Abstract
Self-regulatory failure is a core feature of many social and mental health problems. Self-regulation can by undermined by failures to transcend overwhelming temptations, negative moods, resource depletion, and when minor lapses in self-control snowball into self-regulatory collapse. Cognitive neuroscience research suggests that successful self-regulation is dependent on top-down control from the prefrontal cortex over subcortical regions involved in reward and emotion. We highlight recent neuroimaging research on self-regulatory failure, the findings of which support a balance model of self-regulation whereby self-regulatory failure occurs whenever the balance is tipped in favor of subcortical areas, either due to particularly strong impulses, or when prefrontal function itself is impaired. Such a model is consistent with recent findings in the cognitive neuroscience of addictive behavior, emotion regulation, and decision making.
The Advantages of Self-Control
The ability to control behavior enables humans to live cooperatively, achieve important goals, and maintain health throughout the life span. Self-regulation allows people to make plans, choose from alternatives, control impulses, inhibit unwanted thoughts, and regulate social behavior [1–4]. Although humans have an impressive capacity for self-regulation, failures are common and people lose control of their behavior in a wide variety of circumstances [1, 5]. Such failures are an important cause of several contemporary societal problems—obesity, addiction, poor financial decisions, sexual infidelity, and so on. Indeed, it has been estimated that 40% of deaths are attributable to poor self-regulation [6]. Conversely, those who are better able to self-regulate demonstrate improved relationships, increased job success, and better mental health [7–8] and are less at risk for developing alcohol abuse problems or engaging in risky sexual behavior [9]. An understanding of the circumstances under which people fail at self-regulation -as well as the brain mechanisms associated with those failures- can provide valuable insights into how people regulate and control their thoughts, behaviors, and emotions.
Self-Regulation Failure
The modern world holds many temptations. Everyday people need to resist fattening foods, avoid browsing the internet when they should be working, keep from snapping at annoying coworkers, and curb bad habits, such as smoking or drinking too much. Psychologists have made considerable progress in identifying the individual and situational factors that encourage or impair self-control [4–5, 10]. The most common circumstances under which self-regulation fails are when people are in bad moods, when minor indulgences snowball into full blown binges, when people are overwhelmed by immediate temptations or impulses, and when control itself is impaired (e.g., after alcohol consumption or effort depletion). Researchers have examined each of these and we briefly discuss the major findings, beginning with the behavioral literature and then discussing recent neuroscience findings.
Negative Moods
Among the most important triggers of self-regulation failure are negative emotions [11–12]. When people become upset they sometimes act aggressively [13], spend too much money [14] engage in risky behavior [15] including unprotected sex [16], comfort the self with alcohol, drugs, or food [4, 17], and fail to pursue important life goals. Indeed, negative emotional states are related to relapse for a number of addictive behaviors, such as alcoholism, gambling, and drug addiction [18–19]. Laboratory studies have demonstrated that inducing negative affect leads to heightened cravings among alcoholics [12], increased eating by chronic dieters [20–21] and greater smoking intensity by smokers [22].
A theory by Heatherton and Baumeister [23] provides an explanation for the roles of negative affect in disinhibited eating, which is also applicable to other self-regulatory failures. This theory proposes that dieters hold a negative view of self that is generally unpleasant (especially concerning physical appearance) and that dieters are motivated to escape from these unpleasant feelings by constricting their cognitive attention to the immediate situation while ignoring the long term implications and higher level significance of their current actions. This escape from aversive self-awareness not only helps dieters to forget their unpleasant views of self, but it also disengages long-term planning and meaningful thinking and weakens the inhibitions that normally restrain the dieter’s food intake. This may explain, in part, the lack of insight that occurs in drug addiction [24]. Other behavioral accounts of the impact of negative mood on behavior include the idea that negative affect occupies attention, thereby leading to fewer resources to inhibit behavior [25] or that engaging in appetitive behaviors reduces anxiety and comforts the self and is therefore a form of coping [26].
Lapse Activated Consumption
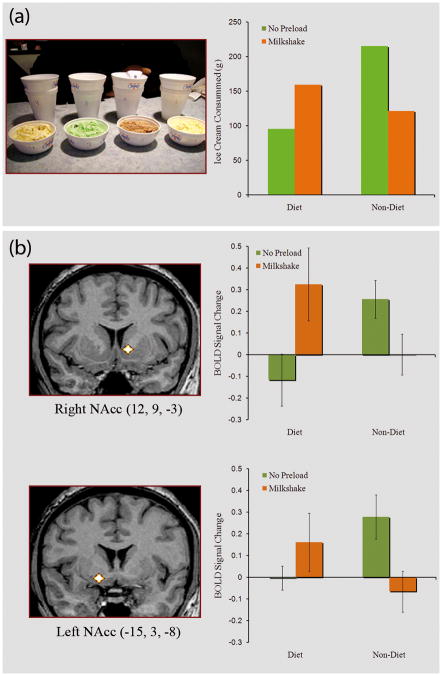
A common pattern of self-regulation failure occurs for addicts and chronic dieters when they “fall off the wagon” by consuming the addictive substance or violating their diets [5]. Marlatt [11] coined the term “abstinence violation effect” to refer to situations in which addicts respond to an initial indulgence by consuming even more of the forbidden substance. In one of the first studies to examine this effect, Herman and Mack [27] experimentally violated the diets of dieters by requiring them to drink a milkshake, a high calorie food, as part of a supposed taste perception study. Although non-dieters ate less after consuming the milkshakes, presumably because they were full, dieters paradoxically ate more after having the milkshake (Figure 1a). This disinhibition of dietary restraint has been replicated numerous times [20, 28] and demonstrates that dieters often eat a great deal after they perceive their diets to be broken. It is currently not clear, however, how a small indulgence, which itself might not be problematic, escalates into a full-blown binge [29].
