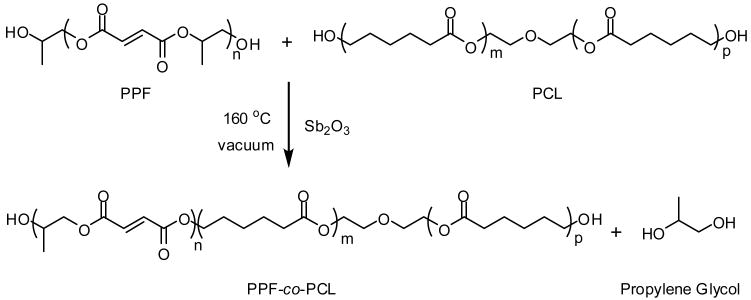
Abstract
In this work, a series of copolymers of polypropylene fumarate-co-polycaprolactone (PPF-co-PCL) were synthesized via a three-step polycondensation reaction of oligomeric polypropylene fumarate (PPF) with polycaprolactone (PCL). The effects of PPF precursor molecular weight, PCL precursor molecular weight, and PCL fraction in the copolymer (PCL feed ratio) on the maximum crosslinking temperature, gelation time, and mechanical properties of the crosslinked copolymers were investigated. The maximum crosslinking temperature fell between 38.2±0.3 and 47.2±0.4 °C, which increased with increasing PCL precursor molecular weight. The gelation time was between 4.2±0.2 and 8.5±0.7 min, and decreased with increasing PCL precursor molecular weight. The compressive moduli ranged from 44±1.8 to 142±7.4 MPa, with enhanced moduli at higher PPF precursor molecular weight and lower PCL feed ratio. The compressive toughness was in the range of 4.1±0.3 and 17.1±1.3 KJ/m3. Our data suggest that the crosslinking and mechanical properties of PPF-co-PCL can be modulated by varying the composition. Therefore the PPF-co-PCL copolymers may offer increased versatility as an injectable, in situ polymerizable biomaterial than the individual polymers of PPF and PCL.
Keywords: Polypropylene fumarate, polycaprolactone, injectable biomaterials, in situ polymerizable
Introduction
Injectable, in situ polymerizable biomaterials hold great promise for use in minimal invasive reconstructive surgeries. Through percutaneous approaches or tiny incisions, the biomaterial can be injected into various defect cavities within the human body. After crosslinking in situ, it can offer mechanical support, a curative effect, or both [1-3]. They are often used in bone or cartilage defects in the areas of orthopedics or craniofacial surgeries. Polymethylmethacrylate (PMMA) is currently the most widely used injectable bone substitute in orthopedic clinical practice, especially for load-bearing sites. After injection into the bone defect, it crosslinks in situ rapidly and provides immediate mechanical support. However, PMMA has a significantly higher modulus than trabecular bone and is non-degradable. Long-term follow-up studies have demonstrated the undesirable stress shielding effect and atrophy of nearby bone after PMMA injection [4-9]. Additionally, PMMA has a relatively short working time for preparation and injection, and exhibits high exothermic heat release during curing that is not ideal for use as injectable bone substitute [4, 5].
Polypropylene fumarate (PPF) is an unsaturated linear polyester that can polymerize in situ through the fumarate carbon-carbon double bonds. It degrades by simple hydrolysis of the ester bonds into the nontoxic products of propylene glycol and fumaric acid [10]. PPF-based composite materials exhibit compressive modulus in the range of 23-265 MPa, which is appropriate for replacement of human trabecular bone [11]. However, the propylene glycol part in each repeating unit of PPF chain provides only one free rotating carbon-carbon single bond. This rigidity of PPF polymer chain limits the accessibility of double bonds during polymerizing and ester bonds during degradation. Due to this rigidity, a crosslinker molecule is often required to crosslink PPF. And the unreacted monomers usually display a dose-dependent cytotoxic response, and may lead to biocompatibility issues [12-14].
Polycaprolactone (PCL) is a widely used FDA-approved biodegradable polymer with excellent flexibility [15], and is increasingly used in making copolymers to enhance the crosslinking and other properties of these materials [16, 17]. We have developed a copolymer polycaprolactone fumarate (PCLF) which has a more flexible backbone than PPF and crosslinks without additional crosslinking agents. However, because of the dominant PCL segment in the copolymer, the crosslinked PCLF is not strong enough for trabecular bone replacement [18].
In an attempt to combine the favorable properties of PPF and PCLF, our laboratory recently designed and synthesized a new injectable copolymer PPF-co-PCL composed of PPF and PCL. The chemical and physical properties of the uncrosslinked copolymers have been characterized [19] and the self-crosslinkability of the copolymer has been demonstrated. In this study, we further evaluated the handling and mechanical properties of the crosslinked copolymer by varying parameters such as PPF precursor molecular weight, PCL precursor molecular weight, and PCL fraction. Maximum crosslinking temperature, gelation time, compressive modulus and compressive toughness of the crosslinked copolymers were measured and compared with those of PPF precursors and PMMA. By optimizing the copolymer formulation, we were trying to tailor PPF-co-PCL for utilization varying from non-biologic to biologic reconstructions. Moreover, based on our findings in this study, future use of the copolymer could be to serve as a delivery vehicle either for local chemotherapeutic agents or bone growth factors. This would be more appropriate in the individualized treatment for a given patient.
Materials and Methods
1. Materials
PCL diols with nominal molecular weights of 530, 1250 and 2000 g/mol were purchased from Aldrich (Milwaukee, WI). All the other chemicals in the present study were also purchased from Aldrich.
2. Experimental design
The three variables we investigated were: PPF number-average molecular weight (Mn) (700 or 2000 g/mol), PCL precursor Mn (530, 1250, 2000 g/mol), and PCL feed ratio (0.3, 0.5, 0.75 by weight). Eighteen copolymers were synthesized and characterized (Table 1). The copolymers were crosslinked by adding benzoyl peroxide and dimethly toluidine. The maximum crosslinking temperature, gelation time, and compressive modulus were measured. All experiments were conducted in triplicates, and data is expressed as means +/- standard deviations.
