Abstract
We sought to investigate the T1 kinetics of blood and myocardium after three infusion schemes of gadobenate dimeglumine (Gd-BOPTA) and subsequently compared contrast-enhanced whole-heart coronary MRI after a bolus Gd-BOPTA infusion with nonenhanced coronary MRI at 1.5T. Blood and myocardium T1 was measured in seven healthy adults, after each underwent three Gd-BOPTA infusion schemes (bolus: 0.2 mmol/kg at 2 ml/sec, hybrid: 0.1 mmol/kg at 2 ml/sec followed by 0.1 mmol/kg at 0.1 ml/sec, and slow: 0.2 mmol/kg at 0.3 ml/sec). Fourteen additional subjects underwent contrast-enhanced coronary MRI with an inversion-recovery steady-state-free-precession sequence after bolus Gd-BOPTA infusion. Images were compared with nonenhanced T2-prepared SSFP whole-heart coronary MRI in signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR), depicted vessel length, vessel sharpness, and subjective image quality. Bolus and slow infusion schemes resulted in similar T1 during coronary MRI, whereas the hybrid infusion method yielded higher T1 values. A bolus infusion of Gd-BOPTA significantly improved SNR, CNR, depicted coronary artery length, and subjective image quality when all segments were collectively compared but not when compared segment by segment. In conclusion, whole-heart SSFP coronary MRI at 1.5T can benefit from a bolus infusion of 0.2 mmol/kg Gd-BOPTA.
Keywords: whole heart coronary MRI, contrast-enhanced, gadobenate dimeglumine, T1 kinetics
INTRODUCTION
Despite considerable advances during the past decade, coronary MRI still faces major challenges including suboptimal spatial resolution, signal-to-noise ratio (SNR), and blood-myocardium contrast-to-noise ratio (CNR). Signal and contrast enhancement methods such as T2 magnetization preparation (1,2) and sublingual isosorbide dinitrate administration (3) have been used in combination with both gradient-recalled echo (GRE) or steady-state free precision (SSFP) imaging to improve SNR and CNR at 1.5 T. Exogenous contrast agent administration is an alternative method to improve SNR and CNR by shortening the T1 relaxation time. Both extracellular (4-6) and intravascular (7-9) contrast agents have been reported. Conventional gadolinium-based extracellular contrast agents (i.e., gadopentetate dimeglumine [Gd-DPTA]) diffuse rapidly into the interstitial space. Therefore, early contrast–enhanced coronary MRI studies focused on breath-hold coronary MRI to take advantage of the first-passage of these agents (5). This approach forces a compromise in spatial resolution and thus in its suitability for whole-heart coronary acquisitions (10).
Gadobenate dimeglumine ([Gd-BOPTA] MultiHance; Bracco Imaging SpA, Milan, Italy) is a weak protein-binding contrast agent with a prolonged plasma half-life compared with that of Gd-DTPA (11). A study that compared breath-hold coronary MRI with use of the two contrast agents showed superior SNR and CNR of Gd-BOPTA (12). Bi and colleagues (6) described improved whole-heart coronary artery MRI at 3T using an inversion recovery (IR) GRE sequence with a slow contrast infusion of Gd-BOPTA at 0.3 ml/s. This infusion method was also used in a single-center coronary MRI study and showed promising sensitivity and specificity (13). However, the kinetics and time course of the T1 shortening effects associated with different Gd-BOPTA injection strategies, such as the slow infusion reported by Bi et al. (6), remain to be systematically studied to better understand the impact of various infusion schemes in coronary MRI. Furthermore, the utility of Gd-BOPTA for whole-heart SSFP coronary MRI at 1.5T, a widely available system for cardiac MR, is still uncertain. Hence, we sought to investigate the kinetics of blood and myocardium T1 with three different Gd-BOPTA infusion schemes. Subsequently, we sought to evaluate a contrast-enhanced whole-heart inversion recovery (IR) SSFP coronary MRI approach at 1.5T with signal enhancement provided by a fast bolus infusion of Gd-BOPTA.
MATERIALS AND METHODS
In this HIPPA-compliant study, written informed consent was obtained from all subjects and the imaging protocol was approved by our institutional review board. All subjects were imaged with a 1.5T Achieva magnet (Philips Healthcare, Best, The Netherlands) with a 32-channel cardiac array coil (In Vivo Corporation, Gainesville, FL) for which an electronic signal combiner was used to form a 16-channel signal (8 from anterior and 8 from posterior) from the 32-channel coil array or a 5-channel phased-array coil.
