Abstract
Summary
The current intake recommendations of 200 to 600 IU vitamin D/d may be insufficient for important disease outcomes reduced by vitamin D.
Introduction
To assess the benefit of higher dose and higher achieved 25-hydroxyvitamin D levels (25(OH)D) versus any associated risk.
Results
Based on double-blind RCTs, 8 for falls (n=2426) and 12 for non-vertebral fractures (n=42,279), there was a significant dose-response relationship between higher dose and higher achieved 25(OH)D and greater fall and fracture prevention. Optimal benefits were observed at the highest dose tested to date for, 700 to 1000 IU vitamin D/d, or mean 25(OH)D between 75 to 110 nmol/l (30–44 ng/ml). Prospective cohort data on cardiovascular health and colo-rectal cancer prevention suggested increased benefits with the highest categories of 25(OH)D evaluated (median levels between 75 and 110 nmol/l). In 25 RCTs, mean serum calcium levels were not related to oral vitamin D up to 100,000 IU /d or achieved 25(OH)D up to 643 nmol/l. Mean levels of 75 to 110 nmol/l were reached in most RCTs with 1800 IU to 4000 IU vitamin D/d without risk.
Conclusion
Our analysis suggests that mean serum 25(OH)D levels of about 75 to 110 nmol/l provide optimal benefits for all investigated endpoints without increasing health risks. These levels can be best obtained with oral doses in the range of 1800 to 4000 IU vitamin D/d; further work is needed, including subject and environment factors, to better define the doses that will achieve optimal blood levels in the large majority of the population.
INTRODUCTION
Voluminous data suggest that higher 25-hydroxyvitamin D (25(OH)D) serum concentrations are advantageous for chronic disease prevention. At present, strong evidence for causality is available for fracture[1] and fall endpoints[2], while promising epidemiologic and mechanistic studies suggest a key role of vitamin D in the preservation of cardiovascular health[3–6], and the prevention of cancer[7] and other common chronic diseases[8]. However benefits of vitamin D on falls, fractures, cardiovascular health, and cancer prevention that have been observed with higher 25(OH)D levels cannot be achieved in the large majority of individuals following current recommendations for vitamin D intake. The current adequate intake (AI) for vitamin D, as defined by the US Institute of Medicine (IOM), of 200 IU per day for adults up to 50 years of age (5 μg), 400 IU per day for adults between age 51 and 70 (10 μg), and 600 IU per day for those aged 70 years and over (15 μg) will achieve a serum 25(OH)D level of up to 50 or 60 nmol/l for 2/3 of adults.
This review draws together recent work by the authors and others on the benefits and risks of higher achieved 25(OH)D levels beyond 60 nmol/l to provide guidance in current efforts to define dose recommendations for vitamin D in the absence of large randomized trials for non-skeletal endpoints. We first examined double-blind RCT data for fall and fracture prevention, as well as mean serum calcium levels by dose and achieved 25(OH)D levels. Specifically, we assessed a dose-response relationship for these endpoints with established causality to explore whether the benefit of higher vitamin D dose and higher 25(OH)D levels is accompanied by an increase in risk of hypercalcemia. Additionally, we assessed whether there were single case reports of hypercalcemia or nephrolithiasis from the same RCTs.
We also evaluated dose-response relationships for non-skeletal outcomes of public health significance, including cardiovascular disease and cancer, especially colorectal cancer, in the context of case reports of hypercalcemia and documented 25(OH)D levels. Our overarching goal was to determine the optimal 25(OH)D level and vitamin D intake that correspond to optimal health without risk of adverse effects.
METHODS
In this review we summarize evidence for optimal serum 25(OH)D levels with respect to benefit and risk. The established benefits of higher vitamin D dose and higher achieved serum 25(OH)D levels were reduction in falls and fractures as summarized in two 2009 meta-analyses of double-blind RCTs[1, 2]. The established risk of higher intakes of vitamin D or higher achieved serum 25(OH)D level that we evaluated in the same RCT's and any available RCTs of vitamin D supplementation and reported 25(OH)D status was hypercalcemia, evaluated as mean serum calcium level. We also examined case reports of hypercalcemia or nephrolithiasis in these trials. Additionally, we review benefits of vitamin D with the strongest evidence today from prospective epidemiological studies that are supported by strong mechanistic evidence, specifically reduction of cardiovascular disease (incident hypertension and cardiovascular mortality) and colo-rectal cancer. Weaker evidence of a beneficial effect of vitamin D exists for other diseases, including multiple sclerosis[9], tuberculosis[10], insulin resistance[11, 12], other cancers[13–16], osteoarthritis[17, 18] and prevalent hypertension [19–21], but these are not considered here. For the assessment of risk in observational studies, we include data on serum calcium levels with any reported 25(OH)D level from case-reports of vitamin D intoxication.
The risk assessment method used is a slight modification of the Institute of Medicine's Tolerable Upper Intake Level (UL) method, as applied in a previous risk assessment on vitamin D by some of the current authors[22]. Our procedure uses the UL protocol exactly, except for a conservative selection of data so that no further correction for uncertainty is required[23]. Details of the entire literature up to that time were described in the previous risk assessment[22].
The benefit and risk that occur with increases in 25(OH)D concentration were placed in context with each other graphically using trend plots for each selected endpoint (decreased risk of falls and non-vertebral fractures) at different doses of vitamin D and achieved 25(OH)D levels. Given the limitations of using mean achieved serum calcium levels reported as a risk endpoint in trials of vitamin D supplementation, we also used individual serum calcium values in published case reports of hypercalcemia alleging vitamin D intoxication to compare with the prospective cohort data relating 25(OH)D concentrations to risks of cardiovascular disease and colorectal cancer.
RESULTS
Benefit and risk data from RCTs
Benefit of higher 25(OH)D levels on non-vertebral fracture prevention
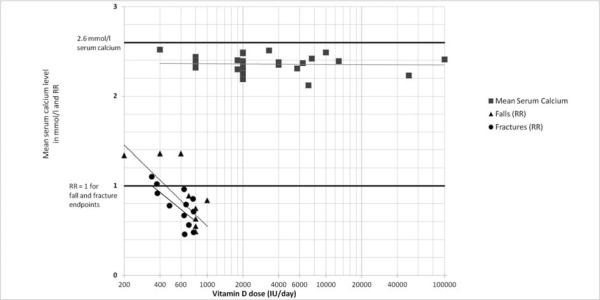
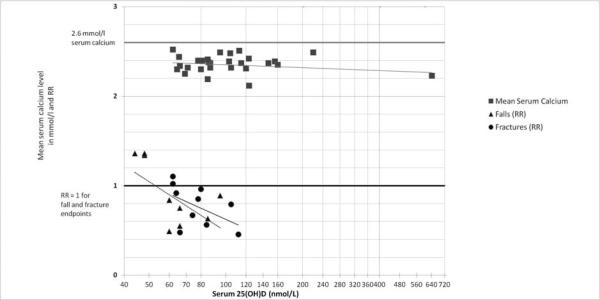
In a recent meta-analysis the efficacy of oral supplemental vitamin D in preventing non-vertebral and hip fractures among older individuals was examined (age 65+)[1]. The analysis included 12 double-blind randomized controlled trials (RCTs) for non-vertebral fractures (n=42,279; [1, 2, 24–32]) and 8 RCTs for hip fractures (n = 40,886; [24, 25, 29, 30, 32–35]) comparing oral vitamin D with or without calcium with calcium or placebo. The pooled relative risk (RR) was 0.86 (95% CI, 0.77–0.96) for prevention of non-vertebral fractures and 0.91 (95% CI, 0.78–1.05) for the prevention of hip fractures, but with significant heterogeneity for both endpoints. Including all trials, anti-fracture efficacy increased significantly with higher dose and higher achieved blood 25(OH)D levels for any non-vertebral fractures and hip fractures separately. Consistently pooling trials with a higher received dose of more than 400 IU/day (received dose of 482 – 770 IU per day) - resolved heterogeneity and resulted in fracture reduction, while the lower received dose (340–380 IU per day) did not reduce fracture risk. For the higher doses, the pooled RR was 0.80 (95% CI, 0.72 –0.89; n = 33,265 persons from 9 trials) for non-vertebral fractures and 0.82 (95% CI, 0.69 –0.97; n = 31,872 from 5 trials) for hip fractures. The higher doses reduced non-vertebral fractures significantly in community-dwelling (−29%) and institutionalized older individuals (−15%), and its effects were independent of additional calcium supplementation. Non-vertebral fracture prevention started with achieved 25(OH)D levels of at least 75 nmol/l in the treatment group. From left to right, Figure 1A indicates increased anti-fracture efficacy with higher dose, and Figure 1 B with higher achieved 25(OH)D levels in the treatment group (meta-regression analyses by dose: p-value = 0.003; by achieved 25(OH)D level: p-value = 0.04; these values are based on 12 trials for dose and 10 trials with measured 25(OH)D levels). From Figure 1A optimal fracture prevention occurred in trials with achieved mean 25(OH)D levels of approximately 75 to 110 nmol/l.
