Abstract
Sensory experience influences brain organization and function. A particularly striking example is in the olfactory bulb where reduction of odorant sensory signals profoundly down-regulates dopamine in glomerular neurons. There are two large populations of glomerular inhibitory interneurons: (1) GABAergic periglomerular (PG) cells, whose processes are limited to a single glomerulus, regulate intraglomerular processing and (2) DAergic-GABAergic short axon (SA) cells, whose processes contact multiple glomeruli, regulate interglomerular processing. The inhibitory neurotransmitter GABA is synthesized from L-glutamic acid by the enzyme glutamic acid decarboxylase (GAD) of which there are two major isoforms: GAD65 and GAD67. GAD65 is expressed in uniglomerular PG cells. GAD67 is expressed by SA cells, which also co-express the rate-limiting enzyme for dopamine synthesis, tyrosine hydroxylase (TH). Deafferentation or sensory deprivation decreases TH expression but it is not known if sensory input alters GAD isoforms. Here we report that either deafferentation or reduction of sensory input by nares occlusion significantly reduced GAD67 protein and the number of SA cells expressing GAD67. However, neither manipulation altered GAD65 protein or the number of GAD65 PG cells. These findings show that sensory experience strongly impacts transmitter regulation in the circuit that controls neural processing across glomeruli but not in the circuit that regulates intraglomerular processing.
Keywords: Olfactory bulb, dopamine, GABA, gene regulation, periglomerular cell, short axon cell
1. Introduction
Neural activity regulates expression of neurotransmitter synthesis enzymes in many brain regions. The inhibitory neurotransmitter GABA is synthesized from L-glutamic acid by the enzyme glutamic acid decarboxylase (GAD), for which there are two major isoforms: 65kDa and 67kDa. These isoforms are differentially expressed by various neuron subpopulations and differentially distributed within cells (Erlander et al., 1991). The two GAD isoforms differ in activity-dependent expression in different brain structures (Rimvall and Martin, 1994). Differential regulation of these two GAD isoforms by experience may lead to plasticity of inhibitory neural circuits. We investigated this possibility in glomerular inhibitory circuits of the olfactory bulb.
Olfactory sensory neurons (OSNs) in the nasal epithelium project axons that terminate on the dendrites of second-order interneurons and output neurons (mitral/tufted cells (M/T)) in glomeruli of the main olfactory bulb. Three principle types of juxtaglomerular (JG) interneurons - external tufted (ET) cells, periglomerular (PG) cells and short axon (SA) cells (Golgi, 1875;Cajal, 1911;Pinching and Powell, 1971;Shao et al., 2009;Kiyokage et al., 2010) - form several distinct glomerular circuits that regulate the glomerular input-output function (Shao et al., 2009;Kiyokage et al., 2010). These interneurons differ in morphotype: ET cells and PG cells processes are predominantly confined to a single parent glomerulus (uniglomerular) while SA cells connect with multiple glomeruli. They also differ in chemotype: PG cells are GABAergic; SA cells use both GABA and dopamine (DA) as transmitters (Kosaka and Kosaka, 2008;Shao et al., 2009;Kiyokage et al., 2010). SA cells express GAD67 whereas PG cells predominantly express GAD65 (Kiyokage et al., 2010). These three JG neurons form two basic glomerular inhibitory circuits: (1) GAD65 GABAergic PG cells mediate intraglomerular inhibition and (2) GAD67 GABAergic-DAergic SA cells mediate interglomerular inhibition.
Afferent sensory input is necessary for maintenance of tyrosine hydroxylase (TH), the rate -limiting enzyme for DA biosynthesis in SA neurons (Kawano and Margolis, 1982;Baker et al., 1983;Kosaka et al., 1987;Baker et al., 1988;Baker et al., 1993;Brunjes, 1994;Cho et al., 1996;Philpot et al., 1998;Saino-Saito et al., 2004). Blocking afferent activity by means of deafferentation or sensory deprivation reduces TH expression (Baker et al., 1983;Baker et al., 1993;Brunjes, 1994;Cho et al., 1996;Philpot et al., 1998). DAergic SA cells predominantly contain GAD67 (Kiyokage et al., 2010) and PG cells predominantly express GAD65 (Parrish-Aungst et al., 2007). Since afferent activity regulates TH we asked if sensory activity alters the expression of GAD67 in SA cells and/or GAD65 expression in PG cells, i.e., are interglomerular and intraglomerular inhibitory circuits differentially impacted by sensory experience?