T1 Kinetics Study
Seven clinically healthy adults (five women; age, 23 ± 2 years) underwent the T1 kinetic study. Exclusion criteria included renal impairment (estimated glomerular filtration rate < 60 ml/min/1.73 m2), any active medical issues, or contraindications to MRI. Each subject underwent MRI three times using a different infusion scheme with each imaging session, There was at least 1 week between successive studies. For each study, a total of 0.2 mmol/kg Gd-BOPTA was administered. In the first infusion scheme (bolus), Gd-BOPTA was injected at 2 ml/sec. In the second scheme (slow), Gd-BOPTA was injected at 0.3 ml/sec (similar to the infusion rate reported by Bi et al. (6)). In the third scheme (hybrid), 0.1 mmol/kg Gd-BOPTA was injected at 2 ml/sec, immediately followed by a very slow infusion of 0.1 mmol/kg at 0.1 ml/sec. The order in which the three schemes were performed on each subject was randomized. The commencement, duration, and completion time of contrast material infusion for each imaging study were recorded.
For each study, scout SSFP images in three orthogonal planes were acquired to localize the anatomy. This was followed by an electrocardiogram (ECG)-triggered free-breathing IR-prepared T1-weighted segmented two-dimensional single-section echo-planar imaging ([EPI] Look-Locker) sequence (14) axially prescribed at the level of mid left ventricle with variable inversion time (TI) for quantitative T1 measurements. The sequence parameters were field of view, 270 × 270 mm2; repetition time msec/echo time msec, 40/4.5; EPI factor, 9; flip angle, 10°; spatial resolution, 2.7 × 2.7 mm2; section thickness, 10 mm. The sequence was repeated continuously 60–80 times, with no time gap in between, for up to 30 minutes after initiation of contrast injection. The Look-Locker sequence was started about 30 seconds before contrast injection to acquire a single data set to obtain a base-line T1 measurement.
Whole-Heart Coronary MRI
Fourteen additional clinically healthy adults (eight women; age, 22 ± 3 years) were subsequently recruited for whole-heart coronary MRI using the Gd-BOPTA administration scheme chosen from the results of the T1 kinetic study. In each imaging session, a breath-held high-temporal-resolution cine SSFP image was obtained in an axial view (TR/TE=3.7/1.8 ms; temporal resolution, 48 msec; spatial resolution, 1.2 × 1.2 mm2; and ×2 acceleration) to visually identify the quiescent period of the right coronary artery (RCA), which was subsequently used as the ECG trigger delay for whole-heart coronary MRI.
A free-breathing ECG-triggered navigator-gated SSFP sequence was used in whole-heart coronary MRI before contrast administration. A T2 magnetization preparation pulse (echo time, 50 msec) (1) and a spectrally selective fat-saturation sequence were used to improve contrast between coronary arteries and surrounding myocardium and fat. To compensate for respiratory motion, a navigator placed on the dome of the right hemi-diaphragm with prospective real-time correction was used with a 5-mm end-expiratory gating window and 0.6 section tracking ratio (15,16). The imaging parameters were TR/TE=3.6/1.8 ms; flip angle, 90°; field of view, 300 × 300 ×120 mm3; spatial resolution, 1.3 × 1.3 × 1.3 mm3; reconstructed to 0.65 × 0.65 × 0.65 mm3. The k-space lines were acquired in a centric radial order in the ky-kz plane (17,18). To suppress flow and SSFP artifacts, a shorter excitation pulse (duration 0.66 msec) with no side lobe was used to enable shorter repetition time. To compensate for the imperfect slab profile of using such excitation pulse, the image encoding to excitation factor was set to 1.6. The reconstructed images outside of the prescribed field of view (FOV) in slice direction were subsequently discarded. The data acquisition was accelerated by a rate of 2 in the phase-encoding (anterior-posterior) direction by using a sensitivity encoding (SENSE) sequence (19).
To measure SNR and CNR, a noise image was obtained immediately after coronary MRI during the same study. The noise image was obtained with the same sequence as the preceding whole-heart MRI except that all radio-frequency pulses were turned off, such that only noise was acquired, and all data were accepted regardless of navigator signal. This is analogous to the previously reported noise measurement where the noise in each channel is acquired before data acquisition in the pre-imaging phase and the reconstruction compensates for g-factor of parallel imaging reconstruction (20). The SENSE regularization parameters between the imaging and noise image reconstructions were identical. This enables accurate comparison of SNR and CNR.
After the nonenhanced whole-heart coronary MRI, Gd-BOPTA 0.2 mmol/kg body weight was injected intravenously, immediately followed by 20 ml of saline flush. Based on the findings of the T1 kinetics study (see Results), Gd-BOPTA was injected as a rapid bolus at 2 ml/sec. Immediately after initiation of contrast injection, a Look-Locker sequence was performed repetitively to determine the optimal TI for the whole-heart acquisition that follows. For each repetition of the Look-Locker sequence, the TI corresponding to the lowest myocardium signal was visually determined. Upon identification of arrival and stability of contrast material in the blood pool, which was determined as similar visual TI between two consecutive repetitions (approximately 60–90 sec after contrast injection), the Look-Locker sequence was aborted. A whole-heart coronary MR image was then acquired with identical imaging parameters as those of the nonenhanced sequence, with the exception of replacing the T2 magnetization preparation with a nonselective inversion pulse with use of the TI determined from the Look-Locker image. The inversion pulse was used to suppress myocardial signal and improve visualization of the coronary arteries. No respiratory navigator was used for the noise images, to allow for shorter acquisition time.
