Abstract
The rapid development of techniques that enable synthesis (and manipulation) of matter on the nanometer scale, as well as the development of new nano-materials, will play a large role in disease diagnosis and treatment, specifically in targeted cancer therapy. Targeted nanocarriers are an intriguing means to selectively deliver high concentrations of cytotoxic agents or imaging labels directly to the cancer site. Often solubility issues and an unfavorable biodistribution can result in a suboptimal response of novel agents even though they are very potent. New nanoparticulate formulations allow simultaneous imaging and therapy (“theranostics”), which can provide a realistic means for the clinical implementation of such otherwise suboptimal formulations. In this review we will not attempt to provide a complete overview of the rapidly enlarging field of nanotechnology in cancer; rather, we will present properties specific to nanoparticles, and examples of their uses, which demonstrate their importance for targeted cancer therapy.
Nanotechnology is defined as the technology developing and working with materials smaller than 100 nm in one dimension. As a reference, the length of a typical carbon-carbon bond is ca 0.12–0.15 nm, the diameter of a DNA-strand is ca 2 nm. As such this includes man-made structures, but also biological entities such as viruses or proteins. It excludes structures with a metabolism, since the smallest cellular life forms (from the genus Mycoplasm) are 100 – 200 nm in size.
The concept of nanotechnology was first described in a talk by the physicist and Nobel laureate Richard Feynman at a meeting of the American Physical Society in 1959 at Caltech [1]. In this talk Feynman described the concept of “plenty of room at the bottom”, considering the possibility to manipulate individual atoms directly. Thirty years later, in September 1989, the physicist Donald M Eigler at the IBM Almaden Research Center precisely arranged 35 xenon atoms to create “IBM” on the atomic scale (Figure 1), using the scanning tunnel microscope he developed [2]. Other important milestones are summarized in a recent review [3]. This also demonstrates that the refinement of tools to explore the nano-scale world (such as electron microscopes, scanning tunnel microscopes or atomic force microscopes) is an important factor to actually drive the field forward.
Figure 1.
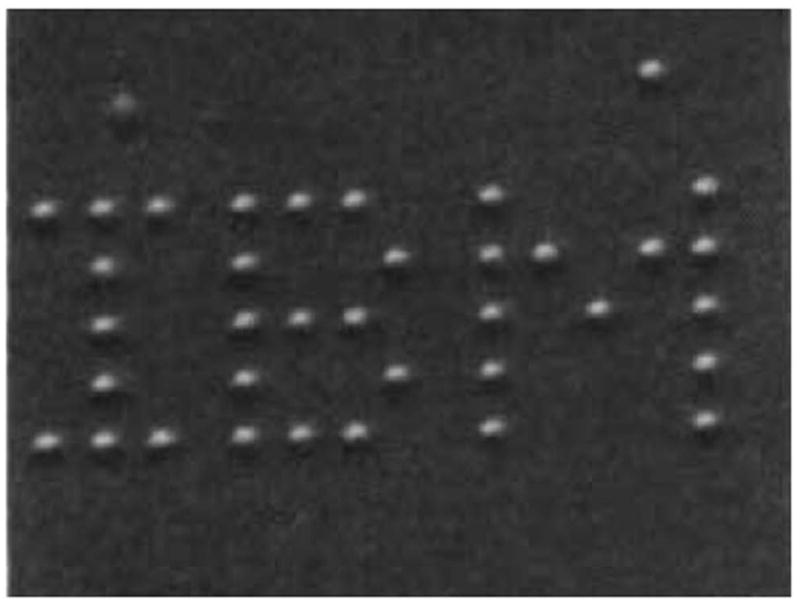
Patterned array of xenon atoms on a nickel surface (atomic structure not resolved). Each letter is 50 nm from top to bottom. Reprinted with permission from [2].
Properties of nanoparticles
Interestingly, a range of properties displayed by nano-sized materials differs significantly from their meso-scale relatives made of the same materials. These include mechanical, thermal, optical, mechanical and catalytic properties. This is of importance as a large portion of the universe of biological activities that proceeds at the level of the cell surface, in glomerular filtration, protein and DNA synthesis, modification and degradation, or hepatic clearance occurs at the nanoscale. Thus there are important differences if these biological structures and processes encounter a macroscopic or even meso-scopic material or one with a size of their own. The very small size of nanomaterials also implicates a large ratio of surface atoms to volume of the material; this allows generation of high specific activity imaging reagents, 10–100 times that possible with conventional agents in current use. In addition it provides a high surface charge in a narrow spatial confinement [4]. These characteristics in turn may substantially modify properties of the bulk materials. For example, metallic nanoparticles can be used as very active catalysts [5], and the large surface area can be modified with a variety of functional groups [6].
An increasing range of nanomaterials is described [3], including targeted nanogenerators for radiotherapy [7] or even nanomachines such as “walking” DNA strands [8] or a DNA based DNA nanomotor [9], paving the way to a molecular robotics. While the latter may play a role in the future (e.g. as the nanosurgeons envisioned by Feynman [1]) we will concentrate on the role of nanoparticles, which offer an immense variety of sizes, shapes, dispersities, surface-charges, valencies and structural designs. Nanoparticles are particularly appealing for several reasons:
1.) Multivalency and Multimodality
Nanoparticles provide very large surface to volume ratios. A single wall carbon nanotube with a total volume similar to a typical large protein (for example, 100–150 kDa) provides a 15-times increased surface compared to a large protein with a comparable volume [3]. The enlarged surface area can be utilized to couple a large amount of targeting ligands to the particle. This increased amount of ligands on the surface may enhance binding of the particle to the desired target significantly (Figure 2a)[6]. A recent report demonstrated that even weak small organic molecules as ligands can significantly enhance the avidity of the particle towards its target by up to 4 orders of magnitude through multivalent interactions [10]. Furthermore, in addition to targeting ligands, imaging labels can be attached to the particle's surface, e.g. fluorochromes for optical imaging, metals for MRI, or radioactive nuclides for nuclear imaging, allowing for simultaneous multimodal imaging (Figure 2b). In this case the same particle can be used for magnetic resonance imaging (MRI) and optical imaging [11, 12], utilizing the high sensitivity of optical imaging in combination with the high resolution and soft tissue contrast of MRI. Adding nuclides to this construct allows for MR / optical / nuclear imaging [13]. With the advancements of clinical multimodality imaging (e.g. the combination of positron emission tomography (PET) with computed tomography (CT) or MRI) this property will become increasingly important. This is particularly valuable in drug development in preclinical imaging and biodistribution studies and also in early phase trials in humans.