2. Results
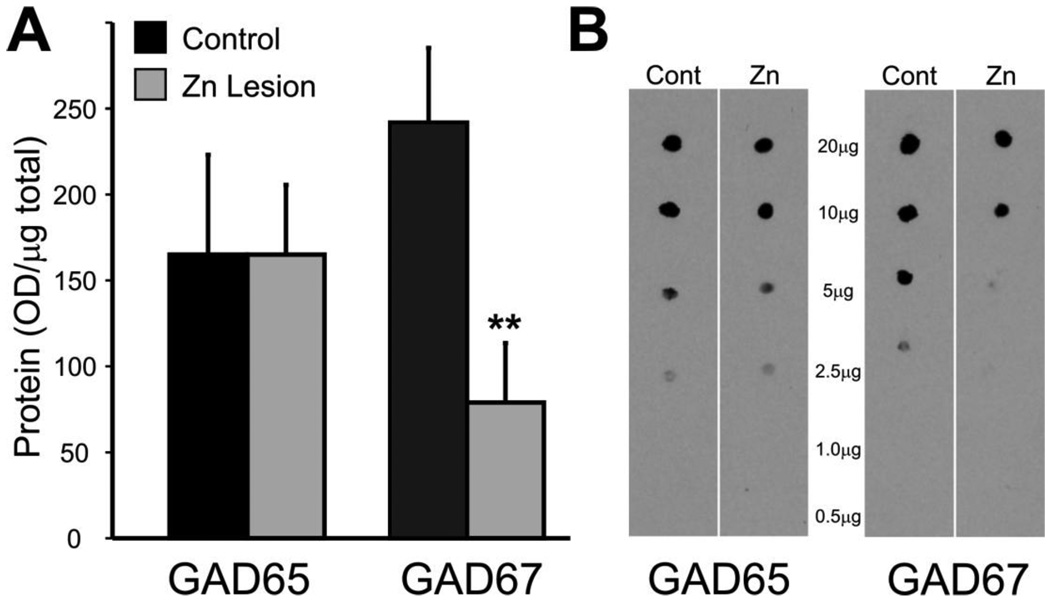
First, bilateral ZnSO4 irrigation of the nasal cavities was used to lesion the olfactory epithelium. This causes rapid degeneration of the olfactory nerve and loss of sensory input to the bulb. Indeed, anosmia occurs as soon as 1hr following ZnSO4 irrigation of the nasal cavities (McBride et al., 2003) and persists for 14–60 days (Harding et al., 1978;Burd, 1993). Tyrosine hydroxylase (TH) exhibits a well characterized downregulation following. At 14 days post lesion TH enzyme activity levels were reduced by 85% (Nadi et al., 1981;Baker et al., 1993); protein levels were not reported. Here, GAD67 and GAD65 protein levels were measured in olfactory bulb from animals 14 days following zinc sulphate lesion; GAD67 protein levels dropped significantly (61 ± 6% reduction; n=4 lesion, 7 control; p<0.01; Fig. 1). By contrast, GAD65 protein expression was unaltered (Fig. 1). This indicates that expression of GAD67, but not GAD65, is dependent on an intact olfactory nerve.
Figure 1.
Quantification of GAD65 and GAD67protein expression in normal and zinc sulphate lesioned mice. (A) GAD65 and GAD67protein expression (optical density/µg total protein) 14 days post zinc sulphate lesion of the olfactory epithelium (n=4, ** p<0.01). (B) Protein dot blots from control and zinc sulphate lesions probed with GAD65 or GAD67 antibodies.
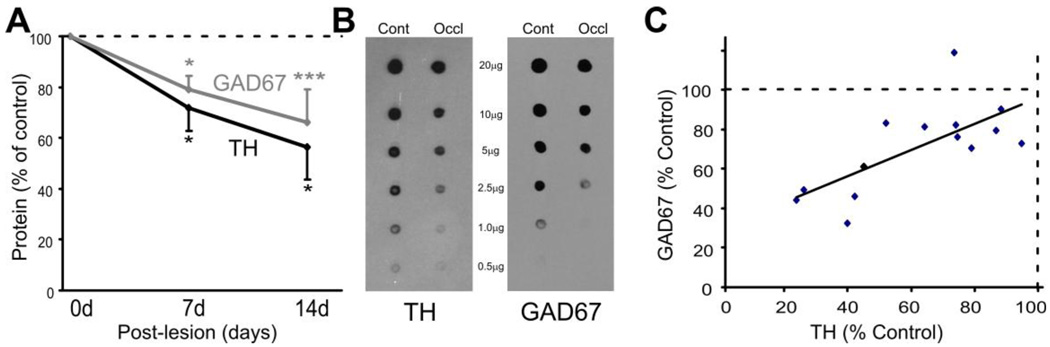
Zinc sulfate irrigation kills olfactory neurons causing degeneration of their axonal projections to the glomeruli. This could entail more than loss of sensory input, e.g., loss of trophic support of glomerular target neurons, reactive gliosis in the glomeruli, etc. To determine if the reduction in GAD67 protein is due to a loss of sensory input per se, as opposed to degenerative factors, we also performed unilateral occlusion of the nasal opening. This reduces odor access to the epithelium of the occluded nasal cavity and reduces sensory-evoked olfactory input to the olfactory bulb ipsilateral to the occlusion without destroying the epithelium. Consistent with previously reported reductions in TH following unilateral nares closure (Cho et al., 1996), occlusion reduced TH protein levels at 7 days (28 ± 8% reduction; n=5; p<0.05; Fig. 2A–B) and 14 days (56 ± 11% reduction; n=9; p<0.001; Fig. 2A–B). GAD67 protein levels decreased by 21 ± 5% at 7 days (n=5; p<0.05; Fig. 2A–B) and 34 ± 11% at 14 days (n=9; p<0.01; Fig. 2A–B) ipsilateral to the occlusion. The relative decrease in GAD67 protein was less than TH, however, decreases in TH and GAD67 within the same animals were positively correlated (coefficient 0.7, p<0.01; Fig. 2C). This suggests that TH and GAD67 expression are co-regulated. Taken together, these data show that the expression of TH and GAD67 are activity-dependent relying on afferent input for production and/or maintenance of the synthetic neurotransmitter enzymes.
