Abstract
Parkinson's disease (PD) presents with extensive heterogeneity in symptomatology, inviting examination of disease subtypes. One significant categorization is by whether patients present at onset with tremor as the dominant symptom (TD) or with nontremor symptoms (NTD). We examined differences in quality of life between TD and NTD patients using the Parkinson's Disease Questionnaire-39 (PDQ-39), correlating performance with aspects of motor function as indexed by the Unified Parkinson's Disease Rating Scale (UPDRS). Participants included 35 nondemented individuals (19 TD, 16 NTD) matched on clinical and demographic characteristics. NTD had significantly lower overall PDQ-39 scores, particularly for the mobility subscale. Several UPDRS subscale scores significantly correlated with quality of life, especially for NTD. Further, the correlations were driven by nontremor type symptoms, even in TD patients. Determining reliable subtypes of PD may aid in prognosis and treatment optimization, thereby enhancing quality of life in afflicted individuals.
1. Introduction
Parkinson's disease (PD) is a degenerative disorder of the central nervous system that demonstrates extensive heterogeneity in symptomatology, leading many investigators to examine disease subtypes [1]. Researchers have used predominance of specific motor symptoms, age of disease onset, laterality of symptoms, disease severity, cognitive performance, and several other categories to define subtypes of PD [2].
The classification of initial symptom has been a major focus of recent research. Individuals displaying primarily rigidity and gait and balance symptoms (nontremor dominant; NTD) and those with mainly tremor symptoms (tremor dominant; TD) at diagnosis present differences in clinical and cognitive profile [1–5]. In addition to exhibiting more severe cardinal motor deficits including problems with gait and balance, NTD individuals have been shown to have increased levels of axial motor symptoms such as freezing, falls, and difficulty with speech and swallowing, as well as nonmotor symptoms such as poorer cognitive functioning (particularly executive deficits) and increased levels of depression [1–3, 5]. NTD individuals have also been shown to be more likely to experience early onset dementia than patients with TD [1].
Clinical differences found between TD and NTD may be related to distinct neuropathological profiles that contribute to tremor or nontremor symptoms. While tremor may implicate pathology in the cortico-striato-thalamocortical circuits caused by progressive dopamine depletion in the substantia nigra, the presence of both axial motor symptoms and nonmotor symptoms may reflect more extensive pathology in additional cortical and subcortical brain regions. A recent pathological analysis demonstrated that NTD cases had significantly wider Lewy body distribution and increased density in the substantia nigra, frontal, and transentorhinal regions than did TD cases. NTD patients also showed significantly more Alzheimer-like neurofibrillary pathology and increased plaque formation in the neocortex as well as more frequent amyloid angiopathy [1].
Motor subtypes may be relevant to understanding specific factors that may contribute to a patient's quality of life, thereby providing additional targets for PD treatments and interventions [5]. The Parkinson's Disease Questionnaire-39 (PDQ-39) is a widely used self-report measure that includes subscales measuring mobility, activities of daily living, emotional well-being, stigma, social support, communication, cognition, and bodily discomfort [6]. Factors that have been shown to affect quality of life in PD include depression, anxiety, cognitive functioning, motor symptom severity, and the presence of additional axial motor symptoms [4, 7, 8].
One study found that patients with the akinetic-rigid disease type of PD endorsed more negative symptoms on the PDQ-39 than did individuals with the TD disease type [9]. A recent report found differences in activities of daily living and quality of life between TD and NTD individuals even prior to their diagnosis of PD [10]. Specifically, this study found that individuals in the NTD group had significant limitations in activities of daily living and a more negative quality of life at their initial neurologist visit prior to any dopaminergic treatment. Even at this first visit to the neurologist, individuals in the NTD group had significantly higher scores on the Unified Parkinson's Disease Rating Scale (UPDRS) and higher Hoehn and Yahr staging than individuals in the TD group. Questions remain as to the specific differences in quality of life for subgroups of PD independent of motor symptom severity and stage of disease.
The Motor Examination (Part III) of the UPDRS includes 26 items with scores ranging from 0 (absent or normal) to 4 (more severe impairment) [11]. Responses on the UPDRS Motor Examination load onto six motor symptom domains including tremor, rigidity, bradykinesia, facial expression, speech, and axial impairments [12]. The six motor domains can also be grouped into two subscales that represent predominantly dopaminergic or nondopaminergic symptoms based on levodopa responsiveness [12]. The dopamine-deficiency (DA) subscale includes the tremor, rigidity, bradykinesia, and facial expression subscale scores, while the nondopamine deficiency (non-DA) subscale includes the speech and axial symptom subscale scores [12].