Table 1.
Full Fractional Design Parameters and Properties Characterization of uncrosslinked PPF-co-PCL. Results are presented as mean ± standard deviation for n=3.
| Copolymer No. | a Copolymer formulations | PCL wt ratio by NMR | Mn (g/mol) by GPC | Tg (°C) by DSC |
|---|---|---|---|---|
| 1 | PPF700-co-PCL530R0.3 | 0.32 | 3141±48 | -19.0±1.3 |
| 2 | PPF700-co-PCL530R0.5 | 0.45 | 3947±278 | -30.4±1.5 |
| 3 | PPF700-co-PCL530R0.75 | 0.76 | 4043±51 | -33.7±2.1 |
| 4 | PPF700-co-PCL1250R0.3 | 0.25 | 3335±84 | -19.9±3.1 |
| 5 | PPF700-co-PCL1250R0.5 | 0.43 | 3133±23 | -35.2±0.8 |
| 6 | PPF700-co-PCL1250R0.75 | 0.66 | 3740±255 | -36.1±0.8 |
| 7 | PPF700-co-PCL2000R0.3 | 0.25 | 3448±76 | -19.7±0.3 |
| 8 | PPF700-co-PCL2000R0.5 | 0.43 | 3649±127 | -35.7±3.5 |
| 9 | PPF700-co-PCL2000R0.75 | 0.66 | 3835±63 | -39.7±2.4 |
| 10 | PPF2000-co-PCL530R0.3 | 0.25 | 4902±165 | -15.0±1.6 |
| 11 | PPF2000-co-PCL530R0.5 | 0.48 | 5907±243 | -23.0±1.1 |
| 12 | PPF2000-co-PCL530R0.75 | 0.65 | 6554±220 | -31.4±0.6 |
| 13 | PPF2000-co-PCL1250R0.3 | 0.37 | 5055±131 | -20.1±1.8 |
| 14 | PPF2000-co-PCL1250R0.5 | 0.52 | 5337±50 | -28.4±0.5 |
| 15 | PPF2000-co-PCL1250R0.75 | 0.68 | 6124±52 | -32.5±1.3 |
| 16 | PPF2000-co-PCL2000R0.3 | 0.28 | 4930±127 | -15.0±1.0 |
| 17 | PPF2000-co-PCL2000R0.5 | 0.44 | 4892±339 | -28.1±1.4 |
| 18 | PPF2000-co-PCL2000R0.75 | 0.63 | 6607±144 | -36.0±1.9 |
Describes copolymers synthesized from PPF (Mn 700 or 2000 g/mol) and PCL (Mn 530, 1250 or 2000 g/mol) with different PCL feed ratios (Ratio 0.3, 0.5 or 0.75). For example, copolymer PPF700-co-PCL530R0.3 is synthesized from PPF700 (Mn 700 g/mol) and PCL530 (Mn 530 g/mol) with PCL feed ratio of 0.3.
3. Synthesis of PPF
PPF was synthesized as described previously [19]. Briefly, diethyl fumarate and 1,2-propylene glycol were mixed together at a molar ratio of 1:3. Hydroquinone was added as a polymerization inhibitor and zinc chloride as a catalyst, in a 0.002:0.01:1 molar ratio to diethyl fumarate. The transesterification reaction obtained the fumaric diester by heating at 100 °C for 1 h and then at 150 °C for 7 h. The solution was allowed to cool to 100 °C and placed under vacuum (<1 mmHg).
The polymerization reaction was run at 150 °C for 1 and 5 h, producing PPF with Mn around 700 and 2000 g/mol, respectively, as measured by gel permeation chromatography (GPC).
4. Synthesis of PPF-co-PCL
The PPF-co-PCL copolymer was synthesized from PPF and PCL with antimony trioxide added as a catalyst (Scheme 1). They were mixed under nitrogen flow at 100 °C for 30 min. The reaction temperature was raised gradually to 160 °C under vacuum (<1 mmHg). The copolymerization took about 5 h and the byproduct 1,2-propylene glycol was removed by condensation. The resulting PPF-co-PCL copolymer was purified by solution precipitation forming a viscous melt or wax-like solid.
Scheme 1.
PPF-co-PCL copolymer synthesis from PPF and PCL
5. Copolymer Characterizations
5.1 GPC
The uncrosslinked copolymer's molecular weight and molecular weight distribution were characterized by gel permeation chromatography (GPC). The GPC was carried out with a Waters 717 Plus autosampler GPC system (Waters, Milford, MA) connected to a model 515 HPLC pump and model 2410 refractive index detector. Polystyrene standards (Polysciences, Warrington, PA) with five molecular weights (474, 1060, 2950, 6690, 18600 g/mol) were used.
5.2 FT-IR
Fourier transform infrared spectroscopy (FT-IR) spectra were obtained on a Nicolet 550 spectrometer. All polymers were analyzed using a zinc selenide ATR crystal. The resolution of the instrument was specified as 4 cm-1 at a wavenumber of 1000 cm-1.
5.3 NMR
Proton nuclear magnetic resonance (1H-NMR) spectra were acquired on a Varian Mercury Plus NMR spectrometer (1H=300.1 MHz) using CDCl3 (δ=7.27 ppm) solutions containing tetramethylsilane (TMS).
5.4 DSC
Differential scanning calorimetry (DSC) was measured on a TA Instruments Q1000 differential scanning calorimeter at a heating rate of 10 °C/min in a nitrogen atmosphere. To keep the same thermal history, each sample was preheated from room temperature to 100 °C and cooled to -90°C at a cooling rate of 5 °C/min. Then the DSC scan was recorded via heating from -90 to 100 °C. The glass transition temperature (Tg) was determined by using the midpoint temperature of the glass transition process.