Figure 1.
(A) When restrained eaters’ diets are broken by consuming a high-calorie milkshake preload, they subsequently show disinhibited eating (e.g. increased grams consumed of ice-cream) compared to control subjects and restrained eaters who did not drink the milkshake (figure based on data from [30]). (B) Restrained eaters whose diets are broken by a milkshake preload show increased activity in the Nucleus Accumbens compared to restrained eaters who did not consume the preload as well as satiated non-dieters [64]. Nacc = Nucleus Accumbens.
Cue Exposure
At the core of self-regulation is impulse control, but how do impulses arise? Both human and animal studies have demonstrated that exposure to drug cues increases the likelihood that the cued substance will be consumed [30–33], and additionally increases cravings, attention, and physiological responses such as changes in heart rate [33–35]. Yet people may be unaware that their environments are influencing them since stimuli can activate goals, cravings, and so forth implicitly [36–37]. Even if people are somewhat aware of cues around them, they are unaware of the process by which exposure to those cues implicitly activates cognitive processes that determine behavior [38]. A recent meta-analysis of 75 articles found that implicit cognition is a strong and reliable predictor of substance use [39]. From this perspective, cognitions that are spontaneously activated by stimuli from the environment alter how people act in a given situation.
The ability to transcend immediate temptations in the service of long-term goals is a key aspect of self-regulation [5, 40]. In an important series of studies, Mischel and colleagues studied how preschoolers responded in the face of temptation and in which delaying gratification led larger rewards [40, 41]. Successful self-control was associated with either redirection of attention away from temptation or cognitively reframing “hot” appetitive features into “cool” representations [40]. A related pattern is found in behavioral economic studies in which people discount future rewards in decision making by choosing less objectively valuable rewards that are immediately available [42]. A common feature of these studies is that people respond to appetizing cues by succumbing to immediate gratification rather than resisting temptation in order to achieve long-term goals.
Self-Regulatory Resource Depletion
Self-regulation, like many other cognitive faculties, is subject to fatigue. One of the more influential theories to emerge from this research is that self-regulation draws upon a common domain-general resource, so that, for example, regulating one’s emotions over an extended period of time impairs subsequent attempts at resisting the temptation to eat appetizing foods resulting in disinhibited eating [43]. Baumeister and Heatherton [5] proposed a strength model of self-regulation in which the ability to effectively regulate behavior was hypothesized to be dependent on a limited resource which is consumed by effortful attempts at self-regulation. In addition, this model also posited that self-regulatory capacity can be built up through practice and training (Box 1).
BOX 1. Can Self-Regulatory Capacity Be Increased?
In addition to postulating that self-regulation relies on a limited domain-general resource, the limited resource account of self-regulatory failure [5] also predicted that that self-regulatory capacity could be increased through practice or training. The first study to examine the effect of self-regulatory training had participants engage in a variety of daily tasks that required exerting small amounts of self-control (e.g. remembering to maintain good posture). Compared to control participants, those who engaged in modest amounts of daily self-control were shown to be more resistant to the effects of self-regulatory depletion [100]. In addition, it has been shown that simple self-control regimens, such as using the non-dominant hand for daily activities, can reduce the depleting effects of suppressing stereotypes [101]. More recently, these results have been extended to health behaviors such as quitting smoking. Engaging in simple daily self-control exercises (e.g. avoiding unhealthy foods) prior to quitting smoking lead to increased abstinent rates at follow-up for those who practice self-control compared to a control group that did not [101]. These findings support the notion that the self-regulatory strength can be increased through practice and that once increased, this newfound capacity to self-regulate can be used not only for comparatively banal tasks such as maintaining posture or using one’s non-dominant hand but also for behaviors with important health consequences such as resisting the temptation to smoke.
If self-regulatory capacity can be increased through simple self-control exercises over relatively short periods of time, what about people whose profession requires constant self-regulation (e.g. professional musicians, air traffic controllers)? The study of self-regulatory capacity in such populations has remained largely unexplored, however related research has shown that a relationship between musical training and grey matter in the dorsolateral prefrontal cortex [103] a brain region that has been implicated in both working memory and self-control [3].