Figure 2.
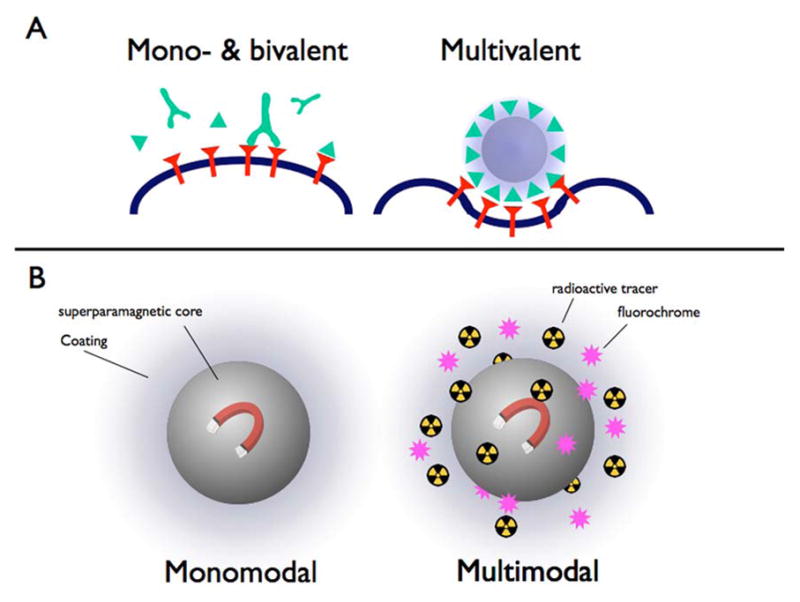
(A) The principle of multivalency. Antibodies are bivalent while small peptides or small molecules are mono-valent. A particle with multiple targeting moieties provides multiple binding sides and is multivalent. Multivalency increases the validity to the target considerably. (B) A mono-modal imaging agent is suitable to be detected with one modality; a multimodal agent can be detected with several imaging modalities as it contains several different labels. This allows to combine the advantages of various modalities in one particle.
In addition, the ability to attach large numbers of imaging agents to a single particle will dramatically enhance sensitivity by increasing signal intensity. In one interesting instance, the containment of gadolinium within a nanotube further enhanced its sensitivity by 40 fold (see subsection 4 below also on this.)
2.) Size
The biodistribution of nanoparticles can be substantially influenced by their surface coating and size. Dendrimers (repeatedly branched, roughly spherical large molecules) or other metallic and organic particles can consist entirely of polymers or crystals, but they can also contain a core material that is usually surrounded by a bio-compatible coating material. The coating permits or increases the solubility of the particle’s central core material and in some cases (such as with Q-dots that contain potentially toxic heavy metals) allows for protection of the recipient. The coating is therefore especially important for materials that are not easily soluble in aqueous solutions and therefore in serum, particularly for metallic particles such as quantum dots, gold- or iron oxide particles [14–17]. Furthermore by choosing the right properties of the coating (foremost charge) the particles can be made relatively invisible to a large non-specific uptake sink in the body, the mononuclear phagocyte system (MPS) or reticulo-endothelial-sytem (RES).
Polyethyleneglycol (PEG) is so far the most frequently used coating material, but numerous other polymeric and monomeric coatings have been used. PEG molecules are thought to form a protective hydrophilic layer on the particle’s surface that inhibits interaction with opsonization-facilitating blood components, thus reducing the uptake of PEG-coated particles in macrophages and Kupffer cells [18]. As a result, the PEG coating reduces uptake by macrophages of the MPS and provides relatively long plasma residence times. However, even the length of the coating PEG chains has an influence on the particle’s properties: varying PEG chain length results in major changes in organ/tissue-selective biodistribution and clearance from the body [19]. On the other hand it is known that positive charges facilitate the uptake of particles in cells, which has been utilized for cell labeling [20–22].
Furthermore, the size of particles has a significant influence on the distribution of the particles. Larger particles tend to have a more rapid uptake into the MPS (especially liver and spleen) and consequently a more rapid clearance from the circulation. This is likely related to the finding that the size of the nanoparticle has a substantial effect on the protein absorption, probably secondary to a higher surface density of the organic coating on the surface of smaller nanoparticles [23]. Small nanoparticles have therefore a longer blood half-life and also a high rate of permeation into tissues, demonstrating the importance of particle size and surface composition for achieving effective, targeted delivery and elimination from the body. On the other hand, very small particles (eg C dots smaller than 5nm or carbon nanotubes with one dimension of 1nm) may rapidly clear through glomerular filtration (see below).
Furthermore the dispersion of the particles, i.e. the variation of the particle sizes, plays an important role [24]. Monodispersed particles are all uniform in size while polydispersed particles show a wide range of size (Figure 3). Polydispersed or even oligodispersed particles tend to have a range of retention times and biodistributions in an organism representative of the different sizes in the particle population, which makes evaluation of the particle's impact complex.
