Abstract
We hypothesized that reduced respiratory neural activity elicits compensatory mechanisms of plasticity that enhance respiratory motor output. In urethane-anesthetized and ventilated rats, we reversibly reduced respiratory neural activity for 25–30 min using: hypocapnia (end tidal CO2 = 30 mmHg), isoflurane (~ 1%) or high frequency ventilation (HFV; ~100 breaths/min). In all cases, increased phrenic burst amplitude was observed following restoration of respiratory neural activity (hypocapnia: 92 ± 22%; isoflurane: 65 ± 22%; HFV: 54 ± 13% baseline), which was significantly greater than time controls receiving the same surgery, but no interruptions in respiratory neural activity (3 ± 5% baseline, p<0.05). Hypocapnia also elicited transient increases in respiratory burst frequency (9 ± 2 versus 1 ± 1 bursts/min, p<0.05). Our results suggest that reduced respiratory neural activity elicits a unique form of plasticity in respiratory motor control which we refer to as inactivity-induced phrenic motor facilitation (iPMF). iPMF may prevent catastrophic decreases in respiratory motor output during ventilatory control disorders associated with abnormal respiratory activity.
Keywords: hypocapnia, isoflurane, activity deprivation, inactivity, respiratory control, phrenic motor facilitation, homeostatic plasticity, control of breathing, phrenic plasticity, reduced neural activity
1. INTRODUCTION
The respiratory control system must produce a regular, rhythmic motor output from birth until death, with only the briefest of pauses. In addition, this motor output must be precisely regulated in order to enable optimal gas exchange, yet maintain sufficient dynamic range to respond to respiratory and metabolic challenges. However, little is known about the neural mechanisms that ensure respiratory neurons continue to function adequately despite changes in neuronal excitability or synaptic inputs that occur throughout life.
Rodent models of spinal injury suggest that reduced respiratory-related inputs elicit compensatory plasticity in the phrenic motor system. For example, while the immediate response to a C2 hemisection is a complete silencing of ipsilateral phrenic motor output as bulbospinal inputs are severed, over time the ipsilateral phrenic nerve will develop modest respiratory-related neural output (Alilain and Goshgarian, 2008; Fuller et al., 2006, 2008, 2009; Golder et al., 2003; Golder and Mitchell, 2005; Nantwi et al., 1999; Vinit et al., 2007), leading to the suggestion that endogenous mechanisms of compensatory plasticity restored partial function to the previously silent phrenic motor pool (Fuller et al., 2006; Goshgarian, 2009; Lane et al., 2009; Mantilla and Sieck, 2009; Mitchell, 2007). Although neurophysiological evidence of this spontaneous partial phrenic recovery typically takes weeks to months following injury to observe, morphological plasticity within the phrenic motor pool can be observed within minutes to hours following injury (Goshgarian et al., 1989; Hadley et al., 1999; Sperry and Goshgarian, 1993). These morphological changes are likely due to disruption of descending respiratory drive to the phrenic motor pool, rather than the injury itself, since reversible C2 axon conduction block induces similar morphological changes in the phrenic motor pool within 4 hours (Castro-Moure and Goshgarian, 1996, 1997). These morphological changes are accompanied by a functional enhancement of diaphragm EMG activity following restoration of spinal axon conduction (Castro-Moure and Goshgarian, 1996), although it is unknown if enhanced diaphragm activity is due to increased phrenic neural output or increased response at the neuromuscular junction. Nevertheless, these data suggest a robust and rapid response to removal of descending respiratory-related inputs to the phrenic motor pool.
Here, we tested the hypothesis that reduced respiratory-related neural activity elicits a rapid and robust form of plasticity in phrenic motor output. We chose three different methods with different mechanisms of action to induce a reversible, global reduction in respiratory-related activity in separate groups of mechanically ventilated, urethane-anesthetized rats: 1) hyperventilation to lower arterial PCO2 below the apneic threshold (hypocapnia), 2) delivery of a volatile anesthetic to depress respiratory drive (isoflurane) and 3) isocapnic high frequency ventilation (HFV) to induce a vagal-reflex inhibition of breathing. Following 25–30 min of reduced respiratory neural activity, baseline conditions were restored, and changes in phrenic burst amplitude and frequency were measured. We demonstrate for the first time that reduced respiratory neural activity elicits a prolonged phrenic motor facilitation (inactivity-induced PMF; iPMF). An understanding of these novel mechanisms may yield insights concerning ventilatory control disorders associated with abnormal respiratory motor activity.
2. METHODS
2.1 Animals
All experiments were performed on male Sprague-Dawley rats (Harlan colony 217). All protocols conformed to the requirements of the Institutional Animal Care and Use Committee at the University of Wisconsin, Madison.
2.2 Surgical Preparation
Rats were induced with isoflurane (2.5–3.5% in 50% O2), tracheotomized and pump ventilated (Harvard Apparatus, Rodent Ventilator 683). Rats exposed to hypocapnia or isoflurane were subjected to bilateral vagotomy, whereas the vagi were left intact in rats experiencing high frequency ventilation. The tail vein and femoral artery were catheterized for fluid infusion and blood sampling, respectively. A dorsal approach to the left phrenic nerve was used; the nerve was cut distally, desheathed and submersed in mineral oil. All rats were then converted slowly to urethane anesthesia (1.6–1.8 g/kg, i.v.) over 15–25 minutes, and then paralyzed with pancuronium bromide (2.5 g/kg, i.v.). A 1.5–2 mL/h fluid infusion of 1:1 lactated ringers and hetastarch (Hespan, 6% hetastarch in 0.9% sodium chloride) solution was then started, and continued through the experiment. Inspired O2 was 50% for all experiments.
2.3 Nerve Recordings
The phrenic nerve was placed on a bipolar silver electrode. Phrenic activity was amplified (10kX), band-pass filtered (0.1–5kHz) and integrated (time constant: 50 ms; CWE, MA-821RSP). Raw and integrated traces were passed to a data acquisition system (WinDAQ, DATAQ Instruments or PowerLab, AD Instruments) for digitization.
2.4 Physiological Measurements
End tidal CO2 was monitored from the expired limb of the ventilator (Novametrix), and used as an index of arterial PCO2 to enable maintenance of isocapnia. These values were confirmed by blood gas analysis (Radiometer Copenhagen, ABL 500) of 0.2 mL arterial blood samples at key points in the protocol. Blood pressure was monitored and used as an indicator of stable conditions and depth of anesthesia by pressor responses to paw pad pinch.