Since its formulation there has been a tremendous surge in research supporting the notion that self-regulation relies on a limited resource. Studies of self-regulatory resource depletion have demonstrated that self-regulatory resources can be depleted by a wide range of activities, from suppressing thoughts [44] and inhibiting emotions [43] to managing the impressions we make [45] and engaging in interracial interactions [46]. A recent meta-analysis of 83 studies of self-regulatory depletion concluded that the limited resource account of self-regulation remains the best explanation for this effect [10]. More recently, it has been suggested that self-regulation relies on adequate levels of circulating blood glucose that are temporarily reduced by tasks that require effortful self-regulation (Box 2).
BOX 2. Self-Regulatory Resource Depletion and Blood Glucose.
One issue with the limited resource model of self-regulation has been the lack of biological specificity in identifying the actual resource that is depleted by acts of self-control. Recently, it has been suggested that self-regulation relies on circulating blood glucose [104]. In a series of experiments, Gailliot and colleagues [105] demonstrated that engaging in effortful self-control reduces blood glucose levels. Moreover, they have also found that artificially raising blood glucose levels eliminates the effects of self-regulatory depletion [105–106].
Although the notion that glucose metabolism affects self-regulation is recent, the impact of glucose on cognitive performance has been known for some time. For example, studies conducted in the 1990s showed that administering glucose improves performance on memory tasks and on tasks requiring response inhibition [107]. In many respects this should come as no surprise as glucose metabolism is the primary contrast in functional neuroimaging with Positron Emission Tomography (PET), which, among numerous other findings, has demonstrated that glucose metabolism increases with task difficulty [108]. In light of this research it seems plausible that self-regulatory failure following resource depletion is at least partly due to a temporary reduction in brain glucose stores. Finally, since self-regulation relies primarily on cognitive functions that are ascribed to the prefrontal cortex, depletion effects should presumably be greatest when both the depleting task and the subsequent self-regulation task recruit the same region of the brain. Although this has yet to be tested, PET neuroimaging, with its ability to directly measure glucose metabolism, is an ideal method for investigating the link between focal glucose depletion in the brain and subsequent impairments in self-regulation.
Functional Neuroimaging Studies of Self-Regulation
Functional neuroimaging studies of self-regulation and its failures suggest that self-regulation involves a balance between brain regions representing the reward, salience and emotional value of a stimulus and prefrontal regions associated self-control. When this balance tips in favor of bottom-up impulses, either due to a failure to engage prefrontal control areas or due to an especially strong impulse (e.g. the sight and smell of cigarettes for an abstinent smoker) then the likelihood of self-regulatory failure increases.
Regulation of Appetitive Behaviors
A universal feature of rewards, including drugs of abuse, is that they activate dopamine receptors in the mesolimbic dopamine system, especially the nucleus accumbens (NAcc) in the ventral striatum [47–49]. Similarly, functional neuroimaging studies have shown that the ingestion of drugs similarly increases activity in NAcc [50]. Earlier we noted that cue exposure is associated with self-regulation failure. Neuroimaging studies provide a plausible mechanism for such effects. When addicted individuals are exposed to visual cues that have become associated with drugs (e.g. images of drugs and drug paraphernalia) they also show cue-related activity in the mesolimbic reward system [51–53] as well as the insula [54]. Likewise, in neuroeconomic studies of decision making, activity in mesolimbic reward structures is associated with choosing immediate monetary rewards [55,56]. Indeed, dopamine agonists increase impulsive behavior in intertemporal choice tasks [57]. Hence, exposure to cues activates reward regions, likely because of learned expectancies that the observed stimulus will be consumed and provide genuine reward. That is, over the course of human evolution food relevant stimuli, for example, were usually real and edible rather than mere visual representations. Thus, cue exposure motivates people to seek out relevant rewards. Interestingly, it seems likely that cue reactivity may influence motivation outside of conscious awareness [24, 37–38, 54]. Indeed, Childress and colleagues [58] found “unseen” stimuli of cocaine (presented for 33 msec. and then backward masked) produced striatal activity for cocaine addicts. This supports the proposition that implicit cognition may be important in part because people are unaware that such unconscious processes are shaping their behavior and therefore unable to resist their influence (see also [59]).
Of particular interest is what happens when participants attempt to regulate their responses to reward cues such as those representing money, food or drugs. When cocaine users [60] or smokers [61–62] are instructed to inhibit craving they show increased activity in regions of the prefrontal cortex (PFC) associated with self-control and reduced cue-reactivity in regions associated with reward processing. Specifically, Volkow and colleagues showed that when cocaine users inhibit their craving in response to cocaine cues they show reduced activity in the orbitofrontal cortex and ventral striatum [60]. Moreover, the magnitude of this reduction is correlated with an increase in activity in lateral PFC [60]. Similarly, in smokers, activity in the dorsolateral PFC during regulation of smoking craving correlated with reduced activity in the ventral striatum to smoking cues and this relationship mediated reductions in self-reported craving [61]. This effect also obtains in healthy participants who are instructed to regulate their response to cues representing monetary rewards, showing that regulating their response to reward cues results in decreased cue-related activity in the ventral striatum [63]. Finally, a recent study extended the above findings by demonstrating that individual differences in activity in the lateral prefrontal cortex during a simple inhibition task were associated with real-world reductions in cigarette craving and consumption among smokers over a three-week period [64].
