Abstract
TCBOPOP (1, 4-bis [2-(3, 5-dichaloropyridyloxy)] benzene) an agonist of the Constitutive Androstane receptor (CAR), produces rapid hepatocyte hyperplasia and hepatomegaly in the absence of hepatic injury. In this study, we demonstrate that integrin linked kinase (ILK) which is involved in transmission of the extracellular matrix [ECM] signaling by way of integrin receptors plays an important role in regulating TCPOBOP-induced proliferation of hepatocytes and hepatomegaly. Hepatocyte specific ILK knockout mice (ILK/liver−/− mice) and wild type mice (WT) were given a single dose of TCPOBOP (3 mg/kg) by oral gavage. Mice were sacrificed at day 1, 2, 5 and 7 after TCPOBOP administration. WT mice showed maximum proliferation on day 1 and 2 which came back to baseline levels by day 5 and 7 after TCPOBOP administration. The ILK/liver−/− mice on the other hand showed a prolonged and a sustained proliferative response as evident by an increased number of PCNA positive cells even at day 5 and 7 after TCPOBOP administration. At day 7 the WT mice showed close to 2.5 fold increase in liver weight while the ILK/liver−/− mice showed a 3.7 fold increase in liver weight. The prolonged proliferative response in the ILK/liver−/− mice seems to be due to sustained induction of CAR leading to sustained induction of c-Myc which is known to be a key mediator of TCPOPOP-CAR induced direct liver hyperplasia.
Conclusion
The data indicates that ECM-mediated signaling by way of ILK is essential for adjustment of final liver size and proper termination of TCPOBOP-induced proliferation of hepatocytes.
The liver responds to specific classes of xenobiotics by inducing members of the nuclear hormone receptor superfamily, particularly the pregnane X receptor and the constitutive Androstane receptor (CAR). (1–3) An ideal candidate for studying xenobiotic metabolism is the halogenated hydrocarbon 1, 4-bis [2-(3, 5-dichaloropyridyloxy)] benzene (TCPOBOP). TCPOBOP is both a nongenotoxic carcinogen on its own and a potent tumor promoter when combined with genotoxic agents. (4–7) It potently induces genes associated with xenobiotic detoxification and hepatocyte proliferation in a CAR-dependent manner. (1, 2, 8) TCPOBOP-induced liver enlargement involves both hypertrophy and proliferation of hepatocytes. The mechanisms involved in termination of hepatocyte proliferation and in precise regulation of the increase in liver size following TCPOBOP-induced hyperplastic response are not understood. The current study is concentrating its focus on this aspect of TCPOBOP-induced hepatocyte proliferation and aims to provide additional understanding of the termination process of hepatocyte proliferation after an acute exposure of a mitogenic stimulus. The basic hypothesis of the study is that the main source of signals leading to termination of hepatocyte proliferation and definition of the final liver size is the hepatic extracellular matrix (ECM). Evaluation of the overall role of ECM in this process has been difficult to assess because of the multiplicity of ECM proteins, their complex interactions, and the redundancy of their roles. Recently, however there has been much progress in determining mechanisms by which integrins, the ECM receptors, deliver their signals inside the cell. The major mediators of the integrin signaling are integrin linked kinase (ILK), focal adhesion kinase (FAK) and Mig2. (9–15) Recently, we have successfully eliminated the ILK gene from mice specifically in hepatocytes. (16, 17) We have shown that hepatocyte-targeted genetic ablation of integrin-linked kinase (ILK), a protein involved in transmitting extracellular matrix (ECM) signals by way of integrins, changes the proliferation kinetics of hepatocytes in normal livers and results in an adapted liver status in which there is a new set of integrins, modified ECM, and a final liver whose size is larger than the normal. These livers when subjected to 70% partial hepatectomy (Phx) show a termination defect. While the wild type livers returned to exactly the same liver weight as pre-hepatectomy, the livers of ILK/liver−/− mice gained additional weight (59% increase). The increase in resting liver weight and the apparent “overgrowth” of the regenerating liver in the ILK/liver−/− mice shows that in absence of matrix signaling (as a result of removal of ILK), liver growth and termination of liver regeneration does not function properly and liver grows to a much larger size. (18) We have also recently shown that ILK/liver−/− undergo sustained and prolonged proliferation in response to chronic administration of phenobarbital, a more modest chemical mitogen. (19) Thus, the objective of the current study is to determine the mechanism of termination of hepatocyte proliferation after an acute mitogenic stimulus using TCPOBOP as the mitogen. We performed this study because TCBOPOP is the strongest chemical mitogen and the response induced by TCBOPOP is primarily due to hyperplasia. The livers obtained in the ILK/liver −/− mice are the largest in terms of liver/body weight ratio ever reported. The extreme results obtained further highlight the important role of the ECM-integrin-ILK system as a regulator of liver size and terminator of regenerative responses in liver.
Materials and Methods
Generation of liver specific ILK/Liver−/− mice
ILK floxed animals were generated as described previously (20) and donated by Drs. René St. Arnaud (Shriners Hospital and McGill University, Montréal) and Shoukat Dedhar (British Columbia Cancer Agency and Vancouver Hospital, Jack Bell Research Center, Vancouver), and mated with AFP-enhancer-albumin-promoter-Cre-recombinase-expressing mice which were kindly provided by Dr. Klaus Kaestner (University of Pennsylvania). The off-spring were genotyped as described previously (20) and the ILK-floxed/floxed Cre-positive mice were considered to be ILK-knock-out (ILK/Liver−/−), while their Cre-negative siblings were used as controls or wild type (WT) (16).
