Abstract
Functional and molecular imaging techniques are increasingly being developed and used to quantitatively map the spatial distribution of parameters such as metabolism, proliferation, hypoxia, perfusion and ventilation, among others, onto anatomically-imaged normal organs and tumor. In radiotherapy optimization, these imaging modalities offer the promise of increased dose sparing to high functioning subregions of normal organs or dose escalation to selected subregions of tumor, as well as the potential to adapt radiotherapy to functional changes that occur during the course of treatment. The practical use of functional/molecular imaging in radiotherapy optimization must take into cautious consideration several factors whose influences are still not clearly quantified or well understood: patient positioning differences between the planning CT and functional/molecular imaging sessions, image reconstruction parameters and techniques, image registration, target/normal organ functional segmentation, the relationship governing the dose escalation/sparing warranted by the functional/molecular image intensity map, and radiotherapy-induced changes in the image intensity map over the course of treatment. The clinical benefit of functional/molecular image guidance in the form of improved local control or decreased normal organ toxicity has yet to be demonstrated and awaits prospective clinical trials addressing this issue.
The Integration of anatomic imaging data directly within radiation therapy treatment planning systems is now widespread and in most cases dictates standard practice. Most common are 3D x-ray computed tomography (CT) datasets, augmented in many cases with magnetic resonance imaging (MRI). Both tumor and normal tissue anatomy delineated (segmented) on these imaging datasets form the backdrop for beam selection, plan optimization and dose computation and display. These same imaging modalities are most often used to assess the results of treatment (tumor shrinkage, some signs of normal tissue changes) either after, or ever more commonly, during the course of treatment. Recent issues of this journal have reviewed the use of anatomic images to guide1 and adapt2 radiation therapy treatments.
More recently, advances in the availability and utility of functional and, to some extent, molecular imaging data has led to great interest within the radiation therapy community related to its use in treatment assessment, and ultimately integration into the treatment planning optimization and adaptive delivery processes. These uses generally fall into the areas of identifying regions of tumors for dose intensification (see for example previous article in this issue), or, as is also now gaining interest, for use in assessing and monitoring the function of normal tissues.3,4
Several recently published articles reviewed the potential of imaging as a biomarker for various mechanisms and processes, and for applications in clinical radiotherapy. Some of these review articles5–8 indicate tumor hallmarks, mechanisms and expression parameters (such as metabolism, proliferation, hypoxia, apoptosis, angiogenesis/vascularity, receptor expression) which have potential for study using imaging biomarkers. Other articles9–16 review clinical sites (such as head and neck, lung, brain, esophagus, cervix, rectum, breast, prostate) where functional MRI, PET and SPECT has been applied in radiation oncology practice. Recent investigations and reports 17–24 address use of this new type of information in optimization strategies for planning and adapting radiotherapy treatments.
In the current article we attempt to review some methodologies used in the planning of functional image guided therapy, indicate areas now using or soon to use functional imaging for adaptive treatment strategies, summarize some expectations related to dosimetric outcome of this planning, and to point out some issues related to the correct use of this type of data in the planning process. Thus, much of this article makes broad assumptions about the applicability of certain functional and molecular imaging data in the selection of “what to treat” or what not to treat. Gregoire et al12 point out how the sensitivity and specificity of functional PET imaging might relate to its applicability for target selection in various tumors. Beyond selection lies the additional task of delineation of targets and normal tissues (or perhaps more meaningful, delineation of subsections of targets and normal tissues) to treat or avoid, respectively. Again, beyond warnings and alerts for concerns, much of this article will of necessity assume that both the selection and delineation questions have (to some extent) been validated prior to use in the planning processes we describe. As such, caution should be applied for use of these techniques in a non-protocol setting within the clinic.
Planning Methodologies for Functional Image-Guided Radiotherapy
The mapping of the spatial variation of function, as identified with functional imaging, can be used to either spare high functioning normal tissue or escalate dose to hyperactive/hypoxic regions of the tumor. These aims can be achieved to differing extents based on whether the plan used is 3D conformal or intensity modulated radiotherapy (IMRT) and also on whether or not beam orientations are selected with this aim in mind. Compared to 3D, IMRT is generally more capable of providing dose to the tumor via less functional intervening normal tissue or, conversely, providing greater dose to hyperactive/hypoxic regions of tumor while not increasing dose to normal tissue. Beam selection can further enhance both 3D and IMRT, provided there is the possibility of aligning beam orientations to exploit delivery through less functional regions of normal tissue. The extent to which functional image-guided dose delivery may be achieved can also depend on the capabilities of the planning system. A planning system that is capable of manipulating the dose to each voxel based on user specifications would be ideal, and perhaps necessary for “dose painting tumor volumes by number” (see article by Bentzen and Gregoire in this issue). However, commercial planning systems typically treat a conglomerate of voxels as one entity, as in the case of organs or tumor, precluding elaborate voxel-specific spatial sculpting of dose. Consequently, it may be easier to treat a grouping of similarly functional voxels within normal organs and tumor as one monolithic functional region. In general, a RTP with some sort of flexible “optimization” engine (which would preferably allow biological cost functions) is likely more important for integration of these types of data for potential reduction in normal tissue function loss than is whether the treatment plans are 3D CRT or IMRT. Their use could permit iso-toxicity based treatment planning25–27 based on predicted loss of function.19,28 Next we illustrate some of the planning methodologies seen in literature.