2.5 Protocols
Baseline
Approximately 1h after conversion to urethane anesthesia, protocols began by determining the apneic and recruitment CO2 thresholds for phrenic activity. The apneic threshold was determined by monitoring end tidal CO2 and slowly lowering inspired CO2 and/or increasing ventilator rate until rhythmic activity ceased. The recruitment threshold was determined by slowly raising end tidal CO2 and/or lowering ventilator rate and noting the value at which phrenic activity resumed. End tidal CO2 was raised a further 1–3 mmHg to establish baseline conditions. After nerve signals were steady for >15 minutes (typically 25–40 minutes after threshold determination), an arterial blood sample was drawn to establish baseline arterial PCO2.
Experimental groups
Six experimental groups were studied. Three groups experienced reduced respiratory neural activity (see below) with: 1) hypocapnia (n = 11); 2) isoflurane (n = 7) or 3) isocapnic high frequency ventilation (HFV; n = 11). A separate group of HFV rats were vagotomized to control for non-vagally mediated changes in phrenic output. Results were compared to “time controls” that received similar surgery and experimental duration, but no neural activity deprivation to control for any time dependent changes in phrenic motor output. One group of time controls were vagotomized (n = 10), while a separate group were vagi-intact (n = 6).
Hypocapnia
Following establishment of baseline, reduced respiratory neural activity was induced by decreasing inspired CO2 and/or increasing ventilator rate until end tidal CO2 was 5–10 mmHg below the apneic threshold. Hypocapnia-induced reduced respiratory neural activity was maintained for 25 minutes, and then phrenic neural activity was resumed by returning arterial PCO2 to baseline values.
Isoflurane
Following establishment of baseline in a separate group of rats, isoflurane was slowly delivered through the inspired gas mixture until respiratory-related neural activity was reduced (final isoflurane ~1%). Isocapnia was maintained by increasing inspired CO2 and/or decreasing ventilator rate; arterial blood samples confirmed that CO2 remained within ~2mmHg of baseline. Following 30 min of reduced respiratory neural activity, baseline conditions were restored by removing isoflurane from the inspired gas line, while reducing inspired CO2 and/or increasing ventilator rate to maintain isocapnia.
High Frequency Ventilation (HFV)
In a subgroup of rats the vagi were left intact and allowed to entrain to the ventilator, which was set at 50 breaths/min with a constant tidal volume (2.5mL). Following establishment of baseline, ventilator frequency was increased (~ 100 breaths/min) to elicit a reflex inhibition of respiratory neural activity. Isocapnia was maintained by increasing inspired CO2 to maintain end-tidal PCO2 at its baseline value; arterial blood samples confirmed that CO2 remained within ~2mmHg of baseline. Following 25 min of reduced respiratory neural activity, baseline conditions were restored by returning ventilator frequency to 50 breaths/min, while reducing inspired CO2 to maintain isocapnia. A separate group of rats were vagotomized prior to HFV to control for non-vagally mediated effects of high ventilation frequencies on phrenic motor output.
Post-stimulus monitoring
Arterial blood samples were analyzed at 15, 30 and 60 minutes after each treatment protocol to ensure adequate maintenance of baseline arterial PCO2, PO2, SBE and pH. At the end of each protocol, a maximal CO2 response (90<PETCO2 <100) was assessed to ensure that observed responses were not influenced by deterioration of the preparation. To be included in the analysis, rats had to meet the following criteria: arterial PCO2 maintained within 1.5 mmHg of baseline, arterial PO2 > 180 mmHg and base excess within 3 mEq/L of baseline values following restoration of respiratory neural activity.
2.6 Statistical Analysis
Integrated phrenic burst amplitude and frequency were analyzed in 30–60 sec bins before (baseline), and 15, 30 and 60 min following reduced respiratory neural activity or an equivalent duration in time controls. Phrenic burst amplitude was expressed as percent change from baseline, which was set at 0%. There were no differences in phrenic burst amplitude at any time point between the two sets of time controls; thus, burst amplitude data from these groups were combined. Phrenic burst frequency (vagotomized rats only) was expressed as an absolute change from baseline. A two-way repeated measures ANOVA was used to detect significant differences (Prism 5, GraphPad Software). Specific group differences were then determined by Bonferroni post-hoc tests at a significance level of 0.05. All data are presented as means ± SEM.