Antibodies
The following primary antibodies were used in this study: rabbit anti-YAP, Rabbit anti-phosphorylated YAP, Rabbit anti-cyclin D1, Rabbit anti-p27 (1:1000 dilution, Cell Signaling Technologies, Danvers, MA); rabbit anti-c-Myc, rabbit anti-TGFβ1 (Promega, Madison, WI), mouse anti-PCNA (Dako, Carpinteria, CA), mouse anti-β-actin (1:5000 dilution, Chemicon, Temecula, CA) and mouse TATA binding protein (Abcam, Cambridge, MA). Goat anti-mouse, donkey anti-goat and donkey anti-rabbit secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA) and were used at 1:50,000 dilution.
TCPOBOP Treatment
TCPOBOP (1 mg/ml dissolved in DMSO/corn oil mixture) was administered to 35 week old ILK/liver−/− and WT mice by oral gavage. Mice were sacrificed and liver excised on day 1, 2, 5 and 7 after TCPOBOP administration.
Protein Isolation and Western Blot
Total protein was isolated from the mouse liver using 1% sodium dodecyl sulfate (SDS) in RIPA buffer (20 mM Tris/Cl pH 7.5, 150 mM NaCl, 0.5% NP-40, 1% TX-100, 0.25% Sodium Deoxycholate (DOC), 0.6– 2 µg/ml aprotinin, 10µM Leupeptin, 1µM Pepstatin). Protein concentrations of all lysates were determined using the bicinchoninic acid protein assay reagents (BCA method) (Pierce Chemical Co., Rockford, IL). Nuclear proteins were prepared using the NE-PER nuclear and cytoplasmic protein isolation kit (Pierce, Rockford IL) according to the manufacturer’s protocol. Pooled sample (n=3) were used for making nuclear and total cell lysates.
Total cell lysates made in Ripa buffer (50 µg) or nuclear preps (20ug) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis in 4% to 12% NuPage Bis-Tris gels with 1 MOPS buffer (Invitrogen, Carlsbad, CA), then transferred to Immobilon-P membranes (Millipore, Bedford,MA) in NuPAGE transfer buffer containing 20% methanol. Membranes were stained with Ponceau S to verify loading and transfer efficiency. Membranes were probed with primary and secondary antibodies in Tris-buffered saline Tween 20 containing 5% nonfat milk, then processed with SuperSignal West Pico chemiluminescence substrate (Pierce, Rockford, IL) and exposed to a X-ray film (Lab Product Sales, Rochester, NY).
Electromobility Shift Assay (EMSA)
EMSA was performed to measure the activation of CAR and c-myc. Pooled nuclear samples (n=3) from ILK/liver−/− and WT mice were used for performing EMSA using a commercial kit from Signosis Inc (Sunnyvale, CA). Biotonalated DNA binding consensus sequence were also purchased from Signosis Inc.
Immunohistochemistry
Paraffin-embedded liver sections (4 µm thick) were used for immunohistochemical staining. Antigen retrieval was achieved by heating the slides in the microwave at high power in 1X citrate buffer for 10 minutes. The tissue sections were blocked in blue blocker for 20 minutes followed by incubation with mouse PCNA antibody overnight at 4°C. The primary antibody was then linked to biotinylated secondary antibody followed by routine avidin-biotin complex method. Diaminobenzidine was used as the chromogen, which resulted in a brown reaction product. PCNA positive cells were counted in low-power fields (200×) in 4 sections from 4 different knockout or control livers.
RNA Isolation and Semi quantitative Reverse Transcription-Polymerase Chain Reaction
RNA was extracted from frozen liver tissues with Trizol (Invitrogen, CA) according to manufacturer’s instructions. RNA, 5ug was reverse transcribed to complementory DNA (cDNA) with Superscript III reverse transcriptase (Invitrogen, CA). standard PCR was performed with Taq polymerase (Qiagen, CA). the primers for HGF were bought for SA Bioscience (Frederick, MD). PCR products were resolved on 2% agarose gels and visualized with ethidium bromide. Expression levels of UGT1A1 were determined by quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR) using SYBR green, and levels were normalized relative to expression of cyclophillin in each sample. Fold change in gene expression was calculated by using the 2(−ΔΔCt) method. Reverse transcribed samples were amplified in parallel on an ABI prism 7000 SDS instrument (Applied Biosystems, Foster city, CA). qRT-PCR for each sample was performed in triplicate in a 20 µl reaction with 50ng of cDNA, 5 picomoles of each primer, and 1X SYBR green PCR mix (Applied Biosystems). The standard conditions for real time PCR were as follows: 2 minutes at 50°C, 10 minutes at 95°C followed by 40 cycles of 15 seconds denaturation at 95°C, and elongation at 60°C for 45 seconds. A dissociation curve analysis was performed at the end of every run. A no RT and a no template control was also included in every run.