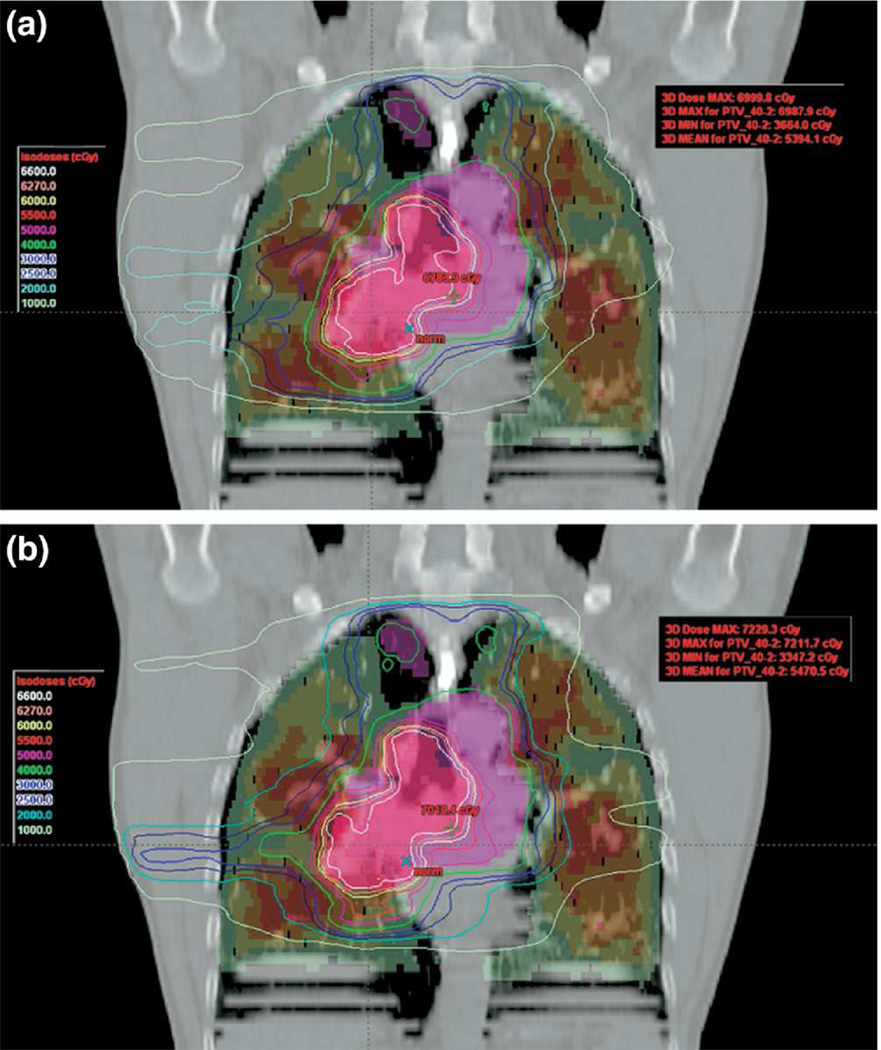
Seppenwoolde et al29 used the U-Mplan system (developed at the University of Michigan) to develop 3D plans with selected beam directions and beam weight optimization to minimize SPECT perfusion-weighted dose to lung. This is an example of a more accessible planning system, with respect to customization. The system allowed the optimization objective function to weight the dose to the voxels by SPECT perfusion. Individual beam directions were selected to minimize the ratio of mean perfused lung dose to mean lung dose. The optimization minimized the mean lung dose, lung volume above 20 Gy as well as the mean SPECT perfusion-weighted lung dose. In a similar context, the work by McGuire et al30 is an example of perfusion SPECT-guided IMRT planning, but conducted within the confines of a commercial planning system (Figure 1). The lung was segmented into four regions based on perfusion, and 9-beam IMRT plans were generated using a hierarchical method. The method consisted of sequential optimizations, starting with dose allowed only through the least functional region. With each subsequent optimization, more functional regions were slowly relaxed to absorb greater dose, until target coverage was met. The sequential optimization process implicitly gave higher priority to more functional lung.
Figure 1.
Coronal view showing plans with (bottom) and without (top) SPECT guidance. The central purple and pink structures are primary and boost targets; lung is shaded by SPECT activity intensity, ranging from red (highest) to green (lowest). Isodose lines demonstrate that SPECT-guidance decreased dose to higher perfusion regions. Reprinted with permission.30
The heterogeneous nature of functional distribution suggests that beam directions can be particularly important in the quest to improving functional sparing. Beam directions that pass through lower functional normal tissue to reach tumor are obviously favorable. However, the issue is not quite as trivial as it appears, as sparing functional tissue has to be balanced against achieving target coverage, which generally requires irradiation from multiple directions, and IMRT for concave target volumes. Thus, in cases where regions of low function are clustered together, it would be necessary for some beams to go through higher functioning tissue to achieve target coverage.
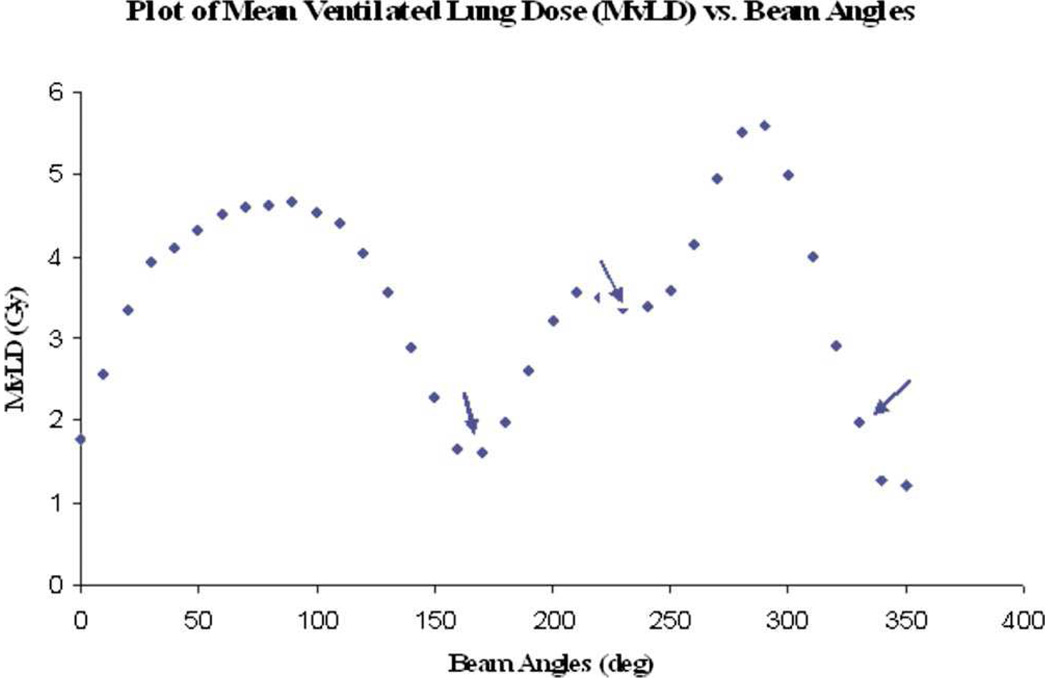
Examples of beam direction selection are as follows. Munawar et al31 used lung ventilation SPECT with 30 MBq 99mTc labeled ultrafine graphite particles to steer dose away from highly ventilated regions. Normal lung was segmented into two functional regions –ventilated lung at greater than 50% and 70% of maximum SPECT intensity. IMRT plans with 9 equally spaced beams were compared to plans with 3 beams that were selected from 36 equally spaced possibilities, such that the 3 beams individually had mean ventilated lung doses close to minima (Figure 2). In cases where the planning target volume was heterogeneously surrounded by well ventilated lung, the 3-beam plans achieved superior ventilation sparing. Shirai et al32 identified hepatocellular carcinoma radiation therapy angles that would least irradiate functional liver (as imaged using Tc-99m-galactosyl human serum albumin SPECT). Radiotherapy was well tolerated, with no cases of grade 3 or higher radiation-induced liver disease. McGuire et al33 demonstrated, for perfusion-weighted lung sparing, that using four selected beam directions can result in substantial functional dose reduction when compared to an equally spaced nine-beam non-functional-guided IMRT plan. The beam orientations used were selected from a larger set of candidate angles, which were individually optimized to minimize functional dose while achieving PTV minimum target coverage. The four beams with greatest functional sparing over the whole dose range were then selected.