3. RESULTS
3.1 Hypocapnia-induced respiratory neural activity deprivation
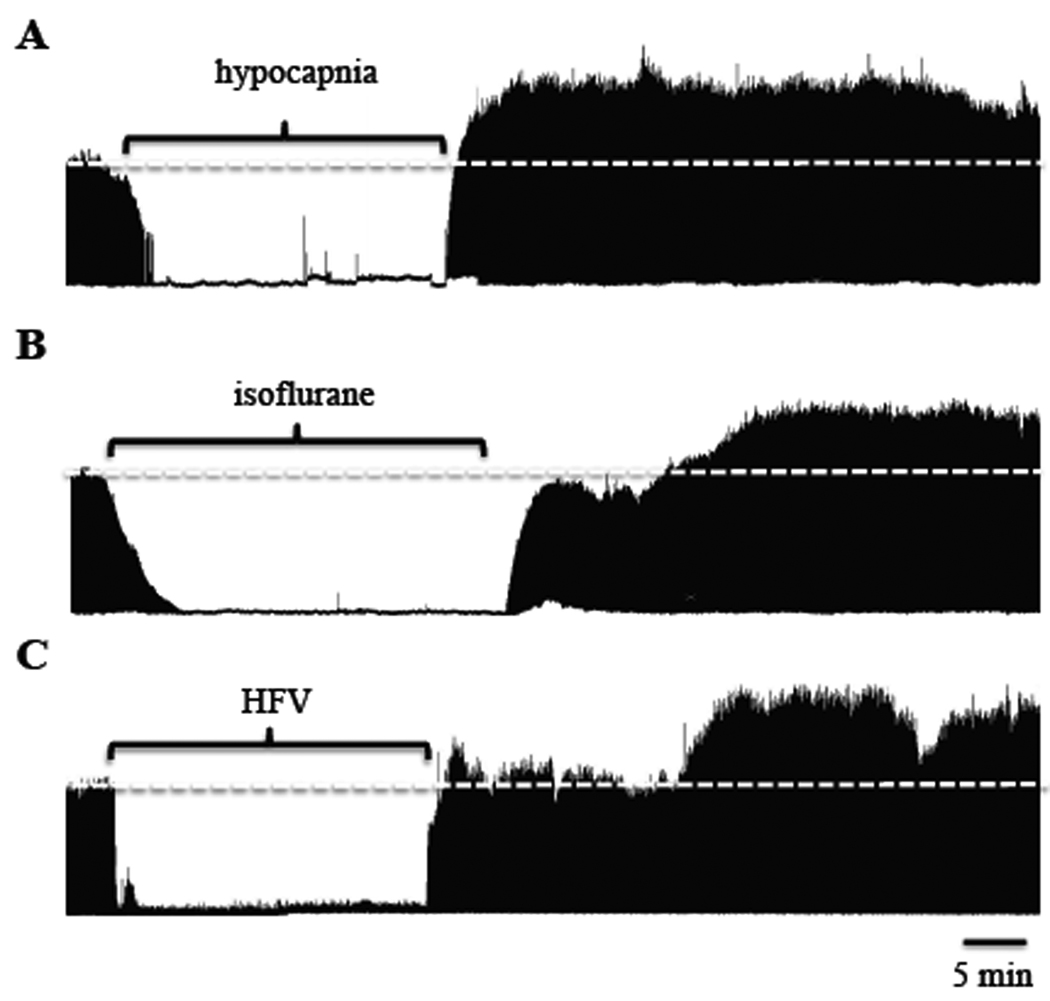
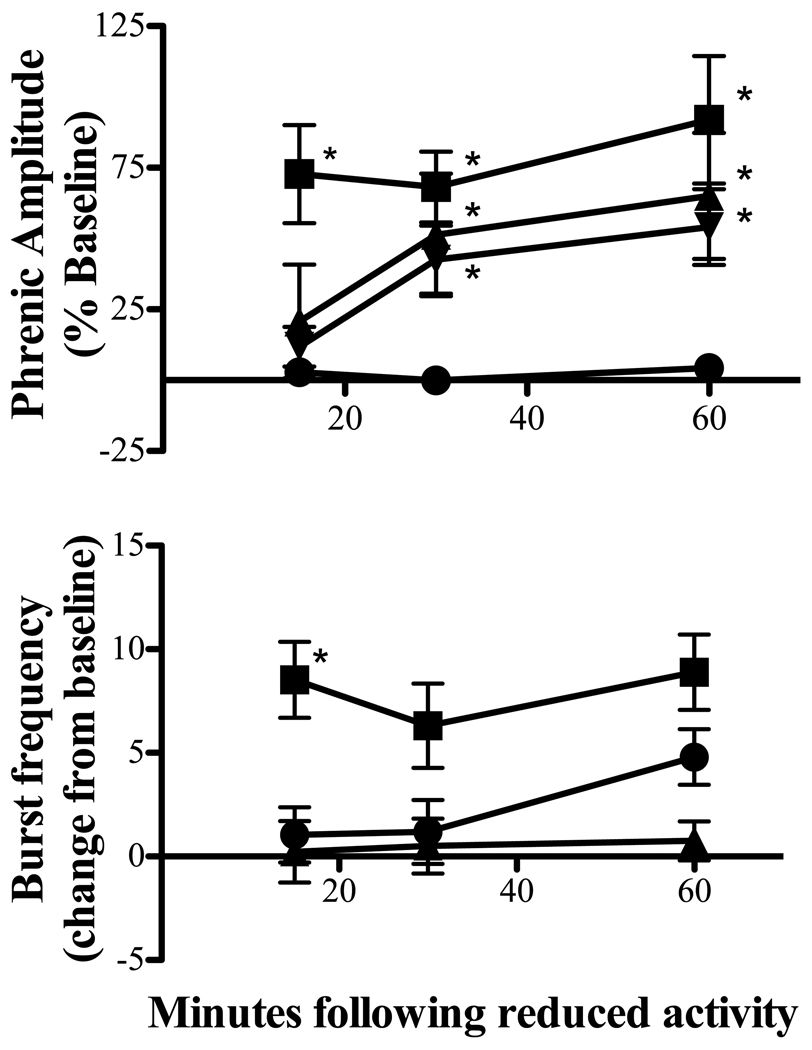
To reversibly reduce respiratory neural activity, rats were hyperventilated to lower arterial PCO2 below the apneic threshold. Baseline end-tidal CO2 was 42.8 ± 1 mmHg, whereas average end-tidal CO2 during hypocapnia was 30 ± 1 mmHg. Figure 1a depicts a representative compressed phrenic neurogram, illustrating baseline phrenic nerve activity, reduced phrenic activity during hypocapnia and restoration of phrenic bursting following resumption of isocapnia. Figure 2a depicts the average change in phrenic burst amplitude from baseline for one hour following resumption of baseline arterial PCO2. In all rats, no respiratory-related phrenic activity was detected during hypocapnia. 15 min following restoration of respiratory neural activity, phrenic burst amplitude was significantly elevated from baseline (73 ± 17% baseline; p<0.005), which persisted for at least 60 min (92 ± 22% baseline; p<0.005), indicating iPMF. Time control rats that remained isocapnic (i.e., no respiratory neural activity deprivation) showed no significant changes in phrenic amplitude relative to baseline at the 15 (0 ± 3% baseline), 30 (−1 ± 5% baseline) or 60 minute time points (3 ± 5% baseline, all p>0.05). At equivalent time points following restoration of respiratory neural activity, hypocapnia-treated rats had significantly higher phrenic burst amplitudes versus time controls (all p<0.001).
Figure 1.
Representative integrated phrenic neurograms before, during and 60 min following respiratory neural activity deprivation with 30 min of hypocapnia (A), 30 min of 0.5% isoflurane (B) or 25 min of high frequency ventilation (HFV; C). The white dashed line represents baseline burst amplitude. In all cases, phrenic burst amplitude was increased above baseline within 30 min of returning to respiratory neural activity. Note that in example B, phrenic burst activity did not completely go to zero during isoflurane treatment, suggesting that complete phrenic inactivity is not required for iPMF expression.
