Abstract
Changes in the intracellular concentration of calcium ([Ca2+]i) represent a vital signaling mechanism enabling communication among cells and between cells and the environment. The initiation of embryo development depends on a [Ca2+]i increase(s) in the egg, which is generally induced during fertilization. The [Ca2+]i increase signals egg activation, which is the first stage in embryo development, and that consist of biochemical and structural changes that transform eggs into zygotes. The spatiotemporal patterns of [Ca2+]i at fertilization show variability, most likely reflecting adaptations to fertilizing conditions and to the duration of embryonic cell cycles. In mammals, the focus of this review, the fertilization [Ca2+]i signal displays unique properties in that it is initiated after gamete fusion by release of a sperm-derived factor and by periodic and extended [Ca2+]i responses. Here, we will discuss the events of egg activation regulated by increases in [Ca2+]i, the possible downstream targets that effect these egg activation events, and the property and identity of molecules both in sperm and eggs that underpin the initiation and persistence of the [Ca2+]i responses in these species.
IP3 receptors and phospholipase C[ζ] help generate characteristic Ca2+ oscillations in eggs at fertilization. These are decoded by enzymes such as CamKII, leading to changes that transform the egg into a zygote.
An increase in the intracellular concentration of calcium ([Ca2+]i) underlies the initiation, progression and/or completion of a wide variety of cellular processes, including fertilization, muscle contraction, secretion, cell division, and apoptosis (Berridge et al. 2000). To survive and proliferate, cells and organisms must communicate, and changes in [Ca2+]i allow them to quickly respond to environmental, nutritional, or ligand challenges with responses that regulate cell fate and function. Cells devote significant amounts of their energy reserves to create and maintain ionic gradients between extracellular and intracellular milieus and also within the latter, thereby allowing brief alterations in these gradients to have profound signaling effects. In the case of Ca2+, myriad proteins have acquired the ability to bind Ca2+, which allows them to interpret and transform these elevations into cellular functions. This review will examine the cellular modifications induced by [Ca2+]i changes during fertilization in mature mammalian oocytes, henceforth referred to as eggs.
Oocytes during maturation ready themselves for fertilization and the initiation of embryogenesis. During this transition, oocytes undergo changes that include the resumption and progression of meiosis, the development of polyspermy-preventing mechanisms, the reorganization of the cytoskeleton with spindle formation and displacement to the cortex, and the translation, accumulation, and degradation of specific mRNAs and proteins involved in development (Horner and Wolfner 2008b). In most species, and in all mammals, a [Ca2+]i signal is responsible for breaking the meiosis-imposed developmental pause, causing egg activation, which is the first stage of embryo development (Whitaker 2006; Horner and Wolfner 2008b). The egg activating [Ca2+]i signal is generally associated with sperm-egg fusion, which occurs at different stages of meiosis depending on the species (Stricker 1999), although in insects, where fertilization is dissociated from activation and where embryos can develop parthenogenetically, the presumed [Ca2+]i increase is thought to be induced by mechanical stimulation during ovulation/oviductal transport (Page and Orr-Weaver 1997; Horner and Wolfner 2008a).
The [Ca2+]i responses that underlie egg activation offer a great deal of diversity regarding their spatiotemporal configuration, reflecting both the plasticity of the Ca2+ signaling machinery as well as the dissimilar Ca2+ requirements for egg activation among species. Generally speaking, species can be categorized either as displaying a single [Ca2+]i increase, which is the case of sea urchins, starfish, frogs, and fish, or showing multiple [Ca2+]i changes, also known as oscillations, which is the case of nemertian worms, ascidians, and mammals (Stricker 1999; Miyazaki and Ito 2006). Elucidation of the signaling cascades and identification of the molecules/receptor(s) that initiate the Ca2+ signal at fertilization has proven elusive, and this review will not dwell on that literature; readers are referred to excellent recent reviews on the subject (Whitaker 2006; Parrington et al. 2007). Nonetheless, research has found that Src-family kinases (SFKs) and phospholipase Cγ (PLCγ) are involved in the activation of the phosphoinositide pathway and production of inositol 1,4,5-trisphosphate (IP3) during fertilization in sea urchins, starfish, and frogs, which reflects the contribution of a plasma membrane receptor/signaling complex (Giusti et al. 1999; Sato et al. 2000). Remarkably, a receptor responsible for recruiting and activating SFKs during fertilization remains undiscovered (Mahbub Hasan et al. 2005). Similarly, it has proved difficult to uncover how the sperm initiates oscillations. Research now suggests that this may be accomplished by a novel mechanism whereby the signaling molecule/cargo, known as the sperm factor (SF), is released by the sperm into the ooplasm after fusion of the gametes. Importantly, the SF is not IP3 or Ca2+ but rather it contains a protein moiety (Swann 1990; Wu et al. 1997; Kyozuka et al. 1998; Harada et al. 2007). To date, only the mammalian SF’s molecular identity has been resolved, and found to be another member of the PLC family, a novel sperm-specific isoform named PLCζ (Saunders et al. 2002). This review will examine the literature on mammalian PLCζs and will focus as well on the egg molecules that are required to initiate and sustain [Ca2+]i oscillations in these species.
EGG ACTIVATION
Following the resumption of meiosis during maturation, vertebrate eggs arrest at the metaphase stage of the second meiosis (MII). Sperm entry induces the resumption and completion of meiosis, release of cortical granules (CG), progression into interphase and pronuclear (PN) formation (Fig. 1A); these phenomena, which make possible the transition from egg to embryo, are collectively known as “egg activation” (Schultz and Kopf 1995; Stricker 1999; Ducibella et al. 2002). As stated earlier, an increase in [Ca2+]i is the universal trigger of egg activation in all species studied to date (Stricker 1999), and in mammals this signal adopts a pattern of brief but periodical increases in [Ca2+]i that last for several hours after sperm entry (Miyazaki et al. 1986). The spatiotemporal pattern of these [Ca2+]i responses is decoded by downstream effectors, underpinning the distinct cellular events. We briefly review the events of egg activation that are controlled by [Ca2+]i increases and the underlying molecular effectors.
Figure 1.
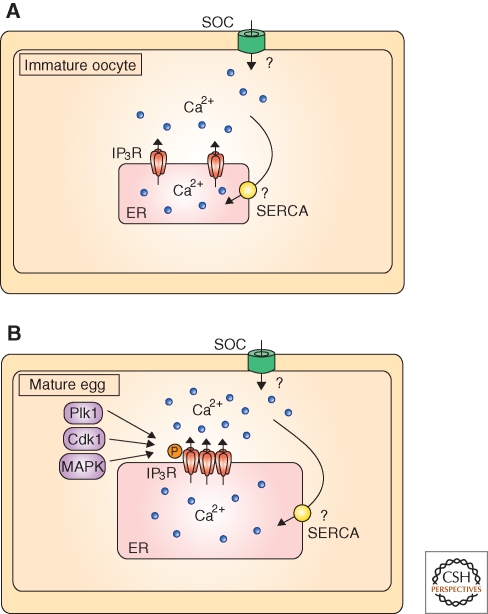
Temporal course of activation events in mouse eggs with a characteristic [Ca2+]i response and candidate molecules involved in [Ca2+]i oscillations and Ca2+ homeostasis. (A) Main cellular events of egg activation and approximate time in hours (hr) required for their completion after sperm entry. (B) A typical pattern of [Ca2+]i oscillations associated with fertilization or with injection of PLCζ cRNA. Note that recordings were terminated prematurely. (C) On fusion, the sperm delivers phospholipase C (PLC)ζ, which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphospahte (IP3) and dyacyl glycerol (DAG). IP3 binds its receptor, IP3R1, causing Ca2+ release out of the endoplasmic reticulum (ER). Following Ca2+ release, basal [Ca2+]i levels are regulated by the combined action of the sarcoendoplasmic reticulum Ca2+ATPase (SERCA), plasma membrane Ca2+ pump (PMCA), Na/Ca2+ exchanger and mitochondria. Store operated Ca2+ channels (SOC) are proposed to mediate Ca2+ influx required to fill the ER and maintain oscillations. Broken lines suggest feedback action of Ca2+ on IP3R1.
Events of Egg Activation Require [Ca2+]i Increases
Release from the MII arrest is an early and necessary event of egg activation, as it allows completion of meiosis, establishment of euploidy and progression into interphase with DNA synthesis. The MII block is imposed at the conclusion of maturation prior to ovulation by the action of the cytostatic factor (CSF) (Masui and Markert 1971). The CSF constrains the activity of the anaphase promoting factor (APC), an E3 ubiquitin ligase (Tunquist and Maller 2003), which is responsible for the ubiquitination and degradation of cyclin B (CycB). CycB and the cyclin-dependent kinase 1 (Cdk1, also known as cdc2) are the molecular components of the maturation promoting factor (MPF) (Swenson et al. 1986; Draetta et al. 1989), and inhibition of CycB degradation by the CSF stabilizes MPF, which results in MII arrest, as MPF activity correlates with metaphase (Masui and Markert 1971). Research has shown that endogenous meiotic inhibitor 2 (Emi2) is an inhibitory component of APC that underlies the MII arrest (Schmidt et al. 2005; Tung et al. 2005; Shoji et al. 2006). In MII oocytes, inhibition of APC also ensures that persistent levels of the separase inhibitor securin prevents sister chromatid separation (Madgwick et al. 2004; Nabti et al. 2008).
The association between intracellular ionic increases and release from meiotic arrest was first proposed at the end of the nineteenth century by Loeb and colleagues who observed that initiation of development in sea urchin eggs was possible simply by varying the concentration and composition of the fertilizing medium (Loeb 1907). Subsequently, it was learned that [Ca2+]i levels dramatically change after fertilization (Mazia 1935), which focused attention on the role of this ion. Steinhardt and colleagues showed the dominant role of Ca2+, as they were able to promote parthenogenetic development in a variety of species by exposing eggs to Ca2+ ionophores (Steinhardt et al. 1974).
How [Ca2+]i increases induce release from the MII arrest remained unknown for decades. Research showed that [Ca2+]i increases per se were unable to induce CycB degradation and that instead it required binding to a calmodulin-sensitive enzyme (Lorca et al. 1993), which was later shown to be Ca2+-calmodulin-dependent protein kinase II (CaMKII). Nonetheless, how CaMKII relieved the CSF-imposed MII arrest remained unknown. Adding to the confusion was the finding that despite the need for a [Ca2+]i increase, Xenopus egg extracts depleted of Polo-like kinase 1 (Plx1), a kinase known until then more for its role on spindle organization, were unable to overcome the MII arrest (Descombes and Nigg 1998). This quandary was solved when it was discovered that Emi2 is a key component of CSF and that Emi2 phosphorylation by Plx1, which causes its degradation, is required for CycB proteasomal degradation (Schmidt et al. 2005). Subsequent studies found that binding of Plx1 to Emi2 requires a preceding phosphorylation by CaMKII, thereby molecularly linking the need for [Ca2+]i and Plx1 to exit MII.
Although the aforementioned pathway was elucidated in Xenopus eggs and extracts, mouse eggs rely on similar mechanisms to enter and exit the MII arrest. Most prominently, it is well documented that CycB undergoes degradation during fertilization (Nixon et al. 2002) and that each of the sperm-induced [Ca2+]i increases is accompanied by a parallel increase in CaMKII activity (Markoulaki et al. 2003). The role of CaMKII on mammalian egg activation was convincingly shown by studies in the mouse, in which expression of constitutive active forms of CaMKII into eggs initiated all events of egg activation, except CG exocytosis, and promoted development to the blastocyst stage (Madgwick et al. 2005; Knott et al. 2006). Conversely, depletion of the CaMKIIγ isoform abrogated the ability of these eggs to exit MII in response to [Ca2+]i stimulation (Backs et al. 2010; Chang et al. 2009), causing infertility. Research also implicated Emi2 in MII arrest in the mouse, as inhibition of Emi2 synthesis prevents cyclin B1 accumulation during maturation (Madgwick et al. 2006), which causes spontaneous activation (Shoji et al. 2006). The role of Plk1 in mouse MII arrest remains unexplored, although our preliminary data show that treatment of eggs with BI2536, a new and selective Plk1 inhibitor, prevents CycB degradation and MII exit in eggs treated with SrCl2 (data not shown). In Xenopus, Plx1 phosphorylates xEmi2 within a phosphodegron motif, after which xEmi2 is rapidly targeted for degradation, but this motif, or its canonical replacements, is absent in the mouse homolog (Perry and Verlhac 2008). Although the role of Ca2+ and the molecular pathways required for MII exit and embryo development are conserved in vertebrate eggs, it is presently unknown how Plk1 regulates Emi2 function in mammals.