Figure 2.
Plot of mean lung dose vs. beam angle, with arrows indicating beams selected. Reprinted with permission.31
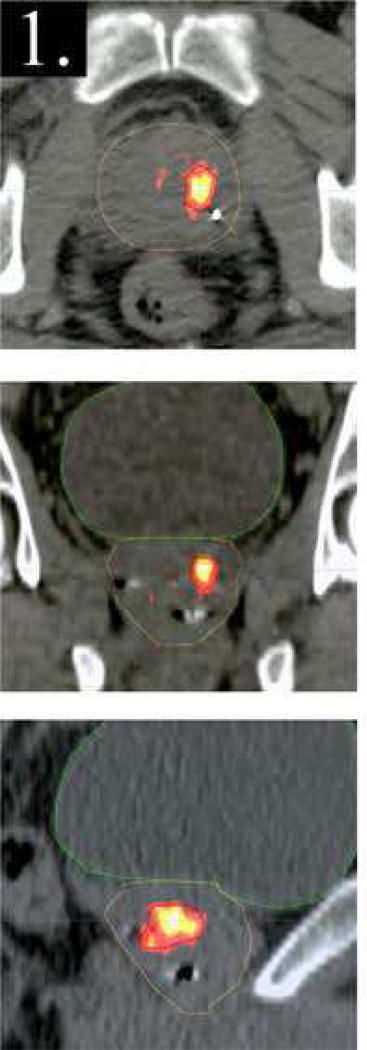
Similarly to the heterogeneous distribution of function in normal organs, tumors can also reflect functional heterogeneity in the form of metabolism, proliferation or hypoxia. Treatment planning to achieve selective spatial dose escalation to specific target regions based on function could presumably improve local control (as discussed in other articles in this issue), but would have to be weighed against the incidental increase in dose to surrounding critical organs. Pinkawa et al34 planned dose boosting to intraprostatic lesions identified using F18-choline PET. A boost of 18 Gy resulted in only minimal increase of the equivalent uniform dose to rectum and bladder. Seppala et al35 used Carbon-11 acetate PET to identify prostate subvolumes for boosting (Figure 3). Comparing standard plans with 77.9 Gy to PTV vs. boosted plans with 72 Gy to PTV and up to 90 Gy to the boosted volume, there was no significant normal tissue complication probability increase in rectum and bladder. Prostate subvolumes may also be identified for dose boosting using magnetic resonance imaging/spectroscopy36,37.
Figure 3.
Distribution of Carbon-11 acetate in axial (top), coronal (middle) and sagittal (bottom) planes, superimposed on the corresponding CT planes through the prostate. Reprinted with permission.35
Lee et al38 used F18 labeled Fluormisonidazole to identify regions of head-and-neck cancers that were hypoxic. Boosting the hypoxia-avid regions by 14 Gy (80 Gy to the gross tumor volume) was possible without exceeding normal tissue constraints. Similarly, reductions in head and neck tumor volumes planned to receive high doses based on PET tumor volume data led to reduced dose to surrounding normal tissues as reported by Geets et al.39 Optimized planning (either conformal 3D or IMRT) based on boosting tumor subvolumes identified via functional imaging could be expected to lead to similar results for treatments of tumors at other anatomic locations as well. These reductions in the overall volumes of tumor required to receive high boost dose could enable the ability to deliver high doses to those subvolumes with no increase in predicted toxicity to normal tissues. However, in some cases the additional information provided by the functional imaging can make composite (anatomic plus functional) target volumes larger as well.40,41
Potential Uses of Functional Imaging for Adaptive IGRT
Geets et al42 showed that the FDG-PET avid volume decreased during the course of radiotherapy in 11 patients with pharyngeo-laryngeal squamous cell carcinoma. They suggest the possibility of adapting radiotherapy dose during the course of radiation, such that the primary pre-RT volume remains unaltered during the course of radiotherapy, but the simultaneously integrated boost volume is reduced based on PET imaging during RT. The main advantage would likely be increased sparing to spinal cord, parotid glands and oral cavity. These functional imaging based adaptive approaches for treatment of head and neck cancers should, of course, be approached with caution, as other authors such as Hentschel et al43 conclude that reduction in treatment volumes may not be possible based on FDG-PET for head and neck cancer due to therapy associated inflammation and possibly on which source to background algorithm is used. On the other hand, Dirix et al44 present evidence for added value (over FDG-PET) of 18F-Fluoromisonidazole PET and suggest potential for both diffusion-weighted and dynamic contrast enhanced MRI in the adaptive treatment of head and neck squamous cell carcinoma. Cao et al45 earlier demonstrated the potential for adaptive treatment planning changes based on blood volume alterations in head and neck cancers as demonstrated using DCE MRI.