Figure 2.
Average iPMF and burst frequency facilitation following reduced respiratory neural activity with hypocapnia (■), isoflurane (▼) or high frequency ventilation (HFV; ▲). Results are compared to time controls not receiving neural activity deprivation (●). * indicates significant increase over time controls (p<0.05).
Burst frequency was increased above baseline at 15, 30 and 60 min following reduced respiratory neural activity with hypocapnia (9 ± 2, 6 ± 2, 9 ± 2 bursts/min, respectively; all p<0.02; figure 2b). Burst frequency was also increased in time control rats at a time point equivalent to 60 min (5 ± 1 bursts/min; p<0.01), but not at 15 or 30 min (1 ± 1, 1 ± 2 bursts/min, respectively; both p>0.4; figure 2b). The change in burst frequency following hypocapnia differed from time controls at 15 (p<0.05), but not 30 or 60 min (p>0.05) following resumption of respiratory neural activity, suggesting that frequency facilitation is only transiently expressed following reduced respiratory neural activity with hypocapnia.
3.2 Isoflurane-induced respiratory neural activity deprivation
The volatile anesthetic, isoflurane, is a profound respiratory depressant (Imai et al., 1999) that has a rapid induction and recovery (Wade and Stevens, 1981; Stachnik, 2006). We took advantage of these properties of isoflurane to induce a reversible reduction in phrenic neural activity, without changes in arterial PCO2. Following establishment of baseline, isoflurane was slowly added to the inspired gas line. In 4/7 rats, respiratory-related phrenic burst activity was eliminated during isoflurane exposure; in the remaining rats, phrenic output was reduced to −73 ± 11% below baseline. After 30 min, isoflurane was removed from the inspired line. Throughout the protocol, end-tidal CO2 was monitored and adjusted to maintain isocapnia, which was confirmed by periodic blood gas analysis. Figure 1b depicts a representative compressed phrenic neurogram, illustrating baseline phrenic nerve activity, reduced phrenic activity during isoflurane and increased phrenic bursting following isoflurane wash-out. Figure 2a depicts the average change in phrenic burst amplitude from baseline for one hour following removal of isoflurane. Phrenic burst amplitude was significantly increased over baseline at 30 and 60 min following recovery of respiratory activity (51 ± 22% and 65 ± 22% baseline, respectively, p<0.05), but not at 15 min (21 ± 20% baseline, p>0.3). Similarly, phrenic burst amplitude at 30 and 60 min following restoration of respiratory neural activity was significantly greater relative to time controls (p<0.05), indicating iPMF.
Phrenic burst frequency was not significantly different from baseline or time controls at any time point following isoflurane (p>0.05; figure 2b), suggesting that frequency facilitation is not apparent in rats following isoflurane-induced reductions in respiratory neural activity.
3.3 Respiratory neural activity deprivation with isocapnic HFV
In a subgroup of rats, we took advantage of vagal reflex inhibition of breathing to induce a reversible reduction in respiratory neural activity. After establishment of baseline, vagi-intact rats were ventilated at high frequencies (>100 breaths/min), while slowly adding CO2 to the inspired line to maintain isocapnia. A separate group of rats were vagotomized prior to HFV to control for non-specific effects of HFV on phrenic motor output. Measurements of end tidal and arterial CO2 levels were used to confirm isocapnia. At the end of the stimulation period, baseline ventilation rate was restored, while maintaining isocapnia. Vagi-intact rats generally exhibited no respiratory-related phrenic activity during HFV, although occasionally short episodes (<2 min) of bursting were detected; when this occurred, ventilator frequency was increased until bursting stopped. Figure 1c depicts a representative compressed phrenic neurogram, illustrating baseline phrenic nerve activity, reduced phrenic activity during HFV and increased phrenic bursting following resumption of baseline ventilation frequency in vagi-intact rats. Figure 2a depicts the average change in phrenic burst amplitude from baseline for one hour following isocapnic HFV in vagi-intact rats. Phrenic burst amplitude was significantly increased from baseline at 30 and 60 min following restoration of respiratory neural activity (43 ± 12%, 54 ± 13% baseline, respectively; both p<0.01), but not at 15 minutes (12 ± 7, p > 0.1). Similarly, the change in phrenic burst amplitude was significantly increased relative to time controls at 30 and 60 min (p<0.05) following HFV in vagi-intact rats, indicating iPMF. As expected, intact vagi were necessary for this effect, since vagotomized rats exposed to isocapnic HFV did not exhibit reduced respiratory neural activity or subsequent iPMF (60 min post-HFV, 13 ± 4% baseline; p > 0.05; data not shown). Since phrenic burst frequency entrains to the ventilator in vagi-intact rats, analysis of burst frequency changes was not relevant in these groups of rats.
3.4 Blood gases and mean arterial pressure
Table 1 lists the average rat body temperature, arterial PO2, arterial PCO2, mean arterial blood pressure, apneic threshold and recruitment threshold in all rat groups. Mean arterial pressure was significantly lower than baseline at 60 min following isoflurane; however, since the change in average mean arterial pressure was <25 mmHg and arterial pressure remained >100 mmHg, we do not consider this difference to be biologically significant. No other time dependent changes were observed within any other group, indicating that the observed iPMF was not due to unintended changes in blood gases or blood pressure.
Table 1.