Figure 1.
Evidence from controlled RCTs: Trend-plots on benefit (fall and non-vertebral fracture prevention) and risk (mean achieved serum calcium levels) by dose of vitamin D and achieved 25(OH)D levels
Figure 1A – by dose of vitamin D
Figure 1B – by achieved 25(OH)D level
Blackcircles represent relative risks (RRs) from 12 double-blind RCTs on vitamin D supplementation and non-vertebral fracture risk as summarized in a 2009 meta-analysis (Bischoff-Ferrari et al; Archives of Internal Medicine 2009[1]). Trendline is based on series of effect sizes (circles). For any non-vertebral fractures, anti-fracture efficacy increased significantly with higher received dose (meta-regression: Beta = - 0.0007; p = 0.003; Figure 1 A) and higher achieved 25-hydroxyvitamin D levels (meta-regression: Beta = - 0.005; p = 0.04; Figure 1 B).
Black triangles represent relative risks (RRs from 8 double-blind RCTs on vitamin D supplementation and fall risk as summarized in a 2009 meta-analysis (Bischoff-Ferrari et al; in press British Medical Journal[2]). Trendline is based on series of effect sizes (triangles). A meta-regression, which included 2426 individuals from 8 RCTs, indicated a significant inverse relationship between higher treatment dose and the risk of sustaining at least one fall (Beta-estimate for dose: 700 IU or higher compared to less = - 0.337; p = 0.02; Figure 1 A).
A meta-regression, which included 1447 individuals from 6 RCTs with reported 25(OH)D levels, indicated a significant inverse relationship between higher achieved 25(OH)D level in the treatment group and the risk of sustaining at least one fall (Beta-estimate for a 25(OH)D of 60 nmol/l or higher compared to lower = - 0.586; p = 0.005; Figure 1 B).
Grey squares represent mean calcium levels in the treatment group from 25 vitamin D supplementation trials (28 data points; one trial with separate report from community-dwelling and hospitalized older individuals[45]). Trendline is based on series of mean serum calcium levels (grey squares). The doses of vitamin D applied in these trials ranged from 400 to 100'000 IU vitamin D per day. There was flat trend line for mean serum calcium levels with higher dose of vitamin D (Figure 1 A) and higher achieved 25-hydroxyvitamin D levels (Figure 1 B).
This threshold of optimal non-vertebral fracture prevention is supported by a large cross-sectional and population-based study that showed a positive dose-response association between higher 25(OH)D levels and hip bone density both in younger and older adults [36]. In the regression plots, higher serum 25(OH)D levels were associated with higher hip bone density throughout the reference range of 22.5 to 94 nmol/l in all subgroups by age and ethnicity. In younger whites and younger Mexican Americans, higher 25(OH)D levels were associated with higher hip bone density even beyond 100 nmol/l.
Benefit of higher 25(OH)D levels on fall prevention
In another meta-analysis the efficacy of oral supplemental vitamin D in preventing falls among older individuals (age 65+) was examined[2]. Only double-blind RCTs with prospective fall assessment were considered (falls were assessed as a primary or secondary endpoint, authors stated how falls were defined and assessed, and falls were assessed over the whole trial period). The analysis included 8 double-blind RCTs (n = 2426; [31, 37–43]) comparing oral vitamin D with or without calcium with calcium or placebo. The pooled relative risk (RR) was 0.87 (95% CI, 0.77–0.99) for prevention of falls, but with significant heterogeneity (Q-test: p = 0.05). Heterogeneity was observed for dose of vitamin D (low-dose: < 700 IU / day versus higher dose: 700 to 1000 IU / day; p-value 0.02) and achieved 25(OH)D level (< 60 nmol/l versus ≤ 60 nmol/l; p-value = 0.005). Higher dose supplemental vitamin D reduced fall risk by 19% (pooled relative risk (RR) = 0.81; 95% CI, 0.71–0.92; n = 1921 from seven trials). Falls were not reduced by low dose supplemental vitamin D (pooled RR = 1.10, 95% CI, 0.89–1.35 from 2 trials) or by achieved serum 25(OH)D concentrations less than 60 nmol/l (pooled RR = 1.35, 95% CI, 0.98–1.84). Fall prevention increased with higher dose (Figure 2A), and with higher achieved 25(OH)D levels in the treatment group (Figure 2 B). Similar to non-vertebral fracture prevention described above, optimal fall prevention appeared to occur in trials with achieved mean 25(OH)D levels of approximately 75 to 100 nmol/l(see Figure 2 B).
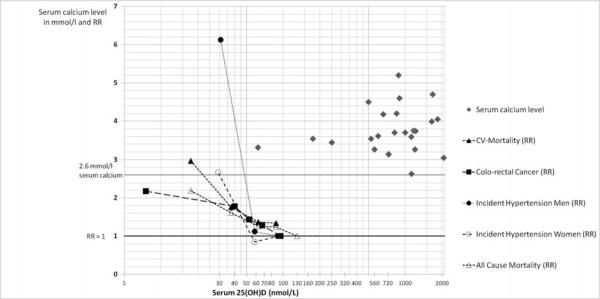
Figure 2.
Trend-plots on benefit from observational studies (cardiovascular disease prevention and colorectal cancer prevention) and risk (case reports of hypercalcemia) by achieved 25(OH)D level
Dashed lines relate to data from epidemiologic studies on the RR of incident hypertension[4], all-cause[90] and cardiovascular[90] mortality and colorectal cancer [91] based on categories of median 25-hydroxyvitamin D levels. For colorectal cancer, we included a quantitative meta-analysis of 5 studies[91]. Based on this summary of non-skeletal endpoints of public health significance, there was a dose-response of better health status with higher median 25(OH)D levels. By visual inspection, the desirable median serum 25(OH)D level to be achieved for all endpoints was approximately 100 nmol/l.
The solid grey diamonds relate to 24 case-reports of hypercalcemia with corresponding 25(OH)D levels. 22 of 24 cases of hypercalcemia were reported at 25(OH)D levels beyond 240 nmol/l 25(OH)D.
This threshold of optimal fall prevention is supported by a large cross-sectional and population-based study that showed a dose-response association between higher 25(OH)D levels and better lower extremity function in older adults[44]. For both tests, performance speed continued to increase throughout the reference range of 25(OH)D (22.5 to 94 nmol/l) with most of the improvement occurring in 25(OH)D levels going from 22.5 to approximately 60 nmol/l. Further improvement was seen in the range of 60–94 nmol, but the magnitude was less dramatic.