Another area under active consideration for functional image based adaptive therapy is non-small cell lung cancer. Reductions in size of PET avid tumor regions have been observed by imaging studies using 18F-FDG46 as well as 18F-FLT47. Such volume reductions suggest the potential to act on these changes while maintaining or reducing predicted lung toxicity.47–49 Care needs to be exercised in these target volume reductions however, as Sonke and Belderbos indicate50 that reduced metabolic activity on PET does not necessarily correspond to geometric tumor volume changes; in some cases erosion (CTV appears to remain constant while the GTV shrinks) takes place instead. These same types of FDG and FLT studies may also help in assessing normal tissue toxicities during treatment,47,51 augmenting or potentially replacing the SPECT perfusion and ventilation studies mentioned above. Lately, there have been indications from Mayr et al52 that longitudinal changes in tumor perfusion of cervical cancer as seen in DCE-MRI might also be used to adapt treatment.
Dosimetric Results of Functional Image-guided Radiotherapy Planning
The ultimate benefit of functional guidance in radiotherapy would either be reduction of normal tissue toxicity, increase in local control rate, or both. This benefit has yet to be demonstrated, primarily due to the lack of full scale trials to address this question. However, the dosimetric advantages of function sparing are evident in the radiotherapy planning papers in the literature. One can only anticipate that this dosimetric advantage will translate into actual clinical benefit in the future.
Seppenwoolde et al29 found that, only in patients with large perfusion defects, perfusion-weighted optimization improved over the geometric optimization. McGuire et al30 was able to show reductions in perfusion-weighted volumes above 20 Gy and 30 Gy of 13.6% ± 5.2% and 10.5% ± 5.8%, respectively. The difference in the conclusions between these two works is likely that Seppenwoolde et al29 used 3D planning as opposed to IMRT planning by McGuire et al.30 To answer the question of 3D vs. IMRT, Lavrenkov et al53 found that in 6/17 patients there was no advantage to using IMRT over 3D to spare SPECT functional lung over 20 Gy. In the remaining cases, the IMRT functional lung volume over 20 Gy was reduced to 74% of the 3D plan value. Shioyama et al54 segmented the highest 50% and 90% of SPECT hyperperfused lung. Compared to IMRT plans without functional avoidance, IMRT plans with functional avoidance lowered mean doses to the 50% and 90% functional lung regions by 2.2 and 4.2 Gy, respectively (prescription dose of 63 Gy). Overall, IMRT does seem to provide improved dosimetric benefit compared to 3D for SPECT perfusion-weighted planning. Radiation therapy planning with ventilation imaging has also been used to demonstrate improved dosimetric benefit. 4DCT derived lung ventilation imaging55 has been used with IMRT planning to demonstrate the possibility of reducing dose to regions of lung with the highest ventilation. Munawar et al31 showed that there was a dosimetric advantage in cases where less than 5% of the highest 50% of ventilation volume was in the PTV.
An important finding by several groups is that the dosimetric gain possible is dependent on the nature of the spatial distribution of function. Munawar et al31 found that the dosimetric gain was lower when the well-ventilated lung completely surrounded the PTV. In examining 3D vs. IMRT, Lavrenkov et al56 showed that IMRT was more capable of perfused lung sparing if highly perfused lung regions were closer to the PTV and the overall functional distribution was more heterogeneous. Shioyama et al54 also saw more sparing was seen in patients with greater spatial heterogeneity in function. The results of these works suggest that functional sparing in the lung shows greatest dosimetric benefit when the functional distribution is heterogeneous and highly functional regions do not completely surround the target.
The clinical significance of changes in tumor volume dose distributions will only result from prospective clinical trials that employ functional image data to redesign dose distributions. These studies are ongoing.
Issues Related to the Utilization of Functional Image Guidance in Radiotherapy Planning
Important issues regarding the utilization of functional image-guidance in radiotherapy include: image registration, delineation of volumes, image reconstruction and the question of the extent to which dose should be escalated to subregions of tumor. Indeed, the ability to make firm conclusions related to the usefulness on defining tumor subregions with functional imaging methods likely relies on the accuracy of these techniques and methods.41,57–59 An informative series of articles have recently been published that highlight the issues related to quantitative imaging for evaluating tumor response60, tumor change measurement, truth data and error sources from MRI61, x-ray CT62 and PET/CT63, as well as statistical considerations related to same.64 Many task groups and initiatives now exist within nearly every medical physics, radiation oncology, radiology and nuclear medicine society or collaborative group with intent to address standardization and quality assurance issues related to the use of functional imaging in planning and assessing cancer treatments.65, 66
Accurate image registration38,67 between the functional image set and the treatment planning set is key to ensuring dosimetric benefit. Inaccurate image registration can, in the worst case, result in the opposite effect being achieved,68 viz., higher dose to more functional normal tissue regions. Modern day functional imagers typically include CT (CT-PET, CT-SPECT), allowing the planning CT images to be easily registered to the CT images from the functional imaging machines, thereby indirectly registering the planning CT and functional image sets. However, this assumes that the CT acquired at the time of functional imaging is exactly aligned with the functional image set. This assumption is not necessarily true if the patient inadvertently moves between the CT and functional imaging acquisitions. To a large extent, the immobilization used for radiotherapy would ensure that the two image sets are aligned, not to mention that this would also improve the quality of image registration between the planning CT and CT acquired at the time of functional imaging. Image reconstruction parameters such as the SPECT attenuation and scatter corrections can influence the SPECT voxel values. Consequently they can also influence the shapes of functional subregions. However, the net dosimetric effect on metrics such as the mean perfused dose or function above certain dose thresholds may be less pronounced because of the aggregate nature of these metrics.69 Indeed, even the evaluation of the quality of the image registration may require consideration of multiple accuracy metrics. For example, Yin et al70 found that while sophisticated non-rigid registrations between the CT components of SPECT/CTs and planning CTs provided a higher degree of accuracy than rigid methods, irregularities in some of the deformation fields, when applied to the SPECT images, resulted in unacceptable changes in the SPECT intensity distributions that would preclude their use in RT planning.