Results
Increased liver to body weight ratio and prolonged hepatocyte proliferation in ILK/Liver−/− mice
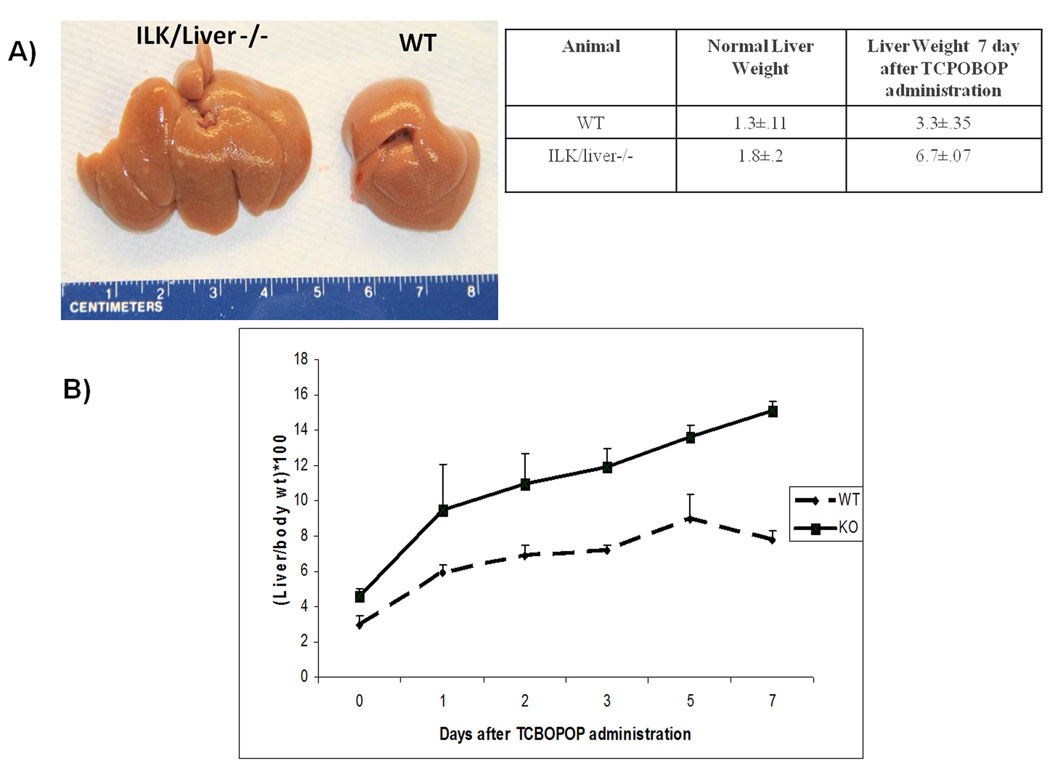
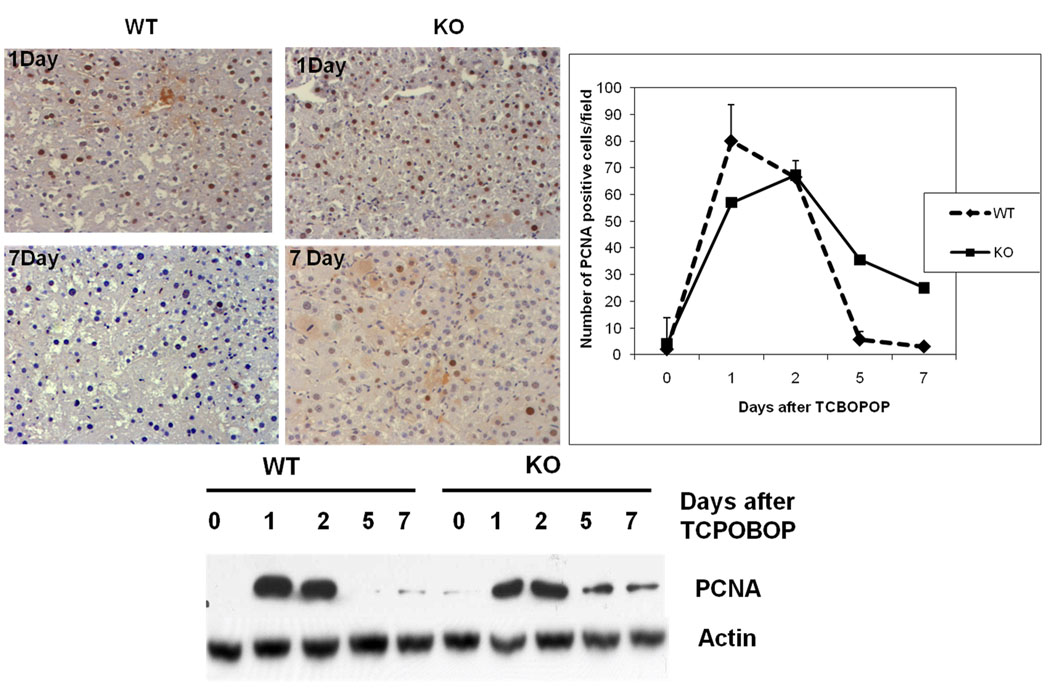
The liver to body weight ratios were measured in WT and ILK/liver−/− mice at day 1, 2, 3, 5 and 7 days after TCPOBOP administration. The WT showed a maximum of 2.5 fold increase (day 5) in liver to body weight ratio as compared to 0 day (Fig 1B). On the other hand the ILK/liver−/− mice showed a maximum of 3.5 fold increase (day 7) in liver to body weight ratio as compared to 0 day. By day 7 the WT mice showed 2.5 fold increase in liver weight while the ILK/liver−/− mice showed a 3.7 fold increase (Fig 1A). We also monitored the cell proliferation kinetics in WT and ILK/Liver−/− mice at 1, 2, 5 and 7 days after TCPOBOP administration. In the WT livers the number of PCNA positive cells increased at day 1 and 2 but came back to baseline levels at day 5 and 7 (Fig 2). On the other hand, livers of ILK/Liver−/− mice showed lower PCNA positive cells as compared to WT at day 1 but higher number of cells at day 5 and 7 (Fig 2). Even though, the number of PCNA positive cells declined after day 2 in the ILK/liver−/− mice, it remained elevated in the ILK/Liver−/− livers as compared to WT suggesting a sustained and a prolonged proliferative response. Western blot analysis of PCNA (Fig 2) also revealed a sustained and prolonged induction in the ILK/liver−/− mice. While the protein levels of PCNA came back to baseline levels at day 5 and 7 after TCPOBOP administration in the WT animals, they remained elevated in the ILK/liver−/− mice even at day 5 and 7 consistent with the observed sustained proliferative response (Fig 1D).
Fig 1.
Liver enlargement after TCPOBOP administration. A) Liver size 7 day after administration of TCPOBOP B) Liver to body weight ratios of WT and ILK/Liver−/− mice at day 0, 1, 2 5 and 7 days after TCPOBOP administration. Each data point is the mean ± SE from more than three measurements per point.
Fig 2.