Average rat body temperature, arterial PO2, arterial PCO2, mean arterial blood pressure (MAP), apneic threshold and recruitment threshold before and 60 min following reduced respiratory neural activity with hypocapnia, isoflurane or high frequency ventilation (HFV). Values are also included for an equivalent duration in control rats not receiving reduced respiratory neural activity (time controls and vagotomized rats receiving HFV). MAP was significantly lower than baseline at 60 min following isoflurane. No other time-dependent changes were observed in any group.
| Temp | PaO2 | PaCO2 | MAP | Apneic threshold |
Recruitment threshold |
||
|---|---|---|---|---|---|---|---|
| hypocapnia | baseline | 36.6 ± 0.1 | 272 ± 4 | 43.0 ± 0.7 | 126 ± 2 | 37.9 ± 1.0 | 41.6 ± 0.8 |
| 60 min | 36.7 ± 0.1 | 266 ± 5 | 43.2 ± 0.7 | 122 ± 3 | |||
| isoflurane | baseline | 37.2 ± 0.1 | 274 ± 7 | 47.1 ± 1.7 | 127 ± 4 | 36.7 ± 2.1 | 45.2 ± 1.4 |
| 60 min | 37.2 ± 0.1 | 270 ± 5 | 47.7 ± 1.7 | 106 ± 7* | |||
| HFV | baseline | 37.2 ± 0.1 | 234 ± 6 | 48.6 ± 0.7 | 120 ± 3 | 44.3 ± 0.5 | 46.6 ± 0.5 |
| 60 min | 37.3 ± 0.1 | 238 ± 5 | 48.3 ± 0.6 | 124 ± 5 | |||
| HFV vagotomized | baseline | 37.3 ± 0.2 | 229 ± 5 | 45.6 ± 0.8 | 114 ± 4 | 39.7 ± 0.4 | 42.4 ± 0.4 |
| 60 min | 37.4 ± 0.2 | 238 ± 5 | 45.2 ± 0.6 | 108 ± 2 | |||
| Time control | baseline | 37.0 ± 0.2 | 249 ± 8 | 44.8 ± 1.4 | 126 ± 4 | 41.2 ± 1.3 | 44.0 ± 1.2 |
| 60 min | 36.9 ± 0.2 | 249 ± 6 | 44.6 ± 1.3 | 126 ± 3 |
significantly different than baseline; p<0.05
4. DISCUSSION
Here, we used three different methods to induce a reversible reduction in respiratory neural activity: hypocapnia, isoflurane or isocapnic high frequency ventilation (HFV). In all three cases, we observed a prolonged increase in phrenic burst amplitude once respiratory neural activity was restored. Since hypocapnia, isoflurane and HFV all have different mechanisms of action in the central nervous system, we argue that the resulting plasticity results, at least in part, from reduced respiratory neural activity per se rather than unintended effects of our treatments. While the physiological significance of inactivity-induced phrenic motor facilitation (iPMF) is not yet known, we hypothesize that this form of plasticity may be important in preventing catastrophic decreases in respiratory neural output in situations in which ventilatory control may be compromised.
4.1 Methodological considerations
Hypocapnia
Rats were hyperventilated to lower arterial CO2 levels below the threshold for breathing (the so-called apneic threshold). This chemoreflex-mediated inhibition of brainstem respiratory activity is easy to implement, readily reversible and the duration/level of respiratory neural activity deprivation can be precisely controlled. However, brain hypocapnia and/or alkalosis elicit other changes in the central nervous system unrelated to chemoreflex control of breathing. For example, brain hypocapnia/alkalosis increases neuronal excitability in the cortex (Balestrino and Somjen, 1988) and spinal cord (King and Rampil, 1994; Zhou and Turndorf, 1998). However, neuronal activity rapidly goes back to baseline levels once normal CO2 levels are restored (Sparing et al., 2007); thus, residual CO2-dependent effects on motor neuron excitability likely does not account for the prolonged increase in phrenic burst amplitude observed here. Hypocapnia also results in decreased cerebral blood flow and reduced oxygen unloading at the tissues (Brian, 1998; Vogel et al., 1996), which could lead to brain hypoxia (Nwaigwe et al., 2000; Schneider et al., 1998), a stimulus known to elicit prolonged increases in respiratory motor output (Bavis and Mitchell, 2003; Blitz and Ramirez, 2002). Thus, it remains possible that increased phrenic motor output following hypocapnia is due to local ischemia, rather than reduced respiratory neural activity per se. To control for these effects, we used two other methods of respiratory neural activity deprivation, isoflurane and vagal-inhibition of respiratory neural activity.
Isoflurane
Inhalation anesthetics depress neuronal activity throughout the central nervous system, including in respiratory neurons (Rampil and King, 1996; Stuth et al., 1992; Takeda and Haji, 1993). In addition to suppression of brainstem respiratory rhythm and pattern formation (Takeda and Haji, 1993), inhalation anesthetics depress motor neuron excitability directly (Antognini et al., 1999; Brandes et al., 2007; Rampil and King, 1996; Sirois et al., 1998). Here, we used isoflurane in urethane-anesthetized rats to induce a reversible reduction in respiratory neural activity without changes in arterial CO2. In some rats, isoflurane resulted in an incomplete loss of phrenic motor output. Despite residual (but sharply reduced) phrenic activity apparent in some rats, increased phrenic motor output was apparent upon washout of isoflurane, suggesting that complete inactivity is not necessary for iPMF. In contrast to hypocapnia, iPMF was only observed at 30 and 60, but not 15 min post-isoflurane; however, the delay to iPMF expression likely reflects the washout time of isoflurane (Bailey, 1997).
Vagal Stimulation by Lung Stretch Receptors
Pulmonary stretch receptors inhibit breathing and can over-ride the eupnic drive to breathe (Kubin et al., 2006). We took advantage of this reflex inhibition of breathing by subjecting the rats to isocapnic HFV. Similar to isoflurane, HFV elicited increased phrenic burst amplitude within 30 min following resumption of respiratory neural activity. Vagal afferents project throughout the central nervous system, including to the medullary raphe (Evans et al., 1993). Since spinal serotonin has been shown to elicit long-lasting increases in respiratory motor output (Baker-Herman and Mitchell, 2002; Bocchiaro and Feldman, 2004; MacFarlane and Mitchell, 2009), it remains possible that serotonin release near phrenic motor neurons plays a key role in increased phrenic burst amplitude following HFV (see below).
4.2 Burst frequency plasticity
In contrast to phrenic burst amplitude, significant changes in burst frequency following reduced respiratory neural activity were only observed with hypocapnia. Although there was a trend for burst frequency to be elevated at all time points post-hypocapnia, significant increases from time controls were only observed up to 15 min following resumption of neural respiratory activity, suggesting that burst frequency plasticity is more transient than burst amplitude plasticity. It is unknown why transient burst frequency plasticity was only observed in rats following hypocapnia. In isoflurane rats, burst frequency plasticity may have been masked by residual isoflurane, since it takes many minutes for isoflurane to reach 90% elimination in neural tissue (Bailey, 1997; Lockwood, 2010). Alternatively, it is possible that competing inhibitory mechanisms masked changes in burst frequency. For example, vagus nerve stimulation elicits a transient (<1 min) depression of phrenic burst discharge following stimulation (Budzinska and Ilasz, 2006; Eldridge and Millhorn, 1986). It is unknown if a longer duration of increased vagal traffic, such as performed here, would result in a longer lasting phrenic depression. Regardless, these data are consistent with previous studies suggesting that burst frequency plasticity tends to be more variable than phrenic burst amplitude plasticity in anesthetized rats (Baker-Herman and Mitchell, 2008).