The above studies indicate that regulating craving requires top-down control of brain reward systems by PFC control regions [60–61, 63]. But what happens when self-control breaks down? As mentioned previously, one common reason self-regulation fails is due to lapse activated consumption, such as when dieters break their diet and temporarily engage in disinhibited eating [20, 27, 65–66]. One possible mechanism for this paradoxical pattern is that the initial intake of the food serves as a hedonic prime, and thereby brain regions involved in reward (i.e., NAcc) are freed from the regulatory influence of PFC, subsequently demonstrating heightened response to appetizing food. A recent study tested this proposition by examining the effect of breaking a diet on neural cue-reactivity to appetizing foods in dieters [67]. Compared to both non-dieters and dieters whose diet remained intact, those who had their diet broken showed increased cue-reactivity to appetizing foods in the NAcc (Figure 1b), echoing the behavioral findings of Herman and Mack[27]; Figure 1b). Interestingly, non-dieters showed the opposite result in which NAcc showed its largest response in the water condition, when subjects might have been hungry, but not in the milkshake condition wherein participants were satiated. Thus, exposure to relevant cues or ingestion of forbidden substances heightens subcortical activity in reward regions, thereby tipping the balance so that frontal mechanisms appear to have less power over behavior.
Self-regulation failure also occurs when frontal executive functions are compromised, such as following alcohol consumption [68] or injury (3). For instance, patients with frontal lobe damage show a preference for immediate rewards in intertemporal choice tasks [69]. Likewise transcranial magnetic stimulation to lateral prefrontal cortex increases choices of immediate over delayed rewards [70]. It is plausible that negative mood and resource depletion interfere with self-regulation because they disrupt frontal control, thereby tipping the balance. Earlier we noted that negative emotional states are associated with self-regulation failure, possibly because they interfere with higher order representations, such as those involved in self-awareness and insight. Sinha and colleagues [71] found that recall of personally distressing episodes led to decreased activity in PFC and increased activity in ventral striatal regions, supporting the idea that stress tips the balance to favor subcortical structures.
Regulation of Emotions
Paralleling studies of appetitive regulation, research on emotion regulation has converged on a top-down model whereby neural responses to emotional material in the amygdala and associated limbic regions are down-regulated by the lateral PFC [72–74]. Analogous to the cue-reactivity research outlined above, a frequent finding in studies of emotion regulation is of an inverse relationship between activity in the lateral PFC and the amygdala, a limbic structure sensitive to emotionally arousing stimuli [74–78]. For instance, Wager and colleagues [79] found that two independent pathways mediated frontal regulation of emotion, such that a frontal-striatal pathway was associated with successful regulation whereas a frontal-amygdala pathway was associated with less successful regulation. Likewise Schardt et al [80] found that increased functional coupling between lateral PFC and amygdala was associated with successful emotion regulation for those whose genotypes have been shown to be associated with hyper-responsivity to negative stimuli.
Research on patients with mood disorders has demonstrated that the reciprocal relationship between PFC and amygdala during emotion regulation breaks down in patients suffering from major depressive disorder and borderline personality disorder [75, 81–82]. Recent studies suggest that this prefrontal-amygdala circuit may be related to differences in brain structure and connectivity. For instance, in contrast to controls, participants with BPD show no coupling of metabolism between the medial PFC and the amygdala [83]. Similarly, reductions in white matter connectivity between the medial PFC and the amygdala, as measured with diffusion tensor imaging, were found for individuals high in anxiety [84]. In the non-clinical population, it has been shown that prolonged sleep deprivation also leads to increased amygdala response to aversive images [85].
Regulation of Attitudes and Prejudice
Social psychological models of person categorization suggest that stereotypes are automatically activated upon encountering outgroup members and that active inhibition is required to suppress stereotypes and thereby avoid prejudicial behavior [86–87]. Functional neuroimaging research on race perception has largely corroborated these models by showing evidence of top-down regulation of the amygdala by the lateral PFC when viewing members of a racial outgroup [88–89]. Echoing the findings on the regulation of craving and emotions outlined above, activity in the lateral PFC was found to be inversely correlated with amygdala activity to racial outgroup members (i.e. African Americans) when viewing faces [88] and when assigning a verbal label to faces [89].
Further evidence that the recruitment of lateral PFC observed in these studies reflects self-regulatory processes comes from a study by Richeson and colleagues [90] that combined functional neuroimaging with a behavioral measure of self-regulatory resource depletion. Activity in the prefrontal cortex (specifically lateral PFC and anterior cingulate cortex) when viewing black vs. white faces was correlated with the degree to which participants experienced self-regulatory resource depletion in a separate behavioral experiment in which they were required to discuss racially charged topic with a black confederate [90]. Put differently, the degree to which participants found the inter-racial interaction cognitively depleting was associated increased activity in lateral prefrontal regions when viewing black vs. white faces during fMRI. Taken together, these findings suggest that, as with emotions and drug cues, regulating attitudes towards outgroup members requires down-regulation of the amygdala by the PFC.