Figure 2.
Quantification of GAD65, GAD67 and TH protein expression in normal and nares occlusion (AS) Quantification of the decrease on GAD67 and TH protein at 7d (n=5, * p<0.05) and 14d (n=9, *** p<0.001) following nares occlusion. (B) Protein dot blots from occluded and control (contralateral to closure) olfactory bulbs probed with GAD65 or GAD67 antibodies. (C) Correlation between the percent reduction in GAD67 and the percent reduction in TH.
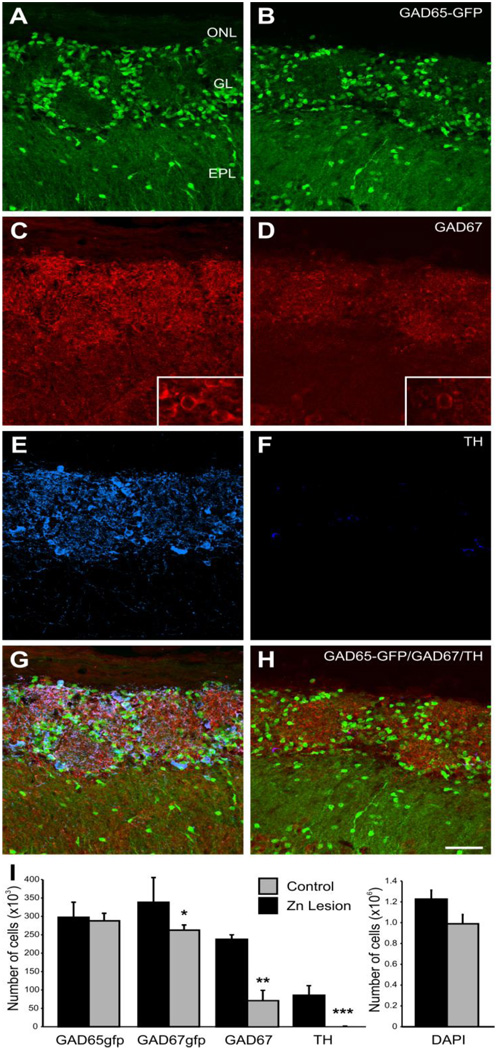
Reduced TH expression in the olfactory bulb following zinc lesion or nares occlusion is not due to the loss of neurons (Baker et al., 1993) but rather to reduced enzyme expression. Thus, we hypothesized decreased GAD67 protein was also due to reduced enzyme rather than cell loss. Using unbiased optical dissector methodology (Russ and Dehoff, 1998;Parrish-Aungst et al., 2007) we counted total cells and the number of cells expressing GAD65-GFP, GAD67 protein, GAD67-GFP, or TH protein 14 days following ZnSO4 lesion of the olfactory epithelium (Fig. 3A–H). The total number of cells in the glomerular layer was unchanged 14 days post lesion compared to control animals (1.0 ± 0.1 and 1.2 ± 0.1 ×106 cells respectively; P=0.1; Fig. 3I). Thus, the ZnSO4 lesions did not cause a loss of cells in the glomeruli. As previously reported (Baker et al., 1983), immunostained TH+ cells were almost completely absent in zinc-lesioned animals (P<0.0001; Fig. 3I). Using antibodies specific to GAD67, we observed a 70 ± 12% decrease in the number of GAD67 stained cells 14 days post lesion compared to control (p<0.01; n=4; Fig. 3I). At the same post lesion time, the number of cells expressing GFP in a GAD67-GFP mouse line was reduced by only 24±9%% (p<0.05 n=5; Fig. 3I). This indicates that sensory deafferentation does not lead to loss of glomerular neurons but does significantly reduce the expression of TH and GAD67 protein.
Figure 3.
Expression of GAD65-GFP (A and B), GAD67 (C and D) and TH (E and F) in a normal (A, C, E, and G) and zinc sulphate lesioned (B, D, F, and H) mouse olfactory bulb (OB). (A) In the normal OB a subpopulation of PG cells express GFP in a mouse line in which the GAD65 promoter drives GFP expression (green). (B) Following zinc sulphate lesion of the olfactory epithelium, glomerular neuropil reduces in volume but the number of GAD65 expressing cells is unchanged. (C) In the normal OB, GAD67 is also expressed by a subpopulation of PG and SA cells (red). Insert shows higher magnification of an individual cell. (D) GAD67 expression (red) following zinc sulphate lesion is reduced. .The insert shows higher magnification of an individual cell. (E) Tyrosine hydroxylase (blue) is expressed by SA cells within the glomerular layer. (F) In animals with the olfactory epithelium zinc lesioned TH expression is almost absent. (G) Overlay of panel A, C, and E showing partially overlapping subsets of PG cells in the normal OB. (H) Overlay of panels B, D, and F from a zinc sulphate lesioned OB. (I) Optical dissector stereology estimate of cell number in the glomerular layer for GAD65-GFP (n=4 animals), GAD67-GFP (n=5), GAD67 immunohistochemistry (n=4), and TH immunohistochemistry (n=4) in the glomerular layer of normal (black) and zinc sulphate lesioned animals (grey). * P<0.05, ** P<0.01, and *** P<0.0001. Scale bar = 50µm.