4.3 Reduced respiratory neural activity as a stimulus to plasticity
Our findings are consistent with other reports of phrenic motor plasticity following reduced neural activity. For example, phrenic inactivity induced by intense stimulation of the vagus nerve elicits a prolonged increase in phrenic motor output upon recovery (Zhang et al., 2003, 2004). Similarly, phrenic inactivity induced by reversible C2 axon conduction block elicits profound morphological changes in the phrenic motor pool that may be associated with enhanced motor neuron excitability (Castro-Moure and Goshgarian, 1996, 1997). This rebound facilitation following suppression of neuronal activity may be a general motor neuron property, since prolonged (> 1 hr) increases in evoked lumbar motor neuron responses are observed following recovery from anesthesia with isoflurane (Savola et al., 1991; Wong et al., 2005), halothane (Wong et al., 2005) or ethanol (Li et al., 2005; Wong et al., 1998).
The mechanisms that give rise to iPMF are almost completely unknown. Evidence suggests that serotonin may play an important role in enabling iPMF. For example, increased phrenic burst amplitude following reduced phrenic activity with high frequency vagal stimulation (Zhang et al., 2003, 2004) or hypocapnia (our unpublished observations) requires activation of non-5-HT2 receptors. However, we hypothesize that rather than directly inducing iPMF, serotonin receptor activation in this case may be important in enabling plasticity (Gu and Singer, 1995; Kojic et al., 1997, 2000; Maya Vetencourt et al., 2008), perhaps by modifying intracellular Ca++ levels (Kirkwood, 2000; Kojic et al., 2000) or NMDA receptor function (Blank et al., 1996; Yuen et al., 2005; Zhong et al., 2008). Indeed, a key role for serotonin in enabling compensatory plasticity following removal of synaptic inputs is illustrated by recent reports suggesting that constitutive 5-HT2C receptor activity is required for spontaneous recovery of motor neuron function following spinal injury (Murray et al., 2010).
4.4 Does iPMF represent “homeostatic plasticity” in respiratory control?
Homeostatic plasticity is emerging as an important principle whereby negative feedback bi-directionally adjusts neuronal activity to maintain neural output in an optimal range (Turrigiano, 2006, 2008). Others have suggested that homeostatic plasticity is a property of neural circuits important for ventilatory control since neurons in the nucleus tractus solitarius receiving peripheral chemoreceptor inputs exhibit synaptic depression following chronic intermittent hypoxia (Kline, 2008; Kline et al., 2007), a stimulus that repetitively activates peripheral chemoreceptors. We do not yet know whether iPMF represents homeostatic plasticity per se since enhanced phrenic activity apparent upon reversal of activity deprivation does not spontaneously return to baseline levels, suggesting that this form of plasticity is not bi-directional. The response to respiratory neuron hyperactivity may operate in longer time domains (versus hypoactivity); indeed, a bias toward a rapid response to reduced activity may be an important feature of respiratory control since constitutive activity is critical to sustain life. Alternatively, factors necessary for adaptation in response to neuronal hyperactivity (e.g., diaphragm sensory inputs) may be missing in our experimental preparation. Regardless of whether iPMF represents “true” homeostatic plasticity, understanding mechanisms of plasticity induced by reduced activity in respiratory motor neurons is an important area of research due to its clear biological and clinical significance.
4.5 Conclusions
Here, we demonstrate that hypocapnia-, isoflurane- or HFV-induced reductions in respiratory neural activity elicit a long-lasting form of respiratory plasticity, which we refer to as iPMF. Although the physiological role for this form of plasticity is completely unknown, iPMF may compensate for long-lasting changes in synaptic inputs or neuronal excitability, thereby preventing catastrophic decreases in respiratory motor output.
ACKNOWLEDGEMENTS
We would like to acknowledge helpful advice from Jerome A. Dempsey and excellent technical assistance from Nathan Baertsch. This work was supported by grants from the National Institutes of Health (HL080209 and HL69064). S. Mahamed was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research.
