in sample buffer (Bio-Rad) containing 80 mM dithiothreitol. Immunoprecipitated proteins were separated by SDS-PAGE using 7.5% gels (Bio-Rad), and analyzed by Western blotting with anti-ubiquitin primary antibody (FK2 clone, BioMol), and a horseradish peroxidase-conjugated anti-mouse secondary antibody. The immunoreactive bands were visualized with Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences). Data were normalized to total immunoprecipitated CFTR (i.e., Ub-CFTR/total immunoprecipitated CFTR) to account for any possible difference in total CFTR abundance and immunoprecipitation efficiency among tissues.
2.5 Q-RT-PCR
Quantitative-RT-PCR (Q-RT-PCR) experiments were conducted to examine the effects of increased salinity on SGK1 mRNA, the only SKG ortholog detected in killifish (Sato, et al., 2007). Gill tissue was obtained as described above and immediately stored in RNAlater (Ambion, Austin, TX). Total RNA was isolated from 30 mg of tissue (from three animals to reduce animal to animal variation (Kendziorski, et al., 2003) per observation using the RNAeasy Mini Kit (Qiagen, Valencia, CA). RNA was treated with DNase (DNA-Free, Ambion, Austin, TX) to remove contaminating DNA. Total RNA was quantified using spectrophotometric measurements (NanoDrop, NanoDrop Technologies, Rockland, DE) and RNA quality was assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies, Wilmington, DE). Two-Step RT-PCR was performed with 1 μg of total RNA (Retroscript Reverse Transcriptase, Ambion, Austin, TX) and random decamers. Primers and probe for real-time PCR were synthesized using the Assays-by-Design service (Applied Biosystems, Foster City, CA). The sequence for killifish SGK (Sato, et al., 2007) was submitted for primer/probe design and the probe target was set to a predicted exon-exon splice junction. Sequence of the primers and probe used (5′ to 3′) were:
SGK Exon 24 Forward: TCCTCAAAGTGATCGGCAAGG
SGK Exon 24 Reverse: TGCGTAAAACTGGTCGTCTGT
SGK Exon 24 Internal probe: CTTCGGCAAGGTGCTGC
The probe was 6-FAM dye-labeled with a minor groove binding modification and non-fluorescent quencher on the 3′ end. The Assays-by-Design primers and probe premixed to a concentration of 18 μM for each primer and 5 μM for each probe (equivalent to a 20× mix) were combined with TaqMan Universal Master Mix (Applied Biosystems, Foster City, CA) and cDNA diluted in RNase-free H2O in a 20 μl reaction and placed in a 96-well format spectrofluorometric thermal cycler (ABI Prism 7500 Sequence Detection System, Foster City, CA). Duplicate and/or triplicate reactions of each sample were incubated at 95° for 10 min, followed by 40 cycles of 15 sec at 95° and 1 min at 60°. In preliminary studies, Q-RT-PCR products were run on an LMP agarose gel to confirm product size, subcloned into pCR4-TOPO (Invitrogen, Carlsbad, CA) and submitted for sequence analysis to confirm identity of the product. Dilutions of SGK1 plasmid DNA prepared from the killifish Q-RT-PCR products were used to construct a standard curve. The standard curves showed a correlation coefficient close to 1 (R2 = >0.99) and were linear over a 6-log range. Equivalent amplification efficiencies of standard and target molecules were observed and melting curve dissociation analysis and sequencing revealed a single PCR product. Raw data were analyzed; baseline and threshold values set and gene expression interpolated using the external standard curves. The cDNA generated during reverse transcription and used as template was quantified (NanoDrop) and data were calculated as gene expression (fg/ng cDNA).
2.6 Analysis of data
Data are presented as mean ± SE. Statistical significance of experimental maneuvers was determined by the paired or unpaired Student's t-test, the Mann-Whitney test or ANOVA and the Tukey-Kramer Multiple Comparison Test, as appropriate, using GraphPad Instat version 3.0a for Macintosh (GraphPad Software, San Diego, California USA). A P-value <0.05 was considered significant.
3. Results
3.1 Arsenic reduces CFTR Cl- secretion
Previous studies demonstrated that arsenic (12,000 ppb) reduced the ability of freshwater acclimated killifish to survive a seawater challenge (Shaw, et al., 2007b). Because acclimation to seawater is mediated by an increase in CFTR Cl- secretion by the gill and the opercular membrane (Hoffmann, et al., 2002; Marshall, et al., 1999; Shaw, et al., 2007b; Stanton, et al., 2006; Wood and Laurent, 2003), studies were conducted to determine if arsenic reduced CFTR Cl- secretion. In these studies CFTR Cl- secretion by the opercular membrane was measured 8 hours after fish were transferred to seawater, a time where ∼100% of the fish survived (Shaw, et al., 2007b). Ussing chamber studies revealed that arsenic reduced CFTR mediated Cl-secretion by ∼60% (Figure 1). This observation suggests that arsenic inhibited the ability of killifish to acclimate to increased salinity by reducing CFTR Cl- secretion.
Figure 1.