The difference between GAD67 immunohistochemistry and GAD67-GFP cell numbers could be due to differences in sensitivity between detection of GAD67 immunohistochemically and the expression of the transgene containing GFP. A second possibility could be due to GFP protein stability resulting in persistent GFP protein even though GAD67 protein is reduced. Alternatively, this difference could be due to regulation at a translational level, i.e. GAD67 mRNA is transcribed but protein translation is reduced. Control at a translational level would be consistent with previous reports indicating GAD mRNA in the glomerular layer is unaltered by Zn lesion (Stone et al., 1991), although these earlier studies used sequences that likely cross-reacted with both GAD65 and GAD67. As neither deafferentation nor occlusion had any effect on GAD65 protein levels, we anticipated that deafferentation would not alter GAD65-positive cells. Indeed, the number of positive cells in the GAD65-GFP mice was unaltered following lesion (0.29±0.20 and 0.30±0.41 ×106 cells respectively; Fig. 3I). Taken together, these data show that expression of both TH and GAD67, but not GAD65, is dependent on sensory activity.
3. Discussion
The present findings show that two juxtaglomerular neuron types are differentially influenced by sensory experience: DAergic-GABAergic SA cells are regulated by sensory signals but GABAergic PG cells are not. Could this differential regulation by afferent activity be due to differences in the number/density of olfactory nerve synaptic contacts? This seems unlikely. EM studies of the glomerular neuropil indicate both GABAergic and DAergic neurons receive ON synapses, indeed, ~80% of all anatomically identifiable synapses on DAergic neurons derive from the ON (Kosaka et al., 1997;Toida et al., 1998;Toida et al., 2000). However, synaptic number does not necessarily reflect synaptic efficacy. Efficacy depends on location of the synapses, their release probability, postsynaptic receptor types and the intrinsic properties of the postsynaptic cells.
ET cells generate spontaneous, bursts of action potentials that persist when ON inputs are pharmacologically blocked. Despite this highly effective ET cell synaptic drive to the majority of DAergic SA cells, they still exhibited reduced enzyme expression following lesion of the olfactory epithelium or nares occlusion. This indicates that neither direct olfactory nerve input, nor indirect activation via ET cells, alone, can maintain normal expression of TH and GAD67, and suggests that additional factors including enhanced synaptic drive by sensory input over and above spontaneous activity is required. However, it is possible that sensory deprivation alters the ligand-gated channels that underlie ET burst firing and thus reduces both spontaneous and sensory-evoked drive.
The interglomerular circuit formed by SA cells connects ten to several hundred glomeruli (Shao et al., 2009;Kiyokage et al., 2010). Reductions of DA and GABA synthetic enzymes in SA cells as a consequence of reduced sensory input might reduce the levels of the respective transmitters. This in turn could reduce presynaptic inhibition of olfactory nerve terminals and, via the interglomerular network, reduce postsynaptic inhibition MT cells. Either or both of these effects would potentially result in higher sensitivity to odors. Consistent with this possibility, olfactory deprivation increases the proportion of MT cells that respond to a given odor (Wilson and Sullivan, 1995;Aylwin et al., 2009), and the proportion of MT cells that respond to more than one odor (Wilson and Sullivan, 1995). This change in MT cell responsiveness is mimicked by D2 antagonist, spiperone (Wilson and Sullivan, 1995). Computational modeling of sensory deprivation suggests decreased inhibitory input to MT cells will decrease MT cell threshold responding to odors (Linster and Cleland, 2009). Thus sensory experience-dependent regulation of DA. and GABA in interglomerular inhibitory circuits, may reduce interglomerular inhibition such as to unmask subthreshold sensory input and increase the odorant receptive range of MT cells.
In contrast to DAergic-GABAergic SA cells that form interglomerular circuits, GAD65+ GABAergic PG cells mediate intraglomerular inhibition and thus, regulate the cohort of MT cells specific to each glomerulus. Neither destruction of the olfactory epithelium nor sensory deprivation (nares occlusion) altered GABA synthesis in these uniglomerular PG inhibitory neurons. This suggests that the main circuits that regulate intraglomerular processing are not influenced by long-term sensory activity and are perhaps less experience-dependent than interglomerular circuits. Long-term sensory experience also modulates the intrabulbar associational projections of CCK positive superficial tufted cells (Liu and Shipley, 1994), which link the pairs of glomeruli expressing the same odorant receptor gene (Belluscio et al., 2002;Lodovichi et al., 2003). Nares occlusion disrupts this precise point-to-point projection between the glomerular pair (Marks et al., 2006), a precision that can be restored following re-opening of the occluded nares (Cummings and Belluscio, 2008). Together these reports taken with the present findings suggest that sensory experience is crucial for intrabulbar links among glomeruli and less so for intraglomerular processing.