Cell proliferation kinetics after TCPOBOP administration (A) Hepatocyte proliferation was quantitated by counting the PCNA positive cells. PCNA positive cells were counted in low-power fields (200×) in 3 sections from 3 different ILK/Liver −/− or WT livers. Each data point is the mean ± SE from more than three measurements per point. (B) Representative photomicrographs of PCNA stained liver section of ILK/Liver−/− and WT mice at day 1, and 7 after TCPOBOP administration. (C) Western Blot analysis of PCNA. Pooled liver samples from at least 3 mice were used for protein analysis.
ILK/liver−/− mice show sustained activation of CAR
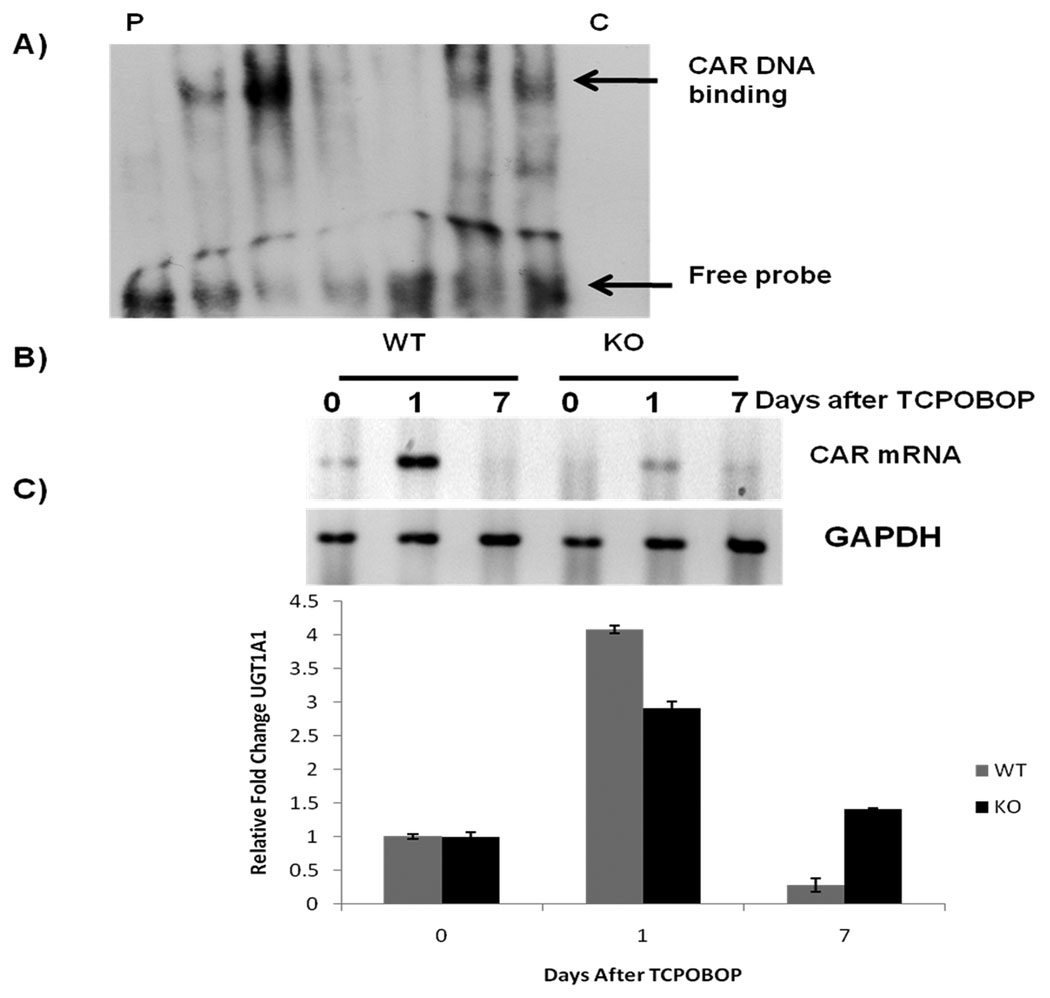
It is well documented that TCPOBOP is a CAR agonist and its activation leads to nuclear localization of CAR. (1, 2, 8) There the protein binds to DNA as a monomer or as a heterodimer with the retinoid X receptor and regulates the transcription of target genes involved in drug metabolism. We measured the activity of CAR by EMSA. WT mice showed activation of CAR at day 1 after TCPOBOP administration while at day 7 it was almost undetectable (Fig 3A). The ILK/liver−/− mice on the other hand showed lower activation of CAR as compared to the WT mice at day1, but overall more sustained CAR activation as evident by presence of CAR in nuclei at day 7 (Fig 3A). These results were also substantiated by measuring CAR mRNA level. Induction of CAR mRNA at day 1 was higher in the WT mice as compared to ILK/liver−/− mice but was undetectable in the WT mice at day 7 while it was still present in the ILK/liver−/− mice suggesting a sustained increased expression of CAR in the ILK/liver−/− mice (Fig 3B). We looked at CAR target UGT1A1 to show that there was a prolonged induction of CAR in the ILK/liver−/− mice. In the ILK/liver−/− mice we saw a lower induction of UGT1A1 at day 1 as compared to WT but was sustained even till day 7 after TCPOBOP administration (Fig 3C). Currently we don’t have an answer to that. It can be speculated that since ILK/liver−/− mice have more matrix deposition in their liver, TCPOBOP is getting absorbed at a lower rate in these mice as a result of which also getting eliminated at a lower rate from the liver. A through pharmacokinetic profile of TCPOBOP in these livers would yield a verification of this possibility.
Fig 3.
A) Gel shift assay for CAR. Pooled liver samples from at least 3 mice were used for preparing nuclear samples. P represents probe only, C represent cold probe (B) mRNA levels of CAR before and after TCPOBOP administration. Pooled liver samples from at least 3 mice were used for preparing RNA. (C) Real time PCR for UGT1A1. Levels were normalized relative to expression of cyclophillin in each sample. Fold change in gene expression was calculated by using the 2(−ΔΔCt) method.