Prefrontal-Subcortical Balance Model of Self-Regulation
A longstanding idea in psychology is that resisting temptations reflects competition between impulses and self-control [2, 5, 40]. More recently, such dual system models have received support from imaging research, with substantial evidence of frontal-subcortical connectivity and reciprocal activity [15, 49, 60, 91–94]. Neuroscientific models of emotion regulation and self-control in drug addiction share conceptual similarities. For instance models of drug addiction posit that brain reward systems are hyper-sensitized to drug cues and become uncoupled from PFC regions involved in top-down regulation [95–96]. Likewise, neuroeconomic studies of decision making find PFC activity associated with long-term outcomes whereas subcortical activity is associated with more immediate outcomes [97]. Similarly, models of emotion regulation and stereotype suppression suggest that prefrontal regions are involved in actively regulating emotion -or prejudicial attitudes- based on the observation of an inverse relationship between PFC and activity in the amygdala [77, 88–89]. Studies of patients with anxiety and mood disorders offer similar evidence in the form of reduced functional [75] and structural [84] connectivity between the PFC and the amygdala. Similarly, alcohol consumption, which is known to disrupt self-regulation, shifts activity from PFC to subcortical limbic structures [98] whereas excessive alcohol use leads to degeneration in cortical areas important for controlling impulsivity [68] which may serve to further undermine attempts to control impulses among alcoholics. During development, when frontal executive functions are still maturing, subcortical structures may more easily tip the balance and overwhelm self-regulatory resources, thereby explaining why adolescents might be prone to heightened emotionality and risk-taking [15].
What these different models have in common is the notion that during successful self-regulation there is a balance between prefrontal regions involved in self-control and subcortical regions involved in representing reward incentives, emotions or attitudes. We propose that the precise subcortical target of top-down control is dependent on the regulatory context that individuals find themselves in, such that when a person regulates their food intake, this involves a prefrontal-striatal circuit, and when this same person later regulates their emotions, they instead invoke a prefrontal-amygdala circuit. From this perspective, the nature of self-regulation is constant across different types of regulation, despite variability in the neural regions that are being regulated (see [49]. Indeed, a recent review of self-control across six different domains found that lateral PFC is involved in exerting control regardless of the specific domain [99]. This supports our conjecture that the mechanism for self-regulation is domain general whereas the subcortical region involved varies depending on the nature of the stimulus, which may explain why the effects of resource depletion are not tied to any one self-regulatory domain.
Why Do People Fail at Self-Regulation?
Giving in to temptations can occur for a variety of reasons; for instance dieters, attempting to control their food intake may find it easy to ignore most foods, but when confronted with their favorite dessert their craving can overpower their resolve. Similarly, bad moods or competing regulatory demands can all conspire to break the hold people have over their impulses and desires. From the perspective of the prefrontal-subcortical balance model outlined above, anything that tips the balance in favor of subcortical regions can lead to self-regulatory collapse. This can occur “bottom-up” when people are confronted with especially potent cues, such as a favorite food, a free drink, or a strong emotion, and also “top-down”, such as when prefrontal functioning is impaired either when self-regulatory resources are depleted, or due to drugs, alcohol or brain damage (e.g. [3]). Successful self-regulation, therefore, requires that current self-regulatory ability withstands the strength of an impulse. To this point researchers have generally neglected to consider the situational factors that influence the balance between activity in subcortical regions and the PFC in self-regulation failure (see Box 3). Our review suggests that some classic self-regulatory failures occur because of their influence on reward (i.e., cue reactivity and lapse activated consumption) whereas others occur due to their influence on PFC (i.e., negative moods, self-regulatory depletion, physiological disruption or damage of PFC).
BOX 3. Outstanding Questions.
Are individual differences in susceptibility to self-regulatory failure related to prefrontal-subcortical connectivity or the integrity of frontal circuitry?
Can direct measurements of brain glucose levels with FDG PET be used to test the glucose model of resource depletion?
Does self-regulatory training alter brain connectivity and morphometry and do these changes predict greater self-regulatory success?
Are patients with prefrontal damage, or adults with age-related cognitive decline, more susceptible to external cues such as appetizing foods or the sight and smell of cigarettes?
Does the frontal-subcortical reciprocal relation change during childhood development or during aging or as a function of substance use?
We also note that self-regulatory failure depends on individualized. That is, the particular domain a person tried to control is that which is most prone to self-regulation failure. For example, self-regulatory resource depletion might lead an abstinent smoker to turn to cigarettes, a dieter to high-calorie foods or a prejudiced individual to make bigoted remarks; though the outcome is different in each case and even the underlying subcortical regions involved may differ (i.e. striatum or amygdala), the overall process is likely the same.