Sources of Support: Supported by: NIH HL80209, NIH HL69064 and CIHR (postdoctoral fellowship to S.M.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alilain WJ, Goshgarian HG. Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp Neurol. 2008;212(2):348–357. doi: 10.1016/j.expneurol.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antognini JF, Carstens E, Buzin V. Isoflurane depresses motoneuron excitability by a direct spinal action: an F-wave study. Anesth Analg. 1999;88:681–685. doi: 10.1097/00000539-199903000-00040. [DOI] [PubMed] [Google Scholar]
- Bailey JM. Context-sensitive half-times and other decrement times of inhaled anesthetics. Anesth Analg. 1997;85:681–686. doi: 10.1097/00000539-199709000-00036. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobio. 2008;162:230–238. doi: 10.1016/j.resp.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL. Phrenic motor facilitation following phrenic inactivity requires spinal atypical PKC activity. SFN. 2009 abstracts. [Google Scholar]
- Balestrino M, Somjen GG. Concentration of carbon dioxide, interstitial pH and synaptic transmission in hippocampal formation of the rat. J Physiol. 1988;396:247–266. doi: 10.1113/jphysiol.1988.sp016961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, Mitchell GS. Intermittent hypoxia induces phrenic long-term facilitation in carotid-denervated rats. J Appl Physiol. 2003;94:399–409. doi: 10.1152/japplphysiol.00374.2002. [DOI] [PubMed] [Google Scholar]
- Blank T, Zwart R, Nijholt I, Spiess J. Serotonin 5-HT2 receptor activation potentiates N-methyl-D-aspartate receptor-mediated ion currents by a protein kinase C-dependent mechanism. J Neurosci Res. 1996;45:153–160. doi: 10.1002/(SICI)1097-4547(19960715)45:2<153::AID-JNR7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Blitz DM, Ramirez JM. Long-term modulation of respiratory network activity following anoxia in vitro. J Neurophysiol. 2002;87:2964–2971. doi: 10.1152/jn.2002.87.6.2964. [DOI] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci U S A. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes IF, Zuperku EJ, Stucke AG, Hopp FA, Jakovcevic D, Stuth EA. Isoflurane depresses the response of inspiratory hypoglossal motoneurons to serotonin in vivo. Anesthesiology. 2007;106:736–745. doi: 10.1097/01.anes.0000264750.93769.99. [DOI] [PubMed] [Google Scholar]
- Brian JE., Jr Carbon dioxide and the cerebral circulation. Anesthesiology. 1998;88:1365–1386. doi: 10.1097/00000542-199805000-00029. [DOI] [PubMed] [Google Scholar]
- Budzinska K, Ilasz R. Short-term depression of inspiratory activity following tonic vagal stimulation. J Physiol Pharmacol. 2006;57 Suppl 4:55–61. [PubMed] [Google Scholar]
- Castro-Moure F, Goshgarian HG. Reversible cervical hemispinalization of the rat spinal cord by a cooling device. 1996;141:102–112. doi: 10.1006/exnr.1996.0143. [DOI] [PubMed] [Google Scholar]
- Castro-Moure F, Goshgarian HG. Morphological plasticity induced in the phrenic nucleus following cervical cold block of descending respiratory drive. Exp Neurol. 1997;147:299–310. doi: 10.1006/exnr.1997.6615. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE. Oscillation, gating and memory in the respiratory control system. Bethesda, MD: Handbook of Physiology: The Respiratory System, American Physiological Society; 1986. [Google Scholar]
- Evans AR, Blair RW. Responses of medullary raphe neurons to electrical and chemical activation of vagal afferent nerve fibers. J Neurophysiol. 1993;70(5):1950–1961. doi: 10.1152/jn.1993.70.5.1950. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211:97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Sandhu MS, Doperalski NJ, Lane MA, White TE, Bishop MD, Reier PJ. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respir Physiol Neurobiol. 2009;165:245–253. doi: 10.1016/j.resp.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci. 2003;23:2494–2501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Singer W. Involvement of serotonin in developmental plasticity of kitten visual cortex. Eur J Neurosci. 1995;7:1146–1153. doi: 10.1111/j.1460-9568.1995.tb01104.x. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Yu XJ, Rafols JA. Neuronal and glial changes in the rat phrenic nucleus occurring within hours after spinal cord injury. J Comp Neurol. 1989;284(4):519–533. doi: 10.1002/cne.902840404. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir Physiol Neurobiol. 2009;169:85–93. doi: 10.1016/j.resp.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley SD, Walker PD, Goshgarian HG. Effects of serotonin inhibition on neuronal and astrocyte plasticity in the phrenic nucleus 4 h following C2 spinal cord hemisection. Exp Neurol. 1999;160(2):433–445. doi: 10.1006/exnr.1999.7238. [DOI] [PubMed] [Google Scholar]
- Hadley SD, Walker PD, Goshgarian HG. Effects of the serotonin synthesis inhibitor p-CPA on the expression of the crossed phrenic phenomenon 4 h following C2 spinal cord hemisection. Exp Neurol. 1999;160(2):479–488. doi: 10.1006/exnr.1999.7240. [DOI] [PubMed] [Google Scholar]
- Imai A, Steffey EP, Farver TB, Ilkiw JE. Assessment of isoflurane-induced anesthesia in ferrets and rats. Am J Vet Res. 1999;60:1577–1583. [PubMed] [Google Scholar]
- King BS, Rampil IJ. Anesthetic depression of spinal motor neurons may contribute to lack of movement in response to noxious stimuli. Anesthesiology. 1994 Dec;81(6):1484–1492. doi: 10.1097/00000542-199412000-00024. 1994. [DOI] [PubMed] [Google Scholar]
- Kirkwood A. Serotonergic control of developmental plasticity. Proc Natl Acad Sci U S A. 2000;97:1951–1952. doi: 10.1073/pnas.070044697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD. Plasticity in glutamatergic NTS neurotransmission. Respir Physiol Neurobiol. 2008;164:105–111. doi: 10.1016/j.resp.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neurosci. 2007;27:4663–4673. doi: 10.1523/JNEUROSCI.4946-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojic L, Dyck RH, Gu Q, Douglas RM, Matsubara J, Cynader MS. Columnar distribution of serotonin-dependent plasticity within kitten striate cortex. Proc Natl Acad Sci U S A. 2000;97:1841–1844. doi: 10.1073/pnas.97.4.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojic L, Gu Q, Douglas RM, Cynader MS. Serotonin facilitates synaptic plasticity in kitten visual cortex: an in vitro study. Brain Res Dev Brain Res. 1997;101:299–304. doi: 10.1016/s0165-3806(97)00083-7. [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006 doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol. 2009;169:123–132. doi: 10.1016/j.resp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HF, Mochly-Rosen D, Kendig JJ. Protein kinase Cgamma mediates ethanol withdrawal hyper-responsiveness of NMDA receptor currents in spinal cord motor neurons. Br J Pharmacol. 