Cell cycle genes
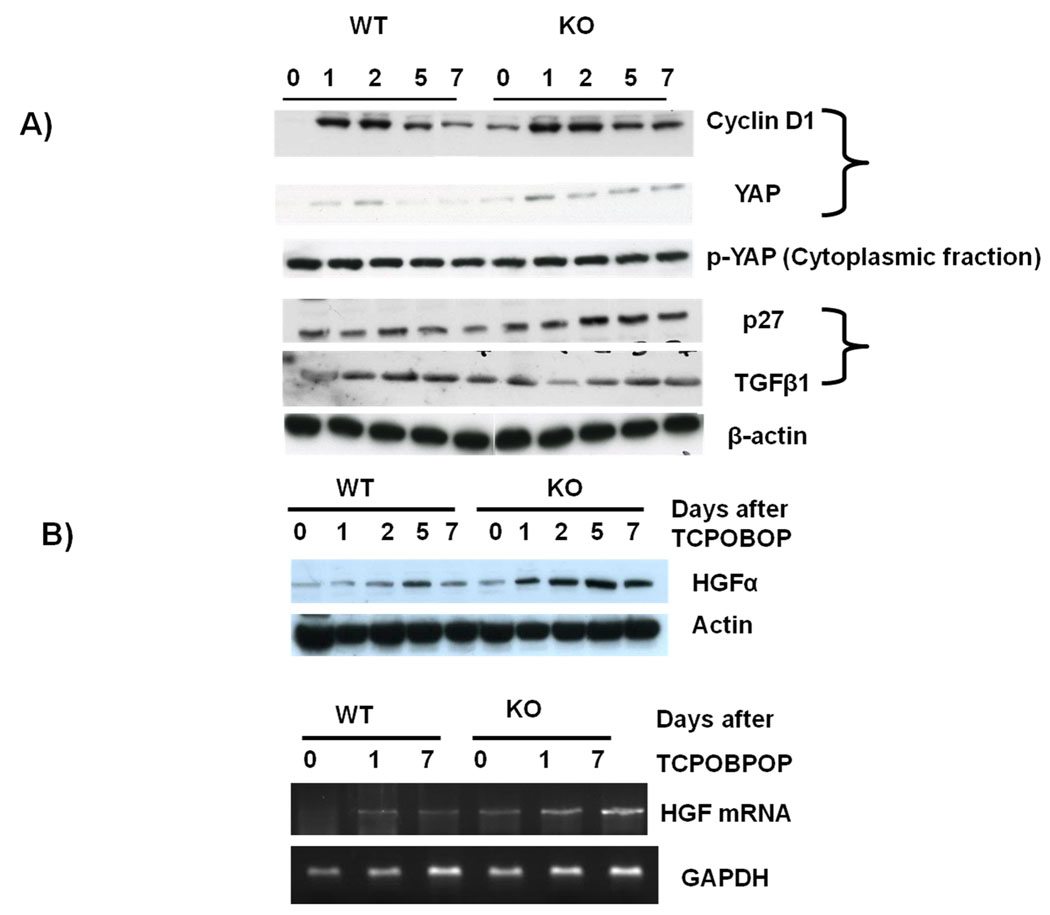
We looked into the key genes that are known to be involved in hepatocyte proliferation. Cyclin D1 has been shown to play an important role in hepatocyte proliferation.(21) There was an induction of cyclin D1 in both the WT and the ILK/liver−/− mice after TCPOBOP administration (Fig 4A). There was no difference in the expression of cyclin D1 between the WT and the ILK/liver−/− between days 2–5. But on day 7 the expression of Cyclin D1 was lower in the WT mice as compared to the ILK/liver−/− mice suggesting a prolonged induction of Cyclin D1. Recently the role of Hippo Kinase pathway in regulation of organ size in Drosophila as well as mammalian liver has been reported (22). The mammalian Hippo Kinase pathway converges on yes-associated protein (YAP), which plays a role in liver size regulation and cancer development (22, 23). YAP is a nuclear protein whose Phosphorylation results in its nuclear export and degradation, which correlates with a decrease in cell proliferation. We investigated whether the absence of hepatocyte ILK affects YAP expression during TCPOBOP-induced liver enlargement. In the WT mice we found an induction of YAP levels at day 1 and 2 after TCPOBOP administration (Fig 4A) suggesting an induction at the time of proliferation. By day 5 and 7 the levels were dramatically down. In the ILK/liver−/− mice there was an induction of YAP after TCPOBOP administration which remained elevated at all time points (Fig 4A) suggesting a sustained and prolonged induction. Thus, there was an overall correlation of YAP with hepatocyte proliferation. Surprisingly, there was no change in the p-YAP levels in the WT mice and the ILK/liver−/− mice after TCPOBOP administration (Fig 4A). There was also no difference in the levels of p-YAP between WT and ILK/liver−/− mice. TGFβ1 and p27 are known to be mitoinhibitory. (21) Thus, we looked at the expression of both proteins. There was no change in the protein levels of p27 in the WT mice throughout the time points (Fig 4A). On the other hand there was an induction of p27 in the ILK/liver−/− mice at days 2–7 after TCPOBOP administration. The levels were also higher in the ILK/liver−/− mice as compared to the WT mice. This was surprising since ILK/liver−/− had more proliferation at days 5–7 as compared to WT animals but still showed higher levels of p27 suggesting a putative negative feedback mechanism. TGFβ1 was induced in the WT mice after TCPOBOP administration. Its expression was particularly higher at day 2 and 5. ILK/liver−/− mice had higher TGFβ1 to start with. Its expression however was reduced at day 1 after TCPOBOP administration. Its expression was increased at day 2 and remained elevated till day 7. The expression of TGFβ1 from day 2 and 5 was lower in the ILK/liver−/− as compared to the WT mice. HGF protein as well as mRNA was also higher and sustained in the ILK/liver/−/− mice (Fig 4B) as compared to the WT mice.
Fig 4.