Figure 3.
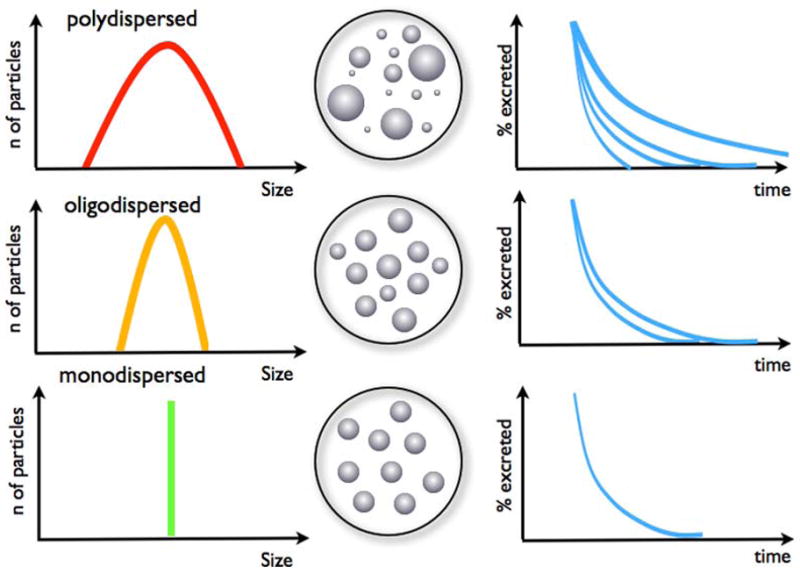
Dispersion of particles as an indicator of the size variability between particles. Monodispersed particles are all uniform and of one size without variability, this is the ideal particle preparation. Oligodispersed particles have some variability in size while polydispersed particles show a large range of sizes. Accordingly, oligo- and polydispersed particles demonstrate different rate of excretions
Furthermore, the shape of a nanoparticle also contributes to its fate in vivo. It especially influences the internalization of particles into cells. For example, it has been demonstrated that rod-like particles have an appreciable advantage over spherical particles in the internalization rates. This is reminiscent of the advantage that many rod-like bacteria have for internalization in non-phagocytic cells [25].
3.) Controlled synthesis
Nanoparticles can be synthesized in a very controlled environment, ideally creating particles which all display the same properties in size, shape, charge and, if functionalized, the same amount of functional groups bound to the particles surface. This is immensely important as only monodispersed particles are expected to display the same distribution, biological half-life and target affinity in vivo, which has become an important criterion for current approval processes. This requirement has lead to very sophisticated production methods for some nanoparticles. A new particle fabrication process, “particle replication in non-wetting templates” (PRINT), has been developed to strictly control physical and chemical traits of the particles [26]. This technology utilizes perfluoropolyether molds, resulting in the production of polymer-based nanoparticles with precise control of size, shape, chemical composition and surface functionality, tailored to the specific properties required to target a variety of diseases [27]. Importantly, all these particles are monodispersed and very well defined. A similar process has been devised to create quantum dots [28].
4.) Core properties
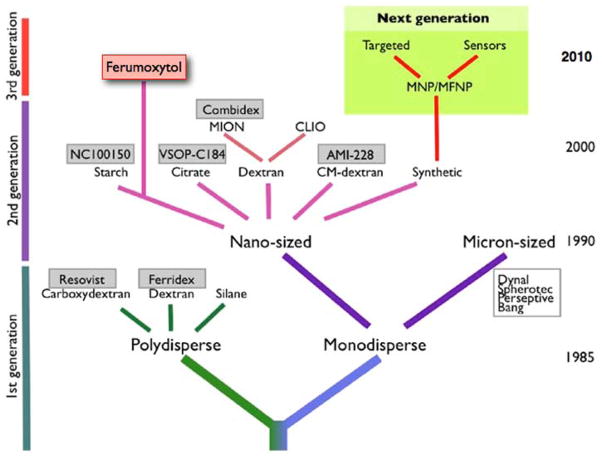
While nanoparticles can consist entirely of a polymer (such as a dendrimer or a carbon nanotube) they can also contain a central core. This can be a semiconductive material in the case of quantum dots or a metallic core as exemplified by iron oxide or gold nanoparticles. An iron oxide core renders the particle superparamagnetic and makes it suitable for MRI [16, 29]. A whole genealogy of iron oxide particles exits (Figure 4)[30]. Resovist® (Bayer-Schering Pharma, Berlin, Germany) was a particle that has been used clinically but has been withdrawn from the market. Several others have made it into clinical trials but never received FDA approval (e.g. Feridex®, AMAG Pharmaceuticals, Lexington, MA). Currently the only solid particle in clinical use is ferumoxytol (Feraheme®, AMAG Pharmaceuticals, Lexington, MA), approved for the treatment of iron deficiency anemia in adult patients with chronic kidney disease – however, these small particles are also suitable for MR imaging [31, 32]. Due to their small size, below 50 nm, they are slowly eliminated out of the circulation and can be used as intravascular contrast agents (they are not currently FDA approved for this application).
Figure 4.
Schematic representation of advances in magnetic nanoparticle (MNP) design. MNP agents currently under development will have significantly higher relaxivities than earlier generations of MNP, and will also have improved synthetic coats for targeted imaging. The red box indicates an approved agent. The green box indicates the next generation of particles. Reprinted with permission from [30].