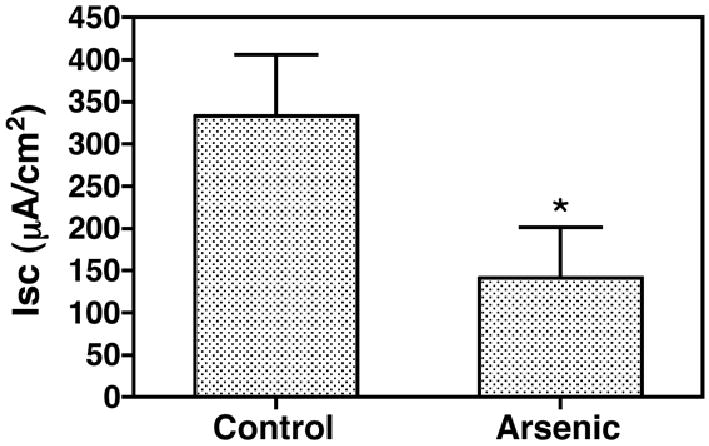
Arsenic (12,000 ppb for 48 hrs) reduces the CFTR Cl- current. Fish were adapted to freshwater (FW) for two weeks, then exposed to arsenic (12,000 ppb) or vehicle for 48 hours and then placed in seawater (SW) containing arsenic (12,000 ppb) or vehicle for 8 hr. Thereafter opercula were isolated and the CFTR Cl- current across the opercula isolated from control and arsenic treated fish was measured as described in methods. N=7/group. *P<0.05 versus Control.
3.2 Arsenic reduces the abundance of CFTR in the plasma membrane
Previously, we reported that arsenic reduced CFTR abundance in gill cell lysates (Shaw, et al., 2007b). To determine whether the decrease in CFTR Cl- secretion observed with arsenic exposure was associated with a decrease in the abundance of CFTR in the plasma membrane, cell surface biotinylation studies were performed. When freshwater adapted fish were transferred to saltwater there was a rapid (1 hr) increase in apical membrane CFTR in control fish (Figure 2). By contrast, arsenic exposure eliminated the increase in apical membrane CFTR (1 and 3 hrs) and decreased membrane CFTR 8 hours after transfer (Figure 2). CFTR in cell lysates did not change in control fish transferred to seawater (1-8 hours), whereas CFTR in arsenic treated fish was reduced at the earliest time point examined (1 hr), and remained lower than controls at 3 and 8 hrs (Figure 3). These studies confirm an earlier report that arsenic reduces CFTR in cell lysates (1-3 days after seawater transfer; Shaw et al., 2007b), and extend these observations by revealing that the decline in CFTR abundance occurs as soon as 1 hour after transfer to seawater, and that the decrease in CFTR is accompanied by a 60% reduction in CFTR Cl- secretion.
Figure 2.
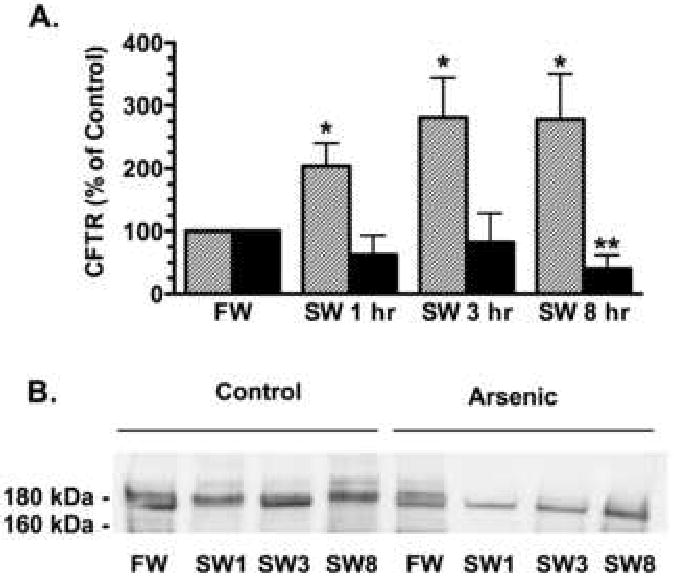
Transfer from freshwater (FW) to seawater (SW) increased the plasma membrane abundance of CFTR in control (1-8 hr) but not in arsenic treated fish. A) Summary of data. Fish were adapted to freshwater for two weeks, then exposed to arsenic (12,000 ppb) or vehicle for 48 hours and then placed in seawater containing arsenic or vehicle for 1 hr, 3 hr, and 8 hr. After the indicated times, opercula membranes were isolated and the amount of CFTR in the apical plasma membrane in control (hatched bars) and arsenic treated fish (solid bars) was measured as described in methods. Data presented as percent of FW. N=6-12/group. *P<0.05 versus FW and **P<0.05 versus FW. B) Representative Western blots of CFTR in the plasma membrane.
Figure 3.
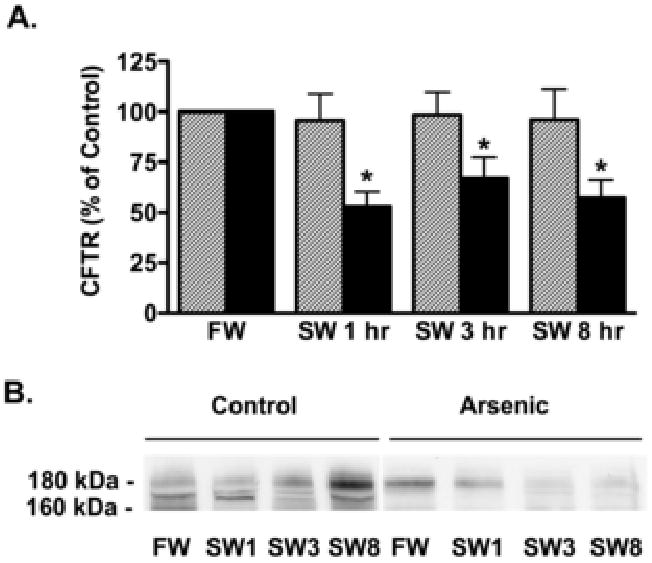
Arsenic reduced the abundance of CFTR in cell lysates of opercular membranes. There was no change in CFTR cell lysates (which include cell membrane and intracellular CFTR) in control fish. A) Summary of data. Fish were adapted to freshwater (FW) for two weeks, then exposed to arsenic (12,000 ppb) or vehicle for 48 hours and then placed in seawater (SW) containing arsenic (12,000 ppb) or vehicle for 1 hr, 3 hr, and 8 hr. After the indicated times, opercula were isolated and the amount of CFTR in control (hatched bars) and arsenic treated fish (solid bars) were measured as described in methods. Data presented as percent of time 0 data. N=7/group. *P<0.05 versus FW. B) Representative Western blots of CFTR.