6. Crosslinking of PPF-co-PCL
As related previously, a typical crosslinking procedure was performed [20]. One and a half grams of copolymer were dissolved in 500 μl of methylene chloride and mixed thoroughly. Fifty microliters of initiator solution (0.2 g of BPO dissolved in 1 ml of NVP) were added into the copolymer solution, and then 40 μl of accelerator solution (20 μl of DMT dissolved in 980 μl of methylene chloride) were added in and mixed thoroughly. Additionally, two other different amounts of accelerator solution (20 and 10 μl) were also used when crosslinking copolymer 1 (PPF700-co-PCL530R0.3).
7. Maximum temperature
The temperature profile was recorded throughout the crosslinking process in a glass vial of 10 mm diameter and 24 mm length immersed into a 37 °C water bath [21]. The copolymer was crosslinked in the glass vial. A thermocouple (Omega, KMTSS-032U-6, Stamford, Connecticut) was inserted into a half depth of the mixture in the glass vial. The temperature was measured every 30 seconds until it returned to 37 °C. Time zero was defined when accelerator was added into the mixture.
8. Gelation time
The gelation time was measured on a rheometer (Model AR2000, TA Instruments). The crosslinked mixture was carefully poured on the center of the temperature controlled Peltier plate of the rheometer. The acquired temperature was 37 °C. A cylindrical, stainless-steel parallel plate geometry of 8 mm in diameter was lowered to the level of 1 mm above the Peltier plate. A flow procedure consisting of a continuous ramp test type at a shear stress range from 0 to 30 Pa was used to monitor the viscosity as the composite cured. Time zero was defined when accelerator was added into the mixture, and the gelation time was defined when the viscosity of the composite suddenly increased as shown on rheometer.
9. Mechanical properties
The crosslinking mixture was injected into cylindrical Teflon molds of 10 mm in diameter and 15 mm in height. Then the scaffolds were put into the oven at 37 °C to simulate the temperature environment in the human body. After 24 hours, the cylinder specimens were removed from the mold and analyzed using a 312 Materials Testing System mechanical testing machine (MTS Systems Corp. Eden Prairie, Minnesota) referencing the guidelines we previously used for testing the crosslinked PPF samples. Samples were compressed at a crosshead speed of 0.1 mm/sec until failure, with the stress versus strain curve recorded throughout. The compressive modulus was calculated as the slope of the initial linear portion of the stress-strain curve. The compressive toughness was calculated as the area under the stress-strain curve before sample failure.
10. Statistical analysis
All experiments were conducted in triplicate and the data were expressed as means ± standard deviation (SD). One or three factors analysis of variance (ANOVA), followed by Duncan's multiple range test, were performed with SAS version 9.1.3 software to identify statistical difference at a significance level of p<0.05.
Results
Copolymer Characterizations
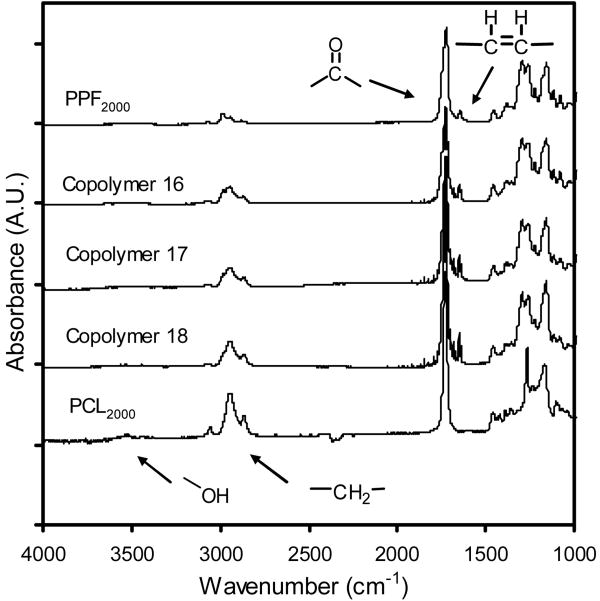
PPF-co-PCL Mn ranged from 3141±48 to 6607±144 g/mol. The polydispersity ranged from 2.6±0.2 to 4.0±0.3. FT-IR of copolymer showed a combination of spectra from its two components. The spectra of copolymer 16, 17 and 18, as well as their starting polymers PPF2000 (Mn 2000 g/mol) and PCL2000 (Mn 2000 g/mol), are presented in Figure 1. From the integration ratio of copolymer's different peaks originated from its PPF precursor and PCL precursor in 1H NMR, respectively, PCL composition in the copolymer was calculated and it showed a good correlation with the actual PCL feed ratio. All the copolymers have a single glass transition temperature which fell between -39.7±2.4 and -15.0±1.6 °C. They decreased significantly with increasing PCL feed ratio (p<0.01).
Figure 1.
FT-IR spectra of PPF2000 (Mn 2000 g/mol), PCL2000 (Mn 2000 g/mol), copolymers 16 (PPF2000-co-PCL2000R0.3), copolymer 17 (PPF2000-co-PCL2000R0.5) and copolymer 18 (PPF2000-co-PCL2000R0.75). As marked, C=C stretching at 1654 cm-1 and carbonyl stretching at 1720 cm-1 were all evident in spectra of PPF2000 and three copolymers. Methylene absorption at 2940 cm-1 can be found in all the polymers with the strongest one in PCL2000 due to the highest methylene content in PCL backbone.