The initial glomerular map appears to be ‘hard-wired’ by axon targeting such that individual glomeruli are innervated by OSNs that express the same odorant receptor gene (Ressler et al., 1993;Vassar et al., 1994;Mombaerts et al., 1996;Wang et al., 1998). Activity-mapping studies have identified clusters of glomeruli that respond to structurally similar odorants, termed molecular-feature clusters (Uchida et al., 2000;Johnson and Leon, 2000a;Johnson and Leon, 2000b;Matsumoto et al., 2010). Other clusters may reflect environmentally significant cues. Interglomerular circuits may play a critical role in forming clusters by linking glomeruli that respond to similar classes of environmentally significant odors. The present findings show that neurotransmitter synthetic enzymes in SA cells that form the interglomerular circuit are dependent on olfactory experience. The sensitivity of intraglomerular circuits to afferent input may allow them to encode learned salient odors as patterns of activity across combinations of more hard-wired individual glomeruli.
4. Experimental Procedure
GFP transgenic mice
Generation of GAD65-GFP and GAD67-GFP transgenic mice has been described in detail elsewhere(Tamamaki et al., 2003;Lopez-Bendito et al., 2004). Briefly, GAD65-GFP transgenic mice contain a random insertion of a 6.5 kb segment of the GAD65 gene (including 5.5 kb of 5’-upstream sequence, the first two exons, and a portion of the third exon with corresponding introns) driving GFP expression in GABAergic neurons in most brain regions (GAD65-GFP from the line GAD65_3e/gfp5.5 #30 on a genetic background of C57BL6 with an F1 backcross to B6CBAF1/J wild-type mice yielding mice heterozygous for the transgene). When staining with GAD65 antibody from Chemicon (Temecula, CA Cat. No. AB5082), we observed a more than 90% overlap with the transgene in the mouse MOB (Parrish-Aungst et al., 2007). GAD67-GFP transgenic mice were generated by homologous recombination of green fluorescent protein and a loxP-flanked neomycin-resistance cassette into the GAD67 loci. The line of mice used in this study had the neomycin cassette recombined out (GAD67-GFP Δneo on a 129/C57BL/6J genetic background backcrossed to C57BL/6J wild type yielding mice heterozygous for the transgene and heterozygous for the endogenous GAD67 gene). All experimental procedures were carried out in accordance with the University of Maryland Institutional Animal Care and Use Committee and National Institute of Health guidelines.
Bilateral zinc sulfate lesion
Adult GAD65-GFP male mice (8 weeks old) were administered bilateral intranasal irrigation with 100 µl of 5% (0.17M) zinc sulfate in dH2O, (Margolis et al., 1974; McBride et al., 2003) . Littermates served as sham controls and were irrigated with 0.01M phosphate buffered saline (PBS), pH 7.4. Mice were allowed to live for 14 days, then deeply anesthetized with Nembutal (50mg/kg body weight) and tissue was processed for immunohistochemistry (IHC) or protein analysis.
Nares occlusion
GAD65-GFP male mice (8 weeks old) were anesthetized by intraperitoneal injection of Nembutal (50 mg/kg body weight), and unilateral nares occlusion of each mouse was done by thermocautery or permanent plug of the nasal opening. After cauterization/plug the animals were monitored and kept warm until full recovery. The contralateral bulb was used as a control for each mouse. Mice were euthanized 7 or 14 days post lesion and tissue was processed for immunohistochemistry (IHC) or protein analysis.
Immunohistochemistry
Mice were deeply anesthetized by intraperitoneal injections of sodium pentobarbital (Nembutal 50 mg/kg body weight in 0.9% saline solution) and transcardially perfused with 0.1M PBS followed by 4% paraformaldehyde (PFA) in PBS for 15 min. Brains were post fixed in 4% PFA for 2 hours at 4°C, embedded in 10% gelatin, and incubated in 30% sucrose for 48 hours. Tissue was then embedded in optimal cutting temperature compound (OCT; Sakura Finetek, Torrance, CA) and frozen. Serial coronal 25 µm sections were cut on a Leica CM3050 cryostat. Immunohistochemistry was performed at room temperature on free-floating sections sequentially incubated in 1% bovine serum albumin (BSA) in TBST (0.1M Tris, pH 7.4, 0.9% saline, 0.3% Triton X-100) for 30 minutes, primary antibodies for GAD67 (mouse; Chemicon-MAB5406; 1:1000) or TH (rabbit; Pel-Freez-P40101; 1:5000) diluted in BSA-TBST for 24 hours, washed (3×5min) in TBST, incubated in Cy3 conjugated secondary antibodies (Jackson Immuno; 1:500)for 1hour at room temperature, washed (3×5min) in TBST and counterstained with the nuclear stain DAPI (5nM) (Molecular Probes, Eugene, OR) in TBST for 30 minutes. Sections were mounted on gelatin coated slides and coverslipped with a DABCO-based anti-fade mounting media.
Photography
Digital microscopy images were captured with a FluoView500 confocal microscope (Olympus Instruments, Center Valley, PA) fitted with standard filters for visualization of GFP, Cy3, Cy5, and DAPI. Images were collected through the full 25 µm thickness of the section at 1 µm optical steps. These images resulted in stacks of ~25 images and were used for cell counting or collapsed into extended focus photographs. Photographs were assembled in to panels using CorelDraw14 (Corel, Ottawa, Ontario) and brightness, color, and contrast were balanced.