CG exocytosis is another event of egg activation that depends on Ca2+ release (Kline and Kline 1992a). CG release underlies, at least in part, the cortical remodeling that occurs after fertilization (Sardet et al. 2002), and modifies the components of zona pellucida to prevent polyspermy, thereby ensuring the formation of a diploid zygote. Although it was believed that the effects of Ca2+ on CGs were transduced by activation of protein kinase C (PKC), as PKC agonists promoted CG release and other activation events, later studies using PKC inhibitors failed to prevent fertilization-associated CG release (Ducibella and LeFevre 1997). Importantly, the widespread expression of PKC isoforms in oocytes (Gallicano et al. 1997; Eliyahu et al. 2001; Page Baluch et al. 2004), along with their distinct cellular distribution (Viveiros et al. 2001; Page Baluch et al. 2004), and the implications of their impact on Ca2+ influx (Halet 2004), suggest important roles for these enzymes in setting off embryo development. CaMKII was also expected to participate in CG exocytosis, although the aforementioned studies using constitutively active forms of the protein (Knott et al. 2006) or eggs devoid of CaMKII have ruled out this possibility (Backs et al. 2010; Chang et al. 2009). Recent studies have implicated myosin light chain kinase (MLCK), another Ca2+-dependent kinase, as being involved in CG exocytosis in mouse fertilization, as pharmacological inhibitors greatly diminished their release in response to Ca2+ stimulation (Matson et al. 2006). The role of MLCK on CG exocytosis is not unexpected, as myosin II, a direct target of MLKC, and actin microfilaments are involved in cortical reorganization in the mouse (Simerly et al. 1998; Deng et al. 2005) and zebrafish eggs (Becker and Hart 1999). Importantly, the molecular regulation of MLCK needs to be determined, as besides its requirement for Ca2+, it is highly sensitive to phosphorylation, and kinases such as ERK and Rho that are active during meiosis might have regulatory roles (Deng et al. 2005). In summary, the molecular effectors for several events of egg activation downstream of Ca2+ have been uncovered over the last decade. Although important gaps remain, the requirement for [Ca2+]i oscillations for initiation of mammalian development is unambiguous.
Single versus Multiple [Ca2+]i Increases
The early ionophore studies hinted to a pivotal role for Ca2+ in the initiation of development, especially with regard to the increases in [Ca2+]i, but not in K+ or pH, induced all early and late events of egg activation (Steinhardt and Epel 1974). Nevertheless, whether or not such changes happened during normal fertilization and how their inhibition affected development was unknown. Evidence soon accumulated, first using the luminescent protein “aequorin” synthesized by Shimomura and colleagues (Shimomura and Johnson 1970) that explosive [Ca2+]i increases accompanied fertilization in medaka fish eggs (Ridgway et al. 1977) and in sea urchin eggs (Steinhardt et al. 1977). Unlike the single [Ca2+]i increases detected in these early recordings, measurements of [Ca2+]i changes in mammals revealed that their eggs displayed [Ca2+]i oscillations (Cuthbertson et al. 1981; Miyazaki and Igusa 1981). Although oscillatory [Ca2+]i responses were subsequently reported in nonmammalian species, mammalian eggs are the only ones whose oscillations extend for over several hours (Stricker 1999). Further, research soon followed demonstrating that abrogation of fertilization-associated [Ca2+]i increases, which was accomplished with the Ca2+ chelator BAPTA, prevented all events of egg activation and prevented the initiation of development (Kline and Kline 1992a). Together, these results confirmed the widespread role of Ca2+ as the activation signal for development.
Although the elevation of [Ca2+]i is ubiquitous in fertilization, the presence of long-lasting oscillations is a hallmark of mammalian fertilization (Fig. 1B). Remarkably, the developmental advantages and underlying molecular changes associated with these oscillations remain unclear. Research by Ducibella et al. underscored the varying sensitivities of egg activation events to [Ca2+]i increases. For instance, most events of egg activation, such as CG exocytosis, meiotic resumption and recruitment of maternal mRNAs require fewer [Ca2+]i increases for initiation than for completion, and early events such as CG exocytosis and release from MII arrest require fewer [Ca2+]i responses for completion than later events, such as PN formation and recruitment of maternal mRNAs (Ducibella et al. 2002). In this context, oscillations make sense, especially to promote CycB degradation and inactivation of MPF, as CycB synthesis is continuous (Nixon et al. 2002; Marangos and Carroll 2004) and a single [Ca2+]i increase would be unable to promote its complete degradation (Nixon et al, 2002). Nevertheless, if exit of MII arrest is overcome either by a single, overwhelming [Ca2+]i increase induced by an electrical pulse (Ozil et al. 2005) or by expression of a constitutively active form of CaMKII, development to the blastocyst stage is only mildly impaired (Madgwick et al. 2005; Ozil et al. 2005; Knott et al. 2006). This apparent lack of impact of [Ca2+]i oscillations on preimplantation development is in contrast to another report showing that both premature termination or excessive Ca2+ stimulation negatively impacts preimplantation and postimplantation development, and alters embryonic gene expression (Ozil et al. 2006). Similar research documented that parthenogenotes generated without a [Ca2+]i increase by exposing eggs to cycloheximide, a protein synthesis inhibitor, showed altered gene expression and poor development to the blastocyst stage (Rogers et al. 2006). Nonetheless, development of these embryos was rescued to the same extent by exposure to a single or multiple [Ca2+]i increases, casting doubts on the beneficial effects of [Ca2+]i oscillations on development.
One way that multiple [Ca2+]i elevations may pose a developmental advantage is by specifically stimulating embryonic gene expression. The recruitment of maternal mRNAs, which mediates new protein synthesis after fertilization, takes place during the period of oscillations and is susceptible to the magnitude of the [Ca2+]i stimulation; more pulses more protein synthesis (Ducibella et al. 2002). To this end, one of the two transcripts identified after fertilization is cyclin A (Oh et al. 1997; Fuchimoto et al. 2001), which participates in the activation of the embryonic genome (Fuchimoto et al. 2001). Thus, based on research in hippocampal neurons, the suggestion was made that pulsatile activation of CaMKII may underlie the enhanced gene expression observed after repeated [Ca2+]i pulses (Ducibella et al. 2006). Subsequent research, however, showed that recruitment of mRNAs could occur independently of this kinase (Backs et al. 2010). Furthermore, it might not be under the exclusive control of Ca2+, as in the absence of cell cycle progression, fertilization-initiated oscillations failed to induce recruitment of mRNAs (Backs et al. 2010). Therefore, it might that the total magnitude of the [Ca2+]i increase, as proposed by Ozil and colleagues (Ducibella et al. 2006; Ozil et al. 2006) rather than the temporal pattern of [Ca2+]i increases is the determinant factor of egg activation in mammals. Nonetheless, oscillations may be necessary, as besides signaling the stepwise completion of all events of egg activation, it might be the only manner whereby mammalian eggs can attain a Ca2+ signal of sufficient magnitude to ensure CycB degradation without undermining other cellular functions.
MOLECULAR PLAYERS RESPONSIBLE FOR [Ca2+]i OSCILLATIONS DURING FERTILIZATION
The [Ca2+]i oscillations that underlie egg activation in mammals rely on molecular players widely characterized in other cellular systems in which they mediate [Ca2+]i responses induced by a variety of agonists such as hormones, growth factors and antigen-presenting mechanisms (Berridge et al. 2000; Clapham 2007). In gametes however, the function and regulation of some of these molecules has been adapted to respond to the unique requirements of fertilization. For instance, oocytes require weeks or months of preparation before being ready for fertilization, because interruptions in the cell cycle are imposed during meiosis to synchronize oocyte and follicular growth before reinitiating meiosis and ovulation. It is believed that during this growth phase, oocytes do not require [Ca2+]i elevations, and Ca2+ release mechanisms are quiescent (Carroll et al. 1994). Importantly, these mechanisms are quickly reactivated in fully-grown oocytes after receiving an LH surge, which is the endocrinological signal that induces oocyte maturation from the germinal vesicle (GV) stage. During this process, which may last from 12 to 48 hr according to the species, the oocytes’ Ca2+ release mechanisms undergo reprogramming and optimization so that fertilization can initiate [Ca2+]i oscillations. The sperm also undergoes a protracted preparation, undergoing changes during transport through the male reproductive tract and more closely as it approaches the site of fertilization in the female tract (Suarez 2008b). Remarkably, some of these changes also involve [Ca2+]i increases (Suarez 2008a), although they occur while preserving the sperms’ Ca2+ activating signal.
Two molecules stand out in mammalian fertilization as central to the initiation and maintenance of [Ca2+]i oscillations; namely, the IP3R1 receptor in eggs and PLCζ in the sperm. Here we will describe the evidence supporting their role in mammalian fertilization, focusing on regulatory mechanisms and highlighting some of the unanswered questions regarding their regulation. We will also review other molecular mechanisms required to maintain oscillations, especially those affecting Ca2+ influx whose function in eggs has not been widely investigated (Fig. 1C).
IP3R1
IP3R1 in MII Eggs
The IP3R is the main intracellular Ca2+-release channel of almost all mammalian cell types and is located in the endoplasmic reticulum (ER), the cells’ main Ca2+ reservoir (reviewed in Berridge et al. [2000]; Bootman et al. [2001]). The IP3R is a large protein (>250 kDa) and functions as a tetramer (>1000 kDa). Each monomer consists of more than 2600 amino acids and can be broadly divided into three regions, a cytosolic amino-terminal domain that binds IP3, a regulatory domain that contains multiple regulatory sites for Ca2+, ATP, and other modulatory molecules/proteins (MacKrill 1999; Patterson et al. 2004) and a carboxy-terminal channel domain that contains six transmembrane domains and a short cytosolic tail. As described by Taylor and Tovey (2010), the activation and opening of the IP3R1 requires binding by both Ca2+ and IP3, and the regulation of IP3-induced Ca2+ release (IICR) by Ca2+ adopts a bell-shape form, as IICR is stimulated at low [Ca2+]i and inhibited at high [Ca2+]i (Taylor and Tovey 2010; Iino 1990; Finch et al. 1991). This dual regulation of IP3R1 by Ca2+ and IP3 makes it especially suited to support long lasting oscillations.
There exists three IP3R isoforms (reviewed in (Berridge et al. [2000]), and mammalian oocytes and eggs and their surrounding cells express all isoforms (Fissore et al. 1999a; Fissore et al. 1999b; Diaz-Munoz et al. 2008). Importantly, oocytes and eggs overwhelmingly express the type I IP3R isoform (Kume et al. 1997; Fissore et al. 1999a; Jellerette et al. 2000; Iwasaki et al. 2002). The initial suggestion that IP3R may play a role during fertilization arose from studies in sea urchin eggs in which an increase in phosphoinositide metabolism accompanied fertilization (Turner et al. 1984), an observation that was soon followed by the demonstration that injection of IP3 triggered Ca2+ release (Clapper and Lee 1985) and cortical granule exocytosis (Turner et al. 1986). Studies followed in hamster oocytes in which injection of IP3 and guanine nucleotides initiated repeated Ca2+ release from intracellular stores (Miyazaki 1988). Purification and identification of the IP3R protein from the cerebellum occurred in the late 1980s (Maeda et al. 1988; Furuichi et al. 1989), and confirmation of its significance in mammalian fertilization took place soon after when both the initiation of [Ca2+]i oscillations (Miyazaki et al. 1992) and egg activation (Xu et al. 1994) were prevented by injection of a functional blocking antibody raised against the Carboxy-terminal end of mouse IP3R1. Subsequent studies confirmed the role of IP3R1 in fertilization in other species (Parys et al. 1994; Thomas et al. 1998; Yoshida et al. 1998; Runft et al. 1999; Goud et al. 2002; Iwasaki et al. 2002).