Target delineation can also present problems, as there is no ground truth related to thresholding of image intensities for segmentation, and the results can change depending upon segmentation technique. Roels et al41 recently reported that, while integration of MRI and FDG-PET into radiotherapy planning appeared to be feasible for use in image-guided radiotherapy of rectal cancer, automated segmentation was recommended for the PET images. Segmentation issues can be especially problematic in assessing PET signals due to the partial volume effect,71 where small volumes may have the signal due to uptake in that region spread over several imaging voxels. Methods continue to be explored to automatically address this problem.72
Even though there is evidence that high and low FDG uptake regions appear to remain stable,73 the reproducibility of image segmentation implementation is very important for prospective clinical studies, especially those that will attempt to adapt treatment plans during the course of treatment. Thus, much attention is being given to application of these methods of target delineation from PET scans,74,75 although similar matters need to be considered in the use of function derived from MRI signals.76 Beyond the issues identified by the series of articles referred to at the end of the first paragraph of this section, consensus recommendations and standards for PET image acquisition,77 monitoring response78 and use in RTP79 have begun to appear.
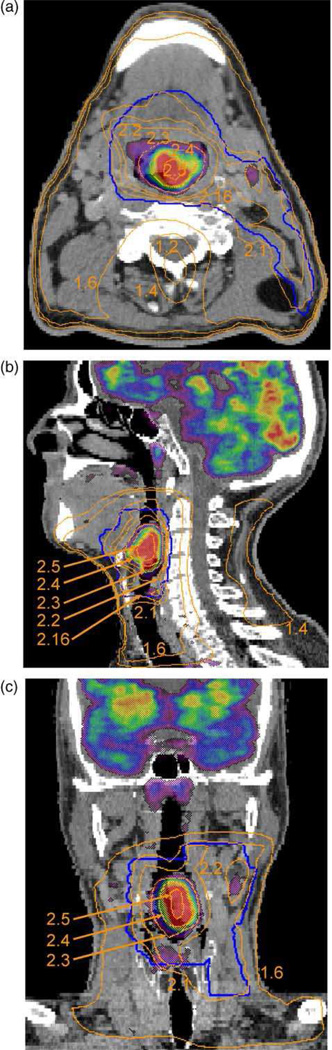
In the context of dose escalation to subregions of the tumor, a yet to be answered question is the extent to which dose should be raised in accordance with the functional image (PET/MRS) intensity. For example, should the dose be linear or non-linear with intensity? Various planning studies have speculatively employed dose-intensity relationships. Das et al80 assigned a linear dose-to-FDG intensity prescription, over and above a baseline uniform therapeutic dose. Vanderstraeten et al81 compared a linear vs. uniform boost prescription from FDG PET (Figure 4). For hypoxia imaging with FMISO PET, Thorwarth et al82 used a dose-escalation scheme that assumed (a) the time needed for reoxygenation was inversely proportional to perfusion and that (b) radioresistance was proportional to tracer retention. In general, the nature of the formulation relating the required dose escalation vs. functional image intensity (to achieve spatially uniform tumor control) awaits basic science and clinical feedback. Once such a formulation is available, planning studies could determine the extent to which achieving the required dose escalation is compromised by toxicity-limiting dose constraints to surrounding normal organs.
Figure 4.
Isodose lines and FDG-PET intensity colormap superimposed on axial (top), sagittal (middle) and coronal (bottom) slices. Numbers indicate the dose in Gy per fraction, reflecting dose escalation to regions of higher FDG uptake. Reprinted with permission.81
Acknowledgments
Dr. Ten Haken’s research is supported in part by NIH P01 CA59827
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jaffray DA. Image-guided radiation therapy: from concept to practice. Semin Radiat Oncol. 2007;17 doi: 10.1016/j.semradonc.2007.08.001. 243-234. [DOI] [PubMed] [Google Scholar]
- 2.Yan D. Adaptive radiotherapy: merging principle into clinical practice. Semin Radiat Oncol. 2010;20:79–83. doi: 10.1016/j.semradonc.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Evans ES, Hahn CA, Kocak Z. The role of functional imaging in the diagnosis and management of late normal tissue injury. Semin Radiat Oncol. 2007;17:72–80. doi: 10.1016/j.semradonc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Jeraj R, Cao Y, Ten Haken RK. Imaging for assessment of radiation-induced normal tissue effects. Int J Radiat Oncol Biol Phys. 2010;76:S140–S144. doi: 10.1016/j.ijrobp.2009.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkacemi Y, Tsoutsou P, Magne N. Metabolic functional imaging for tumor radiosensitivity monitoring. Crit Rev Oncol Hematol. 2007;62:227–239. doi: 10.1016/j.critrevonc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Rudin M. Imaging readouts as biomarkers or surrogate parameters for the assessment of therapeutic interventions. Eur Radiol. 2007;17:2441–2457. doi: 10.1007/s00330-007-0619-9. [DOI] [PubMed] [Google Scholar]
- 7.Guha C, Alfieri A, Blaufox MD. Tumor biology-guided radiotherapy treatment planning: Gross tumor volume versus functional tumor volume. Semin Nucl Med. 2008;38:105–113. doi: 10.1053/j.semnuclmed.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Nimmagadda S, Ford EC, Wong JW. Targeted molecular imaging in oncology: focus on radiation therapy. Semin Radiat Oncol. 2008;18:136–148. doi: 10.1016/j.semradonc.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grosu A-L, Piert M, Weber WA. Positron emission tomography for radiation treatment planning. Strahlenther Onkol. 2005;181:483–499. doi: 10.1007/s00066-005-1422-7. [DOI] [PubMed] [Google Scholar]
- 10.Khoo VS, Joon DL. New developments in MRI for target volume delineation in radiotherapy. Br J Radiol. 2006;79:S2–S15. doi: 10.1259/bjr/41321492. [DOI] [PubMed] [Google Scholar]
- 11.Payne GS, Leach MO. Applications of magnetic resonance spectroscopy in radiotherapy treatment planning. Br J Radiol. 2006;79:S16–S26. doi: 10.1259/bjr/84072695. [DOI] [PubMed] [Google Scholar]
- 12.Gregoire V, Haustermans K, Geets X. PET-based treatment planning in radiotherapy: a new standard? J Nucl Med. 2007;48:68S–77S. [PubMed] [Google Scholar]
- 13.Heron DE, Andrade RS, Beriwal S. PET-CT in radiation oncology: the impact on diagnosis, treatment planning, and assessment of treatment response. Am J Clin Oncol. 2008;31:352–362. doi: 10.1097/COC.0b013e318162f150. [DOI] [PubMed] [Google Scholar]