Levels of 25(OH)D in relation to change in mean serum calcium levels and hypercalcemia in controlled trials
We included the published 22 RCTs involving an oral dose (either D2 or D3) of greater than or equal to 1800 IU/day (or the equivalent in weekly, monthly, bolus doses, etc…), and in which both mean serum calcium and serum 25(OH)D were reported (Appendix; Table 1; [45–66]). The reason for this cut off stems from the purpose of conducting a risk assessment for doses close to or greater than the IOM identification of 2000 IU as the Tolerable Upper Intake level (UL). In addition, in Figure 1 A and B, we plotted mean serum calcium levels from 5 double-blind RCTs that reported serum calcium levels after vitamin D supplementation along with the fall and fracture endpoints[29, 34, 38, 43, 67]. The vitamin D doses in these 5 trials ranged from 400 to 800 IU vitamin D per day. In Figure 1 A and B a total of 28 data points for mean calcium levels are depicted (2 data points stem from one trial for the report by type of dwelling[45]). In Figure 1 A and B we drew a trendline based on this series of mean serum calcium levels. The horizontal trendline indicates that mean serum calcium levels did not increase with higher doses of vitamin D up to 100,000 IU per day (Figure 1 A) or with higher achieved 25(OH)D levels up to 643 nmol/l (Figure 1B). The one trial with 100,000 IU dose of vitamin D per day had a very short duration of 4 days, while the other trials ran for at least one half-life of vitamin D, which is 3 to 6 weeks. We address treatment duration in more detail later in the results section. Notably, in none of the trials was there a shift of mean serum calcium levels above the normal physiologic range (> 2.6 mmol/l) with vitamin D treatment. However, in the Honkanen et al. trial depicted in Figure 1A and B, there was an increase in mean serum calcium levels above 2.6 nmol/l in the institutionalized control group receiving calcium supplementation without vitamin D[45]. Not shown in Figure 1 is one controlled trial with a report of increased mean calcium levels above 2.6 nmol/l as the trial did not measure 25(OH)D levels: the Narang et al. 1984 trial randomized 30 healthy adults to 0, 400, 800, 1200, 2400, 3800 IU/day for 90 days and observed a significant increase in mean serum calcium levels in the two highest dose groups (2.62 and 2.82 mmol/l, respectively)[68]. The limitations of the Narang study include the location and population studied (India), the small sample size (n = 5 per group) and failure to measure serum 25(OH)D levels. Finally, the finding of a significant increase in mean serum calcium levels at these doses has not been replicated in any subsequent published RCT.
For reports of single cases of hypercalcemia, two RCTs of the 28 RCTs identified and depicted in Figure 1 A and B reported single cases where serum calcium levels rose above the normal range (> 2.6 mmol/l): (1) Talwar et al. 2007 with a daily dose of 2000 IU plus 1500 mg calcium supplementation in healthy black women treated for 12 months[48]; (2) Maalouf et al. 2008 with a daily dose of 2000 IU vitamin D in healthy Libanese adolescents treated for 12 months[47]. In the Talwar study 6 isolated incidents of mild hypercalcemia occurred in 8 clinical visits over 3 years in 208 women. The authors report that all 6 cases of hypercalcemia resolved on the repeat fasting sample. In the Maalouf study, hypercalcemia ranging from 2.7 to 2.77 nmol/l was described in 7 of 340 adolescents, 5 cases in the placebo group, 1 with 1400 IU vitamin D per week (equivalent to 400 IU per day), and one with 14, 000 IU vitamin D per week (equivalent to 2000 IU per day).
Of all controlled trials with vitamin D, an increased incidence of nephrolithiasis occurred only in the very large Women's Health Initiative (WHI) trial (involving 400 IU vitamin D plus 1000 mg of calcium per day). [24].
We also identified 10 uncontrolled trials with oral vitamin D supplementation at doses of at least 1800 IU per day and reported serum calcium levels after treatment (Appendix; Table 2; [69–77]). Of these, two reported an increase in mean serum calcium levels above the normal range or single cases with hypercalcemia: (1) Tucci et al reported a mean serum calcium level that exceeded 2.6 mmol/l at baseline and after administration of 7120 IU vitamin D per day in adult primary hyperparathyroidism patients [74].; mean serum calcium levels were 2.73 mmol/l at baseline and after treatment, However, the authors also reported of 2 patients in whom serum calcium levels rose from 2.8 to 3.03 mmol/l and from 2.83 to 3.05 mmol/l, respectively. (2) Restorff et al. reported of 2 single cases of mild hypercalcemia (2.69 mmol/l) at 3 months and normal levels at 6 months in one small uncontrolled trial of 33 elderly rheumatology patients with severe vitamin D deficiency treated with a single oral dose of 300,000 IU vitamin D3[76].
The other remaining clinical trials we identified (both controlled and uncontrolled) did not assess serum calcium and/or serum 25(OH)D [30, 78–81]. In some instances serum calcium was assessed, but only the observation of a lack of hypercalcemia or no significant change in serum calcium was reported ([82–89]. Vitamin D daily (or equivalent) oral doses ranged in these trials from 2000 – 300,000 IU.
Benefit and risk data from observational data
Data from epidemiologic studies on the RR of incident hypertension[4], all-cause[90] and cardiovascular[90] mortality and colorectal cancer [91] based on categories of median 25-hydroxyvitamin D levels are presented in Figure 2. For all-cause and cardiovascular mortality we included a population-based prospective cohort study (National Health and Nutrition Survey III) controlling for age, sex, race/ethnicity, season of blood-draw, income, region, body mass index, physical activity, smoking status, cigarette pack years, asthma, chronic obstructive pulmonary disease, and renal function in community-dwelling individuals age 65 and older [90]. Consistent findings have been reported from a large cohort of patients undergoing angiography[3] and a second report within the National Health and Nutrition Survey III including younger individuals age 20 and older [92], which confirmed an optimal 25(OH)D range of 100 to 120 nmol/l for all-cause mortality.
For colorectal cancer, we included a quantitative meta-analysis of 5 studies [91]. For all non-skeletal endpoints of public health significance illustrated in Figure 2, there was a dose-response of better health status with higher median 25(OH)D levels. By visual inspection, the desirable median serum 25(OH)D level to be achieved for all non-skeletal endpoints included in this report was approximately 100 nmol/l.
The solid grey diamonds in Figure 2 relate to 24 case-reports of hypercalcemia allegedly from vitamin D intoxication with corresponding 25(OH)D levels (Appendix; Table 3[93–115]). Only 2 out of 24 cases with hypercalcemia were reported at 25(OH)D levels below 240 nmol/l 25(OH)D[93, 110], one of which involved a 85 year old woman who reported consuming 400 IU/d vitamin D and had a 25(OH)D of 62 nmol/l[93], the other in a newly arrived international 10 months-old adoptee[110]. These appear to be aberrant cases which have not been replicated in the literature. Extending to a cut-off of 400 nmol/l 25(OH)D a third case with hypercalcemia occurred with 25(OH)D levels of 250 nmol/l. The third case occurred in a 77 year old woman with primary hyperparathyroidism who received 50,000 IU D2 daily instead of weekly for 2 years with a 25(OH)D level of 250 nmol/l[96]. All other cases of hypercalcemia (n = 21) were reported at 25(OH)D levels of 525 to 2070 nmol/l, clearly outside a target range of 75 to 110 nmol/l for optimal health illustrated in Figures 1 B and 2. There are also case reports in the published literature describing individual circumstances where extremely high vitamin D doses have been claimed (ranging from 50,000 IU to 150,000 IU/day vitamin D2 or D3), verified by correspondingly high serum 25(OH)D values (ranging from 107 to 1126 nmol/l), but serum calcium levels were reported to not exceed the threshold for hypercalcemia (2.6 mmol/l)[116] [98, 117].