2005;144:301–307. doi: 10.1038/sj.bjp.0706033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood G. Theoretical context-sensitive elimination times for inhalation anaesthetics. Br J Anaesth. 2010 May;104(5):648–655. doi: 10.1093/bja/aeq051. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol. 2009;587:5469–5481. doi: 10.1113/jphysiol.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Neuromuscular adaptations to respiratory muscle inactivity. Respir Physiol Neurobiol. 2009;169:133–140. doi: 10.1016/j.resp.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, Castrén E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- McGuire M, MacDonald SM, Song G, Poon CS. Phrenic long-term facilitation is robust to hypercapnia and hypocapnia but not hyperventilatory hypotension under PEEP. Respir Physiol Neurobiol. 2007;158:107–111. doi: 10.1016/j.resp.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D'Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med. 2010;16:694–700. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS. Respiratory Neuroplasticity: Life-long Adjustments of a Critical Homeostatic System. Proceedings of the International Union of Biological Sciences. 2007 [Google Scholar]
- Nantwi KD, El-Bohy AA, W SG, Reier PJ, Goshgarian HG. Spontaneous Functional Recovery in a Paralyzed Hemidiaphragm Following Upper Cervical Spinal Cord Injury in Adult Rats. Neurorehabil Neural Repair. 1999;13:225–234. [Google Scholar]
- Nwaigwe CI, Roche MA, Grinberg O, Dunn JF. Effect of hyperventilation on brain tissue oxygenation and cerebrovenous PO2 in rats. Brain Res. 2000;868:150–156. doi: 10.1016/s0006-8993(00)02321-0. [DOI] [PubMed] [Google Scholar]
- Rampil IJ, King BS. Volatile anesthetics depress spinal motor neurons. Anesthesiology. 1996;85(1):129–134. doi: 10.1097/00000542-199607000-00018. [DOI] [PubMed] [Google Scholar]
- Savola MK, Woodley SJ, Maze M, Kendig JJ. Isoflurane and an alpha 2-adrenoceptor agonist suppress nociceptive neurotransmission in neonatal rat spinal cord. Anesthesiology. 1991;75:489–498. doi: 10.1097/00000542-199109000-00017. [DOI] [PubMed] [Google Scholar]
- Schneider GH, Sarrafzadeh AS, Kiening KL, Bardt TF, Unterberg AW, Lanksch WR. Influence of hyperventilation on brain tissue-PO2, PCO2, and pH in patients with intracranial hypertension. Acta Neurochir Suppl. 1998;71:62–65. doi: 10.1007/978-3-7091-6475-4_20. [DOI] [PubMed] [Google Scholar]
- Sirois JE, Pancrazio JJ, III CL, Bayliss DA. Multiple ionic mechanisms mediate inhibition of rat motoneurones by inhalation anaesthetics. J Physiol. 1998;512:851–862. doi: 10.1111/j.1469-7793.1998.851bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparing R, Dafotakis M, Buelte D, Meister IG, Noth J. Excitability of human motor and visual cortex before, during, and after hyperventilation. J Appl Physiol. 2007;102:406–411. doi: 10.1152/japplphysiol.00770.2006. [DOI] [PubMed] [Google Scholar]
- Sperry MA, Goshgarian HG. Ultrastructural changes in the rat phrenic nucleus developing within 2 h after cervical spinal cord hemisection. Exp Neurol. 1993;120(2):233–244. doi: 10.1006/exnr.1993.1058. [DOI] [PubMed] [Google Scholar]
- Stachnik J. Inhaled anesthetic agents. Am J Health Syst Pharm. 2006;63:623–634. doi: 10.2146/ajhp050460. [DOI] [PubMed] [Google Scholar]
- Strey KA, Broytman O, Baker-Herman TL. Increased ventral spinal TNF-alpha following hypocapnia- induced respiratory neural inactivity. FASEB J. 2010 [Google Scholar]
- Strey KA, Baker-Herman TL. Expression of inactivity-induced phrenic motor facilitation differs between substrains of Sprague Dawley rats. Soc for Neurosci. 2010 Abstracts. [Google Scholar]
- Stuth EA, Tonkovic-Capin M, Kampine JP, Zuperku EJ. Dose-dependent effects of isoflurane on the CO2 responses of expiratory medullary neurons and the phrenic nerve activities in dogs. Anesthesiology. 1992;76:763–774. doi: 10.1097/00000542-199205000-00015. [DOI] [PubMed] [Google Scholar]
- Takeda R, Haji A. Cellular effects of isoflurane on bulbar respiratory neurons in decerebrate cats. Jpn J Pharmacol. 1993;62:57–65. doi: 10.1254/jjp.62.57. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. More than a sidekick: glia and homeostatic synaptic plasticity. Trends Mol Med. 2006 Oct;12(10):458–460. doi: 10.1016/j.molmed.2006.08.002. 2006 Epub 2006 Aug 22. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Stamegna JC, Boulenguez P, Gauthier P, Kastner A. Restorative respiratory pathways after partial cervical spinal cord injury: role of ipsilateral phrenic afferents. Eur J Neurosci. 2007;25:3551–3560. doi: 10.1111/j.1460-9568.2007.05619.x. [DOI] [PubMed] [Google Scholar]
- Vogel J, Abounader R, Schröck H, Zeller K, Duelli R, Kuschinsky W. Parallel changes of blood flow and heterogeneity of capillary plasma perfusion in rat brains during hypocapnia. Am J Physiol. 1996;270:H1441–H1445. doi: 10.1152/ajpheart.1996.270.4.H1441. [DOI] [PubMed] [Google Scholar]
- Wade JG, Stevens WC. Isoflurane: an anesthetic for the eighties? Anesth Analg. 1981;60:666–682. [PubMed] [Google Scholar]
- Wong SM, Tauck DL, Fong EG, Kendig JJ. Glutamate receptor-mediated hyperexcitability after ethanol exposure in isolated neonatal rat spinal cord. J Pharmacol Exp Ther. 1998;285:201–207. [PubMed] [Google Scholar]
- Wong SM, Sweitzer SM, Peters MC, Kendig JJ. Hyperresponsiveness on washout of volatile anesthetics from isolated spinal cord compared to withdrawal from ethanol. Anesth Analg. 2005;100:413–436. doi: 10.1213/01.ANE.0000142128.29660.AE. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005;25:5488–5450. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, McGuire M, White DP, Ling L. Episodic phrenic-inhibitory vagus nerve stimulation paradoxically induces phrenic long-term facilitation in rats. J Physiol. 2003;551:981–991. doi: 10.1113/jphysiol.2003.048157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, McGuire M, White DP, Ling L. Serotonin receptor subtypes involved in vagus nerve stimulation-induced phrenic long-term facilitation in rats. Neurosci Lett. 2004;363:108–111. doi: 10.1016/j.neulet.2004.03.067. [DOI] [PubMed] [Google Scholar]
- Zhong P, Yuen EY, Yan Z. Modulation of neuronal excitability by serotonin-NMDA interactions in prefrontal cortex. Mol Cell Neurosci. 2008;38:290–299. doi: 10.1016/j.mcn.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HH, Turndorf H. Hyper- and hypoventilation affects spinal motor neuron excitability during isoflurane anesthesia. Anesth Analg. 1998;87:407–410. doi: 10.1097/00000539-199808000-00033. [DOI] [PubMed] [Google Scholar]