- 14.Ford EC, Herman J, Yorke E. 18F-FDG PET/CT for image-guided and intensity-modulated radiotherapy. J Nucl Med. 2009;50:1655–1665. doi: 10.2967/jnumed.108.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nestle U, Weber W, Hentschel M. Biological imaging in radiation therapy: role of positron emission tomography. Phys Med Biol. 2009;54:R1–R25. doi: 10.1088/0031-9155/54/1/R01. [DOI] [PubMed] [Google Scholar]
- 16.Zaidi H, Vees H, Wissmeyer M. Molecular PET/CT Imaging-Guided Radiation Therapy Treatment Planning. Acad Radiol. 2009;16:1108–1133. doi: 10.1016/j.acra.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Alber M, Paulsen F, Eschmann SM. On biologically conformal boost dose optimization. Phys Med Biol. 2003;48:N31–N35. doi: 10.1088/0031-9155/48/2/404. [DOI] [PubMed] [Google Scholar]
- 18.Brahme A. Biologically optimized 3-dimensional in vivo predictive assay-based radiation therapy using positron emission tomography-computerized tomography imaging. Acta Oncol. 2003;42:123–136. doi: 10.1080/02841860310004986. [DOI] [PubMed] [Google Scholar]
- 19.Nioutsikou E, Partridge M, Bedford JL. Prediction of radiation-induced normal tissue complications in radiotherapy using functional image data. Phys Med Biol. 2005;50:1035–1046. doi: 10.1088/0031-9155/50/6/001. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Xing L. Towards biologically conformal radiation therapy (BCRT): selective IMRT dose escalation under the guidance of spatial biology distribution. Med Phys. 2005;32:1473–1484. doi: 10.1118/1.1924312. [DOI] [PubMed] [Google Scholar]
- 21.Kim Y, Tomé WA. Risk-adaptive optimization: selective boosting of high-risk tumor subvolumes. Int J Radiat Oncol Biol Phys. 2006;66:1528–1542. doi: 10.1016/j.ijrobp.2006.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.South CP, Partridge M, Evans PM. A theoretical framework for prescribing radiotherapy dose distributions using patient-specific biological information. Med Phys. 2008;35:4599–4611. doi: 10.1118/1.2975229. [DOI] [PubMed] [Google Scholar]
- 23.Søvik Å, Malinen E, Olsen DR. Strategies for biologic image-guided dose escalation: a review. Int J Radiat Oncol Biol Phys. 2009;73:650–658. doi: 10.1016/j.ijrobp.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Søvik Å, Malinen E, Olsen DR. Adapting biological feedback in radiotherapy. Semin Radiat Oncol. 2010;20:138–146. doi: 10.1016/j.semradonc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Ten Haken RK, Martel MK, Kessler ML. Use of Veff and iso-NTCP in the implementation of dose escalation protocols. Int J Radiat Oncol Biol Phys. 1993;27:689–695. doi: 10.1016/0360-3016(93)90398-f. [DOI] [PubMed] [Google Scholar]
- 26.McGinn CJ, Ten Haken RK, Ensminger WD. Treatment of intrahepatic cancers with radiation doses based on a normal tissue complication probability model. J Clin Oncol. 1998;16:2246–2252. doi: 10.1200/JCO.1998.16.6.2246. [DOI] [PubMed] [Google Scholar]
- 27.Hayman JA, Martel MK, Ten Haken RK. Dose escalation in non-small-cell lung cancer using three-dimensional conformal radiation therapy: update of a phase I trial. J Clin Oncol. 2001;19:127–136. doi: 10.1200/JCO.2001.19.1.127. [DOI] [PubMed] [Google Scholar]
- 28.van Baardwijk A, Bosmans G, Bentzen SM. Radiation dose prescription for non-small-cell lung cancer according to normal tissue dose constraints: an in silico clinical trial. Int J Radiat Oncol Biol Phys. 2008;71:1103–1110. doi: 10.1016/j.ijrobp.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Seppenwoolde Y, Engelsman M, De Jaeger K. Optimizing radiation treatment plans for lung cancer using lung perfusion information. Radiother Oncol. 2002;63:165–177. doi: 10.1016/s0167-8140(02)00075-0. [DOI] [PubMed] [Google Scholar]
- 30.McGuire SM, Zhou S, Marks LB. A methodology for using SPECT to reduce intensity-modulated radiation therapy (IMRT) dose to functioning lung. Int J Radiat Oncol Biol Phys. 2006;66:1543–1552. doi: 10.1016/j.ijrobp.2006.07.1377. [DOI] [PubMed] [Google Scholar]
- 31.Munawar I, Yaremko BP, Craig J. Intensity modulated radiotherapy of non-small-cell lung cancer incorporating SPECT ventilation imaging. Med Phys. 2010;37:1863–1872. doi: 10.1118/1.3358128. [DOI] [PubMed] [Google Scholar]
- 32.Shirai S, Sato M, Suwa K. Feasibility and efficacy of Single Photon Emission Computed Tomography-based three-dimensional conformal radiotherapy for hepatocellular carcinoma 8 cm or more with portal vein tumor thrombus in combination with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2010;76:1037–1044. doi: 10.1016/j.ijrobp.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 33.McGuire SM, Marks LB, Yin FF. A methodology for selecting the beam arrangement to reduce the intensity-modulated radiation therapy (IMRT) dose to the SPECT-defined functioning lung. Phys Med Biol. 2010;55:403–416. doi: 10.1088/0031-9155/55/2/005. [DOI] [PubMed] [Google Scholar]
- 34.Pinkawa M, Attieh C, Piroth MD. Dose-escalation using intensity-modulated radiotherapy for prostate cancer - Evaluation of the dose distribution with and without 18F-choline PET-CT detected simultaneous integrated boost. Radiother Oncol. 2009;93:213–219. doi: 10.1016/j.radonc.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Seppälä J, Seppänen M, Arponen E. Carbon-11 acetate PET/CT based dose escalated IMRT in prostate cancer. Radiother Oncol. 2009;93:234–240. doi: 10.1016/j.radonc.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Mazaheri Y, Shukla-Dave A, Muellner A. MR imaging of the prostate in clinical practice. Magn Reson Mater Phy. 2008;21:379–392. doi: 10.1007/s10334-008-0138-y. [DOI] [PubMed] [Google Scholar]
- 37.Chang J, Thakur SB, Huang W. Magnetic resonance spectroscopy imaging (MRSI) and brain functional magnetic resonance imaging (fMRI) for radiotherapy treatment planning of glioma. Technol Cancer Res Treat. 2008;7:349–362. [PubMed] [Google Scholar]