To the best of our knowledge, no studies to date have directly examined the relation between the UPDRS and its subscales with the subscales of the PDQ-39. This comparison could elucidate the specific symptoms that most directly contribute to a negative quality of life in individuals with PD, which is important for designing the most effective treatment plans. Because of the primacy of poorer quality of life in NTD relative to TD patients, the present study aimed to examine differences in quality of life in these subtypes using the PDQ-39 and additionally to examine the associations between the subscales of the PDQ-39 and the subscales of the UPDRS motor examination.
2. Methods
2.1. Participants
Thirty-five nondemented patients with PD (22 men, 13 women) were recruited from the outpatient Movement Disorders Clinic of the Department of Neurology, Boston Medical Center. The study was approved by the Boston University Institutional Review Board, and all participants provided informed consent. On the modified Mini-Mental State Examination (mMMSE) [13], a cutoff score of 25 was used for PD participants as this form of the MMSE is particularly sensitive to specific cognitive deficits found in PD without dementia (scores converted from the 57-point scale). This version of the MMSE includes additional executive functioning components, such as forward and backward digit span, as well as additional construction items. Individuals with a history of substance abuse, head injury, or neurologic disorders besides PD were excluded. None met criteria for Dementia with Lewy Bodies as per McKeith [14].
Medication information was obtained for all participants. Levodopa equivalent dosages (LED) were calculated based on previous reports with LED: (regular levodopa dose × 1) + (levodopa controlled-release dose × 0.75) + (pramipexole dose × 67.0) + (ropinirole dose × 16.67) + (rotigotine × 16.67) + (pergolide dose and cabergoline dose × 67.0) + (bromocriptine dose × 10) + ([regular levodopa dose + levodopa controlled-release dose × 0.75) × 0.25]) if taking tolcapone or entacapone [15].
2.2. Self-Report Measures
Motor symptom severity was quantified using the UPDRS and Hoehn and Yahr stage [16]. Information on type of motor symptom at onset was obtained through patient report and confirmed when possible by neurologist review. Based on the response to the UPDRS question addressing the first PD symptom experienced, subjects were categorized as either TD (tremor dominant, n = 16) or NTD (nontremor dominant, n = 19). Mood was assessed using the Beck Depression Inventory (BDI-2) [17] and Beck Anxiety Inventory (BAI) [18]. All patients received the PDQ-39 [6]. Higher scores on all questionnaires indicate more severe symptoms.
2.3. Statistical Analysis
Independent samples t-tests were used to compare demographic and clinical characteristics of the subgroups. The Mann-Whitney U test was used to compare TD and NTD individuals on the PDQ-39 and its subscales. Spearman rank order correlations were used to examine associations between subscales of the PDQ-39 and UPDRS for all the PD participants and for TD and NTD patients separately. A P value of <.01 was considered significant to control for multiple comparisons except for subgroup analyses and where otherwise noted.
3. Results
3.1. Comparison of TD and NTD Patients on PDQ-39
Demographic and clinical characteristics of TD and NTD patients can be found in Table 1. TD and NTD individuals did not significantly differ on age, UPDRS total, disease duration, side of onset, male:female ratio, BAI, BDI, LED, or MMSE score. Groups differed significantly on years of education, with NTD individuals having significantly more years of education (P < .05). Education was accordingly included as a covariate in subsequent group analyses but did not alter any of the findings.
Table 1.
Participant characteristics.
| Age | Female : male | Education, years | UPDRS, total | Duration of Illness, years | H and Y Stage† | MMSE | BDI | BAI | |
|---|---|---|---|---|---|---|---|---|---|
| NTD (n = 19) |
64.0 (8.7) | 7 : 12 | 17.7 (2.5)* | 23.2 (9.1) | 8.0 (4.3) | 2 (2) | 27.9 (1.2) | 9.0 (4.3) | 9.4 (5.9) |
| TD (n = 16) |
68.7 (6.2) | 6 : 10 | 15.8 (1.9) | 27.4 (9.4) | 9.8 (5.9) | 1.5 (2) | 27.8 (1.0) | 7.5 (5.2) | 6.9 (3.2) |
NTD: nontremor dominant; TD: tremor dominant; UPDRS: Unified Parkinson's Disease Rating Scale; MMSE: Mini-Mental State Exam; H and Y: Hoehn and Yahr; BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory. All values reported as means and standard deviations unless otherwise noted. *P < .05; †median and range are reported.