Concluding Remarks
In this review we highlighted a number of threats to self-regulation: from negative mood and potent appetitive cues to lapse activated consumption and self-regulatory resource depletion. Neuroimaging research on self-regulatory failure is still in its infancy. Recently, a small number of studies of drug addicts, patients and healthy individuals have shed light on the neural mechanisms underlying self-regulatory failure. This research corroborates theoretical models of self-control in which the prefrontal cortex is involved in actively regulating subcortical responses to emotions and appetitive cues. This prefrontal-subcortical balance model emphasizes that self-regulatory collapse can occur both due to insufficient top-down control as well as from overwhelming bottom-up impulses.
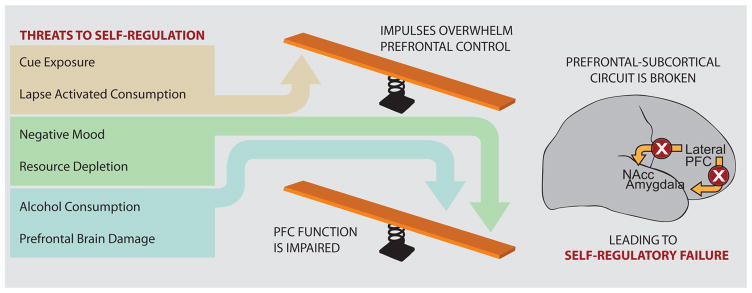
Figure 2.
Schematic of a balance model of self-regulation and its failure highlighting the four threats to self-regulation identified in the text and their putative impact on brain areas involved in self-regulation. This model suggests that self-regulatory failure occurs whenever the balance is tipped in favor of subcortical regions involved in reward and emotion, either due to the strength of an impulse or due to a failure to appropriately engage top-down control mechanisms.
Acknowledgments
We thank Bill Kelley and Paul Whalen for helpful discussions in developing this model. This work was supported by NIH grant R01DA022582.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baumeister RF, et al. Losing control : how and why people fail at self-regulation. Academic Press; 1994. [Google Scholar]
- 2.Hofmann W, et al. Impulse and Self-Control From a Dual-Systems Perspective. Perspect Psychol Sci. 2009;4:162–176. doi: 10.1111/j.1745-6924.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- 3.Wagner DD, Heatherton TF. Giving in to temptation: The emerging cognitive neuroscience of self-regulatory failure. In: Vohs KD, Baumeister RF, editors. Handbook of self-regulation : research, theory, and applications. 2. Guilford Press; 2010. [Google Scholar]
- 4.Heatherton TF. Self and Identity: Neuroscience of Self and Self-Regulation. Annu Rev Psychol. 2011;62:363–390. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumeister RF, Heatherton TF. Self-Regulation Failure: An Overview. Psychol Inq. 1996;7:1–15. [Google Scholar]
- 6.Schroeder SA. We Can Do Better—Improving the Health of the American People. New Eng J Med. 2007;357:1221–1228. doi: 10.1056/NEJMsa073350. [DOI] [PubMed] [Google Scholar]
- 7.Tangney JP, et al. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J Pers. 2004;72:271–324. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- 8.Duckworth AL, Seligman ME. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychol Sci. 2005;16:939–944. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- 9.Quinn PD, Fromme K. Self-regulation as a protective factor against risky drinking and sexual behavior. Psychol Addict Behav. 2010;24:376–385. doi: 10.1037/a0018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagger MS, et al. Ego depletion and the strength model of self-control: a meta-analysis. Psychol Bull. 2010;136:495–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- 11.Marlatt GA, Gordon JR. Relapse prevention : maintenance strategies in the treatment of addictive behaviors. Guilford Press; 1985. [Google Scholar]
- 12.Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27–51. doi: 10.1146/annurev.psych.53.100901.135231. [DOI] [PubMed] [Google Scholar]
- 14.Bruyneel SD, et al. I felt low and my purse feels light: depleting mood regulation attempts affect risk decision making. J Behav Decis Making. 2009;22:153–170. [Google Scholar]
- 15.Somerville LH, et al. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bousman CA, et al. Negative mood and sexual behavior among non-monogamous men who have sex with men in the context of methamphetamine and HIV. J Affect Disord. 2009;119:84–91. doi: 10.1016/j.jad.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magid V, et al. Negative affect, stress, and smoking in college students: unique associations independent of alcohol and marijuana use. Addict Behav. 2009;34:973–975. doi: 10.1016/j.addbeh.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- 19.Witkiewitz K, Villarroel NA. Dynamic association between negative affect and alcohol lapses following alcohol treatment. J Consult Clin Psychol. 2009;77:633–644. doi: 10.1037/a0015647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heatherton TF, et al. Effects of physical threat and ego threat on eating behavior. J Pers Soc Psychol. 1991;60:138–143. doi: 10.1037//0022-3514.60.1.138. [DOI] [PubMed] [Google Scholar]
- 21.Macht M. How emotions affect eating: a five-way model. Appetite. 2008;50:1–11. doi: 10.1016/j.appet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 22.McKee S, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2010 doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heatherton TF, Baumeister RF. Binge eating as escape from self-awareness. Psychol Bull. 1991;110:86–108. doi: 10.1037/0033-2909.110.1.86. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein RZ, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward A, Mann T. Don’t mind if I do: disinhibited eating under cognitive load. J Pers Soc Psychol. 2000;78:753–763. doi: 10.1037//0022-3514.78.4.753. [DOI] [PubMed] [Google Scholar]