Cell Counts
Sections were randomly selected from the middle of the olfactory bulb and analyzed only on the medial wall. Detailed descriptions of cell counting methodology are described in previous work (Parrish-Aungst et al., 2007). In order to ensure consistency with our previous study counts were taken from the medial wall. Image stacks were analyzed using an optical dissector counting methodology (Russ and Dehoff, 1998). The number and position of each cell in the section of the confocal image stack was marked using neurolucida software (MicroBrightField, Colchester, VT). Total cell numbers in the glomerular layer were estimated by counting nuclei stained with DAPI, a highly specific dye that stains nuclei of cell all types, including neurons, glia, and endothelial cells. All cell counts are presented as mean ± standard error of the mean and are derived from at least four animals.
Protein analysis
Mice were deeply anesthetized by intraperitoneal injections of sodium pentobarbital (Nembutal 50 mg/kg body weight in 0.9% saline solution) and the main olfactory bulbs rapidly dissected out in ice-cold phosphate buffered saline (PBS). Tissues were homogenized in ice-cold lysis buffer (Sigma). The protein concentration of each homogenate was determined by Bradford analysis (BioRad, Hercules, CA). A serial dilution of protein from each sample was dot blotted in a series onto nitrocellulose membranes. The membrane was blocked with 5% nonfat dry milk in buffer containing 1M Tris-buffered saline and 0.05% Tween and probed with antibodies against the GAD65 (Chemicon; cat# AB5082 raised against human GAD65 from baculovirus infected cells showing no cross reactivity to GAD67 on western blots; ;1:1000), GAD67 (Chemicon; cat# MAB5406 clone #1G10.2 raised against recombinant GAD 67kDa showing no reactivity on western blot to GAD65kDa; ;1:1000), or TH (ImmunoStar; cat# 22941 raised against rat tyrosine hydroxylase from PC12 cells with specificity to the C-terminal 35kDa catalytic region of the molecule; ;1:5000) at 4°C overnight. After rinses in TBST, the membranes were incubated for 1 hr at room temperature in horseradish peroxidaseconjugated secondary (1:10,000). Antibodies and the blot developed with enhanced chemiluminescence (Amersham) following the manufactures protocols. Samples from individual animals (left/right bulbs) and groups were processed in the same reaction and exposed on the same gel. Quantification of expression levels was calculated from the integrated optical density of each dot series on exposed films and used for statistical analysis. All statistical analyses were performed using NCSS Pass statistical package.
Acknowledgements
Supported by NIH DCCD005676 and DCCD19015. We would also like to thank Dr. Frank Margolis for performing ZnSO4 irrigations of the animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. doi: 10.1038/nature02185. [DOI] [PubMed] [Google Scholar]
- Aylwin ML, Aguilar GA, Flores FJ, Maldonado PE. Odorant modulation of neuronal activity and local field potential in sensory-deprived olfactory bulb. Neuroscience. 2009;162:1265–1278. doi: 10.1016/j.neuroscience.2009.05.051. [DOI] [PubMed] [Google Scholar]
- Baker H, Kawano T, Margolis FL, Joh TH. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J Neurosci. 1983;3:69–78. doi: 10.1523/JNEUROSCI.03-01-00069.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Morel K, Stone DM, Maruniak JA. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res. 1993;614:109–116. doi: 10.1016/0006-8993(93)91023-l. [DOI] [PubMed] [Google Scholar]
- Baker H, Towle AC, Margolis FL. Differential afferent regulation of dopaminergic and GABAergic neurons in the mouse main olfactory bulb. Brain Res. 1988;450:69–80. doi: 10.1016/0006-8993(88)91545-4. [DOI] [PubMed] [Google Scholar]
- Belluscio L, Lodovichi C, Feinstein P, Mombaerts P, Katz LC. Odorant receptors instruct functional circuitry in the mouse olfactory bulb. Nature. 2002;419:296–300. doi: 10.1038/nature01001. [DOI] [PubMed] [Google Scholar]
- Benson DL, Huntsman MM, Jones EG. Activity-dependent changes in GAD and preprotachykinin mRNAs in visual cortex of adult monkeys. Cereb Cortex. 1994;4:40–51. doi: 10.1093/cercor/4.1.40. [DOI] [PubMed] [Google Scholar]
- Bowers G, Cullinan WE, Herman JP. Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. J. Neurosci. 1998;18:5938–5947. doi: 10.1523/JNEUROSCI.18-15-05938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinon JG, Martinez-Guijarro FJ, Bravo IG, Arevalo R, Crespo C, Okazaki K, Hidaka H, Aijon J, Alonso JR. Coexpression of neurocalcin with other calcium-binding proteins in the rat main olfactory bulb. J Comp Neurol. 1999;407:404–414. doi: 10.1002/(sici)1096-9861(19990510)407:3<404::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Brunjes PC. Unilateral naris closure and olfactory system development. Brain Res Brain Res Rev. 1994;19:146–160. doi: 10.1016/0165-0173(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Burd GD. Morphological study of the effects of intranasal zinc sulfate irrigation on the mouse olfactory epithelium and olfactory bulb. Microsc. Res. Tech. 1993;24:195–213. doi: 10.1002/jemt.1070240302. [DOI] [PubMed] [Google Scholar]