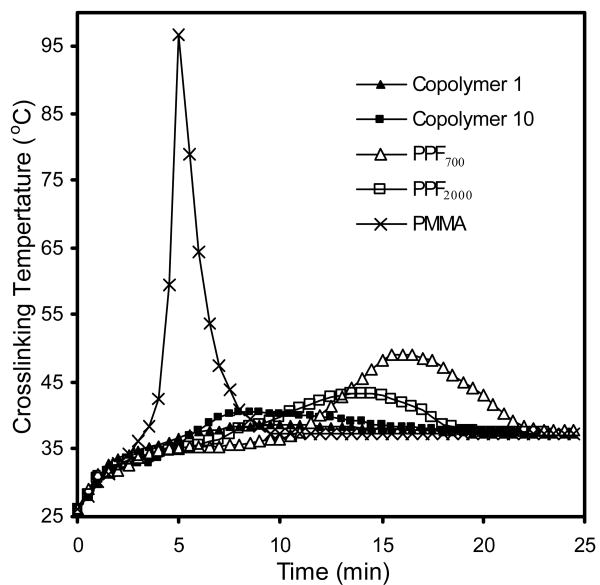
Maximum crosslinking temperature
Figure 2 shows representative temperature profiles of copolymer 1 (PPF700-co-PCL530R0.3), copolymer 10 (PPF2000-co-PCL530R0.3), PPF700 (Mn 700 g/mol), PPF2000 (Mn 2000 g/mol) and PMMA. The maximum crosslinking temperature of crosslinked copolymer fell between 38.2±0.3 and 47.2±0.4 °C. It increased with increasing PCL precursor's molecular weight. The maximum crosslinking temperatures of PPF700, PPF2000 and PMMA were 49.1±2.2 °C, 43.4±1.9 °C and 96.7±3.1 °C respectively. All the values are shown in Table 2.
Figure 2.
Representative temperature profiles for crosslinked copolymer 1 (PPF700-co-PCL530R0.3) (▲), copolymer 10 (PPF2000-co-PCL530R0.3) (■), PPF700 (Mn 700 g/mol) (Δ), PPF2000 (Mn 2000 g/mol) (□) and PMMA (×).
Table 2.
Maximum crosslinking temperature and Gelation time of all the crosslinked PPF-co-PCL, PPF (PPF700, PPF2000) and PMMA. Results are presented as mean ± standard deviation for n=3.
| Polymers | Maximum temperature (°C) | Gelation time (min) |
|---|---|---|
| PPF700-co-PCL530R0.3 | 38.6±0.4 | 8.3±0.5 |
| PPF700-co-PCL530R0.5 | 38.2±0.3 | 8.5±0.7 |
| PPF700-co-PCL530R0.75 | 38.8±1.1 | 7.4±1.5 |
| PPF700-co-PCL1250R0.3 | 43.5±0.6 | 5.9±0.4 |
| PPF700-co-PCL1250R0.5 | 42.9±0.7 | 6.9±0.7 |
| PPF700-co-PCL1250R0.75 | 48.1±0.9 | 4.3±0.5 |
| PPF700-co-PCL2000R0.3 | 44.2±0.6 | 5.2±0.8 |
| PPF700-co-PCL2000R0.5 | 44.3±0.5 | 5.4±0.7 |
| PPF700-co-PCL2000R0.75 | 45.7±0.8 | 4.2±0.2 |
| PPF2000-co-PCL530R0.3 | 40.6±0.5 | 5.9±0.8 |
| PPF2000-co-PCL530R0.5 | 39.7±1.6 | 6.8±0.4 |
| PPF2000-co-PCL530R0.75 | 40.8±0.9 | 6.0±0.3 |
| PPF2000-co-PCL1250R0.3 | 43.3±1.1 | 4.7±0.6 |
| PPF2000-co-PCL1250R0.5 | 40.6±0.7 | 6.9±0.4 |
| PPF2000-co-PCL1250R0.75 | 41.1±0.9 | 6.4±0.7 |
| PPF2000-co-PCL2000R0.3 | 47.2±0.4 | 4.3±0.3 |
| PPF2000-co-PCL2000R0.5 | 46.3±0.3 | 4.7±0.5 |
| PPF2000-co-PCL2000R0.75 | 46.1±0.6 | 4.6±0.6 |
| PPF700 | 49.1±2.2 | 16.4±1.5 |
| PPF2000 | 43.4±1.9 | 14.1±0.8 |
| PMMA | 96.7±3.1 | 3.4±0.2 |
Gelation time
All the gelation time values are shown in Table 2. Gelation time of the crosslinked copolymer fell between 4.2±0.2 and 8.5±0.7 min. It decreased with increasing PCL precursor's molecular weight. The gelation times of PPF700, PPF2000 and PMMA were 16.4±1.5 min, 14.1±0.8 min and 3.4±0.2 min, respectively. All the gelation times of crosslinked copolymers were longer than that of PMMA, and significantly shorter than those of their PPF precursors (p<0.01).