3.3 Arsenic reduces SGK1 mRNA and protein expression
Transfer to seawater increases SGK1 mRNA and protein expression, which precedes the rise in plasma membrane CFTR (Shaw, et al., 2008). Because SGK1 increases the abundance of CFTR Cl- channels in the plasma membrane of Xenopus oocytes and the operculum, in a previous study we concluded that SGK1 mediates the increase in plasma membrane CFTR (Shaw, et al., 2008). Thus, Q-RT-PCR studies were conducted to determine if arsenic blocked the increase in SGK1 mRNA induced by the seawater challenge. Arsenic reduced SGK1 mRNA levels at 60 and 180 minutes after transfer to seawater, compared to control (Figure 4).
Figure 4.
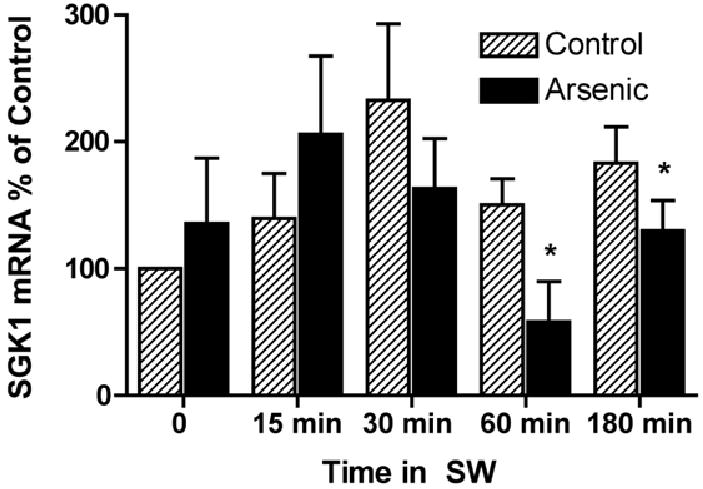
Arsenic reduced the amount of SGK mRNA in the gills of fish transferred from freshwater (FW) to seawater. Fish were adapted to freshwater (FW) for two weeks, then exposed to arsenic (12,000 ppb) or vehicle for 48 hours and then placed in seawater (SW) containing arsenic (12,000 ppb) or vehicle for 1 hr, 3 hr, 8 hr and 24 hrs. After the indicated times gills were isolated and SGK1 mRNA in control (hatched bars) and arsenic treated fish (solid bars) were measured as described in Methods. Data presented as percent of 0 time data. N=7/group. *P<0.05 versus control at each time point.
Before conducting Western blot studies to examine the effect of arsenic on SGK1 protein expression, experiments were conducted to determine if the SGK1 antibody recognized killifish SGK (kfSGK). To this end we expressed kfSGK in HEK cells, and evaluated kfSGK1 protein levels by Western blots analysis. Figure 5A demonstrates that the SGK1 antibody recognized kfSGK1. Representative Western blots on gill tissue using the SGK1 antibody are shown in Figures 5B and C. Although arsenic tended to reduce SGK1 protein abundance in killifish gill as early as 30 minutes after transfer to seawater, the decrease in protein abundance was not significant until 60 and 180 minutes (Figure 5B and C). Taken together these data reveal that arsenic blocks the seawater induced increase in both SGK1 mRNA and protein levels.
3.4 Arsenic increases ubiquitination and degradation of CFTR
SGK1 inhibits the ubiquitination and degradation of numerous ion channels and transporters (Lang, et al., 2006). To determine if arsenite, by down regulating SGK1, increased the amount of ubiquitinated CFTR in killifish, freshwater acclimated fish were exposed to arsenite or vehicle and then placed in seawater containing chloroquine (a lysosomal inhibitor that inhibits the degradation of ubiquitinated proteins) and arsenite or vehicle. After 1 and 3 hrs in seawater, arsenic (12,000 ppb) increased the amount of ubiquitinated CFTR (Ub-CFTR) by 10-fold and 3-fold, respectively (Figure 6A). Moreover, 10 ppb, the EPA drinking water standard that is considered safe for human consumption, and 100 ppb, a concentration estimated by the EPA to negatively impact aquatic communities, of arsenic increased the amount of Ub-CFTR (Figure 6B). It is interesting to note that in fish exposed to 12,000 ppb of arsenic the majority of the Ub-CFTR was ∼200 kDa (figure 6A), a ∼30 kDa increase in the molecular mass of CFTR (from 170 kDa), suggesting that CFTR was multiubiquitinated. By contrast, in fish exposed to 10 and 100 ppb of arsenic the majority of the Ub-CFTR was ≥ 170 kDa (Figure 6B), indicating that CFTR was monoubiquitinated (i.e., Ub is 8 kDa). These observation suggest that the mechanism whereby arsenic enhances the ubituitination of CFTR is dose dependent (i.e., monoubiquitinated at low concentrations and multiubiquitinated at high concentrations). Moreover, these observations support the view that environmentally relevant levels of arsenic reduce CFTR abundance by increasing the ubiquitination and lysosomal degradation of CFTR.
Figure 6.