- 26.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann NY Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman CP, Mack D. Restrained and unrestrained eating. J Pers. 1975;43:647–660. doi: 10.1111/j.1467-6494.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 28.Herman CP, Polivy J. The Self-Regulation of Eating: Theoretical and Practical Problems. In: Vohs KD, Baumeister RF, editors. Handbook of self-regulation : research, theory, and applications. 2. Guilford Press; 2010. [Google Scholar]
- 29.Marlatt GA, et al. Relapse Prevention: Evidence Base and Future Directions. In: Miller PM, editor. Evidence-based addiction treatment. 1. xviii. Elsevier/Academic Press; 2009. p. 465. [Google Scholar]
- 30.Drummond DC, et al. Conditioned learning in alcohol dependence: implications for cue exposure treatment. Br J Addict. 1990;85:725–743. doi: 10.1111/j.1360-0443.1990.tb01685.x. [DOI] [PubMed] [Google Scholar]
- 31.Glautier S, Drummond DC. Alcohol dependence and cue reactivity. J Stud Alcohol. 1994;55:224–229. doi: 10.15288/jsa.1994.55.224. [DOI] [PubMed] [Google Scholar]
- 32.Jansen A. A learning model of binge eating: cue reactivity and cue exposure. Behav Res Ther. 1998;36:257–272. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 33.Stewart J, et al. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- 34.Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. J Abnorm Psychol. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- 35.Payne TJ, et al. Pretreatment cue reactivity predicts end-of-treatment smoking. Addict Behav. 2006;31:702–710. doi: 10.1016/j.addbeh.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson MJ, Bargh JA. How social perception can automatically influence behavior. Trends Cogn Sci. 2004;8:33–39. doi: 10.1016/j.tics.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Stacy AW, Wiers RW. Implicit cognition and addiction: a tool for explaining paradoxical behavior. Annu Rev Clin Psychol. 2010;6:551–575. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bargh JA, Morsella E. The Unconscious Mind. Perspect Psychol Sci. 2008;3:73–79. doi: 10.1111/j.1745-6916.2008.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rooke SE, et al. Implicit cognition and substance use: a meta-analysis. Addict Behav. 2008;33:1314–1328. doi: 10.1016/j.addbeh.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychol Rev. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- 41.Mischel W, et al. “Willpower” over the life span: Mechanisms, consequences, and implications. Soc Cogn Affect Neurosci. 2010 doi: 10.1093/scan/nsq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- 43.Vohs KD, Heatherton TF. Self-regulatory failure: a resource-depletion approach. Psychol Sci. 2000;11:249–254. doi: 10.1111/1467-9280.00250. [DOI] [PubMed] [Google Scholar]
- 44.Muraven M, et al. Self-control and alcohol restraint: an initial application of the self-control strength model. Psychol Addict Behav. 2002;16:113–120. doi: 10.1037//0893-164x.16.2.113. [DOI] [PubMed] [Google Scholar]
- 45.Vohs KD, et al. Self-regulation and self-presentation: regulatory resource depletion impairs impression management and effortful self-presentation depletes regulatory resources. J Pers Soc Psychol. 2005;88:632–657. doi: 10.1037/0022-3514.88.4.632. [DOI] [PubMed] [Google Scholar]
- 46.Richeson JA, Shelton JN. When prejudice does not pay: effects of interracial contact on executive function. Psychol Sci. 2003;14:287–290. doi: 10.1111/1467-9280.03437. [DOI] [PubMed] [Google Scholar]
- 47.Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 49.Volkow ND, et al. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Doherty JP, et al. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 51.Garavan H, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 52.Grant S, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myrick H, et al. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diekhof EK, Gruber O. When desires collide with reason: Functional interactions between anteroventral prefrontal cortex and nucleus accumbens underlie the human ability to resist impulsive desires. J Neurosci. 2010;30:1488–1493. doi: 10.1523/JNEUROSCI.4690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McClure SM, et al. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 57.Pine A, et al. Dopamine, time, and impulsivity in humans. J Neurosci. 2010;30:8888–96. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Childress AR, et al. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS One. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wagner DD, et al. Spontaneous Action Representation in Smokers Watching Movie Smoking. J Neurosci. doi: 10.1523/JNEUROSCI.5174-10.2010. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Volkow ND, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kober H, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brody AL, et al. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delgado MR, et al. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008;11:880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berkman ET, et al. In the trenches of real-world self-control: Neural correlates of breaking the link between craving and smoking. Psychol Sci. doi: 10.1177/0956797611400918. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heatherton TF, et al. Effects of distress on eating: the importance of ego-involvement. J Pers Soc Psychol. 1992;62:801–803. [PubMed] [Google Scholar]