Fertilization-associated [Ca2+]i oscillations in mouse zygotes undergo changes during the transition from the MII stage into interphase, becoming initially less frequent before ceasing altogether at the time of PN formation (Jones et al. 1995; Kono et al. 1996; Deguchi et al. 2000). During this transition, the IP3R1 undergo several modifications and it is possible that either singly or collectively these influence the pattern of oscillations. For example, mammalian eggs richly express IP3R1, as only 20 mouse eggs are required for its detection by Western blotting (Parrington et al. 1998; Jellerette et al. 2000) and within 4 h after sperm entry the IP3R1 mass is reduced approximately to a half (Parrington et al. 1998; Deguchi et al. 2000; Kurokawa and Fissore 2003). Moreover, recent research shows that IP3R1 degradation alone can explain the widening of the [Ca2+]i intervals, although not the termination of oscillations (Lee et al.). Changes in IP3R1 localization may also affect the pattern of oscillations. In eggs, the IP3R1 and the ER are organized in clusters near the cortex, a location that might facilitate the initiation of [Ca2+]i oscillations, as the PLCζ concentration may be higher in this area after sperm-egg fusion. The accumulation of ER clusters in the cortex may also enhance IP3R1 sensitivity, as [Ca2+]i oscillations originate from the hemisphere opposite to the MII spindle where ER/IP3R1 clusters are particularly dense (Kline et al. 1999; Dumollard et al. 2004). Interestingly, in Xenopus, IP3R1s that are more sensitive move to the cortex from the subcortex as oocytes progress to the MII stage (Boulware and Marchant 2005). Importantly, in the mouse, ER (FitzHarris et al. 2003) and possibly IP3R1 (our unpublished data) cortical clusters disappear ahead of the termination of the oscillations, suggesting that they are not required for the persistence of oscillations. Nevertheless, the precise distribution of IP3R1 in eggs suggest an important role during fertilization, which may correspond to the need for localized high amplitude [Ca2+]i increases to facilitate CG release to prevent polyspermy (McAvey et al. 2002).
IP3R1 function may also be regulated by phosphorylation. Not surprisingly, the first report describing IP3R1 phosphorylation in eggs suggested an association with cell cycle kinases (Jellerette et al. 2004), which play a prominent role in the MII arrest. IP3R1 phosphorylation in mouse eggs was first characterized using an antibody that identifies proteins phosphorylated at the MPM-2 epitope, which consists of phosphorylated serines(S)/threonines(T) next to prolines(P) surrounded by hydrophobic amino acids (Westendorf et al. 1994). IP3R1 becomes phosphorylated at a MPM-2-detectable epitope during maturation (Lee et al. 2006) reaching maximal reactivity at the MII stage (Fig. 2). Following egg activation, it becomes gradually dephosphorylated and phosphorylation is not regained at first mitosis (Lee et al. 2006). The responsible kinases for IP3R1 MPM-2 phosphorylation remain to be determined, although several M-phase kinases, such as polo-like kinase 1 (Plk1), mitogen-activated protein kinase (MAPK), and Cdk1 can all phosphorylate this epitope (Joughin et al. 2009). Our studies in mouse oocytes using pharmacological inhibitors revealed that Plk1 might be involved in MPM-2 phosphorylation early during maturation (Ito et al. 2008; Vanderheyden et al. 2009) and MAPK during the MI to MII transition, although whether they directly phosphorylate the receptor remains to be shown. Research in DT40 B-cell lymphocytes showed IP3R1 phosphorylation by MAPK at S436, a residue that lies in the receptor’s ligand binding domain within a consensus site for the kinase; using back cross phosphorylation studies, this group also showed IP3R1 MAPK phosphorylation of mouse MII eggs (Bai et al. 2006). This study did not examine the role of this phosphorylation in eggs, although in microsome preparations MAPK IP3R1 phosphorylation decreased IP3 binding and Ca2+ release (Bai et al. 2006). In contrast to this, phosphorylation of IP3R1 in somatic cells by Cdk1, which was observed to occur in several conserved Cdk1 motifs under in vitro and in vivo conditions, enhanced IP3 binding and Ca2+ release (Malathi et al. 2003; Malathi et al. 2005). IP3R1 phosphorylation within Cdk1 and MAPK consensus sites was reported in Xenopus oocytes and independent activation of these kinases increased IP3R-mediated Ca2+ release (Sun et al. 2009). Notably, an earlier study in mouse zygotes had dismissed the role of MPF and MAPK on [Ca2+]i oscillations, as the continuation was unaltered by the decline in MPF activity, which occurs at the time of second PB extrusion, or after inhibition of the MAPK pathway with U0126 (Marangos et al. 2003). Importantly, the phosphorylation status of IP3R1 was not examined in that study, and subsequent results found that IP3R1 MPM-2 phosphorylation outlasts MPF activity (Lee et al. 2006) and that 4 hours U0126 exposure does not eliminate MPM-2 IP3R1 reactivity (our unpublished observations). A possible interpretation for these results is that IP3R1 phosphorylation, and the phosphorylation of other M-phase substrates in eggs is safeguarded by suppression of phosphatase(s) activity, a function that has been attributed to the Greatwall kinase in mitotic cells and Xenopus egg extracts (Castilho et al. 2009). Therefore, accumulating evidence suggests a regulatory role for phosphorylation on IP3R1 function during fertilization, although the responsible kinases, phosphorylation sites and their impact on IP3R1 function remain to be clarified.
Figure 2.
IP3R1-mediated Ca2+ release increases during mouse oocyte maturation. Several factors may contribute to this, including the increased Ca2+ content of the stores, IP3R1 organization into clusters and IP3R1 phosphorylation. Question marks suggest mechanisms that are suspected to contribute to Ca2+ influx and increased Ca2+ store content. Phosphorylating kinases are Polo-like kinase-1, Cyclin-dependent kinase-1, and Mitogen-associated protein kinase.
IP3R1 in Maturing Oocytes
The precise spatio-temporal pattern of sperm-associated [Ca2+]i responses in vertebrate eggs is established during oocyte maturation. For example, in vitro fertilized mouse GV oocytes show fewer [Ca2+]i oscillations and each [Ca2+]i increase shows reduced duration and amplitude than those observed in fertilized MII eggs (Jones et al. 1995a; Mehlmann et al. 1996). The molecular events underlying these changes are not understood, although changes in IP3R1 sensitivity, i.e., the receptor’s ability to conduct Ca2+ in response to IP3, are thought to be involved. Importantly, studies to elucidate these mechanisms are needed, but given the recalcitrant nature of some of these changes in MII eggs, it is suggested that they should be performed during maturation.
As discussed above, IP3R1 phosphorylation during maturation by M-phase kinases is thought to enhance Ca2+ release in eggs. MPM-2 reactivity, which is used as a marker of their activity, is first evidenced in IP3R1 at the time of GV breakdown (GVBD) and persists until the MII stage, a period that closely coincides with the increased function of IP3R1 during maturation (Mehlmann and Kline 1994). Inhibition of Plk1, a kinase involved in the activation of Cdk1, reduced and delayed MPM-2 IP3R1 reactivity and decreased [Ca2+]i release through IP3R1 at the GVBD stage (Ito et al. 2008a; Vanderheyden et al. 2009). Nonetheless, the persistent presence of BI2556, a nonreversible and specific Plk1 inhibitor, did not eliminate MPM-2 IP3R1 reactivity, which even experienced a partial recovery, suggesting that other kinases, possibly Cdk1, might phosphorylate IP3R1 (our unpublished observation). Investigation of the role of Cdk1 on IP3R1 phosphorylation in mouse oocytes/eggs is hindered by the findings that roscovitine, a specific Cdk1 inhibitor, indirectly inhibits Plk1 activity (our unpublished results) and greatly reduces Ca2+ store content ([Ca2+]ER) (Deng and Shen 2000), thereby compromising the interpretation of the results. Inhibition of the MAPK pathway, which does not affect the aforementioned kinases and only mildly affects IP3R1 MPM-2 phosphorylation after the MI stage, greatly reduces [Ca2+]i responses (Lee et al. 2006). It is therefore possible that IP3R1 phosphorylation by MAPK is not recognized by the MPM-2 antibody or, alternatively, that the MAPK pathway affects other aspects of Ca2+ homeostasis. In this regard, one study found an altered Ca2+ content in U1026-treated oocytes (Matson and Ducibella 2007) whereas the other did not (Lee et al. 2006). Therefore, additional mutational studies are needed to clarify the impact of M-phase kinases on IP3R1-mediated Ca2+ release in mammalian oocytes and eggs.
Besides M-phase kinases, numerous studies in somatic cells have shown that IP3R isoforms can be phosphorylated by various, more wide-ranging kinases that generally increase IP3R-mediated Ca2+ release (Bezprozvanny 2005; Vanderheyden et al. 2009), although phosphorylation by PKB reportedly reduces IP3R-mediated Ca2+ release (Szado et al. 2008). The most commonly implicated kinases include PKA, PKC, and CaMKII, all of which have important physiological functions in oocytes and eggs (Ducibella and Fissore 2008). Extensive phosphopeptide mapping combined with substrate specific antibodies in Xenopus oocytes found that IP3R1 is uniformly phosphorylated throughout maturation in both PKA consensus motifs, whereas PKC sites seemed unperturbed (Sun et al. 2009). Whether or not PKA IP3R1 phosphorylation has functional consequences in oocytes is unknown, although in somatic cells this phosphorylation has been associated with increased IP3R1 activity (DeSouza et al. 2002) and reduced [Ca2+]ER, which may reportedly underlie the antiapoptotic effects of some members of the Bcl-2 family of proteins (Oakes et al. 2005). Moreover, it has been suggested that these proteins modify the PKA-associated IP3R1 phosphorylation status (Oakes et al. 2005), although research from other laboratories has not confirmed this mechanism of action of Bcl-2 family protein on IP3R1 function (Rong et al. 2008; Rong et al. 2009). It is worth noting that [Ca2+]ER is low in GV oocytes in spite of persistent Ca2+ influx, as evident by the continuous spontaneous oscillations at this stage (Carroll and Swann 1992). On GVBD, however, [Ca2+]ER undergoes a marked increase (Jones et al. 1995a), which occurs concurrently with the termination of the oscillations that implies suppression/reduction of Ca2+ influx. Given that cAMP levels decrease at GVBD (Norris et al. 2009), it is therefore possible that a Ca2+ leak mechanism regulated by PKA IP3R1 phosphorylation may be implicated in Ca2+ homeostasis during oocyte maturation. Future studies should examine whether PKA-mediated IP3R1 phosphorylation changes during mouse oocyte maturation.
The differential redistribution of ER/IP3R1 may also enhance IP3R1 function during oocyte maturation. Before the initiation of maturation, the ER in mouse oocytes shows a homogeneous distribution with slight accumulation around the GV, although by the MII stage, the ER displays a fine tubular network appearance with dense accumulation in the cortex (Mehlmann et al. 1995), which is thought to facilitate the initiation of sperm-induced [Ca2+]i oscillations (Kline et al. 1999). The dramatic reorganization ensues at about the time of GVBD and is underpinned by distinct components of the cytoskeleton (FitzHarris et al. 2007), as the migration of the ER toward the condensing chromosomes is dependent on microtubules, whereas its dispersal from the MI spindle to the egg’s cortex relies on actin microfilaments (FitzHarris et al. 2003). The IP3R1 are also organized in cortical clusters at the MII stage (Mehlmann et al. 1996; Fissore et al. 1999a; Ito et al. 2008a), although it remains to be established whether the same cytoskeletal mechanisms that control ER organization control IP3R1 distribution. Curiously, in spite of the large remodeling that the oocyte’s ER undertakes, it is unknown whether this reorganization affects IP3R1’s sensitivity and the ability to initiate and support oscillations in mammalian eggs. Thus, preventing ER and possibly IP3R1 cortical cluster organization with appropriate cytoskeleton inhibitors would help elucidate the influence of their reorganization on IP3R1 function in oocytes.