Data Analysis
T1 Kinetics Study
A region-of-interest (ROI) was drawn in the left ventricular cavity (proximately 10 × 10 mm2) and in the ventricular septum (proximately 5 × 8 mm2). The ROIs were copied to all acquired cardiac phases and repetitions. The ROIs were manually adjusted to avoid misplacement of the copied myocardial ROIs on blood pool due to cardiac motion. The mean signal intensity within the ROI was measured as the blood signal. Because the nonselective inversion pulse was applied immediately after the ECG R-wave, the trigger delay time of the Look-Locker sequence was used as the TI. The blood signal and TI were exported to Matlab (v7.1; The MathWorks, Natick, MA), where an exponential fit was performed on the signal as a function of TI according to the following equation:
The calculated T1 was corrected for the saturation effect of flip angle and repetition time (14). The exponential fit and correction were repeated for every Look-Locker repetition to obtain a time course of the blood T1 values before and up to 30 minutes after the contrast injection. Myocardial T1 changes were also measured similarly.
Whole-Heart Coronary MRI
The SNR and CNR measurements and subjective image quality evaluation were performed on the axial images from the 3D data sets. The visualized vessel length and vessel sharpness measurements were based on coronary images reformatted with use of the SoapBubble tool (21).
All SNR and CNR measurements were performed by using ViewForum (v4.2; Philips Healthcare, Best, The Netherlands). The mean signal intensity of the coronary arteries was measured by drawing an ROI (approximately 2-4 mm wide depending on subject and 10 mm long) in the proximal, middle, and distal segments of each of the three major coronary arteries, respectively. The mean myocardial signal intensity was measured by placing another ROI (proximately 5 × 8 mm2) in the ventricular septum. The ROIs were copied to the corresponding section and position in the noise image. SNR was calculated as the ratio of the mean signal to the standard deviation of the noise. The CNR between the blood and myocardium was calculated as the difference in SNR between blood and myocardium.
The SoapBubble tool (21) was used to quantitatively evaluate the vessel definition by using a Deriche algorithm (22) on the RCA, left anterior descending (LAD), and left circumflex (LCX) coronary arteries. Vessel sharpness scores were calculated for both sides of the vessel, and final normalized sharpness was defined as the average score of both sides divided by the lumen signal. Higher sharpness scores represented a sharper vessel border.
To measure visualized length of the RCA, LAD, and LCX, the coronary arteries were followed and identified on axial sections sequentially starting from the ostia. The identified point coordinates were subsequently imported into the SoapBubble tool, where the length along the course of the coronary artery was calculated.
Qualitative assessment of coronary artery images was performed by an experienced, independent, blinded reader using a four-point scale system (23): 1, indicated poor or uninterpretable (coronary artery visible, with markedly blurred borders or edges); 2, fair (coronary artery visible, with moderately blurred borders or edges); 3, good (coronary artery visible, with mildly blurred borders or edges); or 4, very good (coronary artery visible, with sharply defined borders or edges). For each image, separate scores were given for the proximal, middle, and distal segments of each of the three major coronary arteries.
Statistical Analyses
T1 Kinetics
A linear mixed-effects model with compound symmetry variance-covariance structure for the error matrix and linear contrasts (24) was used for comparing the mean of the T1 measurements by using the three contrast injection strategies. To analyze the difference in the T1 time course between injection methods, the T1 time course data were divided into three segments that were separately analyzed by using the model: 1) 50–100 seconds after initiation of contrast injection, corresponding to the time period before initiation of coronary MRI, 2) 100–800 seconds, corresponding to the average duration of the coronary MRI data acquisition, and 3) 800–1800 seconds. All statistical analyses were performed with SAS (v9.1, SAS Institute Inc., Cary, NC). A two-sided P value of < .05 was considered to indicate a statistically significant difference.
Whole-Heart Coronary MRI
All measurements are presented as mean ± 1 standard deviation. Differences in the means of SNR and CNR were assessed by using a paired t-test. Differences in the means of vessel length, vessel sharpness, and subjective image scores were assessed with use of a linear mixed-effects model with compound symmetry variance-covariance structure for the error matrix and linear contrasts (24).