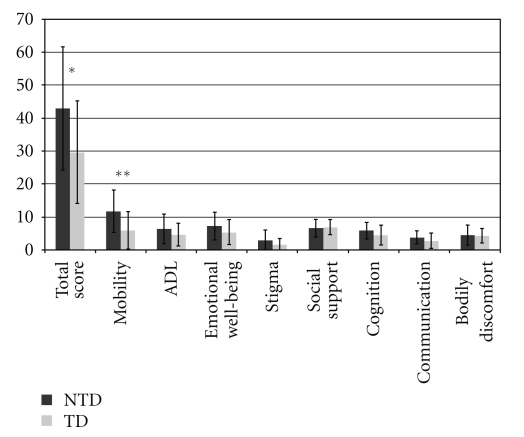
Total PDQ-39 score was significantly higher for NTD individuals than TD individuals (P < .03). A comparison of the subscales of the PDQ-39 indicated that NTD individuals had significantly higher scores for the PDQ-39 mobility subscale than TD individuals (P < .01), as shown in Figure 1. There were no significant differences between groups for the remaining subscales of the PDQ-39.
Figure 1.
Mean subscale scores on the PDQ-39 for NTD and TD participants; **P < .01, *P < .05.
3.2. Correlations between Scores on PDQ-39 and UPDRS
Performance on several subscales of the UPDRS was significantly correlated with scores on the PDQ-39 subscales. In the entire PD group, scores on the Activities of Daily Living (ADL) subscale of the PDQ-39 correlated significantly with those of both the Rigidity (r = .40, P < .01) and Dopamine- (DA-) dependent subscales of the UPDRS (r = .44, P < .01). Scores on the Communication subscale of the PDQ-39 correlated significantly with those of both the Rigidity (r = .53, P < .01) and Facial Expression subscales of the UPDRS (r = .47, P < .01).
When correlations were conducted for TD and NTD patients separately, a different pattern of results emerged for the two groups. For NTD individuals, scores on the ADL subscale of the PDQ-39 correlated significantly with those on the Rigidity (r = .67, P < .02), Bradykinesia (r = .67, P < .01), Speech (r = .54, P < .02), and DA subscales of the UPDRS (r = .69, P < .01). Additionally, scores on the Communication subscale of the PDQ-39 correlated significantly with those on the Bradykinesia (r = .59, P < .01), Facial Expression (r = .66, P < .01), and DA subscales of the UPDRS (r = .61, P < .01). For TD individuals, scores on the Cognition subscale of the PDQ-39 correlated significantly with those on the Axial subscale of the UPDRS (r = .59, P < .02), and scores on the Communication subscale of the PDQ-39 correlated significantly with those on the Rigidity subscale of the UPDRS (r = .74, P < .01). These results are summarized in Table 2. NTD individuals showed a greater number of significant correlations between subscales of the UPDRS and subscales of the PDQ-39 than did TD individuals. Additionally, scores on the subscales of the UPDRS that correlated with scores on the PDQ-39 were primarily those corresponding to nontremor symptoms in both NTD and TD individuals.
Table 2.
Significant correlations between PDQ-39 and UPDRS subscales for NTD and TD subjects.
| UPDRS subscales | NTD | TD | ||
|---|---|---|---|---|
| PDQ-39 subscales | ||||
| ADL | Communication | Cognition | Communication | |
| Rigidity | 0.67* | NS | NS | 0.74** |
| Bradykinesia | 0.67** | 0.59** | NS | NS |
| Speech | 0.54* | NS | NS | NS |
| DA-dependent | 0.69** | 0.61** | NS | NS |
| Facial expression | NS | 0.66** | NS | NS |
| Axial | NS | NS | 0.59* | NS |
**P < .01,
*P < .02.
4. Discussion
We found that individuals with PD who experienced nontremor symptoms such as rigidity and gait and balance impairments at diagnosis reported a significantly worse quality of life than individuals who experienced tremor as their initial symptom. This finding was particularly significant in the domain of issues relating to mobility. Although performance on the mobility subscale alone was significantly different in NTD and TD, NTD patients endorsed a worse quality of life than TD patients on all domains except in the domain of social support, and their overall PDQ score indicated significantly worse self-perceived quality of life.