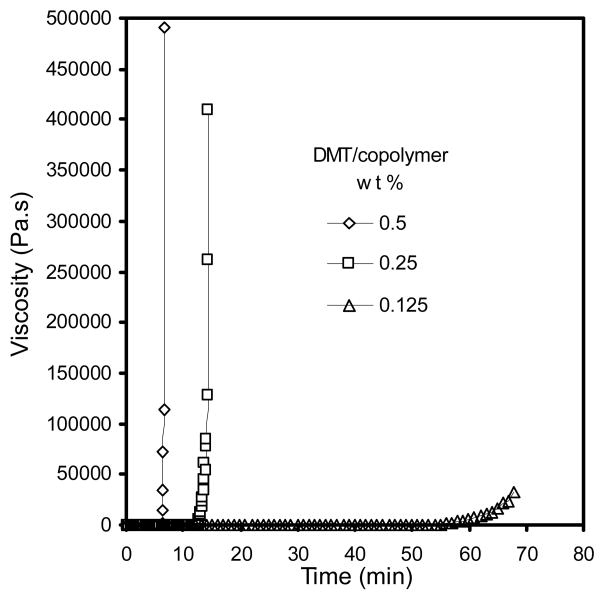
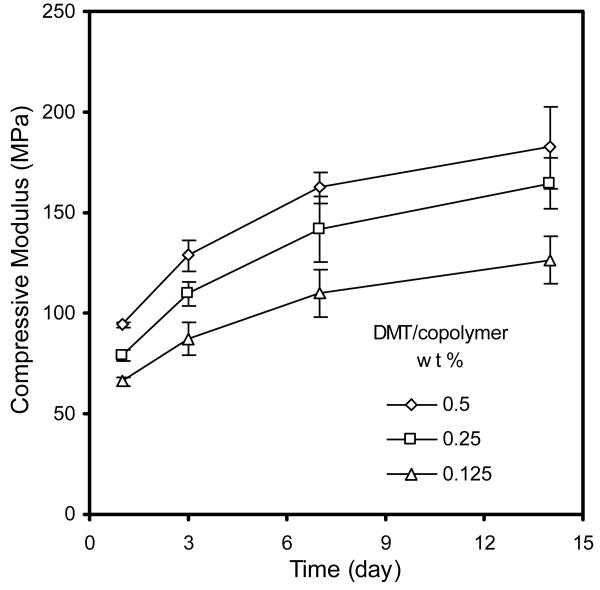
The gelation times of crosslinked copolymer 1 (PPF700-co-PCL530R0.3) with three different amounts of DMT were measured. The compressive modulus of the scaffold was tested at four different time points after setting. Figure 3 shows the representative different gelation times using different amount of DMT as accelerator. When decreasing accelerator, the gelation time greatly increased (from 6 min to over 60 min). Figure 4 shows the changes of compressive modulus under different amount of accelerator. The compressive modulus of the crosslinked scaffold using the lowest amount of accelerator was significantly lower than the other two groups (p<0.05).
Figure 3.
Representative gelation times of crosslinked copolymer 1 (PPF700-co-PCL530R0.3) using three different accelerator amounts: 0.5 (◇), 0.25 (□) and 0.125 (Δ) wt % of DMT/copolymer.
Figure 4.
Compressive moduli changes of crosslinked copolymer 1 (PPF700-co-PCL530R0.3) with time using different accelerator amounts: 0.5 (◇), 0.25 (□) and 0.125 (Δ) wt % of DMT/copolymer. Results are presented as mean ± standard deviation for n=3. The p values between groups by different variables (Time and DMT amount) both were less than 0.01.
Mechanical properties
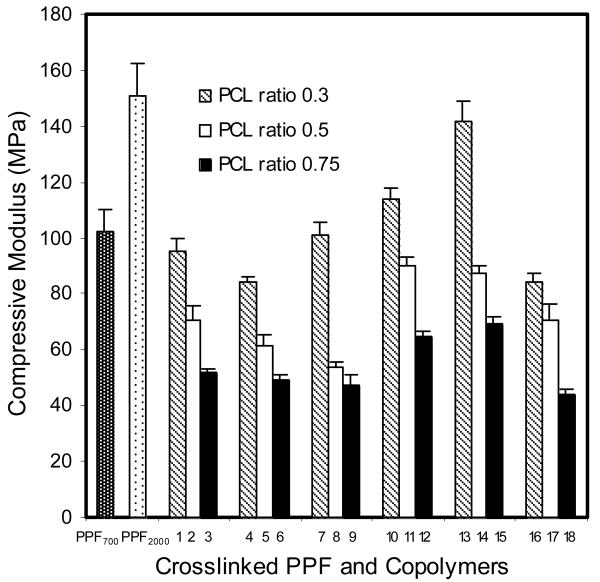
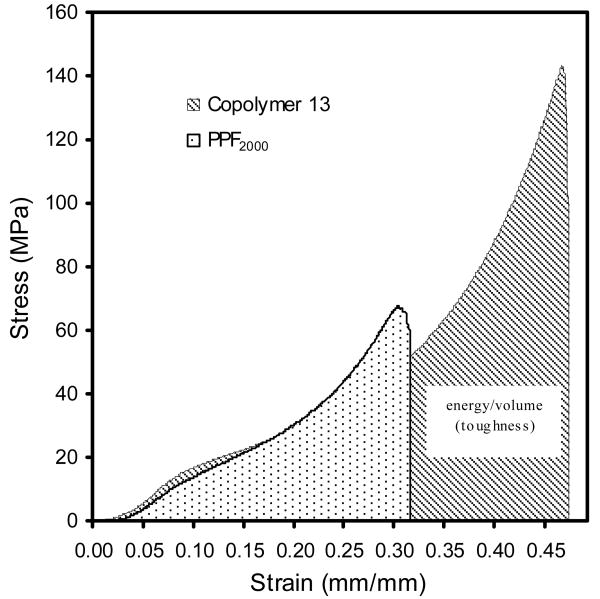
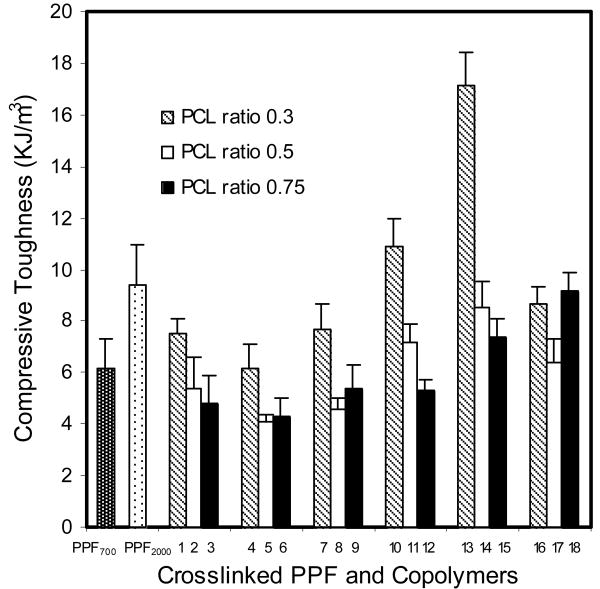
The compressive modulus of all crosslinked copolymers (1 to 18) and PPF (PPF700 and PPF2000) are shown in Figure 5. The compressive modulus ranged between 44±1.8 and 142±7.4 MPa. It increased with increasing PPF precursor's molecular weight and decreasing PCL feed ratio. Stress-strain curves of crosslinked copolymer 13 (PPF2000-co-PCL1250R0.3) and its PPF precursor (PPF2000) are shown in Figure 6. The area under the curve indicates the toughness of the tested material. Copolymer 13 had a much greater compressive toughness than its PPF precursor (PPF2000). The compressive toughness of all crosslinked copolymers and PPF (PPF700 and PPF2000) are shown in Figure 7. The values fell between 4.1±0.3 and 17.1±1.3 KJ/m3.