RESULTS
T1 Kinetics Study
The T1 measurements were successfully completed on all 7 subjects without complications or specific absorption rate issues. Figures 1 and 2 show the blood and myocardium T1 up to 30 minutes after initiation of contrast injection, respectively. The mean blood and myocardium T1 values 50–100 seconds after the bolus contrast injection (157 ± 77 msec for blood and 304 ± 92 msec for myocardium) were significantly lower than that for the hybrid (260 ± 100 msec for blood and 503 ± 107 msec for myocardium) and slow (496 ± 216 msec for blood and 602 ± 114 msec for myocardium) injection methods (P < .01 for both blood and myocardium). The mean blood and myocardium T1 100–800 seconds after initiation of contrast injection using the hybrid method (156 ± 44 msec for blood and 375 ± 79 msec for myocardium) was significantly higher than that for the bolus (119 ± 33 msec for blood and 304 ± 77 msec for myocardium) and slow (118 ± 41 msec for blood and 307 ± 87 msec for myocardium) infusion methods (P < .002 for both blood and myocardium), whereas the bolus and slow injection methods resulted in similar T1 during the same period (P > .7 for both blood and myocardium). The differences between the mean blood and myocardium T1 during 800–1800 seconds after initiation of contrast injection using the bolus (180 ± 29 msec for blood and 381 ± 58 msec for myocardium), hybrid (164 ± 42 msec for blood and 395 ± 62 msec for myocardium), and slow (169 ± 34 msec for blood and 384 ± 69 msec for myocardium) infusion methods were not significant (P > .15 for blood and P > .05 for myocardium). The results show that both slow infusion and bolus infusion yielded similar T1 shortening during the time when coronary MRI was performed, while hybrid infusion resulted in higher blood and myocardium T1, which would subsequently lower the SNR in coronary MRI. A bolus infusion was subsequently chosen for our whole-heart coronary MRI study.
Figure 1.
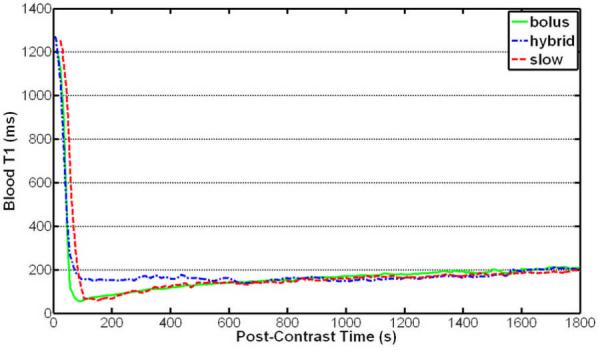
Thirty-minute time course of the average blood T1 of seven healthy subjects after Gd-BOPTA administration using three injection strategies. The hybrid injection method resulted in higher mean T1 than did the bolus and slow injection methods in the first ~10 minutes after injection. The bolus and slow infusion methods resulted in similar blood T1 from 100 seconds to 30 minutes after contrast administration. The bolus injection method was chosen for subsequent coronary MR imaging.
Figure 2.
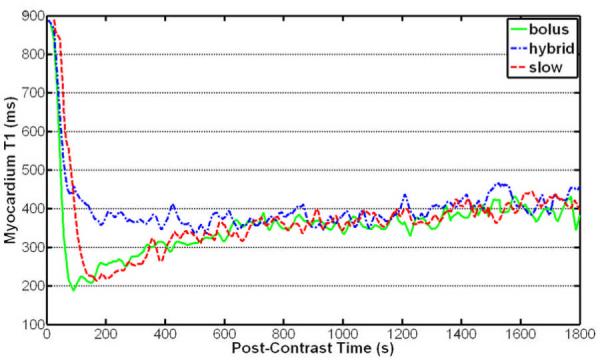
Thirty-minute time course of the average myocardium T1 of seven healthy subjects after Gd-BOPTA administration using three injection strategies. The hybrid injection method resulted in higher mean T1 than did the bolus and slow injection methods in the first ~10 minutes after injection. The bolus and slow infusion methods resulted in similar myocardium T1.
Whole-Heart Coronary MRI
Contrast-enhanced coronary MRI was successfully performed in all 14 subjects, without complications. The major coronary arteries were depicted in all subjects. The contrast injection time was 10–20 seconds depending on the subject’s body weight. The average time delay between contrast injection and initiation of imaging was 100s and the average TI value used in the 14 subjects was 190ms. The total imaging time was 11 ± 2 minutes for the nonenhanced whole-heart acquisition versus 11 ± 2 minutes for the contrast-enhanced acquisition (P = NS), with average navigator efficiency of about 50%.
SNR, CNR, visualized vessel length, vessel sharpness, and subjective image quality for nonenhanced and contrast-enhanced acquisitions are summarized in Tables 1 and 2. The coronary artery SNR and coronary-myocardium CNR were significantly improved in all segments of all three major coronary arteries (P < .05 for all). The SNR and CNR (nonenhanced versus contrast-enhanced) averaged across all coronary arteries and segments were 49 ± 13 versus 65 ± 16 for SNR, and 22 ± 7 versus 44 ± 13 for CNR (P < .001 for both SNR and CNR). The RCA and LAD coronary arteries were visualized longer with Gd-BOPTA (P < .02 for both). The visualized length of LCX was not significantly increased with Gd-BOPTA (P = .06). The sharpness scores of contrast-enhanced and nonenhanced images were similar for all coronary arteries (P = NS). Estimated mean difference (95% confidence interval) between the mean of contrast-enhanced and nonenhanced subjective image scores, adjusted for coronary artery and coronary segment, was 0.14 (0.02, 0.26) (P = .03). The effects of coronary artery and segment on image quality score improvement after Gd-BOPTA administration were statistically significant (P < .002 both for coronary artery and for coronary segment). When compared segment by segment, the contrast-enhanced subjective image scores were not statistically significantly different from nonenhanced image scores.