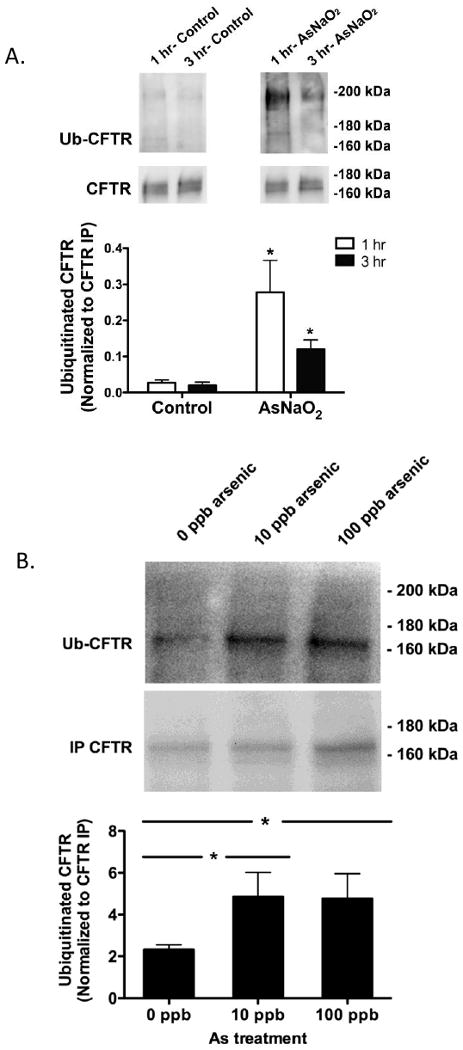
Arsenic increased the amount of ubiquitinated CFTR in the gill of fish transferred from freshwater (FW) to seawater (SW). A) Fish were adapted to freshwater (FW) for two weeks, then exposed to arsenic (12,000 ppb) or vehicle for 48 hours and then placed in seawater (SW) containing arsenic (12,000 ppb) or vehicle for 1 hr or 3 hrs. At the indicated times, gills were isolated and the amount of ubiquitinated CFTR in control (black bars) and arsenic treated fish (white bars) were measured as described in methods. B) Fish were adapted to freshwater (FW) for two weeks, then exposed to arsenic (10 or 100 ppb) or vehicle for 48 hours and then placed in seawater (SW) containing arsenic (10 or 100 ppb) or vehicle for 1 hr. After 1 hr, gills were isolated and the amount of ubiquitinated CFTR in control and arsenic treated fish were measured as described in methods. Data were normalized for total immunoprecipitated CFTR (i.e., Ub-CFTR/total immunoprecipitated CFTR) to account for any possible difference in total CFTR abundance and immunoprecipitation efficiency among tissues. n=5/group. *P<0.05 versus control (0 ppb arsenic).
4. Discussion
There are four major new findings in this manuscript that extend our understanding of SGK1 regulation of plasma membrane CFTR abundance and implicate SGK1 as a target for arsenic. During acclimation to seawater (i.e., direct transfer from freshwater to seawater), arsenic rapidly (hours): (1) inhibits the increase in SGK1 mRNA expression and protein abundance in the gills; (2) increases the amount of ubiquitinated CFTR, leading to the lysosomal degradation of CFTR; (3) decreases the abundance of CFTR in the apical plasma membrane of the opercular epithelium, and (4) inhibits CFTR Cl- secretion by the operculum.
4.1 SGK1 regulation of CFTR
The ability of the gill and opercular membrane of killifish to secrete Cl- is determined in part on the amount of CFTR in the plasma membrane. The membrane abundance of CFTR is dependent on the synthesis and exocytic insertion of CFTR into the membrane as well as its removal from the plasma membrane via endocytosis, and its recycling back to the plasma membrane (Gentzsch, et al., 2004; Swiatecka-Urban, et al., 2005; Ameen, et al., 2007). Previous studies have demonstrated that SGK1 enhances translocation of CFTR from intracellular vesicles to the plasma membrane in Xenous oocytes and killifish gill, and are consistent with the view that SGK1 stimulation of plasma membrane CFTR plays a key role in the rapid increase in gill and opercular Cl- secretion that occurs during seawater acclimation (Sato, et al., 2007; Shaw, et al., 2008; Caohuy, et al., 2009). While SGK1 has been shown to regulate the abundance of CFTR in the plasma membrane, little is known regarding the mechanisms responsible for this regulation. In a recent study in mammalian pancreatic cells Caohuy, et al. (2009) demonstrated that SGK1 increases CFTR membrane abundance by phosphorylating and inactivating Nedd4-2, an E3 ligase that interacts with and ubiquitinates CFTR, targeting it for degradation in the lysosome. Consistent with their observation, an inverse relationship was observed in the present study between SGK1 levels and the amount of ubiquitinated CFTR. These results extend our previous findings in which the abundance of plasma membrane CFTR in killifish was increased under conditions that rapidly enhance SGK1 mRNA and protein levels (Shaw, et al., 2007b).
4.2 Arsenic inhibition of seawater acclimation
Previous studies have demonstrated that our model of acute exposure to 12,000 ppb of arsenic produced environmentally relevant tissue arsenic concentrations (gill 4.27 μg/g) (Shaw, 2007b). This concentration of tissue arsenic is: (1) similar to that observed in killifish exposed to lower, environmentally relevant concentrations (787 ppb) for 14 days that resulted in hepatic tissue arsenic of 9.3 μg/g (Bears, et al., 2006); and (2) ∼7× below those observed in wild killifish (30.7 μg/g) collected in arsenic polluted waters (Moeller et al., 2003). When comparing cellular effects it is important to focus on tissue and intracellular levels rather than external exposure concentrations, and although high concentrations may be required to drive biological responses in short-term studies they produce tissue concentrations that are environmentally relevant. Importantly, we found that 12,000 ppb, as well as environmentally relevant levels of arsenic (10 ppb and 100 ppb), increased the amount of ubiquitinated CFTR in gill. Thus, our data demonstrate that environmentally relevant levels of arsenic enhance the ubiquitination of CFTR in the killifish gill. Because chloroquine, a lysosomal inhibitor, increased the amount of Ub-CFTR in gill, it is reasonable to conclude that Ub-CFTR in the gill is degraded by the lysosome.