Summary of serum 25(OH)D response to oral vitamin D
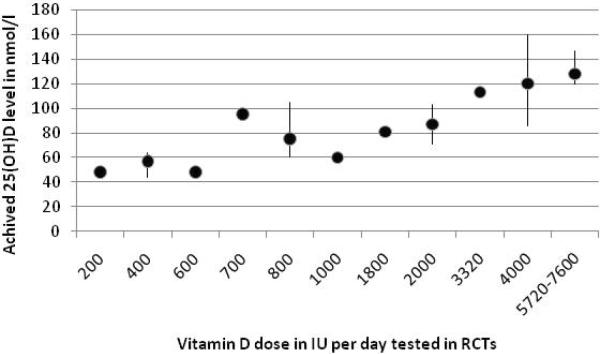
In summary, the data for fall and fracture prevention as well as epidemiologic data on preservation of cardiovascular health, general mortality and colorectal cancer prevention suggest that serum 25(OH)D levels close to 100 nmol/l are desirable and carry no risk of hypercalcemia, as evidenced by controlled trials. Figure 3 illustrates 25(OH)D response to vitamin D doses between 200 to 1000 IU per day in fall and fracture trials, and 1800 IU to 7600 IU from other RCTs included in Figure 1 A and B. With a supplement equal to the AI for young adults of 400 IU per day, data from 3 large RCTs achieved a mean increase in 25(OH)D levels to 56.7 nmol/l (range: 44 to 64 nmol/l) after a mean treatment duration of 653 days leaving more than 50% of individuals below the desirable target range of at least 75 nmol/l for fall and fracture reduction and with very limited chances to reach an optimal range close to 100 nmol/l. With a supplement of 800 IU vitamin D per day, a recommendation 200 IU higher than the highest of the AI values for the oldest segment of the population, tested in 9 RCTs for a mean duration of 697 days resulted in a mean increase in 25(OH)D levels to 75 nmol/l (range: 60 to 105 nmol/l) after a mean treatment duration of 697 days (range: 56 to 1680 days) leaving about 50% of individuals below the desirable target range of at least 75 nmol/l for fall and fracture reduction and a small chance to reach an optimal range close to 100 nmol/l. Most healthy younger and older adults reached the target range of 75 to 110 nmol/l with 1800 IU to 4000 IU vitamin D3 per day treated for at least 42 days.
Figure 3.
Dose of vitamin D and achieved 25(OH)D levels based on RCTs with a duration of at least 4 weeks
Figure 3 A: Lower dose trials (double-blind fall and fracture RCTs)
This graph summarizes data from identified RCTs (as illustrated in Figure 1) with oral doses of vitamin D of less than 10,000 IU vitamin D per day and a treatment duration of at least 4 weeks. Dots either represent the mean 25(OH)D level from a single trial or the mean and the range of several trials.
There were 3 RCTs with 400 IU vitamin D per day with a mean increase in 25(OH)D levels to 56.7 nmol/l (range: 44 to 64 nmol/l) after a mean treatment duration of 653 days (range: 140 to 1148 days). The trial with 700 IU vitamin D and achieved mean serum levels close to 100 nmol/l ay be an outlier due to the high starting levels documented in the trial (84 nmol/l in men and 72 nmol/l in women age 65 and older[26]). There were 9 RCTs for 800 IU per day with a mean increase in 25(OH)D levels to 75 nmol/l (range: 60 to 105 nmol/l) after a mean treatment duration of 697 days (range: 56 to 1680 days).
There were 7 RCTs with 2000 IU vitamin D per day with a mean increase in 25(OH)D levels to 87 nmol/l (range: 71 to 103 nmol/l) after a mean treatment duration of 146 days (range: 42 to 365 days).There were 3 RCTs for 4000 IU per day with a mean increase in 25(OH)D levels to 120 nmol/l (range: 85.5 to 160 nmol/l) after a mean treatment duration of 168 days (range: 56 to 365 days). And there were 4 trials with a treatment dose between 5720 and 7600 IU vitamin D per day with a mean increase in 25(OH)D levels to 128 nmol/l (range: 120–147 nmol/l).
From this summary of available dose-response data from RCTs, most trials among healthy younger and older adults reached a mean value in the target range of 75 to 110 nmol/l with 1800 IU to 4000 IU vitamin D3 per day treated for at least 42 days.
DISCUSSION
Recommendations for consumption of a nutrient are a function of the Tolerable Upper Intake Level (UL) and the Recommended Dietary Allowances (RDA) or for some nutrients the Adequate Intake (AI). For vitamin D, the necessary evidentiary basis for a RDA (i.e., the mean requirement and an estimate of variance) could not be identified by the Institute of Medicine (IOM) in 1997 and, instead, an AI was identified[118]. The AI is an estimated average intake by a group or groups of healthy people and may not reflect the intake needed to achieve a specific health outcome. In order to assess whether a higher vitamin D intake resulting in higher achieved 25(OH)D levels is desirable and safe, we performed a dose-response evaluation bringing together data on benefits and risks of higher doses and higher achieved 25(OH)D levels.
Based on endpoints with established causality from double-blind RCTs as well as epidemiologic data on cardiovascular health (incident hypertension, general mortality and cardiovascular mortality) and colorectal cancer prevention, our review suggests that the target range of 25(OH)D level for these benefits is not accompanied by increased risk of hypercalcemia. Notably, all endpoints evaluated for dose-response with higher 25(OH)D levels point to a similar target range of at least 75 nmol/l and better approximately 100 nmol/l.
Based on our benefit assessment, the current intake recommendations for vitamin D using the AI are insufficient to bring a majority of individuals up to at least 75 nmol/l 25(OH)D and close to 100 nmol/l. Revising recommendations towards a higher dose of vitamin D thus needs an assessment of risk with vitamin D supplementation doses that may bring most of the population into the target range of 75 to 110 nmol/l. This 25(OH)D range was reached in most trials with 1800 to 4000 IU vitamin D per day. Likely, individuals who start with lower 25(OH)D levels will need a supplementation dose at the higher end of this range[59, 119]. Most vulnerable to low vitamin D levels are elderly [120, 121], individuals living in northern latitudes with prolonged winters and thus low UVB exposure [122, 123], obese individuals[124], and African Americans of all ages[36, 125, 126].
In our current risk assessment, hypercalcemia was chosen as the critical effect, the adverse effect occurring at the lowest intake. There were no increases in mean calcium levels with higher vitamin D intakes tested in controlled trials up to 100,000 IU per day. For single cases of hypercalcemia from RCTs, there were cases of mild hypercalcemia from 2 of 28 studies, which resolved on repeating fasting samples in one study[48], and were more frequent in the placebo group in the second study[47].
When we extend our assessment of risk to include case reports of hypercalcemia and associated 25(OH)D levels, hypercalcemia occurred in 22 of 24 cases beyond 240 nmol/l 25(OH)D. Of the two cases that occurred at serum levels below 240 nmol/l 25(OH)D, one case involved a 85 year old woman who reported consuming 400 IU/d vitamin D and had a 25(OH)D of 62 nmol/l[93]. The other was described in a newly arrived adoptee with unknown vitamin D exposure[110]. Notably, 21 of 24 cases of hypercalcemia occurred with 25(OH)D levels beyond 400 nmol/l.
The only RCT that documented an increased risk of nephrolithiasis was the Women's Health Initiative (WHI), which tested 400 IU vitamin D in combination with 1000 mg of calcium (hazard ratio,1.17; 95 percent confidence interval, 1.02 to 1.34)[24]. Whether this was the only trial large enough to detect a small risk of nephrolithiasis with vitamin D supplementation or whether this was caused by the substantial calcium supplement intake taken in combination with the vitamin D and/or the additional calcium and vitamin D supplements taken by the majority of participants outside the study protocol in the WHI trial is unclear. However, the low dose vitamin D used in the WHI argues against a causal role of the increased risk of nephrolithiasis. Based on epidemiologic data, a higher vitamin D intake was not independently associated with nephrolithiasis in one large cohort[127] consistent with findings from a recent study of 18 healthy postmenopausal women with vitamin D deficiency where vitamin D supplementation did not increase urinary calcium excretion [128]. On the other hand, calcium supplementation was associated with a 20% increased risk of nephrolithiasis in the Nurses Health Study I[129], although not in a cohort of younger women [129]. Overall, the data are insufficient to identify nephrolithiasis as the critical effect.