- 38.Lee NY, Mechalakos JG, Nehmeh S. Fluorine-18-Labeled Fluoromisonidazole Positron Emission and Computed Tomography-Guided Intensity-Modulated Radiotherapy for Head and Neck Cancer: A Feasibility Study. Int J Radiat Oncol Biol Phys. 2008;70:2–13. doi: 10.1016/j.ijrobp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geets X, Daisne J-F, Tomsej M. Impact of the type of imaging modality on target volumes delineation and dose distribution in pharyngo-laryngeal squamous cell carcinoma: comparison between pre- and per-treatment studies. Radiother Oncol. 2006;78:291–297. doi: 10.1016/j.radonc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Grills IS, Yan D, Black QC. Clinical implications of defining the gross tumor volume with combination of CT and 18FDG-positron emission tomography in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;67:709–719. doi: 10.1016/j.ijrobp.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 41.Roels S, Slagmolen P, Nuyts J. Biological image-guided radiotherapy in rectal cancer: challenges and pitfalls. Int J Radiat Oncol Biol Phys. 2009;75:782–790. doi: 10.1016/j.ijrobp.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 42.Geets X, Tomsej M, Lee JA. Adaptive biological image-guided IMRT with anatomic and functional imaging in pharyngo-laryngeal tumors: Impact on target volume delineation and dose distribution using helical tomotherapy. Radiother Oncol. 2007;85:105–115. doi: 10.1016/j.radonc.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Hentschel M, Appold S, Schreiber A. Serial FDG-PET on patients with head and neck cancer: implications for radiation therapy. Int J Radiat Biol. 2009;85:796–804. doi: 10.1080/09553000903039180. [DOI] [PubMed] [Google Scholar]
- 44.Dirix P, Vandecaveye V, De Keyzer F. Dose painting in radiotherapy for head and neck squamous cell carcinoma: value of repeated functional imaging with (18)F-FDG PET, (18)F-fluoromisonidazole PET, diffusion-weighted MRI, and dynamic contrast-enhanced MRI. J Nucl Med. 2009;50:1020–1027. doi: 10.2967/jnumed.109.062638. [DOI] [PubMed] [Google Scholar]
- 45.Cao Y, Popovtzer A, Li D. Early prediction of outcome in advanced head-and-neck cancer based on tumor blood volume alterations during therapy: a prospective study. Int J Radiat Oncol Biol Phys. 2008;72:1287–1290. doi: 10.1016/j.ijrobp.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong F-MS, Frey KA, Quint LE. A pilot study of [18F]fluorodeoxyglucose positron emission tomography scans during and after radiation-based therapy in patients with non small-cell lung cancer. J Clin Oncol. 2007;25:3116–3123. doi: 10.1200/JCO.2006.10.3747. [DOI] [PubMed] [Google Scholar]
- 47.Everitt S, Hicks RJ, Ball D. Imaging cellular proliferation during chemoradiotherapy: a pilot study of serial 18F-FLT positron emission tomography/computed tomography imaging for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;75:1098–1104. doi: 10.1016/j.ijrobp.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 48.Gillham C, Zips D, Ponisch F. Additional PET/CT in week 5–6 of radiotherapy for patients with stage III non-small cell lung cancer as a means of dose escalation planning? Radiother Oncol. 2008;88:335–341. doi: 10.1016/j.radonc.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Feng M, Kong F-M, Gross M. Using fluorodeoxyglucose positron emission tomography to assess tumor volume during radiotherapy for non-small-cell lung cancer and its potential impact on adaptive dose escalation and normal tissue sparing. Int J Radiat Oncol Biol Phys. 2009;73:1228–1234. doi: 10.1016/j.ijrobp.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonke J-J, Belderbos J. Adaptive radiotherapy for lung cancer. Semin Radiat Oncol. 2010;20:94–106. doi: 10.1016/j.semradonc.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 51.De Ruysscher D, Houben A, Aerts HJWL. Increased (18)F-deoxyglucose uptake in the lung during the first weeks of radiotherapy is correlated with subsequent Radiation-Induced Lung Toxicity (RILT): a prospective pilot study. Radiother Oncol. 2009;91:415–420. doi: 10.1016/j.radonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Mayr NA, Wang JZ, Zhang D. Longitudinal changes in tumor perfusion pattern during the radiation therapy course and its clinical impact in cervical cancer. Int J Radiat Oncol Biol Phys. 2010;77:502–508. doi: 10.1016/j.ijrobp.2009.04.084. [DOI] [PubMed] [Google Scholar]
- 53.Lavrenkov K, Christian JA, Partridge M. A potential to reduce pulmonary toxicity: The use of perfusion SPECT with IMRT for functional lung avoidance in radiotherapy of non-small cell lung cancer. Radiother Oncol. 2007;83:156–162. doi: 10.1016/j.radonc.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Shioyama Y, Jang SY, Liu HH. Preserving Functional Lung Using Perfusion Imaging and Intensity-Modulated Radiation Therapy for Advanced-Stage Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2007;68:1349–1358. doi: 10.1016/j.ijrobp.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Yaremko BP, Guerrero TM, Noyola-Martinez J. Reduction of Normal Lung Irradiation in Locally Advanced Non-Small-Cell Lung Cancer Patients, Using Ventilation Images for Functional Avoidance. Int J Radiat Oncol Biol Phys. 2007;68:562–571. doi: 10.1016/j.ijrobp.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lavrenkov K, Singh S, Christian JA. Effective avoidance of a functional spect-perfused lung using intensity modulated radiotherapy (IMRT) for non-small cell lung cancer (NSCLC): An update of a planning study. Radiother Oncol. 2009;91:349–352. doi: 10.1016/j.radonc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Bowen SR, Flynn RT, Bentzen SM. On the sensitivity of IMRT dose optimization to the mathematical form of a biological imaging-based prescription function. Phys Med Biol. 2009;54:1483–1501. doi: 10.1088/0031-9155/54/6/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim Y, Tome WA. On the impact of functional imaging accuracy on selective boosting IMRT. Physica Medica. 