Lastly, changes in other cytoplasmic parameters are likely to contribute to increase IP3R1 sensitivity in oocytes. For example, the increase in [Ca2+]ER during maturation (Kline and Kline 1992a; Jones et al. 1995a) may not only increase the amount of available Ca2+ for release, but may also increase the receptor’s sensitivity (Missiaen et al. 1991). The increase in [Ca2+]ER is likely the result of careful regulation of the pathways that control Ca2+ influx and efflux, which in Xenopus oocytes are known to be actively regulated during maturation (El-Jouni et al. 2005; Yu et al. 2009). Remarkably, the molecular identity and significance of these mechanisms in mammalian oocytes remain largely unknown and will be discussed below.
Ca2+ Homeostasis in Oocytes and Eggs
[Ca2+]i Clearing Mechanisms
[Ca2+]i oscillations in mammals continue for long periods that can exceed 20 h (Fissore et al. 1992; Sun et al. 1992). For [Ca2+]i responses to continue without attenuation following a [Ca2+]i increase, [Ca2+]i levels need to be returned to baseline and stores refilled in anticipation of the next [Ca2+]i response. To bring [Ca2+]i to baseline, cells either return free cytosolic Ca2+ into the ER by the action of the sarco-endoplasmic reticulum Ca2+ ATPases (SERCAs), and/or extrude it by the action of plasma membrane (PM) Ca2+ ATPases (PMCAs) and Na+/Ca2+ exchangers (Berridge et al. 2000; Bootman et al. 2001). Few studies have addressed the function of these molecules in mammalian oocytes/eggs, although the presence of SERCA2b can be surmised by the alteration of [Ca2+]i levels caused by exposure to thapsigargin, an inhibitor of SERCA (Kline and Kline 1992b; Lawrence and Cuthbertson 1995; Machaty et al. 2002). Exposure of MII eggs to thapsigargin causes a slow and steady increase in [Ca2+]i followed by a protracted decline, whereas in fertilized eggs it prevents the continuation of oscillations (Kline and Kline 1992b). Importantly, the molecular presence and cellular distribution of SERCA2b has not yet been examined in mammalian oocytes, although transcripts have been found in GV and MII stage oocytes (Su et al. 2007). In Xenopus oocytes, expression of the SERCA2 protein was documented by immunofluorescence and it was shown to undergo reorganization similar to that described for IP3R (El-Jouni et al. 2005). Given that the levels of [Ca2+]ER change dramatically during maturation, it is possible that SERCA activity may be actively regulated during this process. In somatic cells, SERCA activity can be regulated by different mechanisms, including binding to regulatory proteins such phospholamban, sarcolipin, and by several posttranslational modifications (reviewed in Brini and Carafoli [2009]). An earlier report in Xenopus oocytes showed that SERCA2b activity could be regulated by association with the ER chaperone protein, calnexin, which inhibited the pump’s activity; phosphorylation of calnexin relieved both the association with SERCA and its inhibition (Roderick et al. 2000). Although additional studies are needed to understand the conservation of this mechanism, it is worth noting that addition of roscovitine prematurely, albeit reversibly, terminates [Ca2+]i oscillations during fertilization (Deng and Shen 2000). Although the inhibitor’s target was not elucidated, [Ca2+]ER levels were severely depleted, suggesting an effect either on SERCA activity or on the Ca2+ influx mechanism(s). Future studies should explore the pathways involved in regulation of SERCA activity in mammalian eggs, as its function in sustaining long-term [Ca2+]i oscillations after fertilization.
Besides the ER sequestration of Ca2+, cytosolic [Ca2+]i can also be returned to baseline by the action of PMCA and the Na+/Ca2+ exchanger, which release Ca2+ into the external media. The functional activity of Na/Ca2+ exchanger was shown in mouse eggs by two different reports (Pepperell et al. 1999; Carroll 2000). It was shown that elimination of Na+ from the external media caused [Ca2+]i responses, or accelerated existing ones, and these responses were ascribed to reverse mode Na+-Ca2+ exchange. In spite of the initial changes, even in the absence of external Na+, [Ca2+]i levels returned to baseline levels, implying that the action of PMCA may be more physiologically relevant (Carroll 2000). The molecular presence of PMCA has not been documented in mammalian oocytes/eggs, although in Xenopus oocytes PMCA1 seems to contribute to the shaping of [Ca2+]i responses (El-Jouni et al. 2005; El-Jouni et al. 2008). For instance, in GV oocytes the presence of 1 mM La3+, which is known to inhibit PMCA function, delay the half-time decay of a [Ca2+]i increase induced by iononomycin, but the same treatment was without effect in MII eggs, suggesting that the pump’s activity is down regulated during maturation (El-Jouni et al. 2005). Research by these investigators also showed that most of the PMCA in the plasma membrane becomes internalized during maturation explaining, at least in part, the lower [Ca2+]i clearing capacity of eggs versus oocytes (El-Jouni et al. 2005). Nevertheless, it remains to be shown how PMCA plays a role in mammalian fertilization and what are the putative regulatory mechanisms, as complete internalization seems unlikely in this system, given that [Ca2+]i increases occur uninterruptedly for hours in these eggs.
The mitochondria may also contribute to regulate baseline [Ca2+]i in the presence of oscillations (Duchen 2000; Rizzuto et al. 2000), as they can sequester Ca2+ into the matrix thereby decreasing the overall cytosolic Ca2+ load (Rizzuto et al. 1998). Despite early evidence to the contrary (Liu et al. 2001), this does not seem to be the main function in eggs, as inhibition of Ca2+ mitochondrial uptake does not immediately terminate sperm-initiated oscillations (Dumollard et al. 2004). Instead, and possibly because of its vicinity to the IP3R1/ER, the Ca2+-driven ATP output may be the mitochondria’s most critical contribution to Ca2+ homeostasis in MII eggs, as it maintains SERCA activity, which is required to sustain sperm-triggered Ca2+ oscillations (Dumollard et al. 2004).
Ca2+ Influx Mechanisms
Given that a fraction of Ca2+ from each [Ca2+]i increase is secreted out of the egg by the action of PMCA or the Na+/Ca2+ exchanger, external Ca2+ must be taken in to maintain [Ca2+]ER. Ca2+ influx plays a pivotal role in fertilization, as sperm-initiated [Ca2+]i oscillations cease prematurely in the absence of external Ca2+ (Igusa and Miyazaki 1983; Igusa et al. 1983; Winston et al. 1995). Nevertheless, the molecules that mediate Ca2+ influx and their regulation remain poorly characterized. Oocytes/eggs and somatic cells use several Ca2+ influx mechanisms, including receptor-operated channels (ROCs) and voltage-operated Ca2+ channels (VOCs) (Berridge et al. 2000; Smyth et al. 2006), the last of which is active in mammalian oocytes and eggs (Tosti and Boni 2004). Notably, although changes in membrane potential accompany fertilization in mammals, several findings suggest that they might not be causally linked to the replenishment of the stores, as [Ca2+]i increases precede changes in membrane potential (Igusa et al. 1983), Ca2+ influx continues between [Ca2+]i increases (McGuinness et al. 1996) and, in the mouse, the changes in membrane potential are almost imperceptible (Igusa et al. 1983). These findings raise the prospect that Ca2+ influx in oocytes may be attained, at least in part, by a different mechanism(s). Store-operated Ca2+ entry (SOCE), which is associated with [Ca2+]ER levels (Putney 1986), may fulfill this role in oocytes/eggs. The presence of store operated Ca2+ channels (SOC) to mediate SOCE and their electrophysiological properties were surmised in mast cells and in T-cells more than 10 years (Lewis and Cahalan 1989; Zweifach and Lewis 1993), although their molecular identity remained elusive until recently. Using a small RNA interference (RNAi) screen, two groups found that STIM1 was required for SOCE, as its reduction decreased Ca2+ influx in response to thapsigargin (Liou et al. 2005; Roos et al. 2005). Given that STIM1 lacks an obvious channel, the search was on to find the required channel partner protein, one of which was quickly identified as Orai1 (Feske et al. 2006; Vig et al. 2006). Besides the recent demonstration of molecular coupling at the cellular level between STIM1 and Orai1 (Park et al. 2009), spontaneous mutations of these proteins in humans have been linked to related immune response-related diseases, conclusively implicating their function in the same Ca2+ influx pathway (Feske et al. 2005; Picard et al. 2009). For general information about Stim and Orai family of proteins, see Lewis 2011.
Evidence for SOCE in mammalian eggs was first observed after the application of thapsigargin, which caused a large Ca2+ influx after adding Ca2+ back to the media, (Kline and Kline 1992b; Machaty et al. 2002). Subsequent studies implicated SOCE in fertilization, as using the manganese-quenching technique it was found that in mouse eggs the initiation of each [Ca2+]i increase coincided with divalent cation influx (McGuinness et al. 1996). Although SOCE was also described in human eggs (Martin-Romero et al. 2008), the understanding of the molecular underpinning of this influx in mammalian oocytes remains poor.
Transient receptor potential (TRP) ion channels (Venkatachalam and Montell 2007), which show widespread cellular distribution and display numerous regulatory mechanisms, were considered as possible mediators of Ca2+ influx in eggs. Expression at the transcript level was noted in porcine and mouse oocytes for several of the TRP family members (Machaty et al. 2002; Su et al. 2007), although evidence for their involvement in fertilization has yet to materialize. In contrast, two recent manuscripts examined the expression and function of STIM1 in oocytes. In porcine oocytes, STIM1 expression was detected at the mRNA level, and over-expression or knock down of STIM1 enhanced/reduced, respectively, thapsigargin-promoted Ca2+ influx. Expression of YFP-tagged STIM1 suggested ER localization and “puncta” reorganization in these oocytes, although more conclusive studies are needed (Koh et al. 2009). In mouse eggs, STIM1 was detected by western blotting, although the apparent molecular weight (Gomez-Fernandez et al. 2009) seems lower compared to published data in mouse somatic cells (Manji et al. 2000). Further, the detection of endogenous STIM1 by immunofluorescence revealed large patches (Gomez-Fernandez et al. 2009), which seem disproportionate to the reportedly low abundance of this protein in most cell types (Park et al. 2009). Lastly, whereas transcripts of Orai1 and two have been detected in mouse oocytes and eggs (Su et al. 2007), their protein expression has not been confirmed, and therefore their involvement in Ca2+ influx during mammalian fertilization remains to be shown.
A better understanding of SOCE’s molecular effectors and regulatory mechanisms already exists in Xenopus oocytes and eggs (Machaca and Haun 2002; Yu et al. 2009). Initial research showed inactivation of SOCE, which is manifested by the uncoupling of Ca2+ store depletion and Ca2+ influx, around the time of GVBD (Machaca and Haun 2002). Although the inactivating mechanism was not known, it was determined to be associated with the activities of the M-phase kinases that regulate GVBD (Machaca and Haun, 2002). A follow up study found that during GVBD Orai1 is internalized from the plasma membrane, and STIM1’s ability to form clusters and puncta is obliterated, which together disable SOCE (Yu et al. 2009). Earlier, an uncoupling between Ca2+ influx and Ca2+ store content was reported in somatic cells during mitosis (Preston et al. 1991). Those findings were recently extended, and SOCE inactivation during mitosis was associated with Stim1 phosphorylation by Cdk1, which prevents its rearrangement and precludes coupling and activation of Orai1 (Smyth et al. 2009). STIM1 phosphorylation by Cdk1 was also noted in the foregoing Xenopus study, although it was deemed to have minor impact on STIM1 function (Yu et al. 2009). It is noteworthy that a conserved Cdk1 phosphorylation site present in mammalian STIM1 is absent from Xenopus STIM1. Importantly, unlike the previous examples, SOCE is operational during the MII stage of mammalian fertilization. Therefore, future studies should examine the regulatory mechanism(s) that control SOCE during maturation and fertilization in mammals.