The issue of vascular calcification in persons on renal dialysis has also been addressed in detail in the 2007 risk assessment and are not addressed in this review[22]. However, reports in the literature continue to be restricted to extremely high doses of vitamin D3 or administration of the active hormonal form, 1,25(OH)2D3 and/or related analogues, and most of these reports are in animals, not humans. There continues to be no credible evidence to support the notion that oral vitamin D doses up to and even exceeding 10,000 IU per day are associated with vascular calcification in humans, including dialysis patients, and there is no basis for identifying vascular calcification as the critical effect.
There are several limitations to the evaluation of dose-response relationship through a trend-plot approach using mean serum calcium levels after treatment in trials that also report 25(OH)D levels. This approach may miss single cases of hypercalcemia. To address this problem we also assessed the report of hypercalcemia in all available controlled trials without evidence of an increased risk with higher achieved 25(OH)D levels up to 640 nmol/l or a daily dose of 100,000 IU vitamin D from controlled trials. Also, in theory, a direct comparison of the benefit and risk resulting from consumption of vitamin D would require a common metric. As an indicator of risk, we used increases in mean or individual case serum calcium level (a continuous variable, within the range permitted by physiological controls), while the benefit was assessed with the endpoints fall and fracture prevention, as well as endpoints of cardiovascular health and colorectal cancer prevention (categorical outcomes for individuals with population effects identified as relative risk). General limitations to this assessment of benefit and risk of vitamin D are that our findings may not be generalizable to particularly vulnerable subgroups of the population, such as patients of high age in critical care or those with hypersensitivity to vitamin D (e.g. sarcoidosis), as high-dose vitamin D trials are either ongoing or have not been performed in such populations. Also, we used the equivalent daily dose of vitamin D for intermittent dosing of weekly or monthly applications, which may over-estimate the per day dose to some degree[130]. However, the benefit and risk assessment from RCTs is similar if achieved 25(OH)D levels are plotted instead of dose. Finally, we have selected observational data on the benefit of vitamin D, which largely, but may not fully represent the available literature.
The IOM established a UL for vitamin D based on hypercalcemia as the critical effect and two studies have been cited as a matter of concern by the IOM, (1) the trial by Honkanen et al. included in our risk assessment and (2) the trial by Narang et al. not included in our risk assessment as 25(OH)D levels were not available. Notably, in the trial by Honkanen et al. the hypercalcemia observed is not relevant to the risk assessment of vitamin D because it occurred in the institutionalized control group with a mean serum 25(OH)D concentration of 10.4 nmol/l[45]. Both groups of hospitalized and community-dwelling older adults showed no increase in serum calcium levels with 1800 IU vitamin D per day. The trial of Narang et al. [68] that IOM relied upon to identify a no observed adverse effect level of 2400 IU vitamin D per day and establish a UL of 2,000 IU per day is now considered unreliable because several other more recent clinical trials included in our risk analysis have failed to confirm the occurrence of hypercalcemia at intakes of up to more than ten-fold those used in that study[118]. Consequently, the 2007 risk assessment concluded that the UL could be safely adjusted upward to 10,000 IU [22]. Nonetheless, in general the causal relationship between excessive vitamin D intake and hypercalcemia is well established [22]but the dose that will result in this critical effect is higher than any used in the RCTs and prospective cohort studies that have been reported. Thus, the flat-line relationship of serum calcium to the other parameters in Figures 1A and 1B does not contradict the identification of hypercalcemia as the critical effect for excess vitamin D; it indicates only that the effect must occur at higher 25(OH)D concentrations and with higher oral intakes of vitamin D.
As calcium absorption is improved with higher serum 25(OH)D levels[131, 132], future studies may need to evaluate whether current calcium intake recommendations with higher doses of vitamin D beyond 2000 IU per day are safe or require downward adjustment [131]. If dietary calcium is a threshold nutrient, as suggested by Dr. Heaney[119], then that threshold for optimal calcium absorption may be at a lower calcium intake when vitamin D status is adequate.
Regarding relevant endpoints, a downward adjustment of calcium intake recommendations is supported by the recent meta-analysis on non-vertebral fracture prevention where fracture prevention at a vitamin D dose greater than 480 IU per day was independent of additional calcium supplementation. Also two recent epidemiologic studies suggested that both PTH suppression[132] and hip bone density[133] may only depend on a higher calcium intake if serum 25-hydroxyvitamin D levels are very low.
Summary
In this analysis we examined benefits (reductions in fractures and falls) and risks (hypercalcemia) as a function of vitamin D intake and serum concentrations of 25(OH)D in randomized trials. We also used non randomized evidence to evaluate the levels of 25(OH)D at which benefits (reductions in colorectal cancer and cardiovascular disease) and risks (hypercalcemia and nephrolithiasis) are observed. We found no pattern of evidence to suggest that risks are elevated within the ranges of serum 25(OH)D or oral vitamin intake related to increased benefits ( 75–110 nmol/l). Instead, the reliable evidence that excess vitamin D can cause hypercalcemia in generally healthy adults comes from daily intakes of vitamin D greater than 100,000 IU or serum 25(OH)D exceeding 240 nmol/L, which are far higher than those necessary to achieve the benefits. The evidence from randomized trials suggests that the dose of vitamin D supplement needed to bring the large majority of persons to the range of optimal serum 25(OH)D, may be in the range of 1800 to 4000 IU/day. Further work is needed, taking into account subject and environment factors, to better define the doses that will achieve the optimal blood levels in the large majority of the population.
Acknowledgments
Funding: Heike Bischoff-Ferrari is supported by a Swiss National Foundations Professorship Grant (PP00B-114864). This project was supported by an investigator initiated and unrestricted grant provided by DSM and by a Centre Grant of the University of Zurich and the Town of Zurich (Centre on Aging and Mobility).
Footnotes
None of the authors have a conflict of interest. No disclosures.