- 66.Heatherton TF, et al. Self-awareness, task failure, and disinhibition: how attentional focus affects eating. J Pers. 1993;61:49–61. doi: 10.1111/j.1467-6494.1993.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 67.Demos KE, et al. Dietary Restraint Violations Influence Reward Responses in Nucleus Accumbens and Amygdala. J Cogn Neurosci. 2011 doi: 10.1162/jocn.2010.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sellitto M, Ciaramelli E, de Pellegrino G. Myopic discounting of future rewards after medial orbitofrontal damage in humans. J Neurosci. 2010;30:6429–36. doi: 10.1523/JNEUROSCI.2516-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, Weber EU. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010;13:538–39. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- 71.Sinha R, et al. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology. 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- 72.Davidson RJ, et al. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 73.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 74.Hariri AR, et al. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- 75.Johnstone T, et al. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ochsner KN, et al. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 77.Ochsner KN, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 78.Urry HL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wager TD, et al. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schardt DM, et al. Volition diminishes genetically mediated amygdala hyperreactivity. Neuroimage. 2010;53:943–951. doi: 10.1016/j.neuroimage.2009.11.078. [DOI] [PubMed] [Google Scholar]
- 81.Donegan NH, et al. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- 82.Silbersweig D, et al. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. Am J Psychiatry. 2007;164:1832–1841. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- 83.New AS, et al. Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology. 2007;32:1629–1640. doi: 10.1038/sj.npp.1301283. [DOI] [PubMed] [Google Scholar]
- 84.Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoo SS, et al. The human emotional brain without sleep--a prefrontal amygdala disconnect. Curr Biol. 2007;17:R877–878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 86.Devine PG. Stereotypes and Prejudice - Their Automatic and Controlled Components. J Pers Soc Psychol. 1989;56:5–18. [Google Scholar]
- 87.Fiske ST. Stereotyping, prejudice, and discrimination. The handbook of social psychology. 1998;2:357–411. [Google Scholar]
- 88.Cunningham WA, et al. Separable neural components in the processing of black and white faces. Psych Sci. 2004;15:806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- 89.Lieberman MD, et al. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nat Neurosci. 2005;8:720–722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- 90.Richeson JA, et al. An fMRI investigation of the impact of interracial contact on executive function. Nat Neurosci. 2003;6:1323–1328. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- 91.Banks SJ, et al. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Batterink L, et al. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52:1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.MacDonald KB. Effortful control, explicit processing, and the regulation of human evolved predispositions. Psychol Rev. 2008;115:1012–1031. doi: 10.1037/a0013327. [DOI] [PubMed] [Google Scholar]
- 95.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 96.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 97.Heuttel SA. Ten challenges for decision neuroscience. Front Neurosci. 2010;4:1– 7. doi: 10.3389/fnins.2010.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Volkow ND, et al. Moderate doses of alcohol disrupt the functional organization of the human brain. Psychiatry Res. 2008;162:205–213. doi: 10.1016/j.pscychresns.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cohen JR, Lieberman MD. The Common Neural Basis of Exerting Self-Control in Multiple Domains. Self Control in Society, Mind, and Brain. 2010:141–162. [Google Scholar]
- 100.Muraven M, et al. Longitudinal improvement of self-regulation through practice: building self-control strength through repeated exercise. J Soc Psychol. 1999;139:446–457. doi: 10.1080/00224549909598404. [DOI] [PubMed] [Google Scholar]
- 101.Gailliot MT, et al. Increasing self-regulatory strength can reduce the depleting effect of suppressing stereotypes. Pers Soc Psychol Bull. 2007;33:281–294. doi: 10.1177/0146167206296101. [DOI] [PubMed] [Google Scholar]
- 102.Muraven M. Practicing self-control lowers the risk of smoking lapse. Psychol Addict Behav. 2010;24:446–452. doi: 10.1037/a0018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bermudez P, et al. Neuroanatomical correlates of musicianship as revealed by cortical thickness and voxel-based morphometry. Cereb Cortex. 2009;19:1583–1596. doi: 10.1093/cercor/bhn196. [DOI] [PubMed] [Google Scholar]
- 104.Gailliot MT, Baumeister RF. The physiology of willpower: linking blood glucose to self-control. Pers Soc Psychol Rev. 2007;11:303–327. doi: 10.1177/1088868307303030. [DOI] [PubMed] [Google Scholar]
- 105.Gailliot MT, et al. Self-control relies on glucose as a limited energy source: willpower is more than a metaphor. J Pers Soc Psychol. 2007;92:325–336. doi: 10.1037/0022-3514.92.2.325. [DOI] [PubMed] [Google Scholar]
- 106.Gailliot MT, et al. Stereotypes and prejudice in the blood: Sucrose drinks reduce prejudice and stereotyping. J Exp Soc Psychol. 2009;45:288–290. [Google Scholar]
- 107.Benton D, et al. Blood glucose influences memory and attention in young adults. Neuropsychologia. 1994;32:595–607. doi: 10.1016/0028-3932(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 108.Jonides J, et al. Verbal Working Memory Load Affects Regional Brain Activation as Measured by PET. J Cogn Neurosci. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]