In contrast to the standard clinical MR contrast agent (chelated gadolinium), iron oxide particles combine not one contrast producing atom in one molecule, but a cluster of thousands of individual atoms, which is one reason for the higher sensitivity of MR for iron oxide particles. This high concentration of paramagnetic nuclei can perturb the local homogeneity of the magnetic field, which is very sensitively detected by MR scanners. This makes iron oxide particles uniquely suited to detect very low concentrations on a molecular level, as exemplified by multiple publications, using iron oxide particles for targeted approaches [33–35], cell tracking [22, 36] or even activatable imaging agents [37, 38]. Clinically, iron oxide particles have been used as a sensitive method to detect and characterize liver lesions [39] and to detect lymph node metastases [40, 41]. However, there are also approaches to increase the relaxivity of Gadolinium chelates, e.g. by combining them with carbon nanotubes. Similar to iron oxide particles the exterior surface of these nanotubes provides a scaffold for attachment of chemical groups for solubilizing or targeting purposes. However, their internal space within the hollow tube allows for encapsulation of atoms, ions, and even small molecules [42, 43]. Filling ultra-short nanotubes with Gadolinium atoms increased the relaxivity (a measurement of the strength of a MR contrast agent) dramatically compared to clinically used Gadolinium chelates, depending on the magnetic field strength. At the standard MR field strength the relaxivity was nearly 40 times larger but the effect was even more pronounced (nearly 90 times larger) at very low fields [44]. Another interesting new approach is the use of nanodiamonds modified with Gadolinium, resulting in a 10-fold higher relaxivity compared to clinically used Gadolinium chelates. These nanodiamonds may also induce less oxidative stress to cells than other carbon nanomaterials [45].
The exploration of gold nanoparticles began over 150 years ago with colloidal gold. Michael Faraday noted that colloidal gold solutions have properties differing significantly from those of bulk gold. Gold particles have been used for many different applications in cellular biology; for example to detect different mRNA, and also for electron microscopy [46, 47]. Gold nanoparticles are also being studied as contrast agents for CT. Targeted contrast agents for CT are particularly interesting due to the wide availability of CT scanners. However, CT scanners also require very high concentration of the currently used iodine in organic molecules even for non-targeted imaging, making targeted imaging with these molecules not feasible as the local concentrations of targeted agents are way too small to be detected with a CT scanner using iodine. Therefore research is focusing on nanoparticles such as gold [48, 49] or bismuth particles [50], that can deliver dense loads of metallic ions for CT imaging (Figure 5).
Figure 5.
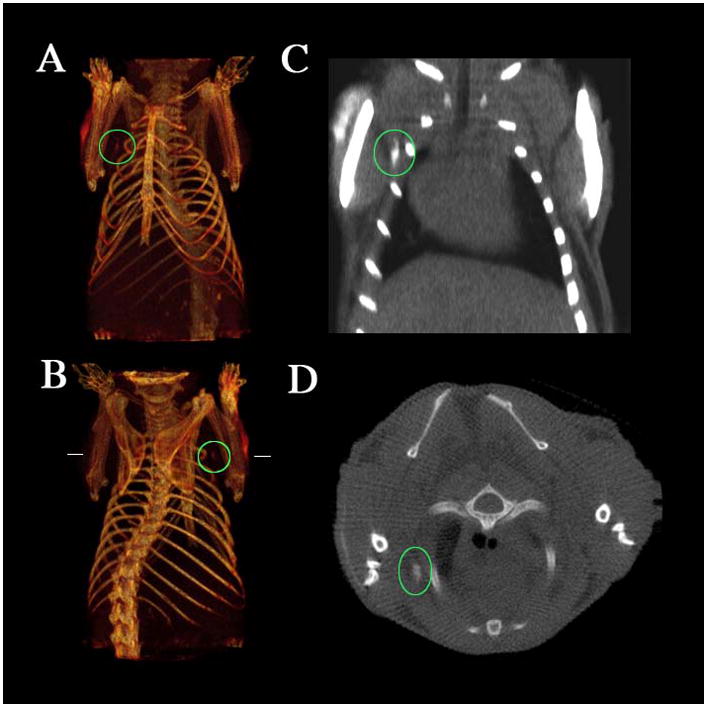
CT imaging of a lymph node of a mouse with a bismuth nanoparticle (a,b) Three-dimensional volume renderings of the CT data set, the length of the reconstruction is 3.8 cm. (c) Coronal slice (length of the slice 2.3 cm). (d) Transverse slice at the height indicated by the horizontal lines in b. The maximal diameter of the mouse is 1.8 cm. The position of the lymph node under the right shoulder is indicated by the ovals, and the arrows show the injection site. Note the lack of contrast in the corresponding contralateral (left shoulder) lymph node. Reprinted with permission from [50].
5.) Beyond imaging – theranostics
Besides using particles solely for imaging purposes they can also carry another payload: a therapeutic agent. In conjunction with the imaging moiety this creates a theranostic agent (Figure 6). These are agents that can be used to diagnose disease foci, deliver targeted therapy, and monitor the response to therapy at the same time [51]. The integration of diagnostic imaging capability with therapeutic efficiency is believed to be critical to addressing the challenges of cancer heterogeneity and adaptation. Therapeutic agents have been used mostly in the form of liposomes to carry antibiotics [52] or anti-cancer agents [53] as well as novel experimental approaches like siRNA [54]. Carbon nanotubes have been employed for radiotherapy. Due to their high aspect ratios (great length and narrow width) they have a high surface area for functionalization, allowing delivering of higher specific activities [55].
Figure 6.