Figure 5.
The compressive modulus of PPF700 (Mn 700 g/mol), PPF2000 (Mn 2000 g/mol) and all the PPF-co-PCL copolymers (No. 1∼18) crosslinking scaffolds. Results are presented as mean ± standard deviation for n=3. The p values of different groups of PPF-co-PCL copolymer by three variables (PPF Mn, PCL Mn and PCL ratio) were 0.0123, 0.127 and <0.01 respectively.
Figure 6.
Representative Stress-Strain curves of crosslinked scaffolds of copolymer 13 (PPF2000-co-PCL1250R0.3) and its precursor PPF2000. The area under the Stress-Strain curve equals the amount of energy/volume which defines the toughness of the tested material.
Figure 7.
The compressive toughness of PPF700 (Mn 700 g/mol), PPF2000 (Mn 2000 g/mol) and all the PPF-co-PCL copolymers (No. 1∼18) crosslinking scaffolds. Results are presented as mean ± standard deviation for n=3. The p values of different groups of PPF-co-PCL copolymer by three variables (PPF Mn, PCL Mn and PCL ratio) were <0.01, 0.5356 and <0.01 respectively.
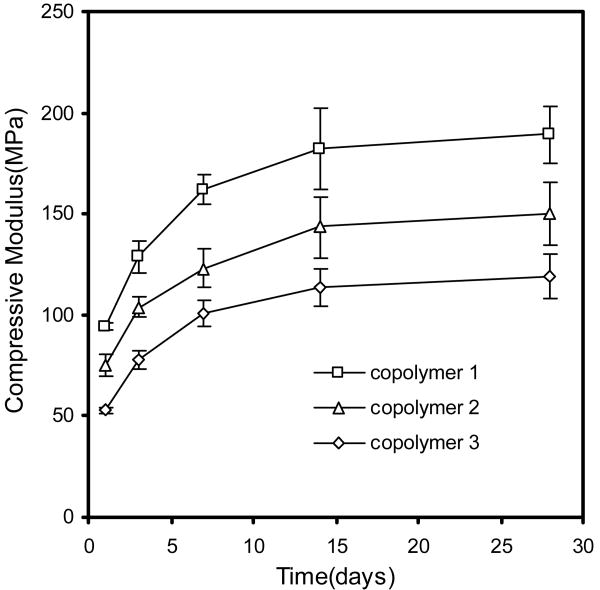
Finally, the compressive modulus of copolymer 1 (PPF700-co-PCL530R0.3), copolymer 2 (PPF700-co-PCL530R0.5) and copolymer 3 (PPF700-co-PCL530R0.75) was tested at different time points after crosslinking. Figure 8 shows the changes of compressive modulus over time. The compressive modulus of all these copolymers kept increasing the end of the second week after setting. It subsequently became stable.
Figure 8.
The compressive modulus changes of copolymer 1 (PPF530-co-PCL530R0.3) (□), copolymer 2 (PPF530-co-PCL530R0.5) (Δ) and copolymer 3 (PPF530-co-PCL530R0.75) (◇) over time. The p values between groups by different variables (Time and PCL ratio) both were less than 0.01.
Discussion
Consistent with the previous studies in our laboratory on the uncrosslinked copolymer PPF-co-PCL [19], the copolymer characterizations including GPC, FT-IR, NMR and DSC, showed a successful synthesis from PPF and PCL precursors. FT-IR confirmed the presence of PCL precursors in the copolymer composition by the appearance of PCL characteristic peaks. 1H NMR showed a good agreement of initial PCL feed ratio with final PCL composition in the copolymer. This allows us to use the ratio as its composition in later discussions for simplicity without affecting the conclusion [19]. All the copolymers had only a single glass transition in DSC curves indicating that copolymerization of semicrystalline PCL with amorphous PPF efficiently decreased the crystallinity of PCL fragment. Moreover, the glass transition for all the copolymers was narrower (less than 15 °C) than PPF precursors. This confirmed that the copolymerization was quite successful since the single glass transition for miscible polymer blends is generally much broader than those of pure polymers. Tg of the uncrosslinked copolymer dramatically decreased with increasing PCL feed ratio. It possibly resulted from an increasing flexibility of copolymer backbone with increasing PCL feed ratio [22].
The heat produced from polymerization of injectable biomaterials is one of the major concerns in the clinical application. The maximum heat produced from PMMA bone cement exceeds 100 °C. This could pose a risk of tissue damage especially when it is injected into the fractured vertebral body where the vulnerable spinal cord is close by [23]. In our study, both PPF and PPF-co-PCL copolymer showed a much lower heat produced during the crosslinking (around 38∼50 °C) than PMMA. These results agree with some previous reports on the PPF-based composites [24-26]. The crosslinking temperature of the copolymer increased slightly with increasing PCL precursor's molecular weight. A higher molecular weight of PCL fragment would introduce more carbon-carbon single bonds into the backbone of copolymer, hence increase the mobility of copolymer chain and the accessibility of double bonds during crosslinking reactions, and lead to a higher speed of crosslinking reaction and heat release.