2009;25:12–24. doi: 10.1016/j.ejmp.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Niyazi M, Bartenstein P, Belka C. Choline PET based dose-painting in prostate cancer--modelling of dose effects. Radiat Oncol. 2010;5:23. doi: 10.1186/1748-717X-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meyer CR, Armatto SG, Fenimore CP. Quantitative imaging to assess tumor response to therapy: common themes of measurement, truth data, and error sources. Transl Oncol. 2009;2:198–210. doi: 10.1593/tlo.09208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jackson EF, Barboriak DP, Bidaut LM. Magnetic resonance assessment of response to therapy: tumor change measurement, truth data and error sources. Transl Oncol. 2009;2:211–215. doi: 10.1593/tlo.09241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McNitt-Gray MF, Bidaut LM, Armatto SG. Computed tomography assessessment of response to theapy: tumor volume change measurement, truth data, and error. Transl Oncol. 2009;2:216–222. doi: 10.1593/tlo.09226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kinahan PE, Doot RK, Wanner-Roybal M. PET/CT assessment of response to therapy: tumor change measeurment, truth data, and error. Transl Oncol. 2009;2:223–230. doi: 10.1593/tlo.09223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barnhart HX, Barboriak DP. Applications of the repeatability of quantitative imaging biomarkers: a review of statistical analysis of repeat data sets. Transl Oncol. 2009;2:231–235. doi: 10.1593/tlo.09268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clarke LP, Sriram RD, Schilling LB. Imaging as a Biomarker: Standards for Change Measurements in Therapy workshop summary. Acad Radiol. 2008;15:501–530. doi: 10.1016/j.acra.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 66.Clarke LP, Croft BS, Nordstrom R. Qualitative imaging for evaluation of response to cancer therapy. Transl Oncol. 2009;2:195–197. doi: 10.1593/tlo.09217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kessler ML. Image registration and data fusion in radiation therapy. Br J Radiol 79 Spec No. 2006;1:S99–S108. doi: 10.1259/bjr/70617164. [DOI] [PubMed] [Google Scholar]
- 68.Papavasileiou P, Divoli A, Hatziioannou K. The importance of the accuracy of image registration of SPECT images for 3D targeted radionuclide therapy dosimetry. Phys Med Biol. 2007;52:N539–N548. doi: 10.1088/0031-9155/52/24/N01. [DOI] [PubMed] [Google Scholar]
- 69.Yin L, Shcherbinin S, Celler A. Incorporating Quantitative Single Photon Emission Computed Tomography into Radiation Therapy Treatment Planning for Lung Cancer: Impact of Attenuation and Scatter Correction on the Single Photon Emission Computed Tomography-Weighted Mean Dose and Functional Lung Segmentation. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2009.11.035. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 70.Yin LS, Tang L, Hamarneh G. Complexity and accuracy of image registration methods in SPECT-guided radiation therapy. Phys Med Biol. 2010;55:237–246. doi: 10.1088/0031-9155/55/1/014. [DOI] [PubMed] [Google Scholar]
- 71.Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48:932–945. doi: 10.2967/jnumed.106.035774. [DOI] [PubMed] [Google Scholar]
- 72.Barbee DL, Flynn RT, Holden JE. A method for partial volume correction of PET-imaged tumor heterogeneity using expectation maximization with a spatially varying point spread function. Phys Med Biol. 2010;55:221–236. doi: 10.1088/0031-9155/55/1/013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aerts HJWL, Bosmans G, van Baardwijk AAW. Stability of 18F-deoxyglucose uptake locations within tumor during radiotherapy for NSCLC: a prospective study. Int J Radiat Oncol Biol Phys. 2008;71:1402–1407. doi: 10.1016/j.ijrobp.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 74.Nestle U, Kremp S, Schaefer-Schuler A. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-Small cell lung cancer. J Nucl Med. 2005;46:1342–1348. [PubMed] [Google Scholar]
- 75.Hatt M, Cheze le Rest C, Descourt P. Accurate automatic delineation of heterogeneous functional volumes in positron emission tomography for oncology applications. Int J Radiat Oncol Biol Phys. 2010;77:301–308. doi: 10.1016/j.ijrobp.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 76.Cao Y, Li D, Shen Z. Sensitivity of quantitative metrics derived from DCE MRI and a pharmacokinetic model to image quality and acquisition parameters. Acad Radiol. 2010;17:468–478. doi: 10.1016/j.acra.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50:11S–20S. doi: 10.2967/jnumed.108.057182. [DOI] [PubMed] [Google Scholar]
- 78.Shankar LK, Hoffman JM, Bacharach S. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–1066. [PubMed] [Google Scholar]
- 79.MacManus M, Nestle U, Rosenzweig KE. Use of PET and PET/CT for radiation therapy planning: IAEA expert report 2006–2007. Radiother Oncol. 2009;91:85–94. doi: 10.1016/j.radonc.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 80.Das SK, Miften MM, Zhou S. Feasibility of optimizing the dose distribution in lung tumors using fluorine-18-fluorodeoxyglucose positron emission tomography and single photon emission computed tomography guided dose prescriptions. Med Phys. 2004;31:1452–1461. doi: 10.1118/1.1750991. [DOI] [PubMed] [Google Scholar]
- 81.Vanderstraeten B, Duthoy W, Gersem WD. [18F]fluoro-deoxy-glucose positron emission tomography ([18F]FDG-PET) voxel intensity-based intensity-modulated radiation therapy (IMRT) for head and neck cancer. Radiother Oncol. 2006;79:249–258. doi: 10.1016/j.radonc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 82.Thorwarth D, Eschmann S-M, Paulsen F. Hypoxia Dose Painting by Numbers: A Planning Study. Int J Radiat Oncol Biol Phys. 2007;68:291–300. doi: 10.1016/j.ijrobp.2006.11.061. [DOI] [PubMed] [Google Scholar]