PLCζ
There has been much debate and speculation as to the mechanism(s) that triggers [Ca2+]i oscillations during mammalian fertilization. Several excellent recent reviews have addressed this topic in depth (Swann et al. 2006; Parrington et al. 2007; Horner and Wolfner 2008b) and therefore only the most salient and outstanding aspects of PLCζ will be discussed here. As noted earlier, research in a variety of species including mammals showed that fertilization-associated [Ca2+]i responses require the same agonists and signaling cascades that cause Ca2+ release in somatic cells (Miyazaki et al. 1993; Miyazaki and Ito 2006). Nevertheless, although stimulation of these pathways induced [Ca2+]i responses, they failed to reproduce the pattern of [Ca2+]i oscillations associated with mammalian fertilization, leaving open the possibility that a different mechanism may underpin oscillations in these species (Swann et al. 1989). Observations first in sea urchin eggs and then in ascidian eggs noted that injection of sperm extracts caused PM currents similar to that observed in fertilization (Dale et al. 1985; Dale 1988). Subsequently, studies in mammals showed that injections of sperm extracts initiated fertilization-like oscillations and egg activation (Stice and Robl 1990; Swann 1990). Based on these results and in light of the protracted nature of the [Ca2+]i oscillations, which can vastly exceed the interaction time of gametes at the PM, a hypothesis was proposed whereby a SF acts as the trigger of oscillations after fusion of the gametes (Swann and Lai 1997). Although this hypothesis was received with skepticism, support for it grew steadily, as injection of sperm extracts initiated oscillations in several mammalian and nonmammalian species (Stricker 1997; Wu et al. 1997). Furthermore, physiological support for this concept was provided both when intracytoplasmic sperm injection into eggs (ICSI) resulted in the birth of young (Palermo et al. 1992), and the subsequent demonstration that ICSI initiated fertilization-like oscillations in several mammalian species (Tesarik and Testart 1994; Nakano et al. 1997; Kurokawa and Fissore 2003; Malcuit et al. 2006). Together, these studies consolidated the concept of the SF as the initiator of oscillations in mammalian eggs, although identification of the active principle would have to wait for another decade.
Identification of PLCζ
The search for the SF’s active component(s) was the subject of intense interest and it was not without some false starts. A turning point came when studies using sea urchin egg extracts and in vitro PLC assays revealed that cytosolic preparations from mammalian sperm possessed high PLC activity, which was nearly twice as high as the activity present in other tissues (Parrington et al. 1999; Jones et al. 2000; Rice et al. 2000). In addition, it was discovered that the sperm’s PLC activity displayed high sensitivity to Ca2+, meaning that it shows near maximal activity in the presence of basal [Ca2+] concentrations (Rice et al. 2000), which in most cells are of ∼100 nM (Clapham 2007). This feature made the putative SF a credible candidate to trigger oscillations, because to attain high specific activity most PLCs require [Ca2+] concentrations in excess of 1 µM (Rebecchi and Pentyala 2000; Nomikos et al. 2005), concentrations that are not compatible with MII arrest. It was therefore not surprising that injection of recombinant proteins representing most of the known isoforms expressed in sperm (Choi et al. 2001; Fukami et al. 2001) failed to initiate oscillations in mouse eggs (Parrington et al. 2002), or if they did, they did so at nonphysiological concentrations (Mehlmann et al. 2001). Hence, it became evident that if a PLC were to be the SF, it had to be a novel PLC. Toward this end, the novel sperm-specific PLCζ (Saunders et al. 2002) was identified in a PLC homology screen of mouse testis expressed sequence tags. Data in the latter study and in follow up reports provided strong evidence to support the concept that PLCζ is the pivotal, and possibly exclusive, initiator of [Ca2+]i oscillations in mammals. Specifically, injection of recombinant PLCζ (Fujimoto et al. 2004; Kouchi et al. 2004) or PLCζ cRNA evoked sperm-like oscillations in mouse (Saunders et al. 2002), rat (Ito et al. 2008b), human (Rogers et al. 2004), bovine (Malcuit et al. 2005; Ross et al. 2008), porcine (Yoneda et al. 2006), and equine (Bedford-Guaus et al. 2008) eggs. In vitro PLC assays, using recombinant PLCζ confirmed the enzyme’s high sensitivity to Ca2+, which render it nearly fully active at basal [Ca2+]i concentrations (Kouchi et al. 2004). Immunolocalization studies localized PLCζ to the postacrosomal region of mouse sperm (Fujimoto et al. 2004) and to the equatorial area of bull and human sperm (Yoon and Fissore 2007; Grasa et al. 2008; Yoon et al. 2008), regions that first come in contact with the ooplasm following gamete fusion, respectively (Sutovsky et al. 2003).
Recent evidence linking PLCζ expression and fertility further strengthened the role of PLCζ as the initiator of [Ca2+]i oscillations in mammals. One study examined the ability of sperm from patients with repeated ICSI failure to initiate [Ca2+]i oscillations in mouse eggs. The sperm from a few of these patients were incapable of initiating [Ca2+]i responses, and examination of PLCζ expression by immunofluorescence and by Western blotting found reduced/absent levels of the enzyme in these sperm (Yoon et al. 2008). The results suggest that the inability of these sperm to activate eggs might be the main cause of their infertility. Consistent with this notion, studies have shown that the infertility of patients with globozoospermia, an affliction where even after ICSI most patients remain sterile, can be overcome by ICSI followed by Ca2+ ionophore-aided egg activation (Taylor et al. 2010; Heindryckx et al. 2005). A second study in patients with ICSI failure found that, in addition to reduced expression of PLCζ, a point mutation was identified that compromises PLCζ’s ability to initiate [Ca2+]i oscillations (Heytens et al. 2009). Collectively, the evidence supporting PLCζ as the mammalian SF is compelling. Nevertheless, questions remain regarding its expression during spermatogenesis and storage in sperm, its mechanism of release into the ooplasm, and mechanism(s) of activation once in the egg.
Despite evidence that PLCζ serves as the principal trigger of oscillations in mammals, research has unearthed species-specific differences that might prove useful in elucidating how PLCζ is regulated during fertilization. For example, although mouse PLCζ, which is the most studied, accumulates into the nucleus following PN formation (Saunders et al. 2002; Yoda et al. 2004), none of the other PLCζ isoforms tested display this localization despite sharing a nuclear localization signal (Cooney et al.; Ito et al. 2008b). There seems also to be significant differences in specific activity. For instance, based on the concentrations of cRNAs required to initiate oscillations, human PLCζ seems ∼40-fold more active than mouse PLCζ, which itself is significantly more active than the rat enzyme (Cox et al. 2002; Rogers et al. 2004; Ito et al. 2008b). Although the role of these species-specific variations has not been explored carefully, it is tempting to speculate that they are the result of adaptations to promote the optimal activation signal. To this end, it is revealing that the species with the weakest PLCζ, the rat, has the easiest oocytes to activate (Zernicka-Goetz 1991; Ito et al. 2007). Future studies should examine whether an inverse association exists between expression levels/activity of PLCζ in sperm and IP3R1 sensitivity/strength of the CSF-arresting machinery in eggs. Similarly, future studies should elucidate the molecular changes that underlie the differences in PLCζ activity among species. For example, despite missing the pleckstrin homology (PH) domain, PLCζ shows the modular organization characteristic of other PLCs, which consists of 4 EF hand Ca2+-binding domains, X and Y catalytic domains, and the Ca2+-dependent phospholipid-binding C2 domain (Rebecchi and Pentyala 2000). The EF-hand domains, and especially the EF3-hand domain, have been suggested to confer the high Ca2+ sensitivity of PLCζ through in vitro studies (Kouchi et al. 2005; Nomikos et al. 2005); whether sequence differences in this or other EF-hand domains underlie PLCζ species-specific differences should be examined.
CONCLUSIONS
The study of the Ca2+ mechanisms that underlie fertilization in mammals has resulted in important contributions to the Ca2+ signaling field in general and to the field of fertilization in particular. For example, the indispensable role of IP3R1-mediated Ca2+ release in regulating cellular functions was unequivocally shown in mouse fertilization (Miyazaki et al. 1992). Likewise, the discovery of the SF’s active component, PLCζ (Saunders et al. 2002), not only provided evidence for a novel way of activating Ca2+ signaling in a host cell, but also added a new member with unique properties to the all important family of PLC enzymes. Importantly, and despite progress in the role of these two molecules in fertilization, we are still unaware of their fine regulatory mechanisms. For example, IP3R1 function is greatly optimized during oocyte maturation, but the precise underlying molecular mechanisms responsible for these changes remain undetermined. Similarly, how the seemingly constitutive activity of PLCζ is provisionally restrained in the sperm and how its expression is regulated during spermatogenesis are questions that need addressing. Lastly, although [Ca2+]i oscillations trigger mammalian development, we remain uninformed of the regulation of SERCA, which recycles Ca2+ into the ER, and of the molecules that underpin Ca2+ influx, which sustain the oscillations. Identification and elucidation of these regulatory mechanisms in oocytes will deepen our understating of fertilization, information that could be then used in the clinic for the diagnosis of infertility, and to enhance developmental competence of embryos generated by a variety of Assisted Reproductive Technology procedures.
ACKNOWLEDGMENTS
This work was supported by grants from the USDA and the NIH/N.I.C.H.D-HD051872 to R.A.F. The authors wish to thank all members of the Fissore lab for their generous contributions and suggestions. We apologize to those whose work was not cited because of space limitations.