In the present study arsenic dramatically increased the amount of Ub-CFTR in killifish gill, an observation that is consistent with the literature demonstrating that arsenic stimulates the ubiquitin-lysosomal pathway (Kirkpatrick, et al., 2003; Zheng, et al., 2003; Bredfeldt, et al., 2004; Shaw, et al., 2008). Arsenic also increases the number of ubiquitin-protein conjugates in arsenic exposed human epithelial kidney cells (HEK293) and urothelial cells (UROtsa), and in slices of rabbit renal cortex (Kirkpatrick, et al., 2003; Bredfeldt, et al., 2004). However, as noted by Kirkpatrick and colleagues (Kirkpatrick, et al., 2003) the effects of arsenic on the ubiquitin-lysosomal pathway are complex and little is known regarding the mechanism whereby arsenic influences this pathway. In a recent study, Tatham and colleagues (Tatham, et al., 2008) demonstrated that arsenic inhibited accumulation of the promyelocytic leukemia (PML) protein by inducing polyubiquitination and its subsequent degradation in the proteosome. They confirmed that arsenic acted through the E3 ubiquitin ligase, ring finger-4 (RNF4), as arsenic failed to induce degradation of PML following knockdown of RNF4 by siRNA. Mattingly (Mattingly, et al., 2009) have also shown in zebrafish that 10 ppb arsenic activated the E3 ligase mindbomb-1 (MIB-1). Although the effect of RNF4 and MIB-1 on the ubiquitination of CFTR has not been examined, the E3 ubiquitin ligase c-Cbl, which is stimulated by arsenic trioxide (Li, et al., 2009), increases the abundance of Ub-CFTR in human airway epithelial cells (Swiatecka-Urban and Stanton, personal communication). These observations raise the interesting possibility that arsenic, by inhibiting SGK1, may increase the activity of E3 ligases (e.g., RNF4, MIB-1 and/or c-CBL) and thereby increase the amount of Ub-CFTR. It is also possible that arsenic, via SGK1, may inhibit the activity of deubiquitinating enzymes, such as USP10, which has been shown by us to regulate the deubiquitination of CFTR (Bomberger et al., 2009). Additional studies, beyond the scope of the present study, are required to identify the E3 ligase(s) and the deubiquitinating enzymes that regulate the amount of Ub-CFTR and to determine if arsenic regulates these proteins either directly or indirectly via SGK1.
4.3 Model for SGK1 regulation and arsenic inhibition of plasma membrane CFTR
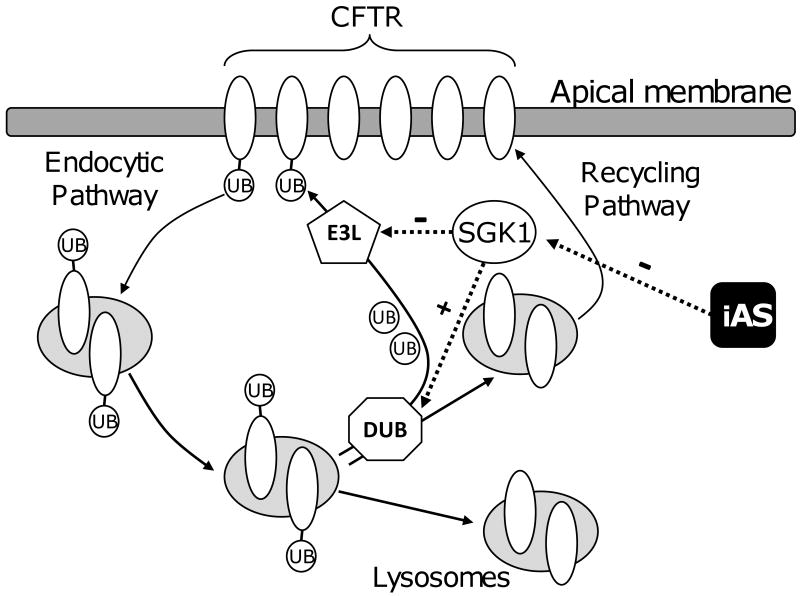
Based on these and previous studies, which demonstrate that SGK1 increases the plasma membrane expression of CFTR (Kannan-Thulasiraman, et al., 2006; Sato, et al., 2007; Ding and Warburton, 2008; Shaw, et al., 2008; Caohuy, et al., 2009; Ghosh, et al., 2009), we propose a model (Figure 7) whereby arsenic reduces CFTR abundance by inhibiting SGK1. According to this hypothetical model, ubiquitination of CFTR in the plasma membrane enhances its endocytic removal from the plasma membrane. If Ub-CFTR is deubiquitinated by a deubiquitinating enzyme (DUB) in the endosomal compartment, CFTR recycles back to the plasma membrane, thereby enhancing the half life of plasma membrane CFTR. However, if Ub-CFTR is not deubiquitinated in the endosomal compartment, it traffics to the lysosome where it is degraded, thereby reducing the half-life of CFTR. We propose that in the absence of arsenic SGK1 decreases the ubiquitination of CFTR in the plasma membrane by inhibiting an E3 ligase (e.g., RNK4, MIB-1 or c-Cbl), and/or stimulating a DUB (e.g., USP10), which increases the recycling of CFTR to the apical plasma membrane thereby increasing the half life of CFTR. We also propose that arsenic, by inhibiting SGK1 expression, enhances the activity of an E3 ligase and/or inhibits a DUB, which results in an increase in the amount of Ub-CFTR, and its degradation by the lysosome, thereby decreasing plasma membrane CFTR abundance and Cl-secretion. Additional experiments, beyond the scope of the present study, are required to test this model and to determine which E3 ligase and DUBs regulate the ubiquitination of killifish CFTR and to determine how arsenic and SGK1 regulate the activity of these enzymes.