The gelation time of all the copolymers decreased approximately to half the time of their PPF precursors. This interesting finding further illustrated the role of PCL on the mobility of the copolymer chain. As a result, a more fully crosslinked copolymer might be achieved and the scaffold's biocompatibility would be increased by reducing the amount of unreacted monomers [14]. Our preliminary in vitro data on the cytotoxicity comparison between crosslinked PPF and PPF-co-PCL did show a more favorable biocompatibility of the copolymer in consequence of a higher double-bond conversion. (Detailed data will be shown in a subsequent paper from our lab.) So far, the in vitro cytotoxicity of parts of our PPF-co-PCL formulations together with PPF and PMMA were already investigated in our lab using MTS Assay. Three time points (1 day, 3 days, and 7 days) were chosen. No cytotoxic response was demonstrated from the different formulations of PPF-co-PCL and PMMA bone cement. Only on the 7 days time point, there was a slight decrease of cell viability in PPF samples compared with the control group. However, no statistical difference was shown. Further studies are needed to exactly assess the crosslinked copolymer's biocompatibility in vitro and in vivo.
The gelation time of the crosslinked copolymer using a specific amount of accelerator fell between 4.2±0.2 and 8.5±0.7 min. Moreover, our study showed that it could be easily adjusted by changing the amount of accelerator for different clinical use. An increase in DMT concentration led to an accelerated production of free radicals which increased the rate of crosslinking and resulted in shorter gelation time. Additionally, we also investigated the mechanical properties changes of the crosslinked scaffold using different amount of accelerator. The results showed that the final compressive modulus of the crosslinked scaffold began to drop when accelerator's amount was decreased to a lower level (0.125 wt % of DMT/copolymer). This was due to a decrease in crosslinking levels that rendered a reduced mechanical property.
Our data revealed that compressive modulus of the crosslinked copolymer increased with a higher PPF precursor molecular weight and lower PCL feed ratio. Both increasing PPF Mn and decreasing PCL ratio led to an increase of fumarate double bonds density in the copolymer. More reacting double bonds in the crosslinked copolymer would yield a stronger material. Furthermore, our preliminary in vitro biocompatibility study showed that the degradation rate of the copolymer increased with increasing PCL feed ratio. The weight loss of copolymer with the highest PCL feed ratio was almost twice as much as that of the copolymer with the lowest PCL feed ratio from the same PPF and PCL precursors. No significant difference of weight loss was found from different formulations varying in PPF or PCL precursor's molecular weights (detailed data not shown). This means that PPF-co-PCL copolymer can be tailored to vary from a higher mechanical property for some non-biological reconstructions where a slow degradability is preferred (like some load-bearing metastatic bone lesions) to a relatively lower mechanical property for cases where bone healing is the goal and a more rapid degradability is preferred (like some non load-bearing upper limb benign bone lesions or defects). This surely offers the copolymer a wider and more individualized application in clinics.
The amount of energy/volume a material can absorb before failure defines the intrinsic toughness of the material. It is calculated as the magnitude of the force times the deformation produced and is equal to the area under stress-strain curve. This concept is very useful in clinics because many injuries may impart a specific energy to the body. For example, a fall from a height may turn the potential energy of the body weight at the original height into the energy of deformation especially in the osteoporotic spine, causing fracture [27]. Wolfe et al reported that the fracture toughness of crosslinked PPF scaffold is much lower than that of human cortical bone. This makes PPF unsuitable for use especially in load-bearing bone defect area [28]. In our study, copolymer 13 (PPF2000-co-PCL1250R0.3) had a much higher compressive toughness than its PPF precursor (PPF2000) even though their compressive moduli were similar (Fig. 6). This indicated that, in this specific formulation of PPF-co-PCL copolymer, the unsaturated PPF part provided enough mechanical stiffness while the flexible PCL part offered a higher toughness than its PPF precursor. This interesting property makes the crosslinked copolymer a more favorable substitute for clinical use than its brittle PPF precursor, especially in loading required bone defect treatment such as vertebroplasty for compressive spinal fractures.
Finally, the changes in the compressive modulus over time were investigated. The compressive moduli of all the tested copolymer scaffolds gradually increased over time. The greatest increase occurred in the first two weeks after setting (Fig. 8). PPF also had the phenomenon of mechanical property enhancement due to the continuous crosslinking reaction over time [29]. However, unlike PPF-co-PCL that showed an increase in mechanical properties up to two weeks, the compressive modulus of PPF kept increasing up to the sixth week even in a degradation environment. This difference between PPF-co-PCL and PPF illustrated that the PCL fragment in the copolymer chain could help to produce a much faster and complete crosslinking process.
Conclusion
Our results show that the maximum crosslinking temperature, the gelation time and the mechanical properties of crosslinked copolymer PPF-co-PCL could be modulated by varying its composition for different applications. Incorporation of PCL in the copolymer composition led to a fully crosslinked copolymer network. By integrating both virtues of its PPF and PCL precursors, PPF-co-PCL appeared to be an optimal injectable, in situ polymerizable biomaterial with a more favorable characteristic.
Acknowledgments
This work was funded by the National Institutes of Health (AR056212-01) and 1T32AR056950. We thank Stephen Cha for his help in statistical analysis of the data.