Footnotes
Editors: Martin D. Bootman, Michael J. Berridge, James W. Putney, and H. Llewelyn Roderick
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Backs J, Stein P, Backs T, Duncan FE, Grueter CE, McAnally J, Qi X, Schultz RM, Olson EN 2010. The gamma isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption. Proc Natl Acad Sci 107: 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai GR, Yang LH, Huang XY, Sun FZ 2006. Inositol 1,4,5-trisphosphate receptor type 1 phosphorylation and regulation by extracellular signal-regulated kinase. Biochem Biophys Res Commun 348: 1319–1327 [DOI] [PubMed] [Google Scholar]
- Becker KA, Hart NH 1999. Reorganization of filamentous actin and myosin-II in zebrafish eggs correlates temporally and spatially with cortical granule exocytosis. J Cell Sci 112 ( Pt 1): 97–110 [DOI] [PubMed] [Google Scholar]
- Bedford-Guaus SJ, Yoon SY, Fissore RA, Choi YH, Hinrichs K 2008. Microinjection of mouse phospholipase C zeta complementary RNA into mare oocytes induces long-lasting intracellular calcium oscillations and embryonic development. Reprod Fertil Dev 20: 875–883 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD 2000. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I 2005. The inositol 1,4,5-trisphosphate receptors. Cell Calcium 38: 261–272 [DOI] [PubMed] [Google Scholar]
- Bootman M, Collins T, Peppiatt C, Prothero L, MacKenzie L, De Smet P, Travers M, Tovey S, Seo J, Berridge M, et al. 2001. Calcium signalling—an overview. Semin Cell Dev Biol 12: 3–10 [DOI] [PubMed] [Google Scholar]
- Boulware MJ, Marchant JS 2005. IP3 receptor activity is differentially regulated in endoplasmic reticulum subdomains during oocyte maturation. Curr Biol 15: 765–770 [DOI] [PubMed] [Google Scholar]
- Brini M, Carafoli E 2009. Calcium pumps in health and disease. Physiol Rev 89: 1341–1378 [DOI] [PubMed] [Google Scholar]
- Carroll J 2000. Na+-Ca2+ exchange in mouse oocytes: modifications in the regulation of intracellular free Ca2+ during oocyte maturation. J Reprod Fertil 118: 337–342 [DOI] [PubMed] [Google Scholar]
- Carroll J, Swann K 1992. Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. J Biol Chem 267: 11196–11201 [PubMed] [Google Scholar]
- Carroll J, Swann K, Whittingham D, Whitaker M 1994. Spatiotemporal dynamics of intracellular [Ca2+]i oscillations during the growth and meiotic maturation of mouse oocytes. Development 120: 3507–3517 [DOI] [PubMed] [Google Scholar]
- Castilho PV, Williams BC, Mochida S, Zhao Y, Goldberg ML 2009. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol Biol Cell 20: 4777–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Minahan K, Merriman JA, Jones KT 2009. Calmodulin-dependent protein kinase gamma 3 (CamKIIgamma3) mediates the cell cycle resumption of metaphase II eggs in mouse. Development 136: 4077–4081 [DOI] [PubMed] [Google Scholar]
- Choi D, Lee E, Hwang S, Jun K, Kim D, Yoon BK, Shin HS, Lee JH 2001. The biological significance of phospholipase C beta 1 gene mutation in mouse sperm in the acrosome reaction, fertilization, and embryo development. J Assist Reprod Genet 18: 305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE 2007. Calcium signaling. Cell 131: 1047–1058 [DOI] [PubMed] [Google Scholar]
- Clapper DL, Lee HC 1985. Inositol trisphosphate induces calcium release from nonmitochondrial stores in sea urchin egg homogenates. J Biol Chem 260: 13947–13954 [PubMed] [Google Scholar]
- Cooney MA, Malcuit C, Cheon B, Holland MK, Fissore RA, D’Cruz NT 2010. Species-specific differences in the activity and nuclear localization of murine and bovine phospholipase C, Zeta 1. Biol Reprod 83: 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, Lai FA 2002. Sperm phospholipase Czeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction 124: 611–623 [DOI] [PubMed] [Google Scholar]
- Cuthbertson KS, Whittingham DG, Cobbold PH 1981. Free Ca2+ increases in exponential phases during mouse oocyte activation. Nature 294: 754–757 [DOI] [PubMed] [Google Scholar]
- Dale B 1988. Primary and secondary messengers in the activation of ascidian eggs. Exp Cell Res 177: 205–211 [DOI] [PubMed] [Google Scholar]
- Dale B, DeFelice LJ, Ehrenstein G 1985. Injection of a soluble sperm fraction into sea-urchin eggs triggers the cortical reaction. Experientia 41: 1068–1070 [DOI] [PubMed] [Google Scholar]
- Deguchi R, Shirakawa H, Oda S, Mohri T, Miyazaki S 2000. Spatiotemporal analysis of Ca(2+) waves in relation to the sperm entry site and animal-vegetal axis during Ca(2+) oscillations in fertilized mouse eggs. Dev Biol 218: 299–313 [DOI] [PubMed] [Google Scholar]
- Deng MQ, Shen SS 2000. A specific inhibitor of p34(cdc2)/cyclin B suppresses fertilization-induced calcium oscillations in mouse eggs. Biol Reprod 62: 873–878 [DOI] [PubMed] [Google Scholar]
- Deng M, Williams CJ, Schultz RM 2005. Role of MAP kinase and myosin light chain kinase in chromosome-induced development of mouse egg polarity. Dev Biol 278: 358–366 [DOI] [PubMed] [Google Scholar]
- Descombes P, Nigg EA 1998. The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. Embo J 17: 1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza N, Reiken S, Ondrias K, Yang YM, Matkovich S, Marks AR 2002. Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J Biol Chem 277: 39397–39400 [DOI] [PubMed] [Google Scholar]
- Diaz-Munoz M, de la Rosa Santander P, Juarez-Espinosa AB, Arellano RO, Morales-Tlalpan V 2008. Granulosa cells express three inositol 1,4,5-trisphosphate receptor isoforms: cytoplasmic and nuclear Ca2+ mobilization. Reprod Biol Endocrinol 6: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G, Luca F, Westendorf J, Brizuela L, Ruderman J, Beach D 1989. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell 56: 829–838 [DOI] [PubMed] [Google Scholar]
- Duchen MR 2000. Mitochondria and calcium: from cell signalling to cell death. J Physiol 529 Pt 1: 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducibella T, Fissore R 2008. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol 315: 257–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducibella T, LeFevre L 1997. Study of protein kinase C antagonists on cortical granule exocytosis and cell-cycle resumption in fertilized mouse eggs. Mol Reprod Dev 46: 216–226 [DOI] [PubMed] [Google Scholar]
- Ducibella T, Schultz RM, Ozil JP 2006. Role of calcium signals in early development. Semin Cell Dev Biol 17: 324–332 [DOI] [PubMed] [Google Scholar]
- Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP 2002. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev Biol 250: 280–291 [PubMed] [Google Scholar]
- Dumollard R, Marangos P, Fitzharris G, Swann K, Duchen M, Carroll J 2004. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development 131: 3057–3067 [DOI] [PubMed] [Google Scholar]
- El-Jouni W, Haun S, Machaca K 2008. Internalization of plasma membrane Ca2+-ATPase during Xenopus oocyte maturation. Dev Biol 324: 99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Jouni W, Jang B, Haun S, Machaca K 2005. Calcium signaling differentiation during Xenopus oocyte maturation. Dev Biol 288: 514–525 [DOI] [PubMed] [Google Scholar]
- Eliyahu E, Kaplan-Kraicer R, Shalgi R 2001. PKC in eggs and embryos. Front Biosci 6: D785–D791 [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A 2006. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185 [DOI] [PubMed] [Google Scholar]
- Feske S, Prakriya M, Rao A, Lewis RS 2005. A severe defect in CRAC Ca2+ channel activation and altered K+ channel gating in T cells from immunodeficient patients. J Exp Med 202: 651–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch EA, Turner TJ, Goldin SM 1991. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science 252: 443–446 [DOI] [PubMed] [Google Scholar]
- Fissore RA, Dobrinsky JR, Balise JJ, Duby RT, Robl JM 1992. Patterns of intracellular Ca2+ concentrations in fertilized bovine eggs. Biol Reprod 47: 960–969 [DOI] [PubMed] [Google Scholar]
- Fissore RA, Reis MM, Palermo GD 1999b. Isolation of the Ca2+ releasing component(s) of mammalian sperm extracts: the search continues. Mol Hum Reprod 5: 189–192 [DOI] [PubMed] [Google Scholar]
- Fissore RA, Longo FJ, Anderson E, Parys JB, Ducibella T 1999a. Differential distribution of inositol trisphosphate receptor isoforms in mouse oocytes. Biol Reprod 60: 49–57 [DOI] [PubMed] [Google Scholar]
- FitzHarris G, Marangos P, Carroll J 2003. Cell cycle-dependent regulation of structure of endoplasmic reticulum and inositol 1,4,5-trisphosphate-induced Ca2+ release in mouse oocytes and embryos. Mol Biol Cell 14: 288–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzHarris G, Marangos P, Carroll J 2007. Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev Biol 305: 133–144 [DOI] [PubMed] [Google Scholar]
- Fuchimoto D, Mizukoshi A, Schultz RM, Sakai S, Aoki F 2001. Posttranscriptional regulation of cyclin A1 and cyclin A2 during mouse oocyte meiotic maturation and preimplantation development. Biol Reprod 65: 986–993 [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Yoshida N, Fukui T, Amanai M, Isobe T, Itagaki C, Izumi T, Perry AC 2004. Mammalian phospholipase Czeta induces oocyte activation from the sperm perinuclear matrix. Dev Biol 274: 370–383 [DOI] [PubMed] [Google Scholar]
- Fukami K, Nakao K, Inoue T, Kataoka Y, Kurokawa M, Fissore R, Nakamura K, Katsuki M, Mikoshiba K, Yoshida N, et al. 2001. Requirement of phospholipase Cdelta4 for the zona pellucida-induced acrosome reaction. Science 292: 920–923 [DOI] [PubMed] [Google Scholar]
- Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K 1989. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature 342: 32–38 [DOI] [PubMed] [Google Scholar]
- Gallicano GI, Yousef MC, Capco DG 1997. PKC—a pivotal regulator of early development. Bioessays 19: 29–36 [DOI] [PubMed] [Google Scholar]
- Giusti AF, Carroll DJ, Abassi YA, Terasaki M, Foltz KR, Jaffe LA 1999. Requirement of a Src family kinase for initiating calcium release at fertilization in starfish eggs. J Biol Chem 274: 29318–29322 [DOI] [PubMed] [Google Scholar]
- Gomez-Fernandez C, Pozo-Guisado E, Ganan-Parra M, Perianes MJ, Alvarez IS, Martin-Romero FJ 2009. Relocalization of STIM1 in mouse oocytes at fertilization: early involvement of store-operated calcium entry. Reproduction 138: 211–221 [DOI] [PubMed] [Google Scholar]
- Goud PT, Goud AP, Leybaert L, Van Oostveldt P, Mikoshiba K, Diamond MP, Dhont M 2002. Inositol 1,4,5-trisphosphate receptor function in human oocytes: calcium responses and oocyte activation-related phenomena induced by photolytic release of InsP(3) are blocked by a specific antibody to the type I receptor. Mol Hum Reprod 8: 912–918 [DOI] [PubMed] [Google Scholar]
- Grasa P, Coward K, Young C, Parrington J 2008. The pattern of localization of the putative oocyte activation factor, phospholipase Czeta, in uncapacitated, capacitated, and ionophore-treated human spermatozoa. Hum Reprod 23: 2513–2522 [DOI] [PubMed] [Google Scholar]
- Halet G 2004. PKC signaling at fertilization in mammalian eggs. Biochim Biophys Acta 1742: 185–189 [DOI] [PubMed] [Google Scholar]
- Harada Y, Matsumoto T, Hirahara S, Nakashima A, Ueno S, Oda S, Miyazaki S, Iwao Y 2007. Characterization of a sperm factor for egg activation at fertilization of the newt Cynops pyrrhogaster. Dev Biol 306: 797–808 [DOI] [PubMed] [Google Scholar]
- Heindryckx B, Van der Elst J, De Sutter P, Dhont M 2005. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod 20: 2237–2241 [DOI] [PubMed] [Google Scholar]
- Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon S, Fissore R, Hamer R, Deane C, Ruas M, et al. 2009. Reduced amounts and abnormal forms of phospholipase C zeta (PLCzeta) in spermatozoa from infertile men. Hum Reprod 24: 2417–2428 [DOI] [PubMed] [Google Scholar]
- Horner VL, Wolfner MF 2008a. Mechanical stimulation by osmotic and hydrostatic pressure activates Drosophila oocytes in vitro in a calcium-dependent manner. Dev Biol 316: 100–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner VL, Wolfner MF 2008b. Transitioning from egg to embryo: triggers and mechanisms of egg activation. Dev Dyn 237: 527–544 [DOI] [PubMed] [Google Scholar]