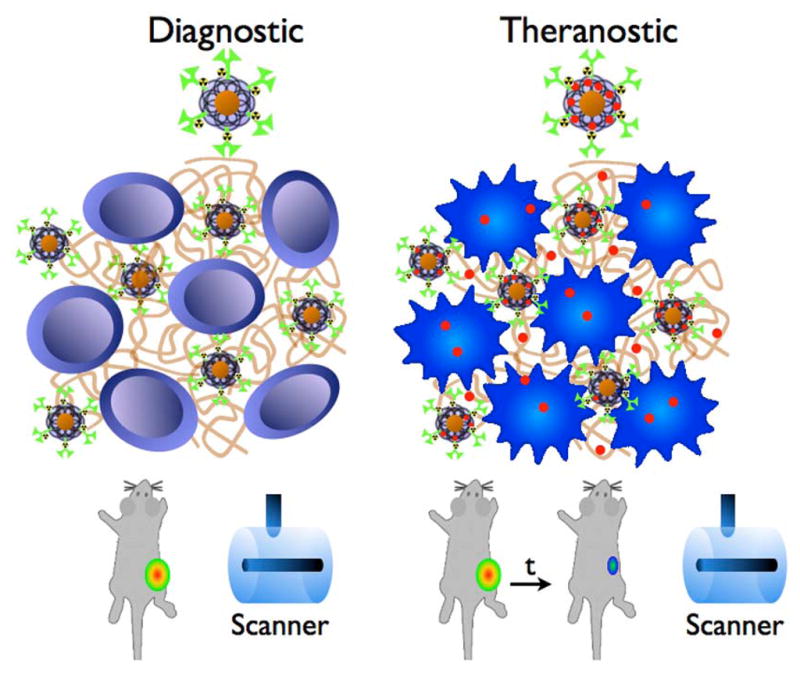
Schematic drawing of the theranostic approach. The diagnostic particle consists of a diagnostic moiety and a coating, modified with a nuclide (green), targeting an antigen (brown), here deposited between the tumor cells. Imaging is performed with MRI and PET. The particle can carry a payload drug (red) that is released in the tumor creating a theranostic agent, allowing for imaging and therapy of the tumor.
Because liposomes carry their payload inside of the particle mechanisms need to be provided for a release of the drug, ideally triggered by the target tissue. For liposomes, for example, an enzymatic trigger mechanism has been developed, in which phospholipase releases the drug [56]. Some formulations are built to release the drug under environmental conditions found in tumors. A biocompatible polymeric multimodal, multivalent theranostic nanoparticle was constructed that released the drug at a lower pH. This particle allowed for MR and optical imaging (via an iron oxide core and fluorochromes as payload), carried multiple folate molecules as targeting ligands and a therapeutic payload of taxol [57].
Enhanced permeability and retention
The enhanced permeability and retention (EPR) effect describes the relative and passive accumulation of macromolecules inside of tumors. This is generally explained by the presence of high permeability of the tumor vasculature in combination with compromised lymphatic drainage, which allows the accumulation and subsequent retention of macromolecules. Consequently, in such areas (mostly tumors and inflamed or infarcted tissues) large molecules and particles ranging from 10 to 500 nm in size can leave the leaky neovasculature and accumulate in the interstitial space [58, 59]. Small molecules and particles do not accumulate in tumors likely because of their ability to diffuse back to the circulation [60]. EPR-mediated drug delivery is seen as an effective way to bring drugs to tumors, especially macromolecular drugs and drug-loaded pharmaceutical nanocarriers. This process is therefore also called “passive targeting”. It is advantageous to use EPR when appropriate targeting ligands are not available and even when they are available, EPR may enhance selective delivery to the target site.
Toxicity issues
The safety of nanoparticles is attracting the attention of the FDA, and also the public and especially the EPA, which is starting to look into regulation of nanotechnology. As nanotechnology progresses to commercialization and more widespread applications it is likely that nanomaterials and nanoproducts will be released into the environment, potentially causing harm to the public. It is conceivable that hazards introduced by nanoparticles are different from those posed by conventional chemicals, partially due to the ability of the very small nanoparticles to move readily cross the various biological barriers and their somewhat different physicochemical properties [61].
A first reminder of possible hazards associated with the excessive exposure to nanoparticles was published in late 2009 [62]. Seven female workers were exposed in China to polyacrylate nanoparticles at the workplace and presented with shortness of breath and pleural effusions. Lung biopsies revealed nonspecific pulmonary inflammation, pulmonary fibrosis and pleural foreign-body granulomas. Electron microscopy demonstrated nanoparticles in pulmonary epithelial and mesothelial cells, but also located in the chest fluid. These cases raised concern that long-term exposure to at least some nanoparticles without protective measures may cause serious damage to the lungs. This concern has not been diminished by other reports on the toxicity of these materials in animals. Around 2009 reports on pulmonary toxicity of carbon nanotubes emerged [63, 64], as well as a report on the induction of mesotheliomas in rats [65] and on liver damage [66] by multi-walled carbon nanotubes. In addition metal nanoparticles, metal-oxide nanoparticles, quantum dots, fullerenes, and fibrous nanomaterials were found to cause chromosomal fragmentation, DNA strand breakages, point mutations, oxidative DNA adducts and alterations in gene expression profiles – even though inconsistencies in many studies were also found [67]. These reports lead to the calls for more governmental oversight, nano-dosimetry, maximum tolerated doses and the generation of the field of nanotoxicology.