References
- 1.Bischoff-Ferrari HA, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169(6):551–61. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff-Ferrari HA, et al. Fall prevention with supplemental and alpha-hydroxylated vitamin D: a meta-analysis of randomized controlled trials. BMJ. 2009 Oct 1;339:b3692. doi: 10.1136/bmj.b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobnig H, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25- dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 4.Forman JP, et al. Plasma 25-Hydroxyvitamin D Levels and Risk of Incident Hypertension. Hypertension. 2007;19:19. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, et al. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20(7):713–9. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 8.Bouillon R, Bischoff-Ferrari H, Willett W. Vitamin D and health: perspectives from mice and man. J Bone Miner Res. 2008;23(7):974–9. doi: 10.1359/jbmr.080420. [DOI] [PubMed] [Google Scholar]
- 9.Munger KL, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60–5. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 10.Liu PT, et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science. 2006;23:23. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 11.Chiu KC, et al. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820–5. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 12.Borissova AM, et al. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract. 2003;57(4):258–61. [PubMed] [Google Scholar]
- 13.John EM, et al. Vitamin D and breast cancer risk: the NHANES I Epidemiologic follow-up study, 1971–1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999;8(5):399–406. [PubMed] [Google Scholar]
- 14.Robsahm TE, et al. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway) Cancer Causes Control. 2004;15(2):149–58. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- 15.Lokeshwar BL, et al. Inhibition of prostate cancer metastasis in vivo: a comparison of 1,23- dihydroxyvitamin D (calcitriol) and EB1089. Cancer Epidemiol Biomarkers Prev. 1999;8(3):241–8. [PubMed] [Google Scholar]
- 16.Trump DL, et al. Anti-tumor activity of calcitriol: pre-clinical and clinical studies. J Steroid Biochem Mol Biol. 2004;89–90:519–26. doi: 10.1016/j.jsbmb.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 17.McAlindon TE, et al. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125(5):353–9. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 18.Lane NE, et al. Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: a longitudinal study. Study of Osteoporotic Fractures Research Group. Arthritis Rheum. 1999;42(5):854–60. doi: 10.1002/1529-0131(199905)42:5<854::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 19.Pfeifer M, et al. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86(4):1633–7. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 20.Lind L, et al. Hypertension in primary hyperparathyroidism--reduction of blood pressure by long-term treatment with vitamin D (alphacalcidol). A double-blind, placebo-controlled study. Am J Hypertens. 1988;1(4 Pt 1):397–402. doi: 10.1093/ajh/1.4.397. [DOI] [PubMed] [Google Scholar]
- 21.Li YC, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin- angiotensin system. J Clin Invest. 2002;110(2):229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hathcock JN, et al. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85(1):6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 23.Hathcock JN, Shao A. Expanded approach to tolerable upper intake guidelines for nutrients and bioactive substances. J Nutr. 2008;138(10):1992S–1995S. doi: 10.1093/jn/138.10.1992S. [DOI] [PubMed] [Google Scholar]
- 24.Jackson RD, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 25.Grant AM, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365(9471):1621–8. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 26.Dawson-Hughes B, et al. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–6. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 27.Meyer HE, et al. Can vitamin D supplementation reduce the risk of fracture in the elderly? A randomized controlled trial. J Bone Miner Res. 2002;17(4):709–15. doi: 10.1359/jbmr.2002.17.4.709. [DOI] [PubMed] [Google Scholar]
- 28.Chapuy MC, et al. Effect of calcium and cholecalciferol treatment for three years on hip fractures in elderly women. Bmj. 1994;308(6936):1081–2. doi: 10.1136/bmj.308.6936.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapuy MC, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002;13(3):257–64. doi: 10.1007/s001980200023. [DOI] [PubMed] [Google Scholar]
- 30.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. Bmj. 2003;326(7387):469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeifer M, et al. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. 2009;20(2):315–22. doi: 10.1007/s00198-008-0662-7. [DOI] [PubMed] [Google Scholar]
- 32.Lips P, et al. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124(4):400–6. doi: 10.7326/0003-4819-124-4-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 33.Lyons RA, et al. Preventing fractures among older people living in institutional care: a pragmatic randomised double blind placebo controlled trial of vitamin D supplementation. Osteoporos Int. 2007;18(6):811–8. doi: 10.1007/s00198-006-0309-5. [DOI] [PubMed] [Google Scholar]
- 34.Chapuy MC, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327(23):1637–42. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 35.Meyer HE, et al. Dietary factors and the incidence of hip fracture in middle-aged Norwegians. A prospective study. Am J Epidemiol. 1997;145(2):117–23. doi: 10.1093/oxfordjournals.aje.a009082. [DOI] [PubMed] [Google Scholar]
- 36.Bischoff-Ferrari HA, et al. Positive association between 25-hydroxy vitamin d levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116(9):634–9. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 37.Broe KE, et al. A higher dose of vitamin d reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc. 2007;55(2):234–9. doi: 10.1111/j.1532-5415.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 38.Bischoff HA, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343–51. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 39.Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Arch Intern Med. 2006;166(4):424–30. doi: 10.1001/archinte.166.4.424. [DOI] [PubMed] [Google Scholar]
- 40.Prince RL, et al. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med. 2006;166(8):869–75. doi: 10.1001/archinte.166.8.869. [DOI] [PubMed] [Google Scholar]
- 41.Flicker L, et al. Should all older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. JBMR. 2004;19(Suppl. 1):S99. doi: 10.1111/j.1532-5415.2005.00468.x. abstract F459. [DOI] [PubMed] [Google Scholar]
- 42.Graafmans WC, et al. Falls in the elderly: a prospective study of risk factors and risk profiles. Am J Epidemiol. 1996;143(11):1129–36. doi: 10.1093/oxfordjournals.aje.a008690. [DOI] [PubMed] [Google Scholar]
- 43.Pfeifer M, et al. Effects of a short-term vitamin D andcalcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15(6):1113–8. doi: 10.1359/jbmr.2000.15.6.1113. [DOI] [PubMed] [Google Scholar]
- 44.Bischoff-Ferrari HA, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged >=60 y. Am J Clin Nutr. 2004;80(3):752–8. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 45.Honkanen R, et al. The necessity and safety of calcium and vitamin D in the elderly. J Am Geriatr Soc. 1990;38(8):862–6. doi: 10.1111/j.1532-5415.1990.tb05700.x. [DOI] [PubMed] [Google Scholar]
- 46.Stefikova K, et al. Intensive vitamin D supplementation in the treatment of osteoporosis. Vnitr Lek. 2004;50(4):286–90. [PubMed] [Google Scholar]
- 47.Maalouf J, et al. Short- and long-term safety of weekly high-dose vitamin D3 supplementation in school children. J Clin Endocrinol Metab. 2008;93(7):2693–701. doi: 10.1210/jc.2007-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talwar SA, et al. Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr. 2007;86(6):1657–62. doi: 10.1093/ajcn/86.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schleithoff SS, et al. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 50.Aloia JF, et al. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. 2005;165(14):1618–23. doi: 10.1001/archinte.165.14.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Himmelstein S, et al. Vitamin D supplementation in elderly nursing home residents increases 25(OH)D but not 1,25(OH)2D. Am J Clin Nutr. 1990;52(4):701–6. doi: 10.1093/ajcn/52.4.701. [DOI] [PubMed] [Google Scholar]