Figure 7.
Model for SGK1 regulation and arsenic inhibition of plasma membrane CFTR during the acute phase of seawater acclimation in killifish. Deubiquinating enzyme (DUB), E3 ubiquitin ligase (E3L), inorganic arsenic (iAs), Ubiquitin (UB).
In conclusion, the data in this manuscript support a model whereby arsenic reduces the ability of killifish to acclimate to seawater by blocking the seawater induced increase in SGK1, which results in increased ubiquitination and degradation of CFTR, thereby reducing the ability of killifish to excrete Cl-.
Acknowledgments
This study was supported by NIEHS Superfund Basic Research Program Project grant P42 ESO7373 (BAS, JRS), NIEHS Center for Membrane Toxicity Studies at MDIBL P30-ES03828 (BAS and JRS), a Research Development Program grant from the Cystic Fibrosis Foundation (BAS), and a MDIBL New Investigator Award and funds from the DOD SERDP program ER1503 (JRS). We thank Dawoon Jung, J. Denry Sato, Chris Chapline, Kristen Gabor, Caitlin Stanton, Lydia Durant, and Renee Thibodeau for valuable support and advice. Comments by two anonymous reviewers improved this manuscript. Conflict of interest: none disclosed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joseph R. Shaw, Email: joeshaw@indiana.edu.
Jennifer M. Bomberger, Email: Jennifer.M.Bomberger@Dartmouth.edu.
John VanderHeide, Email: jdv8@calvin.edu.
Taylor LaCasse, Email: taylorjlacasse@yahoo.com.
Sara Stanton, Email: sstanton1989@gmail.com.
Bonita Coutermarsh, Email: Bonita.A.Coutermarsh@Dartmouth.EDU.
Roxanna Barnaby, Email: Roxanna.L.Barnaby@Dartmouth.EDU.
Bruce A. Stanton, Email: bas@Dartmouth.edu.
References
- Abernathy CO, Liu YP, Longfellow D, Aposhian HV, Beck B, Fowler B, Goyer R, Menzer R, Rossman T, Thompson C, Waalkes M. Arsenic: health effects, mechanisms of actions, and research issues. Environ Health Perspect. 1999;107:593–597. doi: 10.1289/ehp.99107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameen N, Silvis M, Bradbury NA. Endocytic trafficking of CFTR in health and disease. J Cys Fibrosis. 2007;6:1–14. doi: 10.1016/j.jcf.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Society for Testing and Materials. Annual Book of ASTM Standards Water and Environmental Technology Water (I) American Society for Testing and Materials; Philadelphia, PA: 1985. p. v. [Google Scholar]
- Bears H, Richards JG, Schulte PM. Arsenic exposure alters hepatic arsenic species composition and stress-mediated gene expression in the common killifish (Fundulus heteroclitus) Aquat Toxicol. 2006;77:257–266. doi: 10.1016/j.aquatox.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Bhalla V, Soundararajan R, Pao AC, Li H, Pearce D. Disinhibitory pathways for control of sodium transport: regulation of ENaC by SGK1 and GILZ. Am J Physiol Renal Physiol. 2006;291:F714–721. doi: 10.1152/ajprenal.00061.2006. [DOI] [PubMed] [Google Scholar]
- Bomberger JM, Barnaby RL, Stanton BA. The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. J Biol Chem. 2009;284:18778–18789. doi: 10.1074/jbc.M109.001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle RW, Jonasson IR. In: Geochemistry of Arsenic and Antimony. Canada GSo., editor. National Research Council of Canada; Ottawa, Canada: 1973. [Google Scholar]
- Bredfeldt TG, Kopplin MJ, Gandolfi AJ. Effects of arsenite on UROtsa cells: low-level arsenite causes accumulation of ubiquitinated proteins that is enhanced by reduction in cellular glutathione levels. Toxicol Appl Pharmacol. 2004;198:412–418. doi: 10.1016/j.taap.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Caohuy H, Jozwik C, Pollard HB. Rescue of DeltaF508-CFTR by the SGK1/Nedd4-2 signaling pathway. J Biol Chem. 2009;284:25241–25253. doi: 10.1074/jbc.M109.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Warburton D. Down-regulation of Sprouty2 via p38 MAPK plays a key role in the induction of cellular apoptosis by tumor necrosis factor-alpha. Biochem Biophys Res Commun. 2008;375:460–464. doi: 10.1016/j.bbrc.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst SA, Dodson WC, Karnaky KJ., Jr Structural diversity of occluding junctions in the low-resistance chloride-secreting opercular epithelium of seawater-adapted killifish (Fundulus heteroclitus) J Cell Biol. 1980;87:488–497. doi: 10.1083/jcb.87.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzsch M, Chang X, Cui L, Wu Y, Ozols VV, Choudhury A, Pagano RE, Riordan JR. Endocytic trafficking routes of wild-type and ΔF508 CFTR. Mol Biol Cell. 2004;15:2684–2696. doi: 10.1091/mbc.E04-03-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh J, Das J, Manna P, Sil PC. Taurine prevents arsenic-induced cardiac oxidative stress and apoptotic damage: role of NF-kappa B, p38 and JNK MAPK pathway. Toxicol Appl Pharmacol. 2009;240:73–87. doi: 10.1016/j.taap.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Henke KR. Arsenic : environmental chemistry, health threats and waste treatment. Wiley; Chichester, U.K.: 2009. p. xviii.p. 569. [Google Scholar]