As a potential solution to the problem of the toxicity of nanomaterials (most obvious in the example of cadmium-containing quantum dots) a rapid elimination of these materials from the body by renal clearance has been suggested. The requirements for renal filtration and urinary excretion of inorganic, metal-containing nanoparticles have been defined [68]. A final hydrodynamic diameter below 5.5 nm resulted in rapid and efficient urinary excretion and elimination of quantum dots from the body. Zwitterionic or neutral organic coating of the particles prevented their adsorption to serum proteins, thus stabilizing the hydrodynamic diameter to below 15 nm and promoting renal excretion. The same principle has been recently utilized for silica coated fluorochrome nanoparticles [69] and even carbon nanotubes [70]
Conclusion
Will nanotechnology influence targeted cancer therapy? In this review we focused on nanoparticles and have discussed that these particles can be applied to fit the requirements and needs for individual applications. By choosing the right size, shape, coating and charge as well as targeting moiety (Figure 7) the fate of the particles in the body can be well predicted. Targeting of the particles can already be achieved via the EPR effect and can be made more efficient by utilizing targeting moieties on the surface, increasing the binding affinity and avidity further. Drugs can be delivered this way by specifically targeting them to the tumor, where the release of the drug is triggered by the tumor's environment (pH- or enzyme-mediated). Adding an imaging label to the particle enables direct monitoring of the biodistribution, predictions of toxicity and therapeutic effects (and might in the future also aid in visualizing the triggered release of the payload). On the other hand, the particles can be used entirely for imaging as exemplified with iron oxide nanoparticles. Toxicity issues have been raised for some particles and materials, but can probably be circumvented by constructing particles that are eliminated quickly from the body without any retention. Taking all this into account we believe that the answer to the opening question is a sound “yes, in a positive way”.
Figure 7.
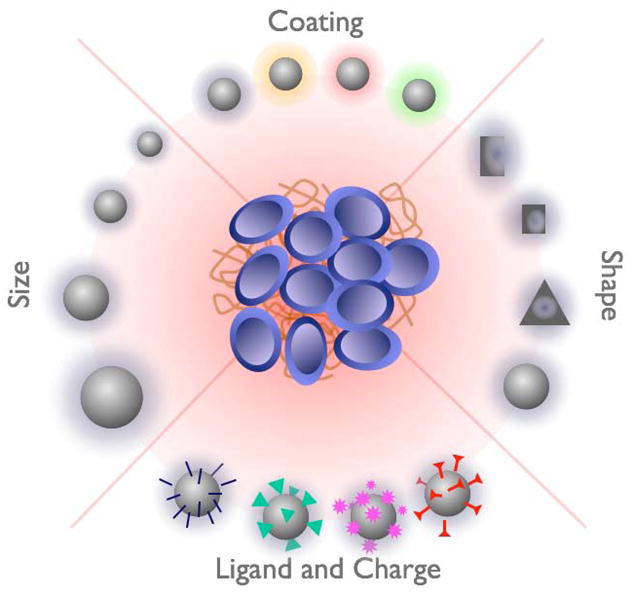
Main properties influencing the distribution, elimination and the targeting of particles to tumors. Refer to the main text for details.
Footnotes
No financial disclosures for JG; DAS is a consultant to Encyse pharmaceuticals, a nanotechnology company.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jan Grimm, Email: grimmj@mskcc.org.
David A. Scheinberg, Email: d-scheinberg@mskcc.org.
References
- 1.Feynman RP, Robbins J, Dyson FJ. The pleasure of finding things out. Cambridge, MA: Perseus Books; 1999. [Google Scholar]
- 2.Eigler DM, Schweizer EK. Positioning single atoms with a scannin tunnelling microscope. Nature. 1990;344(6266):524–526. [Google Scholar]
- 3.Scheinberg DA, Villa CH, Escorcia FE, et al. Conscripts of the infinite armada: systemic cancer therapy using nanomaterials. Nat Rev Clin Oncol. 2010;7(5):266–76. doi: 10.1038/nrclinonc.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao G. Nanostructures & Nanomaterials: Synthesis, Properties & Applications. London: Imperial College Press; 2004. [Google Scholar]
- 5.Gao L, Zhuang J, Nie L, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2(9):577–83. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 6.Montet X, Funovics M, Montet-Abou K, et al. Multivalent effects of RGD peptides obtained by nanoparticle display. J Med Chem. 2006;49(20):6087–93. doi: 10.1021/jm060515m. [DOI] [PubMed] [Google Scholar]
- 7.McDevitt MR, Ma D, Lai LT, et al. Tumor therapy with targeted atomic nanogenerators. Science. 2001;294(5546):1537–40. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 8.Shin JS, Pierce NA. A synthetic DNA walker for molecular transport. J Am Chem Soc. 2004;126(35):10834–5. doi: 10.1021/ja047543j. [DOI] [PubMed] [Google Scholar]
- 9.Klapper Y, Sinha N, Ng TW, et al. A rotational DNA nanomotor driven by an externally controlled electric field. Small. 2010;6(1):44–7. doi: 10.1002/smll.200901106. [DOI] [PubMed] [Google Scholar]
- 10.Tassa C, Duffner JL, Lewis TA, et al. Binding affinity and kinetic analysis of targeted small molecule-modified nanoparticles. Bioconjug Chem. 2010;21(1):14–9. doi: 10.1021/bc900438a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Josephson L, Kircher MF, Mahmood U, et al. Near-infrared fluorescent nanoparticles as combined MR/optical imaging probes. Bioconjug Chem. 2002;13(3):554–60. doi: 10.1021/bc015555d. [DOI] [PubMed] [Google Scholar]
- 12.Tannous BA, Grimm J, Perry KF, et al. Metabolic biotinylation of cell surface receptors for in vivo imaging. Nat Methods. 2006;3(5):391–6. doi: 10.1038/nmeth875. [DOI] [PubMed] [Google Scholar]
- 13.Nahrendorf M, Zhang H, Hembrador S, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117(3):379–87. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimm J, Karger N, Lusse S, et al. Characterization of ultrasmall magnetite [correction of paramagnetic magnetite] particles as superparamagnetic contrast agents in MRI. Invest Radiol. 2000;35(9):553–6. doi: 10.1097/00004424-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Romberg B, Hennink WE, Storm G. Sheddable coatings for long-circulating nanoparticles. Pharm Res. 2008;25(1):55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wunderbaldinger P, Josephson L, Weissleder R. Crosslinked iron oxides (CLIO): a new platform for the development of targeted MR contrast agents. Acad Radiol. 2002;9(Suppl 2):S304–6. doi: 10.1016/s1076-6332(03)80210-6. [DOI] [PubMed] [Google Scholar]