Table 1.
Comparison of SNR, CNR and subjective image scores between nonenhanced (NE) and contrast-enhanced (CE) coronary MR imaging
| PROXIMAL | MIDDLE | DISTAL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RCA | LAD | LCX | RCA | LAD | LCX | RCA | LAD | LCX | |
| NE-SNR | 45±22 | 55±24 | 48±16 | 59±24 | 53±24 | 45±11 | 44±14 | 49±18 | 43±11 |
| CE-SNR | 69±20* | 71±32* | 55±25* | 79±26* | 64±17* | 57±15* | 54±13* | 73±31* | 61±28* |
|
| |||||||||
| NE-CNR | 19±14 | 28±20 | 20±14 | 31±19 | 25±19 | 17±6 | 17±16 | 21±13 | 15±8 |
| CE-CNR | 47±18* | 50±29* | 33±20* | 58±25* | 43±16* | 36±12* | 33±14* | 52±26* | 40±27* |
|
| |||||||||
| NE-SIS | 3.1±.6 | 3.1±.7 | 2.9±.7 | 2.6±.7 | 2.5±.7 | 2.3±.7 | 1.8±.8 | 2.1±1 | 1.6±.9 |
| CE-SIS | 3.2±.7 | 3.1±.7 | 2.9±.8 | 2.8±.5 | 2.7±.7 | 2.4±.7 | 2.1±.9 | 2.2±1 | 1.9±.8 |
RCA: right coronary artery. LAD: left anterior descending coronary artery
LCX: left circumflex coronary artery. NE: nonenhanced. CE: contrast-enhanced. SIS: subjective image score. SNR: signal to noise ratio. CNR: contrast to noise ratio.
P<0.05 compared with NC.
Table 2.
Comparison of depicted vessel length and vessel sharpness between nonenhanced and contrast-enhanced coronary MRI
| NONENHANCED | CONTRAST-ENHANCED | |||||
|---|---|---|---|---|---|---|
| RCA | LAD | LCX | RCA | LAD | LCX | |
| Length (mm) | 86±27 | 65±22 | 43±14 | 96±30* | 76±18* | 53±16 |
| Sharpness (%) |
65±9 | 56±8 | 58±14 | 66±8 | 56±8 | 59±7 |
P<0.05 comparing with nonenhanced.
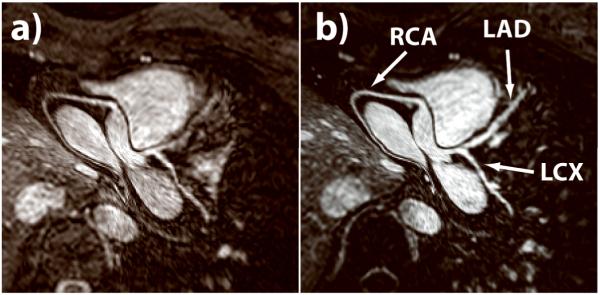
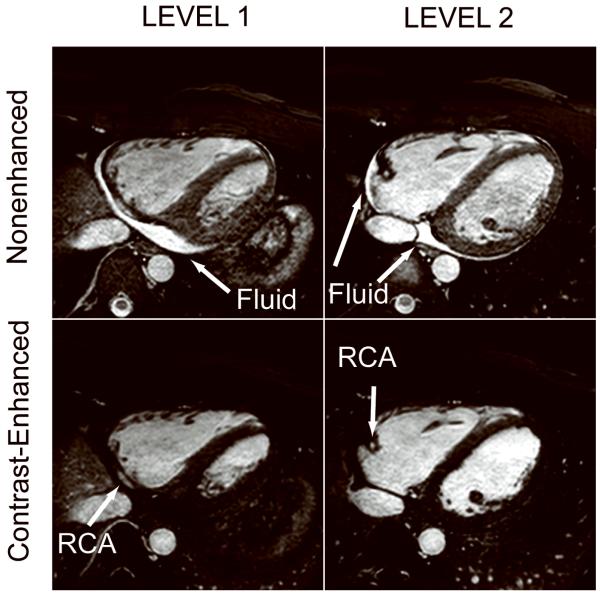
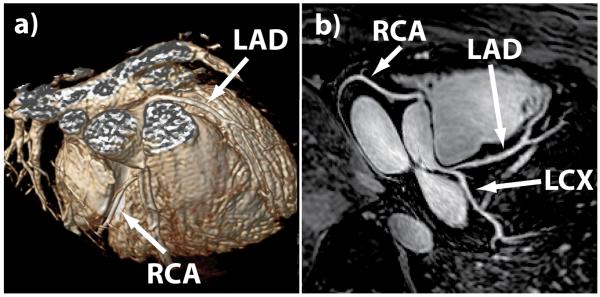
Figure 3 shows a comparison of reformatted nonenhanced and contrast-enhanced coronary images acquired on the same healthy subject. The depiction of the major coronary arteries, especially the LAD, was visually improved with Gd-BOPTA administration due to gains in coronary signal and improved suppression of the myocardial signal by the nonselective inversion pulse. An additional benefit of the contrast-enhanced SSFP sequence is the suppression of pericardial fluid, which appears brighter than the coronary arteries on nonenhanced SSFP coronary MR images. As shown in Figure 4, the pericardial fluid may compromise the depiction of the distal RCA and was completely suppressed on the contrast-enhanced images. An example of reformatted whole-heart coronary artery images and corresponding volume rendering is shown in Figure 5. The major coronary arteries are readily depicted, including distal segments and branches in all three major coronary arteries.