- Cajal RS. Histologie du Systeme Neurneux de l'Hommes et des Vertebres. Paris: Maloine; 1911. [Google Scholar]
- Cho JY, Min N, Franzen L, Baker H. Rapid down-regulation of tyrosine hydroxylase expression in the olfactory bulb of naris-occluded adult rats. J Comp Neurol. 1996;369:264–276. doi: 10.1002/(SICI)1096-9861(19960527)369:2<264::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Belluscio L. Charting plasticity in the regenerating maps of the mammalian olfactory bulb. Neuroscientist. 2008;14:251–263. doi: 10.1177/1073858408315026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drengler SM, Oltmans GA. Rapid increases in cerebellar Purkinje cell glutamic acid decarboxylase (GAD67) mRNA after lesion-induced increases in cell firing. Brain Res. 1993;615:175–179. doi: 10.1016/0006-8993(93)91129-g. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Freichel C, Potschka H, Ebert U, Brandt C, Loscher W. Acute changes in the neuronal expression of GABA and glutamate decarboxylase isoforms in the rat piriform cortex following status epilepticus. Neuroscience. 2006;141:2177–2194. doi: 10.1016/j.neuroscience.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Golgi C. Sulla Fina Struttura dei Bulbi Olfactorii. Rome: Reggio-Emilia; 1875. [Google Scholar]
- Harding JW, Getchell TV, Margolis FL. Denervation of the primary olfactory pathway in mice. V. Long-term effect of intranasal ZnSO4 irrigation on behavior, biochemistry and morphology. Brain Res. 1978;140:271–285. doi: 10.1016/0006-8993(78)90460-2. [DOI] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Neuronal circuit-dependent alterations in expression of two isoforms of glutamic acid decarboxylase in the hippocampus following electroconvulsive shock: A stereology-based study. Hippocampus. 2009;19:1130–1141. doi: 10.1002/hipo.20576. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 2000a;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Odorant molecular length: One aspect of the olfactory code. J Comp Neurol. 2000b;426:330–338. doi: 10.1002/1096-9861(20001016)426:2<330::aid-cne12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kawano T, Margolis FL. Transsynaptic regulation of olfactory bulb catecholamines in mice and rats. J Neurochem. 1982;39:342–348. doi: 10.1111/j.1471-4159.1982.tb03953.x. [DOI] [PubMed] [Google Scholar]
- Kiyokage E, Pan YZ, Shao Z, Kobayashi K, Szabo G, Yanagawa Y, Obata K, Okano H, Toida K, Puche AC, Shipley MT. Molecular identity of periglomerular and short axon cells. J. Neurosci. 2010;30:1185–1196. doi: 10.1523/JNEUROSCI.3497-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, Mori K, Sakano H. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- Kosaka K, Toida K, Aika Y, Kosaka T. How simple is the organization of the olfactory glomerulus?: the heterogeneity of so-called periglomerular cells. Neurosci Res. 1998;30:101–110. doi: 10.1016/s0168-0102(98)00002-9. [DOI] [PubMed] [Google Scholar]
- Kosaka K, Toida K, Margolis FL, Kosaka T. Chemically defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb--II. Prominent differences in the intraglomerular dendritic arborization and their relationship to olfactory nerve terminals. Neuroscience. 1997;76:775–786. doi: 10.1016/s0306-4522(96)00308-9. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K. Tyrosine hydroxylase-positive GABAergic juxtaglomerular neurons are the main source of the interglomerular connections in the mouse main olfactory bulb. Neurosci. Res. 2008;60:349–354. doi: 10.1016/j.neures.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K, Hama K, Wu JY, Nagatsu I. Differential effect of functional olfactory deprivation on the GABAergic and catecholaminergic traits in the rat main olfactory bulb. Brain Res. 1987;413:197–203. doi: 10.1016/0006-8993(87)90171-5. [DOI] [PubMed] [Google Scholar]
- Linster C, Cleland TA. Glomerular microcircuits in the olfactory bulb. Neural Netw. 2009;22:1169–1173. doi: 10.1016/j.neunet.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwak J, Mercugliano M, Chesselet MF, Oltmans GA. Increased glutamic acid decarboxylase (GAD) mRNA and GAD activity in cerebellar Purkinje cells following lesion-induced increases in cell firing. Neurosci. Lett. 1990;116:179–183. doi: 10.1016/0304-3940(90)90406-y. [DOI] [PubMed] [Google Scholar]
- Liu WL, Shipley MT. Intrabulbar associational system in the rat olfactory bulb comprises cholecystokinin-containing tufted cells that synapse onto the dendrites of GABAergic granule cells. J Comp Neurol. 1994;346:541–558. doi: 10.1002/cne.903460407. [DOI] [PubMed] [Google Scholar]