- 17.Yu WW. Semiconductor quantum dots: synthesis and water-solubilization for biomedical applications. Expert Opin Biol Ther. 2008;8(10):1571–81. doi: 10.1517/14712598.8.10.1571. [DOI] [PubMed] [Google Scholar]
- 18.Bazile D, Prud'homme C, Bassoullet MT, et al. Stealth Me. PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J Pharm Sci. 1995;84(4):493–8. doi: 10.1002/jps.2600840420. [DOI] [PubMed] [Google Scholar]
- 19.Choi HS, Ipe BI, Misra P, et al. Tissue- and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett. 2009;9(6):2354–9. doi: 10.1021/nl900872r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimm J, Kircher MF, Weissleder R. Cell tracking : Principles and applications. Radiologe. 2007;47(1):25–33. doi: 10.1007/s00117-006-1449-5. [DOI] [PubMed] [Google Scholar]
- 21.Josephson L, Tung CH, Moore A, et al. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjug Chem. 1999;10(2):186–91. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- 22.Kircher MF, Allport JR, Graves EE, et al. In vivo high resolution three-dimensional imaging of antigen-specific cytotoxic T-lymphocyte trafficking to tumors. Cancer Res. 2003;63(20):6838–46. [PubMed] [Google Scholar]
- 23.Fang C, Shi B, Pei YY, et al. In vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. Eur J Pharm Sci. 2006;27(1):27–36. doi: 10.1016/j.ejps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Alexis F, Pridgen E, Molnar LK, et al. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505–15. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gratton SE, Ropp PA, Pohlhaus PD, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105(33):11613–8. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolland JP, Maynor BW, Euliss LE, et al. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc. 2005;127(28):10096–100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 27.Jeong W, Napier ME, DeSimone JM. Challenging nature's monopoly on the creation of well-defined nanoparticles. Nanomedicine (Lond) 2010;5(4):633–9. doi: 10.2217/nnm.10.34. [DOI] [PubMed] [Google Scholar]
- 28.Hampton MJ, Templeton JL, DeSimone JM. Direct patterning of CdSe quantum dots into sub-100 nm structures. Langmuir. 2010;26(5):3012–5. doi: 10.1021/la904787k. [DOI] [PubMed] [Google Scholar]
- 29.Weissleder R, Elizondo G, Wittenberg J, et al. Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology. 1990;175(2):489–93. doi: 10.1148/radiology.175.2.2326474. [DOI] [PubMed] [Google Scholar]
- 30.Sosnovik DE, Nahrendorf M, Weissleder R. Magnetic nanoparticles for MR imaging: agents, techniques and cardiovascular applications. Basic Res Cardiol. 2008;103(2):122–30. doi: 10.1007/s00395-008-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balakrishnan VS, Rao M, Kausz AT, et al. Physicochemical properties of ferumoxytol, a new intravenous iron preparation. Eur J Clin Invest. 2009;39(6):489–96. doi: 10.1111/j.1365-2362.2009.02130.x. [DOI] [PubMed] [Google Scholar]
- 32.Provenzano R, Schiller B, Rao M, et al. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4(2):386–93. doi: 10.2215/CJN.02840608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allkemper T, Bremer C, Matuszewski L, et al. Optimization of iron oxides for contrast-enhanced MRA: Results of an expereiemental study (Abstract) Eur Radiol. 1999;9(suppl):76. [Google Scholar]
- 34.Lee JH, Huh YM, Jun YW, et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007;13(1):95–9. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 35.Montet X, Montet-Abou K, Reynolds F, et al. Nanoparticle imaging of integrins on tumor cells. Neoplasia. 2006;8(3):214–22. doi: 10.1593/neo.05769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arbab AS, Yocum GT, Kalish H, et al. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104(4):1217–23. doi: 10.1182/blood-2004-02-0655. [DOI] [PubMed] [Google Scholar]
- 37.Grimm J, Perez JM, Josephson L, et al. Novel nanosensors for rapid analysis of telomerase activity. Cancer Res. 2004;64(2):639–43. doi: 10.1158/0008-5472.can-03-2798. [DOI] [PubMed] [Google Scholar]
- 38.Perez JM, Josephson L, O'Loughlin T, et al. Magnetic relaxation switches capable of sensing molecular interactions. Nat Biotechnol. 2002;20(8):816–20. doi: 10.1038/nbt720. [DOI] [PubMed] [Google Scholar]
- 39.Reimer P, Balzer T. Ferucarbotran (Resovist): a new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: properties, clinical development, and applications. Eur Radiol. 2003;13(6):1266–76. doi: 10.1007/s00330-002-1721-7. [DOI] [PubMed] [Google Scholar]
- 40.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348(25):2491–9. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 41.Harisinghani MG, Weissleder R. Sensitive, Noninvasive Detection of Lymph Node Metastases. PLoS Med. 2004;1(3):e66. doi: 10.1371/journal.pmed.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackeyev YA, Marks JW, Rosenblum MG, et al. Stable containment of radionuclides on the nanoscale by cut single-wall carbon nanotubes. J Phys Chem B. 2005;109(12):5482–4. doi: 10.1021/jp0456436. [DOI] [PubMed] [Google Scholar]