(A) Protein levels of various mitogenic and mitoinhibitory molecules. Pooled liver samples from at least 3 mice were used for preparing nuclear, cytoplasmic and total cell lysates. (B) HGF protein and mRNA levels after TCPOBOP administration.
Mechanism of increased and sustained proliferation in ILK/liver−/− mice
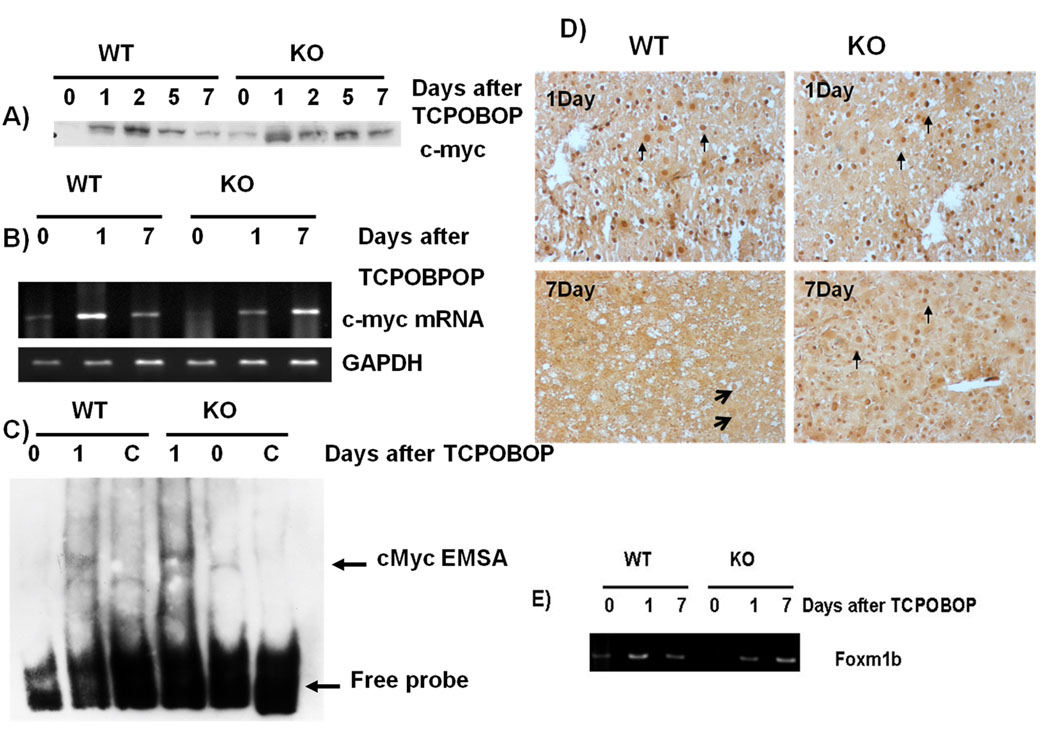
c-Myc and FoxM1 are known to be a key mediator of TCPOBOP-CAR induced direct liver hyperplasia.(1) Thus, we examined if c-Myc and FoxM1 levels are differentially expressed in the ILK/liver−/− mice. C-myc mRNA was induced in both the WT and ILK/liver−/− mice 1 day after TCPOBOP (Fig 5B). Expression levels were higher in the WT as compared to the ILK/liver−/− mice. By day 7 the mRNA level of c-myc went down in WT mice as compared to day 1 while it was upregulated in the ILK/liver−/− mice as compared to day 1. The mRNA level of c-myc was also higher in the ILK/liver−/− mice as compared to the WT mice at day 7. Since c-myc is regulated posttranscriptionally, (24) we analyzed the protein expression of c-myc after TCPOBOP administration. In the WT mice c-myc expression was induced as early as day 1 (Fig 5A). Its expression however was maximum at day 2. By day 5 and 7 its expression had started to go down. In the ILK/liver−/− mice the expression levels were higher as compared to the WT mice at all time points except for day 2 suggesting a sustained induction of c-myc. Overall, there seemed to be a higher and more sustained induction of cmyc in the ILK/liver−/− mice. Transcriptional activity of c-myc was also higher in the ILK/liver−/− mice as compared to the WT mice at day 1 after TCPOBOP administration (Fig 5C). To further corroborate the findings that there was a sustained induction of c-myc in the ILK/liver−/− mice we performed c-myc immunohistochemistry at day 1 and 7 after TCPOBOP administration. At day 1 both WT and ILK/liver−/− mice showed nuclear staining. By day 7 the WT mice showed minimal nuclear staining while the ILK/liver−/− still had plenty of hepatocytes that showed nuclear staining suggesting a sustained induction of c-myc (Fig 5D). Studies have shown FoxM1 to be a key target of c-Myc (1) which is turn in involved in promoting hepatocyte proliferation (25). We looked at the levels of FoxM1 mRNA in the WT and ILK/liver−/− mice after TCPOBOP administration. FoxM1 mRNA levels Eventhough lower in the ILK/liver−/− mice at day one after TCPOBOP administration were more sustained as compared to the WT mice (Fig 5E).
Fig 5.
A) Protein levels of c-myc after TCPOBOP administration. B) mRNA levels of c-myc after TCPOBOP administration. C) Gel shift assay for c-myc after TCPOBOP administration. C represents cold competition (20 fold more as compared to biotinalated probe). 24 h sample for the WT and 24 h sample for the KO were used for cold competition D) Representative photomicrographs of Immunohistochemistry for c-myc in WT and ILK/liver−/− mice at day 1 and 7 after TCPOBOP administration. Arrows indicate nuclear localization of c-myc. NOTE: Pooled liver samples from at least 3 mice were used for preparing RNA and nuclear extracts. (E) FoxM1 mRNA level after TCPOBOP administration.