These results are in line with those from Hariz and Forsgren, who found significant differences in PDQ-39 scores in individuals with the TD and NTD subtypes of PD [10]. Specifically, in their study the subgroup with NTD had significantly worse scores than the TD subgroup for the mobility, ADL, Communication, and Bodily Discomfort subscales as well as for total scores [10]. We found significant differences between NTD and TD for the total PDQ-39 score as well as for the mobility subscale score. Hariz and Forsgren reported significant differences between the NTD and TD subgroups for total UPDRS scores and Hoehn and Yahr staging, with the NTD group exhibiting more severe deficits than the TD subgroup [10]. The present study extended these findings by showing that group differences in PDQ-39 scores were independent of motor symptom severity or stage of disease, as no significant differences were found between NTD and TD individuals for scores on the UPDRS examination or Hoehn and Yahr staging in our sample.
Our results also demonstrated that subscales derived from a commonly used quality of life measure, the PDQ-39, correlated significantly with subscales of the UPDRS. In the entire PD group, scores on the Rigidity subscale of the UPDRS were significantly correlated with those on both the ADL and Communication subscales of the PDQ-39, whereas scores on the Tremor subscale did not correlate significantly with those on any PDQ-39 subscale. A separate analysis for TD and NTD patients revealed that the correlations between performance on the PDQ-39 and UPDRS were primarily driven by nontremor symptoms even in TD patients. This finding indicates that nontremor symptoms in general more negatively impact quality of life, regardless of initial motor symptom. The subscales of the UPDRS that pointed to significant relations with the quality of life subscales of the PDQ-39 for TD individuals were for axial symptoms and symptoms of rigidity. That is, for both TD and NTD individuals, nontremor type symptoms appear to negatively impact multiple domains of their quality of life. These results provide evidence that tremor as a singular symptom may not significantly compromise quality of life, whereas symptoms of rigidity and bradykinesia may act as more substantial contributors to deficits in self-reported quality of life.
Our findings provide insight into how the common motor symptoms of PD negatively affect specific aspects of quality of life. For example, performance on the Communication subscale of the PDQ-39 was significantly related to performance on the Facial Expression subscale of the UPDRS, suggesting that difficulties with displaying facial expressions contribute to problems with communication for patients with PD. A recent study examining the effectiveness of a randomized controlled rehabilitation trial for PD used the PDQ-39 to evaluate improvements in quality of life [19]. Patients demonstrated the greatest improvements in the communication domain of the PDQ-39 [19]. This finding demonstrated that communication may be significantly related to quality of life and can be particularly receptive to rehabilitation techniques. Accordingly, health care providers should focus on efforts to improve speech and communication skills in individuals with PD. Understanding the relation between specific symptoms of PD and quality of life may inform intervention strategies in order to most effectively improve life quality for individuals with PD.
Additionally, for the entire PD group, scores on the ADL subscale of the PDQ-39 also correlated significantly with Dopamine- (DA-) dependent subscales of the UPDRS, which includes the tremor, rigidity, bradykinesia, and facial expression subscale scores. The non-DA-dependent subscale includes the speech and axial symptom subscale scores of the UPDRS. This finding provides evidence that the physical symptoms of PD may have a larger effect on self-reported quality of life than axial symptoms. This correlation was driven by the NTD patients, indicating that quality of life is more affected by symptoms in NTD individuals rather than individuals with a tremor-dominant symptoms at diagnosis.
Poorer scores on the PDQ-39 may reflect that individuals with the NTD symptom profile (i.e., more rigidity, gait, and balance problems) experience more extensive disease pathology than TD individuals [1]. While the presence of a tremor may indicate the dopamine depletion in the substantia nigra that negatively affects cortico-striato-thalamocortical circuits, the presence of nontremor symptoms may indicate pathology in additional cortical and subcortical structures of the brain, such as frontal regions and the transentorhinal cortex [1]. Individuals with NTD may have a wider distribution and greater density of Lewy bodies in the brain than TD individuals, which may contribute to more frequent or more severe nontremor symptoms that ultimately have a negative effect on quality of life. These symptoms may not respond as well to the dopaminergic treatments that have been shown to significantly improve the quality of life in individuals with PD [1].
In summary, the present study provides evidence that individuals with nontremor initial symptoms of PD endorse a more negative quality of life than individuals who experience tremor as the initial symptom, despite lack of differences in disease duration or severity. A novel finding of this study is the demonstration of significant correlations between performance on specific subscales of quality of life and specific motor symptom domains. These correlations were driven by nontremor symptoms, even in individuals with tremor as the initial symptom. Determining reliable subtypes and specific motor symptom profiles of PD may aid in prognosis and individualized treatment plans, as well as assist researchers in advancing the understanding of the etiology of PD.