Figure 3.
Comparison between (a) nonenhanced and (b) contrast-enhanced whole-heart SSFP coronary MRI. Depiction of all three major coronary arteries (right coronary artery [RCA], left anterior descending [LAD], and left circumflex [LCX]), especially the left coronary arteries, is improved on the contrast-enhanced MR image because of higher signal intensity and better suppression of surrounding myocardium.
Figure 4.
Example of nonenhanced (top row) and contrast-enhanced (bottom row) whole-heart SSFP coronary images acquired in a subject. The epicardial fluid has high signal intensity on the nonenhanced SSFP coronary MRI, which may compromise depiction of adjacent middle and distal RCA. The fluid is completely suppressed on the contrast-enhanced MR images.
Figure 5.
Example of (a) three-dimensional volume-rendered and (b) corresponding reformatted whole-heart SSFP coronary images acquired with a bolus injection of Gd-BOPTA. All three major coronary arteries (RCA, LAD, and LCX) and their distal branches are clearly depicted.
DISCUSSION
In this study of healthy adult subjects, a bolus infusion of Gd-BOPTA provided significant improvements in coronary artery SNR, CNR, depicted vessel length, vessel sharpness, and image quality compared to nonenhanced acquisitions. Although both slow and bolus infusion can be used based on the result from T1 kinetic study, we have chosen to investigate a bolus infusion for coronary MRI. While there are studies on a bolus infusion to assess viability, there are no data on the impact of a slow infusion or a hybrid infusion protocol in assessing viability. Furthermore, a bolus infusion could potentially simplify the initiation of imaging for patients with low cardiac output and allow for contrast administration without a power injector.
Despite widespread use of 1.5-T systems, contrast-enhanced coronary artery MRI at 3 T (6,25-27) is promising, owing to the increased SNR. Although T1 at 3 T is longer than at 1.5 T, we expect the kinetics of the T1 shortening effect to be similar. Therefore, the findings in our T1 kinetics study should apply to 3 T, but needs to be confirmed. To our knowledge, there is currently no report of coronary MRI at 3 T with use of a bolus infusion of Gd-BOPTA as described in this report. Further studies comparing coronary MRI at 3 T with slow infusion (6) or bolus injection are therefore warranted.
Although the main goal of this study was to evaluate contrast-enhanced coronary artery MRI, the sequence and contrast injection strategies used in this study may also be used to visualize the coronary veins. Several nonenhanced and contrast-enhanced methods to evaluate coronary vein anatomy for guidance of cardiac resynchronization therapy have been proposed (28-30). Our proposed method will be another alternative to evaluate coronary veins, although the timing should be adjusted to acquire the data in systolic phase to take advantage of larger venous diameter.
Our study has limitations. Only a small number of healthy adult subjects were studied. Further studies are needed to study the clinical evaluation of the proposed coronary MRI in a cohort known or suspected to have coronary artery disease to determine the clinical benefit. In addition, we focused on the T1 shortening effect of Gd-BOPTA. The signal in our SSFP acquisitions is also dependent on the T2 of blood. Therefore, a study of the impact of Gd-BOPTA on the blood T2 (30) and on SSFP whole-heart contrast-enhanced coronary MRI would be necessary. As T2-prepared SSFP has been working reasonably well at 1.5T, additional limitations of using Gd-BOPTA, such as the added cost, need for intravenous access and potential side effects, should also be considered against the benefits demonstrated in this study.
CONCLUSION
The kinetic of Gd-BOPTA yield similar low and stable T1 for both bolus and slow infusion, whereas a hybrid infusion resulted in higher T1. The objective image quality measures were significantly improved after a bolus infusion of 0.2 mmol/kg Gd-BOPTA. The subjective image quality was significantly improved when all segments were collectively compared but not when compared segment by segment.
ACKNOWLEDGEMENTS
The authors acknowledge Dr. Peter Kellman from National Institutes of Health for valuable discussions on SNR measurements with parallel imaging. The project described was supported by Grant NIH R01EB008743-01A2, AHA SDG-0730339N and UL1 RR025758-01, Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health or AHA.