Discussion
Despite the dramatic effects of specific chemical mitogens such as TCPOBOP on the liver, the signaling pathways responsible for limiting the chemically induced hypertrophic and hyperplastic responses remain largely unknown. Studies in our laboratory have shown the role of ECM in inhibiting hepatocyte proliferation. (16, 18, 26) Because it is practically impossible to eliminate ECM from an intact organ, elimination of the proteins responsible for transmission of the ECM signals to hepatocytes became a feasible alternative when ILKloxP/loxP mice became available. FAK (focal adhesion kinase), Mig2 and ILK are three proteins, primarily involved with transmission of the integrin signals. Our results demonstrate that the final size of the liver due to the TCPOBOP-induced hypertrophic and hyperplastic response is to a significant level dependent on ILK. Livers deficient in ILK show prolonged and sustained proliferative response to TCPOBOP. They also show higher liver/body weight ratios as compared to the WT counterpart given the same treatment.
TCBOPOP is the strongest chemical mitogen (5–7) for the liver. The final liver sizes in normal mice following stimulation by TCBOPOP are the largest know in terms of liver to body weight ratio. The observed further enhancements in liver size and weight after TCBOPOP in the ILK/Liver −/− mice is to our knowledge the largest recorded for mice of that age. Numerous studies have demonstrated that the size of the liver, though highly susceptible to hormonal and nutritional responses, is overall adjusted to appropriate levels for the size of the body of the animal. We have used the term “hepatostat” to characterize this phenomenon.(27) Our recent studies have implicated extracellular and pericellular matrix as involved in this process. Interference with ECM/integrin signaling by elimination of hepatocyte ILK has led to a higher “hepatostat” in three different models of growth, such as liver regeneration after partial hepatectomy (18), phenobarbital (19) and now TCBOPOP. On the other hand, over-expression of the pericellular protein glypican 3 (GPC3) in hepatocytes led to a lower hepatostat (Am. J. Pathology, in press), consistent with the growth suppressing effects of GPC3.(28) Our current studies underscore the important role of ECM as overall regulator of the hepatostat by mechanisms which need to further studied.
The hepatomegaly induced by TCPOBOP is known to be CAR dependent. (1, 8) We found considerable differences in the activation of CAR in the WT and ILK/liver−/− mice. While the WT mice showed an early strong activation of CAR the ILK/liver−/− mice showed a lower but a prolonged activation. It is very likely that the prolonged activation of CAR in the ILK/liver−/− mice is to compensate for the lower activation of CAR at early time points. Why removal of ILK from the hepatocytes leads to lower activation of CAR is worthy of further investigation.
We next investigated the mechanisms behind this prolonged proliferative response in the ILK/liver−/− mice. Promitogenic proteins like cyclin d1, HGF and Yap show sustained induction in the ILK/liver−/− mice. The protein c-myc has been implicated in various aspects of liver proliferation such as that observed in liver regeneration, growth and tumorigenesis. (29–31) Recent study have shown(1) c-myc as a key component of the TCPOBOP-induced hepatocyte proliferation. In our study also we saw increased and sustained induction of c-myc in the ILK/liver−/− mice as compared to the WT mice. It is possible that the increased and sustained proliferation seen in the ILK/liver−/− is in part c-myc dependent. Mitoinhibitory molecule like TGFβ1 was also lower (day 2 and 5) in the ILK/liver−/− mice as compared to WT mice. Taken together the ILK/liver−/− mice have a sustained and prolonged induction of Promitogenic signaling. It is important to understand that given the multiplicity of changes accompanying removal of ILK, it is not easy to assign the defect in termination of TCPOBOP-induced hepatocyte proliferation to any specific single signaling system. The cybernetic interconnections between the different signaling systems are quite complex. It is reasonable to postulate, however, that the “hyperproliferation” seen in these mice is a consequence of the adaptations resulting from one central event, the disruption or alteration of the signaling of ECM to hepatocytes because of the removal of ILK.
Our studies show that the overall CAR function and expression are different in the ILK/liver−/− mice. While it is possible that there is a direct interaction between ILK and CAR, the changes in hepatocyte differentiation and function after elimination of ILK are so complex that it is highly likely that the effects on CAR are indirect. For a perspective, please note Figure 6 of reference 16.
In summary, these results demonstrate a central role of ECM signaling via ILK in terminating TCPOBOP-induced hepatocyte proliferation. Overall, these studies provide critical information on the mechanisms by which matrix defines and controls hepatocyte proliferation the liver. This work, however, has implications, not just for liver, but also for all tissue biology. Matrix defines the extracellular environment and regulates cellular function and growth in all tissues including liver, which has been one of the best tissue paradigms to investigate the complex interactions between matrix and different aspects of growth and differentiation.