- Lodovichi C, Belluscio L, Katz LC. Functional topography of connections linking mirror-symmetric maps in the mouse olfactory bulb. Neuron. 2003;38:265–276. doi: 10.1016/s0896-6273(03)00194-6. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Sturgess K, Erdelyi F, Szabo G, Molnar Z, Paulsen O. Preferential Origin and Layer Destination of GAD65-GFP Cortical Interneurons. Cereb. Cortex. 2004 doi: 10.1093/cercor/bhh072. [DOI] [PubMed] [Google Scholar]
- Marks CA, Cheng K, Cummings DM, Belluscio L. Activity-dependent plasticity in the olfactory intrabulbar map. J. Neurosci. 2006;26:11257–11266. doi: 10.1523/JNEUROSCI.2805-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Kobayakawa K, Kobayakawa R, Tashiro T, Mori K, Sakano H, Mori K. Spatial arrangement of glomerular molecular-feature clusters in the odorant-receptor class domains of the mouse olfactory bulb. J. Neurophysiol. 2010;103:3490–3500. doi: 10.1152/jn.00035.2010. [DOI] [PubMed] [Google Scholar]
- McBride K, Slotnick B, Margolis FL. Does intranasal application of zinc sulfate produce anosmia in the mouse? An olfactometric and anatomical study. Chem. Senses. 2003;28:659–670. doi: 10.1093/chemse/bjg053. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Nadi NS, Head R, Grillo M, Hempstead J, Grannot-Reisfeld N, Margolis FL. Chemical deafferentation of the olfactory bulb: plasticity of the levels of tyrosine hydroxylase, dopamine and norepinephrine. Brain Res. 1981;213:365–377. doi: 10.1016/0006-8993(81)90241-9. [DOI] [PubMed] [Google Scholar]
- Parrish-Aungst S, Shipley MT, Erdelyi F, Szabo G, Puche AC. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. J. Comp Neurol. 2007;501:825–836. doi: 10.1002/cne.21205. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Men D, McCarty R, Brunjes PC. Activity-dependent regulation of dopamine content in the olfactory bulbs of naris-occluded rats. Neuroscience. 1998;85:969–977. doi: 10.1016/s0306-4522(97)00667-2. [DOI] [PubMed] [Google Scholar]
- Pinching AJ, Powell TP. The neuron types of the glomerular layer of the olfactory bulb. J Cell Sci. 1971;9:305–345. doi: 10.1242/jcs.9.2.305. [DOI] [PubMed] [Google Scholar]
- Price JL, Powell TP. The mitral and short axon cells of the olfactory bulb. J Cell Sci. 1970;7:631–651. doi: 10.1242/jcs.7.3.631. [DOI] [PubMed] [Google Scholar]
- Ramirez M, Gutierrez R. Activity-dependent expression of GAD67 in the granule cells of the rat hippocampus. Brain Res. 2001;917:139–146. doi: 10.1016/s0006-8993(01)02794-9. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Vaughn JE, Saito K, Barber R, Roberts E. Glutamate decarboxylase localization in neurons of the olfactory bulb. Brain Res. 1977;126:1–18. doi: 10.1016/0006-8993(77)90211-6. [DOI] [PubMed] [Google Scholar]
- Rimvall K, Martin DL. The level of GAD67 protein is highly sensitive to small increases in intraneuronal gamma-aminobutyric acid levels. J. Neurochem. 1994;62:1375–1381. doi: 10.1046/j.1471-4159.1994.62041375.x. [DOI] [PubMed] [Google Scholar]
- Russ JC, Dehoff RT. Practical Stereology. New York: Plenum Press; 1998. [Google Scholar]
- Saino-Saito S, Sasaki H, Volpe BT, Kobayashi K, Berlin R, Baker H. Differentiation of the dopaminergic phenotype in the olfactory system of neonatal and adult mice. J. Comp Neurol. 2004;479:389–398. doi: 10.1002/cne.20320. [DOI] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Kiyokage E, Szabo G, Shipley MT. Two GABAergic intraglomerular circuits differentially regulate tonic and phasic presynaptic inhibition of olfactory nerve terminals. J. Neurophysiol. 2009;101:1988–2001. doi: 10.1152/jn.91116.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DM, Grillo M, Margolis FL, Joh TH, Baker H. Differential effect of functional olfactory bulb deafferentation on tyrosine hydroxylase and glutamic acid decarboxylase messenger RNA levels in rodent juxtaglomerular neurons. J Comp Neurol. 1991;311:223–233. doi: 10.1002/cne.903110205. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Toida K, Kosaka K, Aika Y, Kosaka T. Chemically defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb--IV. Intraglomerular synapses of tyrosine hydroxylase-immunoreactive neurons. Neuroscience. 2000;101:11–17. doi: 10.1016/s0306-4522(00)00356-0. [DOI] [PubMed] [Google Scholar]
- Toida K, Kosaka K, Heizmann CW, Kosaka T. Chemically defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb: III. Structural features of calbindin D28K-immunoreactive neurons. J Comp Neurol. 1998;392:179–198. doi: 10.1002/(sici)1096-9861(19980309)392:2<179::aid-cne3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Wang F, Nemes A, Mendelsohn M, Axel R. Odorant receptors govern the formation of a precise topographic map. Cell. 1998;93:47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]
- White EL. Synaptic organization in the olfactory glomerulus of the mouse. Brain Res. 1972;37:69–80. doi: 10.1016/0006-8993(72)90346-0. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. The D2 antagonist spiperone mimics the effects of olfactory deprivation on mitral/tufted cell odor response patterns. J Neurosci. 1995;15:5574–5581. doi: 10.1523/JNEUROSCI.15-08-05574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]