Acknowledgments
This study was supported by a Ruth L. Kirschstein National Research Service Award from the National Institute of Neurological Disorders and Stroke (1F31NS061555) and a Clara Mayo Foundation Fellowship from the Department of Psychology, Boston University (K. Stavitsky), and by NINDS grant R01 NS050446 (A. Cronin-Golomb). Marie Saint-Hilaire, M.D., F.R.C.P.C., Cathi Thomas, RN, M.S., and Denyse Turpin, RN, M.P.H. of the Department of Neurology, Boston Medical Center provided valuable support of the authors' participant recruitment efforts. Chase Ansok, Sophie Blease, Eric Griffith, Patricia Johnson, Barrett Phillips, and Jessica Saurman assisted with testing participants and scoring data. The authors acknowledge with gratitude the efforts of these colleagues and especially the efforts of all of the individuals who participated in this study.
References
- 1.Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ. A clinico-pathological study of subtypes in Parkinson’s disease. Brain. 2009;132(11):2947–2957. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- 2.Lewis SJG, Foltynie T, Blackwell AD, Bobbins TW, Owen AM, Barker RA. Heterogeneity of Parkinson’s disease in the early clinical stages using a data driven approach. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76(3):343–348. doi: 10.1136/jnnp.2003.033530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Rooden SM, Visser M, Verbaan D, Marinus J, van Hilten JJ. Motor patterns in Parkinson’s disease: a data-driven approach. Movement Disorders. 2009;24(7):1042–1047. doi: 10.1002/mds.22512. [DOI] [PubMed] [Google Scholar]
- 4.Visser M, van Rooden SM, Verbaan D, Marinus J, Stiggelbout AM, van Hilten JJ. A comprehensive model of health-related quality of life in Parkinson’s disease. Journal of Neurology. 2008;255(10):1580–1587. doi: 10.1007/s00415-008-0994-4. [DOI] [PubMed] [Google Scholar]
- 5.Reijnders JSAM, Ehrt U, Lousberg R, Aarsland D, Leentjens AFG. The association between motor subtypes and psychopathology in Parkinson’s disease. Parkinsonism and Related Disorders. 2009;15(5):379–382. doi: 10.1016/j.parkreldis.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Quality of Life Research. 1995;4(3):241–248. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- 7.Klepac N, Trkulja V, Relja M, Babić T. Is quality of life in non-demented Parkinson’s disease patients related to cognitive performance? A clinic-based cross-sectional study. European Journal of Neurology. 2008;15(2):128–133. doi: 10.1111/j.1468-1331.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- 8.Montel S, Bonnet AM, Bungener C. Quality of life in relation to mood, coping strategies, and dyskinesia in parkinson’s disease. Journal of Geriatric Psychiatry and Neurology. 2009;22(2):95–102. doi: 10.1177/0891988708328219. [DOI] [PubMed] [Google Scholar]
- 9.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? Journal of Neurology Neurosurgery and Psychiatry. 2000;69(3):308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hariz G-M, Forsgren L. Activities of daily living and quality of life in persons with newly diagnosed Parkinson's disease according to subtype of disease, and in comparison to healthy controls. Acta Neurologica Scandinavica. 2011;123(1):20–27. doi: 10.1111/j.1600-0404.2010.01344.x. [DOI] [PubMed] [Google Scholar]
- 11.Fahn S, Elton R. Unified Parkinson's disease rating scale. In: Fahn S, Mardsen CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. Florham Park, NJ, USA: Macmillan Health Care Information; 1987. [Google Scholar]
- 12.Levy G, Louis ED, Cote L, et al. Contribution of aging to the severity of different motor signs in Parkinson disease. Archives of Neurology. 2005;62(3):467–472. doi: 10.1001/archneur.62.3.467. [DOI] [PubMed] [Google Scholar]
- 13.Stern Y, Sano M, Paulson J, Mayeux R. Modified mini-mental state examination:validity and reliability. Neurology. 1987;37, article 179 [Google Scholar]
- 14.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Journal of Alzheimer’s Disease. 2006;9(3):417–423. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- 15.Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen JP. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson’s disease over time. Journal of Neurology, Neurosurgery and Psychiatry. 2008;79(4):387–391. doi: 10.1136/jnnp.2007.116830. [DOI] [PubMed] [Google Scholar]
- 16.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 17.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 19.Tickle-Degnen L, Ellis T, Saint-Hilaire MH, Thomas CA, Wagenaar RC. Self-management rehabilitation and health-related quality of life in Parkinson’s disease: a randomized controlled trial. Movement Disorders. 2010;25(2):194–204. doi: 10.1002/mds.22940. [DOI] [PMC free article] [PubMed] [Google Scholar]