- 52.Wagner CL, et al. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed Med. 2006;1(2):59–70. doi: 10.1089/bfm.2006.1.59. [DOI] [PubMed] [Google Scholar]
- 53.Wagner D, et al. The bioavailability of vitamin D from fortified cheeses and supplements is equivalent in adults. J Nutr. 2008;138(7):1365–71. doi: 10.1093/jn/138.7.1365. [DOI] [PubMed] [Google Scholar]
- 54.Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73(2):288–94. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- 55.Zittermann A, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89(5):1321–7. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 56.Sneve M, Figenschau Y, Jorde R. Supplementation with cholecalciferol does not result in weight reduction in overweight and obese subjects. Eur J Endocrinol. 2008;159(6):675–84. doi: 10.1530/EJE-08-0339. [DOI] [PubMed] [Google Scholar]
- 57.Chandra P, et al. Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot study. Endocr Pract. 2008;14(1):10–7. doi: 10.4158/EP.14.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berlin T, Emtestam L, Bjorkhem I. Studies on the relationship between vitamin D3 status and urinary excretion of calcium in healthy subjects: effects of increased levels of 25- hydroxyvitamin D3. Scand J Clin Lab Invest. 1986;46(8):723–9. doi: 10.3109/00365518609084043. [DOI] [PubMed] [Google Scholar]
- 59.Heaney RP, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 60.Rickers H, et al. Corticosteroid-induced osteopenia and vitamin D metabolism. Effect of vitamin D2, calcium phosphate and sodium fluoride administration. Clin Endocrinol (Oxf) 1982;16(4):409–15. doi: 10.1111/j.1365-2265.1982.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 61.Barger-Lux MJ, et al. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int. 1998;8(3):222–30. doi: 10.1007/s001980050058. [DOI] [PubMed] [Google Scholar]
- 62.Stern PH, et al. Demonstration that circulating 1 alpha, 25-dihydroxyvitamin D is loosely regulated in normal children. J Clin Invest. 1981;68(5):1374–7. doi: 10.1172/JCI110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fu L, et al. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem. 2009;42(10–11):1174–7. doi: 10.1016/j.clinbiochem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Smith SM, et al. Vitamin D supplementation during Antarctic winter. Am J Clin Nutr. 2009;89(4):1092–8. doi: 10.3945/ajcn.2008.27189. [DOI] [PubMed] [Google Scholar]
- 65.Hillman LS, et al. Percent true calcium absorption, mineral metabolism, and bone mass in children with arthritis: effect of supplementation with vitamin D3 and calcium. Arthritis Rheum. 2008;58(10):3255–63. doi: 10.1002/art.23809. [DOI] [PubMed] [Google Scholar]
- 66.Hillman LS, et al. Percent true calcium absorption, mineral metabolism, and bone mineralization in children with cystic fibrosis: effect of supplementation with vitamin D and calcium. Pediatr Pulmonol. 2008;43(8):772–80. doi: 10.1002/ppul.20863. [DOI] [PubMed] [Google Scholar]
- 67.Ooms ME, et al. Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double-blind trial. J Clin Endocrinol Metab. 1995;80(4):1052–8. doi: 10.1210/jcem.80.4.7714065. [DOI] [PubMed] [Google Scholar]
- 68.Narang NK, Gupta RC, Jain MK. Role of vitamin D in pulmonary tuberculosis. J Assoc Physicians India. 1984;32(2):185–8. [PubMed] [Google Scholar]
- 69.Kimball SM, et al. Safety of vitamin D3 in adults with multiple sclerosis. Am J Clin Nutr. 2007;86(3):645–51. doi: 10.1093/ajcn/86.3.645. [DOI] [PubMed] [Google Scholar]
- 70.Jean G, Souberbielle JC, Chazot C. Monthly cholecalciferol administration in haemodialysis patients: a simple and efficient strategy for vitamin D supplementation. Nephrol Dial Transplant. 2009;21:21. doi: 10.1093/ndt/gfp370. [DOI] [PubMed] [Google Scholar]
- 71.Tjellesen L, et al. Serum concentration of vitamin D metabolites during treatment with vitamin D2 and D3 in normal premenopausal women. Bone Miner. 1986;1(5):407–13. [PubMed] [Google Scholar]
- 72.Goldner WS, et al. Finding the optimal dose of vitamin D following Roux-en-Y gastric bypass: a prospective, randomized pilot clinical trial. Obes Surg. 2009;19(2):173–9. doi: 10.1007/s11695-008-9680-y. [DOI] [PubMed] [Google Scholar]
- 73.Mocanu V, et al. Long-term effects of giving nursing home residents bread fortified with 125 microg (5000 IU) vitamin D(3) per daily serving. Am J Clin Nutr. 2009;89(4):1132–7. doi: 10.3945/ajcn.2008.26890. [DOI] [PubMed] [Google Scholar]
- 74.Tucci JR. Vitamin D therapy in patients with primary hyperparathyroidism and hypovitaminosis D. Eur J Endocrinol. 2009;161(1):189–93. doi: 10.1530/EJE-08-0901. [DOI] [PubMed] [Google Scholar]
- 75.Blair D, et al. Prevalence of vitamin D [25(OH)D] deficiency and effects of supplementation with ergocalciferol (vitamin D2) in stage 5 chronic kidney disease patients. J Ren Nutr. 2008;18(4):375–82. doi: 10.1053/j.jrn.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 76.von Restorff C, Bischoff-Ferrari HA, Theiler R. High-dose oral vitamin D3 supplementation in rheumatology patients with severe vitamin D3 deficiency. Bone. 2009;45(4):747–9. doi: 10.1016/j.bone.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 77.Thacher TD, et al. The effect of vitamin D2 and vitamin D3 on intestinal calcium absorption in Nigerian children with rickets. J Clin Endocrinol Metab. 2009;94(9):3314–21. doi: 10.1210/jc.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berlin T, Bjorkhem I. Lack of effects of an increased pool of 25-hydroxyvitamin D3 on urinary excretion of calcium in healthy subjects. Contrib Nephrol. 1987;58:143–7. doi: 10.1159/000414504. [DOI] [PubMed] [Google Scholar]
- 79.Hiremath GS, et al. Vitamin D status and effect of low-dose cholecalciferol and high-dose ergocalciferol supplementation in multiple sclerosis. Mult Scler. 2009;15(6):735–40. doi: 10.1177/1352458509102844. [DOI] [PubMed] [Google Scholar]
- 80.Boas SR, et al. Very high-dose ergocalciferol is effective for correcting vitamin D deficiency in children and young adults with cystic fibrosis. J Cyst Fibros. 2009;8(4):270–2. doi: 10.1016/j.jcf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 81.Nordin BE, et al. A prospective trial of the effect of vitamin D supplementation on metacarpal bone loss in elderly women. Am J Clin Nutr. 1985;42(3):470–4. doi: 10.1093/ajcn/42.3.470. [DOI] [PubMed] [Google Scholar]
- 82.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79(5):717–26. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 83.Vieth R, et al. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J. 2004;3(1):8. doi: 10.1186/1475-2891-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aloia JF, et al. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr. 2008;87(6):1952–8. doi: 10.1093/ajcn/87.6.1952. [DOI] [PubMed] [Google Scholar]
- 85.Khan QJ, et al. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat. 2009;5:5. doi: 10.1007/s10549-009-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ilahi M, Armas LA, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr. 2008;87(3):688–91. doi: 10.1093/ajcn/87.3.688. [DOI] [PubMed] [Google Scholar]
- 87.Hasling C, et al. Safety of osteoporosis treatment with sodium fluoride, calcium phosphate and vitamin D. Miner Electrolyte Metab. 1987;13(2):96–103. [PubMed] [Google Scholar]
- 88.Leventis P, Kiely PD. The tolerability and biochemical effects of high-dose bolus vitamin D2 and D3 supplementation in patients with vitamin D insufficiency. Scand J Rheumatol. 2009;38(2):149–53. doi: 10.1080/03009740802419081. [DOI] [PubMed] [Google Scholar]
- 89.Romagnoli E, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93(8):3015–20. doi: 10.1210/jc.2008-0350. [DOI] [PubMed] [Google Scholar]