- Hoffmann EK, Hoffmann E, Lang F, Zadunaisky JA. Control of Cl- transport in the operculum epithelium of Fundulus heteroclitus: long- and short-term salinity adaptation. Biochim Biophys Acta. 2002;1566:129–139. doi: 10.1016/s0005-2736(02)00587-4. [DOI] [PubMed] [Google Scholar]
- Kannan-Thulasiraman P, Katsoulidis E, Tallman MS, Arthur JS, Platanias LC. Activation of the mitogen- and stress-activated kinase 1 by arsenic trioxide. J Biol Chem. 2006;281:22446–22452. doi: 10.1074/jbc.M603111200. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Tosteson TD, Blum J, Morris JS, Baron JA, Klaue B. Design of an epidemiologic study of drinking water arsenic exposure and skin and bladder cancer risk in a U.S. population. Environ Health Perspect. 1998;106 4:1047–1050. doi: 10.1289/ehp.98106s41047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnaky KG, Jr, Kinter WB. Killifish opercular skin: a flat epithelium with a high density of chloride cells. J Exp Zool. 1977;199:355–364. doi: 10.1002/jez.1401990309. [DOI] [PubMed] [Google Scholar]
- Kendziorski CM, Zhang Y, Lan H, Attie AD. The efficiency of pooling mRNA in microarray experiments. Biostatistics. 2003;4:465–477. doi: 10.1093/biostatistics/4.3.465. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick DS, Dale KV, Catania JM, Gandolfi AJ. Low-level arsenite causes accumulation of ubiquitinated proteins in rabbit renal cortical slices and HEK293 cells. Toxicol Appl Pharmacol. 2003;186:101–109. doi: 10.1016/s0041-008x(02)00019-4. [DOI] [PubMed] [Google Scholar]
- Lang F, Henke G, Embark HM, Waldegger S, Palmada M, Bohmer C, Vallon V. Regulation of channels by the serum and glucocorticoid-inducible kinase - implications for transport, excitability and cell proliferation. Cell Physiol Biochem. 2003;13:41–50. doi: 10.1159/000070248. [DOI] [PubMed] [Google Scholar]
- Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- Li Y, Qu X, Qu J, Zhang Y, Liu J, Teng Y, Hu X, Hou K, Liu Y. Arsenic trioxide induces apoptosis and G2/M phase arrest by inducing Cbl to inhibit PI3K/Akt signaling and thereby regulate p53 activation. Cancer Lett. 2009;284:208–215. doi: 10.1016/j.canlet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Loffing J, Flores SY, Staub O. Sgk kinases and their role in epithelial transport. Annu Rev Physiol. 2006;68:461–490. doi: 10.1146/annurev.physiol.68.040104.131654. [DOI] [PubMed] [Google Scholar]
- Marshall WS, Emberley TR, Singer TD, Bryson SE, McCormick SD. Time course of salinity adaptation in a strongly euryhaline estuarine teleost, fundulus heteroclitus: a multivariable approach. J Exp Biol. 1999;202(Pt 11):1535–1544. doi: 10.1242/jeb.202.11.1535. [DOI] [PubMed] [Google Scholar]
- Marshall WS. Na(+), Cl(-), Ca(2+) and Zn(2+) transport by fish gills: retrospective review and prospective synthesis. J Exp Zool. 2002;293:264–283. doi: 10.1002/jez.10127. [DOI] [PubMed] [Google Scholar]
- Marshall WS, Singer TD. Cystic fibrosis transmembrane conductance regulator in teleost fish. Biochim Biophys Acta. 2002;1566:16–27. doi: 10.1016/s0005-2736(02)00584-9. [DOI] [PubMed] [Google Scholar]
- Marshall WS. Rapid regulation of NaCl secretion by estuarine teleost fish: coping strategies for short-duration freshwater exposures. Biochim Biophys Acta. 2003;1618:95–105. doi: 10.1016/j.bbamem.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Marshall WS, Cozzi RR, Pelis RM, McCormick SD. Cortisol receptor blockade and seawater adaptation in the euryhaline teleost Fundulus heteroclitus. J Exp Zool A Comp Exp Biol. 2005;303:132–142. doi: 10.1002/jez.a.129. [DOI] [PubMed] [Google Scholar]
- Mattingly CJ, Hampton TH, Brothers KM, Griffin NE, Planchart A. Perturbation of defense pathways by low-dose arsenic exposure in zebrafish embryos. Environ Health Perspect. 2009;117:981–987. doi: 10.1289/ehp.0900555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller A, MacNeil SD, Ambrose RF, Que Hee SS. Elements in fish of Malibu Creek and Malibu Lagoon near Los Angeles, California. Mar Pollut Bull. 2003;46:424–429. doi: 10.1016/S0025-326X(02)00466-6. [DOI] [PubMed] [Google Scholar]
- Moyer BD, Loffing J, Schwiebert EM, Loffing-Cueni D, Halpin PA, Karlson KH, Ismailov II, Guggino WB, Langford GM, Stanton BA. Membrane trafficking of the cystic fibrosis gene product, cystic fibrosis transmembrane conductance regulator, tagged with green fluorescent protein in madin-darby canine kidney cells. J Biol Chem. 1998;273:21759–21768. doi: 10.1074/jbc.273.34.21759. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.). Subcommittee on Arsenic in Drinking Water., NetLibrary Inc. Arsenic in drinking water. National Academy Press; Washington, D.C.: 1999. p. xvii.p. 310. [Google Scholar]