REFERENCES
- 1.Brittain JH, Hu BS, Wright GA, Meyer CH, Macovski A, Nishimura DG. Coronary angiography with magnetization-prepared T2 contrast. Magn Reson Med. 1995;33(5):689–696. doi: 10.1002/mrm.1910330515. [DOI] [PubMed] [Google Scholar]
- 2.Botnar RM, Stuber M, Danias PG, Kissinger KV, Manning WJ. Improved coronary artery definition with T2-weighted, free-breathing, three-dimensional coronary MRA. Circulation. 1999;99(24):3139–3148. doi: 10.1161/01.cir.99.24.3139. [DOI] [PubMed] [Google Scholar]
- 3.Hu P, Chuang ML, Ngo LH, Stoeck CT, Peters DC, Kissinger KV, Goddu B, Goepfert LA, Manning WJ, Nezafat R. Coronary MR imaging: effect of timing and dose of isosorbide dinitrate administration. Radiology. 2010;254(2):401–409. doi: 10.1148/radiol.09090483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng J, Bae KT, Woodard PK, Haacke EM, Li D. Efficacy of slow infusion of gadolinium contrast agent in three-dimensional MR coronary artery imaging. J Magn Reson Imaging. 1999;10(5):800–805. doi: 10.1002/(sici)1522-2586(199911)10:5<800::aid-jmri26>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Goldfarb JW, Edelman RR. Coronary arteries: breath-hold, gadolinium-enhanced, three-dimensional MR angiography. Radiology. 1998;206(3):830–834. doi: 10.1148/radiology.206.3.9494509. [DOI] [PubMed] [Google Scholar]
- 6.Bi X, Carr JC, Li D. Whole-heart coronary magnetic resonance angiography at 3 Tesla in 5 minutes with slow infusion of Gd-BOPTA, a high-relaxivity clinical contrast agent. Magn Reson Med. 2007;58(1):1–7. doi: 10.1002/mrm.21224. [DOI] [PubMed] [Google Scholar]
- 7.Knuesel PR, Nanz D, Wolfensberger U, Saranathan M, Lehning A, Luescher TF, Marincek B, von Schulthess GK, Schwitter J. Multislice breath-hold spiral magnetic resonance coronary angiography in patients with coronary artery disease: effect of intravascular contrast medium. J Magn Reson Imaging. 2002;16(6):660–667. doi: 10.1002/jmri.10202. [DOI] [PubMed] [Google Scholar]
- 8.Herborn CU, Barkhausen J, Paetsch I, Hunold P, Mahler M, Shamsi K, Nagel E. Coronary arteries: contrast-enhanced MR imaging with SH L 643A--experience in 12 volunteers. Radiology. 2003;229(1):217–223. doi: 10.1148/radiol.2291021033. [DOI] [PubMed] [Google Scholar]
- 9.Prompona M, Cyran C, Nikolaou K, Bauner K, Reiser M, Huber A. Contrast-enhanced whole-heart MR coronary angiography at 3.0 T using the intravascular contrast agent gadofosveset. Invest Radiol. 2009;44(7):369–374. doi: 10.1097/rli.0b013e3181a40d1d. [DOI] [PubMed] [Google Scholar]
- 10.Weber OM, Martin AJ, Higgins CB. Whole-heart steady-state free precession coronary artery magnetic resonance angiography. Magn Reson Med. 2003;50(6):1223–1228. doi: 10.1002/mrm.10653. [DOI] [PubMed] [Google Scholar]
- 11.Cavagna FM, Maggioni F, Castelli PM, Dapra M, Imperatori LG, Lorusso V, Jenkins BG. Gadolinium chelates with weak binding to serum proteins. A new class of high-efficiency, general purpose contrast agents for magnetic resonance imaging. Invest Radiol. 1997;32(12):780–796. doi: 10.1097/00004424-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Nassenstein K, Breuckmann F, Hunold P, Barkhausen J, Schlosser T. Magnetic Resonance Coronary Angiography: Comparison between a Gd-BOPTA- and a Gd-DTPA-Enhanced Spoiled Gradient-Echo Sequence and a Non-Contrast-Enhanced Steady-State Free-Precession Sequence. Acta Radiol. 2009:1–6. doi: 10.1080/02841850902838066. [DOI] [PubMed] [Google Scholar]
- 13.Yang Q, Li K, Liu X, Bi X, Liu Z, An J, Zhang A, Jerecic R, Li D. Contrast-enhanced whole-heart coronary magnetic resonance angiography at 3.0-T: a comparative study with X-ray angiography in a single center. J Am Coll Cardiol. 2009;54(1):69–76. doi: 10.1016/j.jacc.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Look DC, Locker DR. Time saving in measurement of NMR and EPR relaxation times. Rev Sci Instrum. 1970;41:250–251. [Google Scholar]