Acknowledgments
The work is supported by R01 grant (5R01CA035373-26)
Abbreviations
- CAR
constitutive Androstane receptor
- ILK
integrin linked kinase
- PCNA
proliferative cell nuclear antigen assay
- TCPOBOP
1, 4-bis [2-(3, 5-dichaloropyridyloxy)] benzene
- AFP
alpha-fetoprotein
- ECM
extracellular matrix
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Blanco-Bose WE, Murphy MJ, Ehninger A, Offner S, Dubey C, Huang W, Moore DD, et al. C-Myc and its target FoxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology. 2008;48:1302–1311. doi: 10.1002/hep.22475. [DOI] [PubMed] [Google Scholar]
- 2.Qatanani M, Moore DD. CAR, the continuously advancing receptor, in drug metabolism and disease. Curr Drug Metab. 2005;6:329–339. doi: 10.2174/1389200054633899. [DOI] [PubMed] [Google Scholar]
- 3.Waxman DJ. P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR. Arch Biochem Biophys. 1999;369:11–23. doi: 10.1006/abbi.1999.1351. [DOI] [PubMed] [Google Scholar]
- 4.Diwan BA, Lubet RA, Ward JM, Hrabie JA, Rice JM. Tumor-promoting and hepatocarcinogenic effects of 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) in DBA/2NCr and C57BL/6NCr mice and an apparent promoting effect on nasal cavity tumors but not on hepatocellular tumors in F344/NCr rats initiated with N-nitrosodiethylamine. Carcinogenesis. 1992;13:1893–1901. doi: 10.1093/carcin/13.10.1893. [DOI] [PubMed] [Google Scholar]
- 5.Columbano A, Ledda-Columbano GM. Mitogenesis by ligands of nuclear receptors: an attractive model for the study of the molecular mechanisms implicated in liver growth. Cell Death Differ. 2003;10 Suppl 1:S19–S21. doi: 10.1038/sj.cdd.4401113. [DOI] [PubMed] [Google Scholar]
- 6.Ledda-Columbano GM, Pibiri M, Loi R, Perra A, Shinozuka H, Columbano A. Early increase in cyclin-D1 expression and accelerated entry of mouse hepatocytes into S phase after administration of the mitogen 1, 4-Bis[2-(3,5-Dichloropyridyloxy)] benzene. Am J Pathol. 2000;156:91–97. doi: 10.1016/S0002-9440(10)64709-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locker J, Tian J, Carver R, Concas D, Cossu C, Ledda-Columbano GM, Columbano A. A common set of immediate-early response genes in liver regeneration and hyperplasia. Hepatology. 2003;38:314–325. doi: 10.1053/jhep.2003.50299. [DOI] [PubMed] [Google Scholar]
- 8.Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 9.Golubovskaya VM, Kweh FA, Cance WG. Focal adhesion kinase and cancer. Histol Histopathol. 2009;24:503–510. doi: 10.14670/HH-24.503. [DOI] [PubMed] [Google Scholar]
- 10.Joshi MB, Ivanov D, Philippova M, Erne P, Resink TJ. Integrin-linked kinase is an essential mediator for T-cadherin-dependent signaling via Akt and GSK3beta in endothelial cells. Faseb J. 2007;21:3083–3095. doi: 10.1096/fj.06-7723com. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Hua ZC. FAK expression regulation and therapeutic potential. Adv Cancer Res. 2008;101:45–61. doi: 10.1016/S0065-230X(08)00403-X. [DOI] [PubMed] [Google Scholar]
- 12.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase - essential roles in physiology and cancer biology. J Cell Sci. 2008;121:3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 13.van Nimwegen MJ, van de Water B. Focal adhesion kinase: a potential target in cancer therapy. Biochem Pharmacol. 2007;73:597–609. doi: 10.1016/j.bcp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Zachary I. Focal adhesion kinase. Int J Biochem Cell Biol. 1997;29:929–934. doi: 10.1016/s1357-2725(97)00008-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Zhang Y, Ithychanda SS, Tu Y, Chen K, Qin J, Wu C. Migfilin interacts with Src and contributes to cell-matrix adhesion-mediated survival signaling. J Biol Chem. 2009;284:34308–34320. doi: 10.1074/jbc.M109.045021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gkretsi V, Apte U, Mars WM, Bowen WC, Luo JH, Yang Y, Yu YP, et al. Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology. 2008 doi: 10.1002/hep.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gkretsi V, Mars WM, Bowen WC, Barua L, Yang Y, Guo L, St-Arnaud R, et al. Loss of integrin linked kinase from mouse hepatocytes in vitro and in vivo results in apoptosis and hepatitis. Hepatology. 2007;45:1025–1034. doi: 10.1002/hep.21540. [DOI] [PubMed] [Google Scholar]
- 18.Apte U, Gkretsi V, Bowen WC, Mars WM, Luo JH, Donthamsetty S, Orr A, et al. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology. 2009 doi: 10.1002/hep.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donthamsetty S, Bowen W, Mars W, Bhave V, Luo JH, Wu C, Hurd J, et al. Liver-specific ablation of integrin-linked kinase in mice results in enhanced and prolonged cell proliferation and hepatomegaly after phenobarbital administration. Toxicol Sci. 113:358–366. doi: 10.1093/toxsci/kfp281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terpstra L, Prud'homme J, Arabian A, Takeda S, Karsenty G, Dedhar S, St-Arnaud R. Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J Cell Biol. 2003;162:139–148. doi: 10.1083/jcb.200302066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson DG, Walker CL. Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol. 1999;39:295–312. doi: 10.1146/annurev.pharmtox.39.1.295. [DOI] [PubMed] [Google Scholar]
- 22.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20:638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saucedo LJ, Edgar BA. Filling out the Hippo pathway. Nat Rev Mol Cell Biol. 2007;8:613–621. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]
- 24.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6:635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Kiyokawa H, Dennewitz MB, Costa RH. The Forkhead Box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci U S A. 2002;99:16881–16886. doi: 10.1073/pnas.252570299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalopoulos GK, Bowen WC, Mule K, Luo J. HGF-, EGF-, and dexamethasone-induced gene expression patterns during formation of tissue in hepatic organoid cultures. Gene Expr. 2003;11:55–75. doi: 10.3727/000000003108748964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu B, Paranjpe S, Bowen WC, Bell AW, Luo JH, Yu YP, Mars WM, et al. Investigation of the role of glypican 3 in liver regeneration and hepatocyte proliferation. Am J Pathol. 2009;175:717–724. doi: 10.2353/ajpath.2009.081129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baena E, Gandarillas A, Vallespinos M, Zanet J, Bachs O, Redondo C, Fabregat I, et al. c-Myc regulates cell size and ploidy but is not essential for postnatal proliferation in liver. Proc Natl Acad Sci U S A. 2005;102:7286–7291. doi: 10.1073/pnas.0409260102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauleon I, Lombard MN, Munoz-Alonso MJ, Canelles M, Leon J. Kinetics of myc-max-mad gene expression during hepatocyte proliferation in vivo: Differential regulation of mad family and stress-mediated induction of c-myc. Mol Carcinog. 2004;39:85–90. doi: 10.1002/mc.20000. [DOI] [PubMed] [Google Scholar]
- 31.Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, Bachmann MH, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]