- Neff JM. Ecotoxicology of arsenic in the marine environment. Environmental Toxicology and Chemistry. 1997;16:917–927. [Google Scholar]
- Pearce D. SGK1 regulation of epithelial sodium transport. Cell Physiol Biochem. 2003;13:13–20. doi: 10.1159/000070245. [DOI] [PubMed] [Google Scholar]
- Sato JD, Chapline MC, Thibodeau R, Frizzell RA, Stanton BA. Regulation of human cystic fibrosis transmembrane conductance regulator (CFTR) by serum- and glucocorticoid-inducible kinase (SGK1) Cell Physiol Biochem. 2007;20:91–98. doi: 10.1159/000104157. [DOI] [PubMed] [Google Scholar]
- Scott GR, Richards JG, Forbush B, Isenring P, Schulte PM. Changes in gene expression in gills of the euryhaline killifish Fundulus heteroclitus after abrupt salinity transfer. Am J Physiol Cell Physiol. 2004;287:C300–309. doi: 10.1152/ajpcell.00054.2004. [DOI] [PubMed] [Google Scholar]
- Shaw JR, Jackson B, Gabor K, Stanton S, Hamilton JW, Stanton BA. The influence of exposure history on arsenic accumulation and toxicity in the killifish, Fundulus heteroclitus. Environ Toxicol Chem. 2007a;26:2704–2709. doi: 10.1897/07-032.1. [DOI] [PubMed] [Google Scholar]
- Shaw JR, Gabor K, Hand E, Lankowski A, Durant L, Thibodeau R, Stanton CR, Barnaby R, Coutermarsh B, Karlson KH, Sato JD, Hamilton JW, Stanton BA. Role of glucocorticoid receptor in acclimation of killifish (Fundulus heteroclitus) to seawater and effects of arsenic. Am J Physiol Regul Integr Comp Physiol. 2007b;292:R1052–1060. doi: 10.1152/ajpregu.00328.2006. [DOI] [PubMed] [Google Scholar]
- Shaw JR, Sato JD, VanderHeide J, LaCasse T, Stanton CR, Lankowski A, Stanton SE, Chapline C, Coutermarsh B, Barnaby R, Karlson K, Stanton BA. The role of SGK and CFTR in acute adaptation to seawater in Fundulus heteroclitus. Cell Physiol Biochem. 2008;22:69–78. doi: 10.1159/000149784. [DOI] [PubMed] [Google Scholar]
- Singer TD, Tucker SJ, Marshall WS, Higgins CF. A divergent CFTR homologue: highly regulated salt transport in the euryhaline teleost F. heteroclitus. Am J Physiol. 1998;274:C715–723. doi: 10.1152/ajpcell.1998.274.3.C715. [DOI] [PubMed] [Google Scholar]
- Stanton CR, Thibodeau R, Lankowski A, Shaw JR, Hamilton JW, Stanton BA. Arsenic inhibits CFTR-mediated chloride secretion by killifish (Fundulus heteroclitus) opercular membrane. Cell Physiol Biochem. 2006;17:269–278. doi: 10.1159/000094139. [DOI] [PubMed] [Google Scholar]
- Swiatecka-Urban A, Brown A, Moreau-Marquis S, Coutermarsh B, Barnaby R, Karlson KH, Flotte TR, Fukuda M, Langford G, Stanton BA. The short apical membrane half-life of resuced Δ508-cystic fibrosis transmembrane conductance regulator (CFTR) results from accelerated encocytosis of Δ508-CFTR in polarized human airway epithelial cells. J Biol Chem. 2005;280:36762–36772. doi: 10.1074/jbc.M508944200. [DOI] [PubMed] [Google Scholar]
- Tatham MH, Geoffroy MC, Shen L, Plechanovova A, Hattersley N, Jaffray EG, Palvimo JJ, Hay RT. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat Cell Biol. 2008;10:538–546. doi: 10.1038/ncb1716. [DOI] [PubMed] [Google Scholar]
- Tessier M, Woodgett JR. Serum and glucocorticoid-regulated protein kinases: variations on a theme. J Cell Biochem. 2006;98:1391–1407. doi: 10.1002/jcb.20894. [DOI] [PubMed] [Google Scholar]
- Thomas CP, Itani OA. New insights into epithelial sodium channel function in the kidney: site of action, regulation by ubiquitin ligases, serum- and glucocorticoid-inducible kinase and proteolysis. Curr Opin Nephrol Hypertens. 2004;13:541–548. doi: 10.1097/00041552-200409000-00010. [DOI] [PubMed] [Google Scholar]
- United States. Environmental Protection Agency. Office of Water Regulations and Standards. Criteria & Standards Division. Ambient water quality criteria for arsenic. Washington, D.C. Springfield, Va.: 1980. p. 211. in various pagings. [Google Scholar]
- Vallon V, Wulff P, Huang DY, Loffing J, Volkl H, Kuhl D, Lang F. Role of Sgk1 in salt and potassium homeostasis. Am J Physiol Regul Integr Comp Physiol. 2005;288:R4–10. doi: 10.1152/ajpregu.00369.2004. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Nepomuceno IB, Messner AH, Moran ML, Batson EP, Dimiceli S, Brown BW, Desch JK, Norbash AM, Conrad CK, Guggino WB, Flotte TR, Wine JJ, Carter BJ, Reynolds TC, Moss RB, Gardner P. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum Gene Ther. 2002;13:1349–1359. doi: 10.1089/104303402760128577. [DOI] [PubMed] [Google Scholar]
- Wood CM, Laurent P. Na+ versus Cl- transport in the intact killifish after rapid salinity transfer. Biochim Biophys Acta. 2003;1618:106–119. doi: 10.1016/j.bbamem.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Zheng XH, Watts GS, Vaught S, Gandolfi AJ. Low-level arsenite induced gene expression in HEK293 cells. Toxicology. 2003;187:39–48. doi: 10.1016/s0300-483x(03)00025-8. [DOI] [PubMed] [Google Scholar]