- Igusa Y, Miyazaki S 1983. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J Physiol 340: 611–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igusa Y, Miyazaki S, Yamashita N 1983. Periodic hyperpolarizing responses in hamster and mouse eggs fertilized with mouse sperm. J Physiol 340: 633–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M 1990. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol 95: 1103–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J, Shimada M, Hochi S, Hirabayashi M 2007. Involvement of Ca2+-dependent proteasome in the degradation of both cyclin B1 and Mos during spontaneous activation of matured rat oocytes. Theriogenology 67: 475–485 [DOI] [PubMed] [Google Scholar]
- Ito M, Shikano T, Oda S, Horiguchi T, Tanimoto S, Awaji T, Mitani H, Miyazaki S 2008b. Difference in Ca2+ oscillation-inducing activity and nuclear translocation ability of PLCZ1, an egg-activating sperm factor candidate, between mouse, rat, human, and medaka fish. Biol Reprod 78: 1081–1090 [DOI] [PubMed] [Google Scholar]
- Ito J, Yoon SY, Lee B, Vanderheyden V, Vermassen E, Wojcikiewicz R, Alfandari D, De Smedt H, Parys JB, Fissore RA 2008a. Inositol 1,4,5-trisphosphate receptor 1, a widespread Ca2+ channel, is a novel substrate of polo-like kinase 1 in eggs. Dev Biol 320: 402–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Chiba K, Uchiyama T, Yoshikawa F, Suzuki F, Ikeda M, Furuichi T, Mikoshiba K 2002. Molecular characterization of the starfish inositol 1,4,5-trisphosphate receptor and its role during oocyte maturation and fertilization. J Biol Chem 277: 2763–2772 [DOI] [PubMed] [Google Scholar]
- Jellerette T, He CL, Wu H, Parys JB, Fissore RA 2000. Down-regulation of the inositol 1,4,5-trisphosphate receptor in mouse eggs following fertilization or parthenogenetic activation. Dev Biol 223: 238–250 [DOI] [PubMed] [Google Scholar]
- Jellerette T, Kurokawa M, Lee B, Malcuit C, Yoon SY, Smyth J, Vermassen E, De Smedt H, Parys JB, Fissore RA 2004. Cell cycle-coupled [Ca(2+)](i) oscillations in mouse zygotes and function of the inositol 1,4,5-trisphosphate receptor-1. Dev Biol 274: 94–109 [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Whittingham DG 1995a. Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J Biol Chem 270: 6671–6677 [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Merriman JA, Whittingham DG, Kono T 1995. Repetitive sperm-induced Ca2+ transients in mouse oocytes are cell cycle dependent. Development 121: 3259–3266 [DOI] [PubMed] [Google Scholar]
- Jones KT, Matsuda M, Parrington J, Katan M, Swann K 2000. Different Ca2+-releasing abilities of sperm extracts compared with tissue extracts and phospholipase C isoforms in sea urchin egg homogenate and mouse eggs. Biochem J 346 (Pt 3): 743–749 [PMC free article] [PubMed] [Google Scholar]
- Joughin BA, Naegle KM, Huang PH, Yaffe MB, Lauffenburger DA, White FM 2009. An integrated comparative phosphoproteomic and bioinformatic approach reveals a novel class of MPM-2 motifs upregulated in EGFRvIII-expressing glioblastoma cells. Mol Biosyst 5: 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D, Kline JT 1992a. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol 149: 80–89 [DOI] [PubMed] [Google Scholar]
- Kline D, Kline JT 1992b. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem 267: 17624–17630 [PubMed] [Google Scholar]
- Kline D, Mehlmann L, Fox C, Terasaki M 1999. The cortical endoplasmic reticulum (ER) of the mouse egg: localization of ER clusters in relation to the generation of repetitive calcium waves. Dev Biol 215: 431–442 [DOI] [PubMed] [Google Scholar]
- Knott JG, Gardner AJ, Madgwick S, Jones KT, Williams CJ, Schultz RM 2006. Calmodulin-dependent protein kinase II triggers mouse egg activation and embryo development in the absence of Ca2+ oscillations. Dev Biol 296: 388–395 [DOI] [PubMed] [Google Scholar]
- Koh S, Lee K, Wang C, Cabot RA, Machaty Z 2009. STIM1 regulates store-operated Ca(2+) entry in oocytes. Dev Biol 330: 368–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T, Jones KT, Bos-Mikich A, Whittingham DG, Carroll J 1996. A cell cycle-associated change in Ca2+ releasing activity leads to the generation of Ca2+ transients in mouse embryos during the first mitotic division. J Cell Biol 132: 915–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S 2004. Recombinant phospholipase Czeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem 279: 10408–10412 [DOI] [PubMed] [Google Scholar]
- Kouchi Z, Shikano T, Nakamura Y, Shirakawa H, Fukami K, Miyazaki S 2005. The role of EF-hand domains and C2 domain in regulation of enzymatic activity of phospholipase Czeta. J Biol Chem 280: 21015–21021 [DOI] [PubMed] [Google Scholar]
- Kume S, Muto A, Okano H, Mikoshiba K 1997. Developmental expression of the inositol 1,4,5-trisphosphate receptor and localization of inositol 1,4,5-trisphosphate during early embryogenesis in Xenopus laevis. Mech Dev 66: 157–168 [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Fissore RA 2003. ICSI-generated mouse zygotes exhibit altered calcium oscillations, inositol 1,4,5-trisphosphate receptor-1 down-regulation, and embryo development. Mol Hum Reprod 9: 523–533 [DOI] [PubMed] [Google Scholar]
- Kyozuka K, Deguchi R, Mohri T, Miyazaki S 1998. Injection of sperm extract mimics spatiotemporal dynamics of Ca2+ responses and progression of meiosis at fertilization of ascidian oocytes. Development 125: 4099–4105 [DOI] [PubMed] [Google Scholar]
- Lawrence YM, Cuthbertson KS 1995. Thapsigargin induces cytoplasmic free Ca2+ oscillations in mouse oocytes. Cell Calcium 17: 154–164 [DOI] [PubMed] [Google Scholar]
- Lee B, Vermassen E, Yoon SY, Vanderheyden V, Ito J, Alfandari D, De Smedt H, Parys JB, Fissore RA 2006. Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: a role for the MAP kinase pathway. Development 133: 4355–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Yoon SY, Malcuit C, Parys JB, Fissore RA Inositol 1,4,5-trisphosphate receptor 1 degradation in mouse eggs and impact on [Ca2+]i oscillations. J Cell Physiol 222: 238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS, Cahalan MD 1989. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul 1: 99–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS 2011. Store-operated calcium channels: New perspectives on mechanism and function. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a003970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T 2005. STIM is a Ca2+ sensor essential for Ca2+ store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Hammar K, Smith PJ, Inoue S, Keefe DL 2001. Mitochondrial modulation of calcium signaling at the initiation of development. Cell Calcium 30: 423–433 [DOI] [PubMed] [Google Scholar]
- Loeb J 1907. On the chemical character of the process of fertilization and its bearing upon the theory of life phenomena. Science 26: 425–437 [DOI] [PubMed] [Google Scholar]
- Lorca T, Cruzalegui FH, Fesquet D, Cavadore JC, Mery J, Means A, Doree M 1993. Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature 366: 270–273 [DOI] [PubMed] [Google Scholar]
- Machaca K, Haun S 2002. Induction of maturation-promoting factor during Xenopus oocyte maturation uncouples Ca(2+) store depletion from store-operated Ca(2+) entry. J Cell Biol 156: 75–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaty Z, Ramsoondar JJ, Bonk AJ, Bondioli KR, Prather RS 2002. Capacitative calcium entry mechanism in porcine oocytes. Biol Reprod 66: 667–674 [DOI] [PubMed] [Google Scholar]
- MacKrill JJ 1999. Protein-protein interactions in intracellular Ca2+-release channel function. Biochem J 337 ( Pt 3): 345–361 [PMC free article] [PubMed] [Google Scholar]
- Madgwick S, Levasseur M, Jones KT 2005. Calmodulin-dependent protein kinase II, and not protein kinase C, is sufficient for triggering cell-cycle resumption in mammalian eggs. J Cell Sci 118: 3849–3859 [DOI] [PubMed] [Google Scholar]
- Madgwick S, Hansen DV, Levasseur M, Jackson PK, Jones KT 2006. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J Cell Biol 174: 791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madgwick S, Nixon VL, Chang HY, Herbert M, Levasseur M, Jones KT 2004. Maintenance of sister chromatid attachment in mouse eggs through maturation-promoting factor activity. Dev Biol 275: 68–81 [DOI] [PubMed] [Google Scholar]
- Maeda N, Niinobe M, Nakahira K, Mikoshiba K 1988. Purification and characterization of P400 protein, a glycoprotein characteristic of Purkinje cell, from mouse cerebellum. J Neurochem 51: 1724–1730 [DOI] [PubMed] [Google Scholar]
- Mahbub Hasan AK, Sato K, Sakakibara K, Ou Z, Iwasaki T, Ueda Y, Fukami Y 2005. Uroplakin III, a novel Src substrate in Xenopus egg rafts, is a target for sperm protease essential for fertilization. Dev Biol 286: 483–492 [DOI] [PubMed] [Google Scholar]
- Malathi K, Kohyama S, Ho M, Soghoian D, Li X, Silane M, Berenstein A, Jayaraman T 2003. Inositol 1,4,5-trisphosphate receptor (type 1) phosphorylation and modulation by Cdc2. J Cell Biochem 90: 1186–1196 [DOI] [PubMed] [Google Scholar]
- Malathi K, Li X, Krizanova O, Ondrias K, Sperber K, Ablamunits V, Jayaraman T 2005. Cdc2/cyclin B1 interacts with and modulates inositol 1,4,5-trisphosphate receptor (type 1) functions. J Immunol 175: 6205–6210 [DOI] [PubMed] [Google Scholar]
- Malcuit C, Knott JG, He C, Wainwright T, Parys JB, Robl JM, Fissore RA 2005. Fertilization and inositol 1,4,5-trisphosphate (IP3)-induced calcium release in type-1 inositol 1,4,5-trisphosphate receptor down-regulated bovine eggs. Biol Reprod 73: 2–13 [DOI] [PubMed] [Google Scholar]
- Malcuit C, Maserati M, Takahashi Y, Page R, Fissore RA 2006. Intracytoplasmic sperm injection in the bovine induces abnormal [Ca2+]i responses and oocyte activation. Reprod Fertil Dev 18: 39–51 [DOI] [PubMed] [Google Scholar]
- Manji SS, Parker NJ, Williams RT, van Stekelenburg L, Pearson RB, Dziadek M, Smith PJ 2000. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta 1481: 147–155 [DOI] [PubMed] [Google Scholar]
- Marangos P, Carroll J 2004. Fertilization and InsP3-induced Ca2+ release stimulate a persistent increase in the rate of degradation of cyclin B1 specifically in mature mouse oocytes. Dev Biol 272: 26–38 [DOI] [PubMed] [Google Scholar]
- Marangos P, FitzHarris G, Carroll J 2003. Ca2+ oscillations at fertilization in mammals are regulated by the formation of pronuclei. Development 130: 1461–1472 [DOI] [PubMed] [Google Scholar]
- Markoulaki S, Matson S, Abbott AL, Ducibella T 2003. Oscillatory CaMKII activity in mouse egg activation. Dev Biol 258: 464–474 [DOI] [PubMed] [Google Scholar]
- Martin-Romero FJ, Ortiz-de-Galisteo JR, Lara-Laranjeira J, Dominguez-Arroyo JA, Gonzalez-Carrera E, Alvarez IS 2008. Store-operated calcium entry in human oocytes and sensitivity to oxidative stress. Biol Reprod 78: 307–315 [DOI] [PubMed] [Google Scholar]
- Masui Y, Markert CL 1971. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool 177: 129–145 [DOI] [PubMed] [Google Scholar]
- Matson S, Ducibella T 2007. The MEK inhibitor, U0126, alters fertilization-induced [Ca2+]i oscillation parameters and secretion: differential effects associated with in vivo and in vitro meiotic maturation. Dev Biol 306: 538–548 [DOI] [PubMed] [Google Scholar]
- Matson S, Markoulaki S, Ducibella T 2006. Antagonists of myosin light chain kinase and of myosin II inhibit specific events of egg activation in fertilized mouse eggs. Biol Reprod 74: 169–176 [DOI] [PubMed] [Google Scholar]
- Mazia D 1937. The release of calcium in Arbacia eggs on fertilization. J Cell Comp Physiol 10: 291–304 [Google Scholar]
- McAvey BA, Wortzman GB, Williams CJ, Evans JP 2002. Involvement of calcium signaling and the actin cytoskeleton in the membrane block to polyspermy in mouse eggs. Biol Reprod 67: 1342–1352 [DOI] [PubMed] [Google Scholar]
- McGuinness OM, Moreton RB, Johnson MH, Berridge MJ 1996. A direct measurement of increased divalent cation influx in fertilised mouse oocytes. Development 122: 2199–2206 [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Kline D 1994. Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biology of Reproduction 51: 1088–1098 [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Mikoshiba K, Kline D 1996. Redistribution and increase in cortical inositol 1,4,5-trisphosphate receptors after meiotic maturation of the mouse oocyte. Dev Biol 180: 489–498 [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Chattopadhyay A, Carpenter G, Jaffe LA 2001. Evidence that phospholipase C from the sperm is not responsible for initiating Ca(2+) release at fertilization in mouse eggs. Dev Biol 236: 492–501 [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Terasaki M, Jaffe LA, Kline D 1995. Reorganization of the endoplasmic reticulum during meiotic maturation of the mouse oocyte. Dev Biol 170: 607–615 [DOI] [PubMed] [Google Scholar]