- 90.Ginde AA, et al. Prospective Study of Serum 25-Hydroxyvitamin D Level, Cardiovascular Disease Mortality, and All-Cause Mortality in Older U.S. Adults. J Am Geriatr Soc. 2009;22:22. doi: 10.1111/j.1532-5415.2009.02359.x. [DOI] [PubMed] [Google Scholar]
- 91.Gorham ED, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32(3):210–6. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 92.Melamed ML, et al. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jansen TL, Janssen M, de Jong AJ. Severe hypercalcaemia syndrome with daily low-dose vitamin D supplementation. Br J Rheumatol. 1997;36(6):712–3. doi: 10.1093/rheumatology/36.6.712. [DOI] [PubMed] [Google Scholar]
- 94.Hatun S, Cizmecioglu F. Use of alendronate in the treatment of vitamin D intoxication in infants. Turk J Pediatr. 2005;47(4):373–5. [PubMed] [Google Scholar]
- 95.Drinka PJ, Moore J, Boushon MC. Severe hypercalcemia after transition from calcium carbonate to calcium citrate in an elderly woman treated with ergocalciferol 50,000 IU per day. Am J Geriatr Pharmacother. 2006;4(1):70–4. doi: 10.1016/j.amjopharm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 96.Taskapan H, Vieth R, Oreopoulos DG. Unusually prolonged vitamin D intoxication after discontinuation of vitamin D: possible role of primary hyperparathyroidism. Int Urol Nephrol. 2008;40(3):801–5. doi: 10.1007/s11255-008-9404-1. [DOI] [PubMed] [Google Scholar]
- 97.Atabek ME, Pirgon O, Sert A. Oral alendronate therapy for severe vitamin D intoxication of the infant with nephrocalcinosis. J Pediatr Endocrinol Metab. 2006;19(2):169–72. doi: 10.1515/jpem.2006.19.2.169. [DOI] [PubMed] [Google Scholar]
- 98.Kimball S, Vieth R. Self-prescribed high-dose vitamin D3: effects on biochemical parameters in two men. Ann Clin Biochem. 2008;45(Pt 1):106–10. doi: 10.1258/acb.2007.007074. [DOI] [PubMed] [Google Scholar]
- 99.Jacobus CH, et al. Hypervitaminosis D associated with drinking milk. N Engl J Med. 1992;326(18):1173–7. doi: 10.1056/NEJM199204303261801. [DOI] [PubMed] [Google Scholar]
- 100.Klontz KC, Acheson DW. Dietary supplement-induced vitamin D intoxication. N Engl J Med. 2007;357(3):308–9. doi: 10.1056/NEJMc063341. [DOI] [PubMed] [Google Scholar]
- 101.Mawer EB, et al. Vitamin D metabolism in patients intoxicated with ergocalciferol. Clin Sci (Lond) 1985;68(2):135–41. doi: 10.1042/cs0680135. [DOI] [PubMed] [Google Scholar]
- 102.Selby PL, et al. Vitamin D intoxication causes hypercalcaemia by increased bone resorption which responds to pamidronate. Clin Endocrinol (Oxf) 1995;43(5):531–6. doi: 10.1111/j.1365-2265.1995.tb02916.x. [DOI] [PubMed] [Google Scholar]
- 103.Blank S, et al. An outbreak of hypervitaminosis D associated with the overfortification of milk from a home-delivery dairy. Am J Public Health. 1995;85(5):656–9. doi: 10.2105/ajph.85.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barrueto F, Jr., et al. Acute vitamin D intoxication in a child. Pediatrics. 2005;116(3):e453–6. doi: 10.1542/peds.2004-2580. [DOI] [PubMed] [Google Scholar]
- 105.Doneray H, et al. Intragastric alendronate therapy in two infants with vitamin D intoxication: a new method. Clin Toxicol (Phila) 2008;46(4):300–2. doi: 10.1080/15563650701455361. [DOI] [PubMed] [Google Scholar]
- 106.Vieth R, et al. Vitamin D poisoning by table sugar. Lancet. 2002;359(9307):672. doi: 10.1016/S0140-6736(02)07814-5. [DOI] [PubMed] [Google Scholar]
- 107.Pettifor JM, et al. Serum levels of free 1,25-dihydroxyvitamin D in vitamin D toxicity. Ann Intern Med. 1995;122(7):511–3. doi: 10.7326/0003-4819-122-7-199504010-00006. [DOI] [PubMed] [Google Scholar]
- 108.Koutkia P, Chen TC, Holick MF. Vitamin D intoxication associated with an over-the-counter supplement. N Engl J Med. 2001;345(1):66–7. doi: 10.1056/NEJM200107053450115. [DOI] [PubMed] [Google Scholar]
- 109.Chatterjee M, Speiser PW. Pamidronate treatment of hypercalcemia caused by vitamin D toxicity. J Pediatr Endocrinol Metab. 2007;20(11):1241–8. doi: 10.1515/jpem.2007.20.11.1241. [DOI] [PubMed] [Google Scholar]
- 110.Chan W, et al. Hypercalcemia in a newly arrived international adoptee. J Pediatr Endocrinol Metab. 2006;19(10):1249–50. doi: 10.1515/jpem.2006.19.10.1249. [DOI] [PubMed] [Google Scholar]
- 111.Gurkan F, et al. Pamidronate treatment in acute vitamin D intoxication. J Endocrinol Invest. 2004;27(7):680–2. doi: 10.1007/BF03347503. [DOI] [PubMed] [Google Scholar]
- 112.Bereket A, Erdogan T. Oral bisphosphonate therapy for vitamin D intoxication of the infant. Pediatrics. 2003;111(4 Pt 1):899–901. doi: 10.1542/peds.111.4.899. [DOI] [PubMed] [Google Scholar]
- 113.Orbak Z, et al. Vitamin D intoxication and therapy with alendronate (case report and review of literature) Eur J Pediatr. 2006;165(8):583–4. doi: 10.1007/s00431-005-0069-9. [DOI] [PubMed] [Google Scholar]
- 114.Titan SM, et al. Acute renal failure and hypercalcemia in an athletic young man. Clin Nephrol. 2009;71(4):445–7. doi: 10.5414/cnp71445. [DOI] [PubMed] [Google Scholar]
- 115.v Muhlendahl KE, Nawracala J. Vitamin D intoxication. Eur J Pediatr. 1999;158(3):266. doi: 10.1007/s004310051067. [DOI] [PubMed] [Google Scholar]
- 116.McKiernan FE, Wiley C. Vitamin D2, vitamin D3, and the tolerable upper intake level. J Bone Miner Res. 2008;23(12):2060–1. doi: 10.1359/jbmr.080801. [DOI] [PubMed] [Google Scholar]
- 117.Stephenson DW, Peiris AN. The lack of vitamin D toxicity with megadose of daily ergocalciferol (D2) therapy: a case report and literature review. South Med J. 2009;102(7):765–8. doi: 10.1097/SMJ.0b013e3181a8d1e4. [DOI] [PubMed] [Google Scholar]
- 118.Dietary reference intakes: calcium, phosphorus, magnesium, vitamin D, and fluoride. National Academy Press; Washington, D.C.: 1997. [PubMed] [Google Scholar]
- 119.Heaney RP. The Vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;(15):15. doi: 10.1016/j.jsbmb.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 120.McKenna MJ. Differences in vitamin D status between countries in young adults and the elderly. Am J Med. 1992;93(1):69–77. doi: 10.1016/0002-9343(92)90682-2. [DOI] [PubMed] [Google Scholar]
- 121.Theiler R, et al. Calcidiol, calcitriol and parathyroid hormone serum concentrations in institutionalized and ambulatory elderly in Switzerland. Int J Vitam Nutr Res. 1999;69(2):96–105. doi: 10.1024/0300-9831.69.2.96. [DOI] [PubMed] [Google Scholar]
- 122.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 123.Dawson-Hughes B, Harris SS, Dallal GE. Plasma calcidiol, season, and serum parathyroid hormone concentrations in healthy elderly men and women. Am J Clin Nutr. 1997;65(1):67–71. doi: 10.1093/ajcn/65.1.67. [DOI] [PubMed] [Google Scholar]
- 124.Parikh SJ, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89(3):1196–9. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 125.Looker AC, et al. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–7. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 126.Nesby-O'Dell S, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187–92. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 127.Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15(12):3225–32. doi: 10.1097/01.ASN.0000146012.44570.20. [DOI] [PubMed] [Google Scholar]
- 128.Penniston KL, et al. Vitamin D repletion does not alter urinary calcium excretion in healthy postmenopausal women. BJU Int. 2009;(15):15. doi: 10.1111/j.1464-410X.2009.08559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Curhan GC, et al. Dietary factors and the risk of incident kidney stones in younger women: Nurses' Health Study II. Arch Intern Med. 2004;164(8):885–91. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]
- 130.Chel V, et al. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos Int. 2008;19(5):663–71. doi: 10.1007/s00198-007-0465-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Heaney RP, et al. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22(2):142–6. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 132.Steingrimsdottir L, et al. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. Jama. 2005;294(18):2336–41. doi: 10.1001/jama.294.18.2336. [DOI] [PubMed] [Google Scholar]
- 133.Bischoff-Ferrari HA, et al. Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J Bone Miner Res. 2009;24(5):935–42. doi: 10.1359/JBMR.081242. [DOI] [PMC free article] [PubMed] [Google Scholar]