- 43.Monthioux M. Filling single-wall carbon nanotubes. Carbon. 2002;40(10):1809–1823. [Google Scholar]
- 44.Sitharaman B, Kissell KR, Hartman KB, et al. Superparamagnetic gadonanotubes are high–performance MRI contrast agents. Chem Commun (Camb) 2005;31:3915–7. doi: 10.1039/b504435a. [DOI] [PubMed] [Google Scholar]
- 45.Manus LM, Mastarone DJ, Waters EA, et al. Gd(III)-nanodiamond conjugates for MRI contrast enhancement. Nano Lett. 2010;10(2):484–9. doi: 10.1021/nl903264h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giljohann DA, Seferos DS, Daniel WL, et al. Gold nanoparticles for biology and medicine. Angew Chem Int Ed Engl. 2010;49(19):3280–94. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seferos DS, Giljohann DA, Hill HD, et al. Nano-flares: probes for transfection and mRNA detection in living cells. J Am Chem Soc. 2007;129(50):15477–9. doi: 10.1021/ja0776529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park JA, Kim HK, Kim JH, et al. Gold nanoparticles functionalized by gadolinium-DTPA conjugate of cysteine as a multimodal bioimaging agent. Bioorg Med Chem Lett. 2010;20(7):2287–91. doi: 10.1016/j.bmcl.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Aydogan B, Li J, Rajh T, et al. AuNP-DG: Deoxyglucose-Labeled Gold Nanoparticles as X-ray Computed Tomography Contrast Agents for Cancer Imaging. Mol Imaging Biol. 2010 doi: 10.1007/s11307-010-0299-8. [DOI] [PubMed] [Google Scholar]
- 50.Rabin O, Manuel Perez J, Grimm J, et al. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat Mater. 2006;5(2):118–22. doi: 10.1038/nmat1571. [DOI] [PubMed] [Google Scholar]
- 51.Sumer B, Gao J. Theranostic nanomedicine for cancer. Nanomedicine (Lond) 2008;3(2):137–40. doi: 10.2217/17435889.3.2.137. [DOI] [PubMed] [Google Scholar]
- 52.Drulis-Kawa Z, Dorotkiewicz-Jach A. Liposomes as delivery systems for antibiotics. Int J Pharm. 2010;387(1–2):187–98. doi: 10.1016/j.ijpharm.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 53.Alexis F, Pridgen EM, Langer R, et al. Nanoparticle technologies for cancer therapy. Handb Exp Pharmacol. 2010;(197):55–86. doi: 10.1007/978-3-642-00477-3_2. [DOI] [PubMed] [Google Scholar]
- 54.Ozpolat B, Sood AK, Lopez-Berestein G. Nanomedicine based approaches for the delivery of siRNA in cancer. J Intern Med. 2010;267(1):44–53. doi: 10.1111/j.1365-2796.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 55.Escorcia FE, McDevitt MR, Villa CH, et al. Targeted nanomaterials for radiotherapy. Nanomedicine (Lond) 2007;2(6):805–15. doi: 10.2217/17435889.2.6.805. [DOI] [PubMed] [Google Scholar]
- 56.Pedersen PJ, Adolph SK, Subramanian AK, et al. Liposomal formulation of retinoids designed for enzyme triggered release. J Med Chem. 2010;53(9):3782–92. doi: 10.1021/jm100190c. [DOI] [PubMed] [Google Scholar]
- 57.Santra S, Kaittanis C, Grimm J, et al. Drug/dye-loaded, multifunctional iron oxide nanoparticles for combined targeted cancer therapy and dual optical/magnetic resonance imaging. Small. 2009;5(16):1862–8. doi: 10.1002/smll.200900389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–92. [PubMed] [Google Scholar]
- 59.Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2010 doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release. 2001;74(1–3):47–61. doi: 10.1016/s0168-3659(01)00309-1. [DOI] [PubMed] [Google Scholar]
- 61.Shvedova AA, V, Kagan E. The role of nanotoxicology in realizing the 'helping without harm' paradigm of nanomedicine: lessons from studies of pulmonary effects of single-walled carbon nanotubes. J Intern Med. 2010;267(1):106–18. doi: 10.1111/j.1365-2796.2009.02188.x. [DOI] [PubMed] [Google Scholar]
- 62.Song Y, Li X, Du X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur Respir J. 2009;34(3):559–67. doi: 10.1183/09031936.00178308. [DOI] [PubMed] [Google Scholar]
- 63.Ryman-Rasmussen JP, Cesta MF, Brody AR, et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009;4(11):747–51. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ryman-Rasmussen JP, Tewksbury EW, Moss OR, et al. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am J Respir Cell Mol Biol. 2009;40(3):349–58. doi: 10.1165/rcmb.2008-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakamoto Y, Nakae D, Fukumori N, et al. Induction of mesothelioma by a single intrascrotal administration of multi–wall carbon nanotube in intact male Fischer 344 rats. J Toxicol Sci. 2009;34(1):65–76. doi: 10.2131/jts.34.65. [DOI] [PubMed] [Google Scholar]
- 66.Zhang D, Deng X, Ji Z, et al. Long-term hepatotoxicity of polyethylene-glycol functionalized multi-walled carbon nanotubes in mice. Nanotechnology. 2010;21(17):175101. doi: 10.1088/0957-4484/21/17/175101. [DOI] [PubMed] [Google Scholar]
- 67.Singh N, Manshian B, Jenkins GJ, et al. NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials. 2009;30(23–24):3891–914. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 68.Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burns AA, Vider J, Ow H, et al. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano Lett. 2009;9(1):442–8. doi: 10.1021/nl803405h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruggiero A, Villa CH, Bander E, et al. Paradoxical glomerular filtration of carbon nanotubes. Proceedings of the National Academy of Sciences of the United States of America. 2010 doi: 10.1073/pnas.0913667107. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]