- 15.Wang Y, Riederer SJ, Ehman RL. Respiratory motion of the heart: kinematics and the implications for the spatial resolution in coronary imaging. Magn Reson Med. 1995;33(5):713–719. doi: 10.1002/mrm.1910330517. [DOI] [PubMed] [Google Scholar]
- 16.Danias PG, Stuber M, Botnar RM, Kissinger KV, Edelman RR, Manning WJ. Relationship between motion of coronary arteries and diaphragm during free breathing: lessons from real-time MR imaging. AJR Am J Roentgenol. 1999;172(4):1061–1065. doi: 10.2214/ajr.172.4.10587147. [DOI] [PubMed] [Google Scholar]
- 17.Kotys MS, Herzka DA, Vonken EJ, Ohayon J, Heroux J, Gharib AM, Stuber M, Pettigrew RI. Profile order and time-dependent artifacts in contrast-enhanced coronary MR angiography at 3T: origin and prevention. Magn Reson Med. 2009;62(2):292–299. doi: 10.1002/mrm.21997. [DOI] [PubMed] [Google Scholar]
- 18.Bhat H, Lai P, Li D. Self-tracking of contrast kinetics for automatic triggering of contrast-enhanced whole-heart coronary magnetic resonance angiography. J Magn Reson Imaging. 2009;29(4):809–816. doi: 10.1002/jmri.21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pruessmann KP, Weiger M, Boesiger P. Sensitivity encoded cardiac MRI. J Cardiovasc Magn Reson. 2001;3(1):1–9. doi: 10.1081/jcmr-100000143. [DOI] [PubMed] [Google Scholar]
- 20.Kellman P, McVeigh ER. Image reconstruction in SNR units: a general method for SNR measurement. Magn Reson Med. 2005;54(6):1439–1447. doi: 10.1002/mrm.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etienne A, Botnar RM, Van Muiswinkel AM, Boesiger P, Manning WJ, Stuber M. “Soap-Bubble” visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magn Reson Med. 2002;48(4):658–666. doi: 10.1002/mrm.10253. [DOI] [PubMed] [Google Scholar]
- 22.Deriche R. Fast algorithms for low-level vision. IEEE Trans Pattern Anal Mach Intell. 1990;12(1):78–87. [Google Scholar]
- 23.Kim WY, Danias PG, Stuber M, Flamm SD, Plein S, Nagel E, Langerak SE, Weber OM, Pedersen EM, Schmidt M, Botnar RM, Manning WJ. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med. 2001;345(26):1863–1869. doi: 10.1056/NEJMoa010866. [DOI] [PubMed] [Google Scholar]
- 24.Laird MN, Ware HJ. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 25.Stuber M, Botnar RM, Fischer SE, Lamerichs R, Smink J, Harvey P, Manning WJ. Preliminary report on in vivo coronary MRA at 3 Tesla in humans. Magn Reson Med. 2002;48(3):425–429. doi: 10.1002/mrm.10240. [DOI] [PubMed] [Google Scholar]
- 26.Nezafat R, Stuber M, Ouwerkerk R, Gharib AM, Desai MY, Pettigrew RI. B1-insensitive T2 preparation for improved coronary magnetic resonance angiography at 3 T. Magn Reson Med. 2006;55(4):858–864. doi: 10.1002/mrm.20835. [DOI] [PubMed] [Google Scholar]
- 27.Bi X, Li D. Coronary arteries at 3.0 T: Contrast-enhanced magnetization-prepared three-dimensional breathhold MR angiography. J Magn Reson Imaging. 2005;21(2):133–139. doi: 10.1002/jmri.20250. [DOI] [PubMed] [Google Scholar]
- 28.Nezafat R, Han Y, Peters DC, Herzka DA, Wylie JV, Goddu B, Kissinger KK, Yeon SB, Zimetbaum PJ, Manning WJ. Coronary magnetic resonance vein imaging: imaging contrast, sequence, and timing. Magn Reson Med. 2007;58(6):1196–1206. doi: 10.1002/mrm.21395. [DOI] [PubMed] [Google Scholar]
- 29.Rasche V, Binner L, Cavagna F, Hombach V, Kunze M, Spiess J, Stuber M, Merkle N. Whole-heart coronary vein imaging: a comparison between non-contrast-agent- and contrast-agent-enhanced visualization of the coronary venous system. Magn Reson Med. 2007;57(6):1019–1026. doi: 10.1002/mrm.21228. [DOI] [PubMed] [Google Scholar]
- 30.Chiribiri A, Kelle S, Kohler U, Tops LF, Schnackenburg B, Bonamini R, Bax JJ, Fleck E, Nagel E. Magnetic resonance cardiac vein imaging: relation to mitral valve annulus and left circumflex coronary artery. JACC Cardiovasc Imaging. 2008;1(6):729–738. doi: 10.1016/j.jcmg.2008.06.009. [DOI] [PubMed] [Google Scholar]