References
- 1.Deramond H, Depriester C, Galibert P, Le Gars D. Radiologic Clinics of North America. 1998;36:533. doi: 10.1016/s0033-8389(05)70042-7. [DOI] [PubMed] [Google Scholar]
- 2.Jensen ME, Evans AJ, Mathis JM, Kallmes DF, Cloft HJ, Dion JE. American Journal of Neuroradiology. 1997;18:1897. [PMC free article] [PubMed] [Google Scholar]
- 3.Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Spine. 2001;26:1631. doi: 10.1097/00007632-200107150-00026. [DOI] [PubMed] [Google Scholar]
- 4.Einhorn TA. Spine. 2000;25:1051. doi: 10.1097/00007632-200005010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Deramond H, Wright NT, Belkoff SM. Bone. 1999;25:17S. doi: 10.1016/s8756-3282(99)00127-1. [DOI] [PubMed] [Google Scholar]
- 6.Tseng YY, Yang TC, Tu PH, Lo YL, Yang ST. Spine. 2009;34:1917. doi: 10.1097/BRS.0b013e3181ac8f07. [DOI] [PubMed] [Google Scholar]
- 7.Grafe IA, Baier M, Noldge G, Weiss C, Da Fonseca K, Hillmeier J, Libicher M, Rudofsky G, Metzner C, Nawroth P, Meeder PJ, Kasperk C. Spine. 2008;33:1284. doi: 10.1097/BRS.0b013e3181714a84. [DOI] [PubMed] [Google Scholar]
- 8.Legroux-Gerot I, Lormeau C, Boutry N, Cotten A, Duquesnoy B, Cortet B. Clinical Rheumatology. 2004;23:310. doi: 10.1007/s10067-004-0914-7. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Higueras A, Alvarez L, Rossi RE, Quinones D, Al-Assir I. Neuroradiology. 2002;44:950. doi: 10.1007/s00234-002-0856-1. [DOI] [PubMed] [Google Scholar]
- 10.He S, Timmer MD, Yaszemski MJ, Yasko AW, Engel PS, Mikos AG. Polymer. 2001;42:1251. [Google Scholar]
- 11.Peter SJ, Miller ST, Zhu GM, Yasko AW, Mikos AG. Journal of Biomedical Materials Research. 1998;41:1. doi: 10.1002/(sici)1097-4636(199807)41:1<1::aid-jbm1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Gresser JD, Hsu SH, Nagaoka H, Lyons CM, Nieratko DP, Wise DL, Barabino GA, Trantolo DJ. Journal of Biomedical Materials Research. 1995;29:1241. doi: 10.1002/jbm.820291011. [DOI] [PubMed] [Google Scholar]
- 13.Timmer MD, Shin H, Horch RA, Ambrose CG, Mikos AG. Biomacromolecules. 2003;4:1026. doi: 10.1021/bm0300150. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ZX, Feng XL, Mao J, Xiao JZ, Liu CM, Qiu JJ. Biochem Biophys Res Commun. 2009;379:557. doi: 10.1016/j.bbrc.2008.12.084. [DOI] [PubMed] [Google Scholar]
- 15.Elfick APD. Biomaterials. 2002;23:4463. doi: 10.1016/s0142-9612(02)00163-1. [DOI] [PubMed] [Google Scholar]
- 16.Wu QX, Yoshino T, Sakabe H, Zhang HK, Isobe S. Polymer. 2003;44:3909. [Google Scholar]
- 17.Kweon H, Yoo MK, Park IK, Kim TH, Lee HC, Lee HS, Oh JS, Akaike T, Cho CS. Biomaterials. 2003;24:801. doi: 10.1016/s0142-9612(02)00370-8. [DOI] [PubMed] [Google Scholar]
- 18.Jabbari E, Wang SF, Lu LC, Gruetzmacher JA, Ameenuddin S, Hefferan TE, Currier BL, Windebank AJ, Yaszemski MJ. Biomacromolecules. 2005;6:2503. doi: 10.1021/bm050206y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Lu L, Gruetzmacher JA, Currier BL, Yaszemski MJ. Macromolecules. 2005;38:7358. [Google Scholar]
- 20.Wang SF, Lu LC, Gruetzmacher JA, Currier BL, Yaszemski MJ. Biomaterials. 2006;27:832. doi: 10.1016/j.biomaterials.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Pascual B, Vazquez B, Gurruchaga M, Goni I, Ginebra MP, Gil FJ, Planell JA, Levenfeld B, Roman JS. Biomaterials. 1996;17:509. doi: 10.1016/0142-9612(96)82725-6. [DOI] [PubMed] [Google Scholar]
- 22.Odian G. Principles of Polymerization. 4th. John Wiley & Sons, Inc.; Hoboken, NJ: 2004. [Google Scholar]
- 23.Lewis G. J Biomed Mater Res Part B. 2006;76B:456. doi: 10.1002/jbm.b.30398. [DOI] [PubMed] [Google Scholar]
- 24.He SL, Yaszemski MJ, Yasko AW, Engel PS, Mikos AG. Biomaterials. 2000;21:2389. doi: 10.1016/s0142-9612(00)00106-x. [DOI] [PubMed] [Google Scholar]
- 25.Peter SJ, Kim P, Yasko AW, Yaszemski MJ, Mikos AG. Journal of Biomedical Materials Research. 1999;44:314. doi: 10.1002/(sici)1097-4636(19990305)44:3<314::aid-jbm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.Suggs LJ, Mikos AG. Cell Transplant. 1999;8:345. doi: 10.1177/096368979900800402. [DOI] [PubMed] [Google Scholar]
- 27.T AE, Joseph A Buckwalter, Sheldon R Simon. Orthopaedic Basic Science. 2nd. American Academy of Orthopaedic Surgeons; Rosemont, IL: 2000. [Google Scholar]
- 28.Wolfe MS, Dean D, Chen JE, Fisher JP, Han SH, Rimnac CM, Mikos AG. Journal of Biomedical Materials Research. 2002;61:159. doi: 10.1002/jbm.10058. [DOI] [PubMed] [Google Scholar]
- 29.Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG. Biomaterials. 1996;17:2127. doi: 10.1016/0142-9612(96)00008-7. [DOI] [PubMed] [Google Scholar]