- Missiaen L, Taylor CW, Berridge MJ 1991. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature 352: 241–244 [DOI] [PubMed] [Google Scholar]
- Miyazaki S 1988. Inositol 1,4,5-trisphosphate-induced calcium release and guanine nucleotide-binding protein-mediated periodic calcium increases in golden hamster eggs. J Cell Biol 106: 345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S, Igusa Y 1981. Fertilization potential in golden hamster eggs consists of recurring hyperpolarizations. Nature 290: 702–704 [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Ito M 2006. Calcium signals for egg activation in mammals. J Pharmacol Sci 100: 545–552 [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Hashimoto N, Yoshimoto Y, Kishimoto T, Igusa Y, Hiramoto Y 1986. Temporal and spatial dynamics of the periodic increase in intracellular free calcium at fertilization of golden hamster eggs. Dev Biol 118: 259–267 [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Shirakawa H, Nakada K, Honda Y 1993. Essential role of the inositol 1,4,5-trisphosphate receptor/ Ca2+ release channel in Ca2+waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol 158: 62–78 [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K 1992. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science 257: 251–255 [DOI] [PubMed] [Google Scholar]
- Nabti I, Reis A, Levasseur M, Stemmann O, Jones KT 2008. Securin and not CDK1/cyclin B1 regulates sister chromatid disjunction during meiosis II in mouse eggs. Dev Biol 321: 379–386 [DOI] [PubMed] [Google Scholar]
- Nakano Y, Shirakawa H, Mitsuhashi N, Kuwabara Y, Miyazaki S 1997. Spatiotemporal dynamics of intracellular calcium in the mouse egg injected with a spermatozoon. Mol Hum Reprod 3: 1087–1093 [DOI] [PubMed] [Google Scholar]
- Nixon VL, Levasseur M, McDougall A, Jones KT 2002. Ca(2+) oscillations promote APC/C-dependent cyclin B1 degradation during metaphase arrest and completion of meiosis in fertilizing mouse eggs. Curr Biol 12: 746–750 [DOI] [PubMed] [Google Scholar]
- Nomikos M, Blayney LM, Larman MG, Campbell K, Rossbach A, Saunders CM, Swann K, Lai FA 2005. Role of phospholipase C-zeta domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations. J Biol Chem 280: 31011–31018 [DOI] [PubMed] [Google Scholar]
- Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA 2009. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 136: 1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ 2005. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci 102: 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh B, Hwang SY, Solter D, Knowles BB 1997. Spindlin, a major maternal transcript expressed in the mouse during the transition from oocyte to embryo. Development 124: 493–503 [DOI] [PubMed] [Google Scholar]
- Ozil JP, Banrezes B, Toth S, Pan H, Schultz RM 2006. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol 300: 534–544 [DOI] [PubMed] [Google Scholar]
- Ozil JP, Markoulaki S, Toth S, Matson S, Banrezes B, Knott JG, Schultz RM, Huneau D, Ducibella T 2005. Egg activation events are regulated by the duration of a sustained [Ca2+]cyt signal in the mouse. Dev Biol 282: 39–54 [DOI] [PubMed] [Google Scholar]
- Page AW, Orr-Weaver TL 1997. Activation of the meiotic divisions in Drosophila oocytes. Dev Biol 183: 195–207 [DOI] [PubMed] [Google Scholar]
- Page Baluch D, Koeneman BA, Hatch KR, McGaughey RW, Capco DG 2004. PKC isotypes in post-activated and fertilized mouse eggs: association with the meiotic spindle. Dev Biol 274: 45–55 [DOI] [PubMed] [Google Scholar]
- Palermo G, Joris H, Devroey P, Van Steirteghem AC 1992. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 340: 17–18 [DOI] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS 2009. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136: 876–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrington J, Brind S, De Smedt H, Gangeswaran R, Lai FA, Wojcikiewicz R, Carroll J 1998. Expression of inositol 1,4,5-trisphosphate receptors in mouse oocytes and early embryos: the type I isoform is upregulated in oocytes and downregulated after fertilization. Dev Biol 203: 451–461 [DOI] [PubMed] [Google Scholar]
- Parrington J, Davis LC, Galione A, Wessel G 2007. Flipping the switch: how a sperm activates the egg at fertilization. Dev Dyn 236: 2027–2038 [DOI] [PubMed] [Google Scholar]
- Parrington J, Jones KT, Lai A, Swann K 1999. The soluble sperm factor that causes Ca2+ release from sea-urchin (Lytechinus pictus) egg homogenates also triggers Ca2+ oscillations after injection into mouse eggs. Biochem J 341 ( Pt 1): 1–4 [PMC free article] [PubMed] [Google Scholar]
- Parrington J, Jones ML, Tunwell R, Devader C, Katan M, Swann K 2002. Phospholipase C isoforms in mammalian spermatozoa: potential components of the sperm factor that causes Ca2+ release in eggs. Reproduction 123: 31–39 [DOI] [PubMed] [Google Scholar]
- Parys JB, McPherson SM, Mathews L, Campbell KP, Longo FJ 1994. Presence of inositol 1,4,5-trisphosphate receptor, calreticulin, and calsequestrin in eggs of sea urchins and Xenopus laevis. Dev Biol 161: 466–476 [DOI] [PubMed] [Google Scholar]
- Patterson RL, Boehning D, Snyder SH 2004. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem 73: 437–465 [DOI] [PubMed] [Google Scholar]
- Pepperell JR, Kommineni K, Buradagunta S, Smith PJ, Keefe DL 1999. Transmembrane regulation of intracellular calcium by a plasma membrane sodium/calcium exchanger in mouse ova. Biol Reprod 60: 1137–1143 [DOI] [PubMed] [Google Scholar]
- Perry AC, Verlhac MH 2008. Second meiotic arrest and exit in frogs and mice. EMBO Rep 9: 246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C, McCarl C, Papolos A, Khalil S, Luthy K, Hivroz C, LeDeist F, Rieux-Laucat F, Rechavi G, Rao A, et al. 2009. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med 360: 1971–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SF, Sha’afi RI, Berlin RD 1991. Regulation of Ca2+ influx during mitosis: Ca2+ influx and depletion of intracellular Ca2+ stores are coupled in interphase but not mitosis. Cell Regul 2: 915–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW Jr 1986. A model for receptor-regulated calcium entry. Cell Calcium 7: 1–12 [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN 2000. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev 80: 1291–1335 [DOI] [PubMed] [Google Scholar]
- Rice A, Parrington J, Jones KT, Swann K 2000. Mammalian sperm contain a Ca(2+)-sensitive phospholipase C activity that can generate InsP(3) from PIP(2) associated with intracellular organelles. Dev Biol 228: 125–135 [DOI] [PubMed] [Google Scholar]
- Ridgway EB, Gilkey JC, Jaffe LF 1977. Free calcium increases explosively in activating medaka eggs. Proc Natl Acad Sci 74: 623–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Bernardi P, Pozzan T 2000. Mitochondria as all-round players of the calcium game. J Physiol 529 Pt 1: 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T 1998. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280: 1763–1766 [DOI] [PubMed] [Google Scholar]
- Roderick HL, Lechleiter JD, Camacho P 2000. Cytosolic phosphorylation of calnexin controls intracellular Ca(2+) oscillations via an interaction with SERCA2b. J Cell Biol 149: 1235–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NT, Halet G, Piao Y, Carroll J, Ko MS, Swann K 2006. The absence of a Ca(2+) signal during mouse egg activation can affect parthenogenetic preimplantation development, gene expression patterns, and blastocyst quality. Reproduction 132: 45–57 [DOI] [PubMed] [Google Scholar]
- Rogers NT, Hobson E, Pickering S, Lai FA, Braude P, Swann K 2004. Phospholipase Czeta causes Ca2+ oscillations and parthenogenetic activation of human oocytes. Reproduction 128: 697–702 [DOI] [PubMed] [Google Scholar]
- Rong Y, Aromolaran A, Bultynck G, Zhong F, Li X, McColl K, Matsuyama S, Herlitze S, Roderick H, Bootman MW, et al. 2008. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2’s inhibition of apoptotic calcium signals. Mol Cell 31: 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong YP, Bultynck G, Aromolaran AS, Zhong F, Parys JB, De Smedt H, Mignery GA, Roderick HL, Bootman MD, Distelhorst CW 2009. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc Natl Acad Sci 106: 14397–14402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio P, Yeromin A, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak J, Wagner S, Cahalan MA, et al. 2005. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PJ, Beyhan Z, Iager AE, Yoon SY, Malcuit C, Schellander K, Fissore RA, Cibelli JB 2008. Parthenogenetic activation of bovine oocytes using bovine and murine phospholipase C zeta. BMC Dev Biol 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runft LL, Watras J, Jaffe LA 1999. Calcium release at fertilization of Xenopus eggs requires type I IP(3) receptors, but not SH2 domain-mediated activation of PLCgamma or G(q)-mediated activation of PLCbeta. Dev Biol 214: 399–411 [DOI] [PubMed] [Google Scholar]
- Sardet C, Prodon F, Dumollard R, Chang P, Chenevert J 2002. Structure and function of the egg cortex from oogenesis through fertilization. Dev Biol 241: 1–23 [DOI] [PubMed] [Google Scholar]
- Sato K, Tokmakov AA, Iwasaki T, Fukami Y 2000. Tyrosine kinase-dependent activation of phospholipase Cgamma is required for calcium transient in Xenopus egg fertilization. Dev Biol 224: 453–469 [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA 2002. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development 129: 3533–3544 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Duncan PI, Rauh NR, Sauer G, Fry AM, Nigg EA, Mayer TU 2005. Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev 19: 502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RM, Kopf GS 1995. Molecular basis of mammalian egg activation. Curr Top Dev Biol 30: 21–62 [DOI] [PubMed] [Google Scholar]
- Shimomura O, Johnson FH 1970. Calcium binding, quantum yield, and emitting molecule in aequorin bioluminescence. Nature 227: 1356–1357 [DOI] [PubMed] [Google Scholar]
- Shoji S, Yoshida N, Amanai M, Ohgishi M, Fukui T, Fujimoto S, Nakano Y, Kajikawa E, Perry AC 2006. Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J 25: 834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly C, Nowak G, de Lanerolle P, Schatten G 1998. Differential expression and functions of cortical myosin IIA and IIB isotypes during meiotic maturation, fertilization, and mitosis in mouse oocytes and embryos. Mol Biol Cell 9: 2509–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW Jr 2006. Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta 1763: 1147–1160 [DOI] [PubMed] [Google Scholar]
- Smyth JT, Petranka JG, Boyles RR, DeHaven WI, Fukushima M, Johnson KL, Williams JG, Putney JW Jr 2009. Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat Cell Biol 11: 1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt RA, Epel D 1974. Activation of sea-urchin eggs by a calcium ionophore. Proc Natl Acad Sci 71: 1915–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R, Zucker R, Schatten G 1977. Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol 58: 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt RA, Epel D, Carroll EJ Jr, Yanagimachi R 1974. Is calcium ionophore a universal activator for unfertilised eggs? Nature 252: 41–43 [DOI] [PubMed] [Google Scholar]
- Stice SL, Robl JM 1990. Activation of mammalian oocytes by a factor obtained from rabbit sperm. Mol Reprod Dev 25: 272–280 [DOI] [PubMed] [Google Scholar]
- Stricker SA 1997. Intracellular injections of a soluble sperm factor trigger calcium oscillations and meiotic maturation in unfertilized oocytes of a marine worm. Dev Biol 186: 185–201 [DOI] [PubMed] [Google Scholar]
- Stricker SA 1999. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol 211: 157–176 [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Woo Y, Wigglesworth K, Kamdar S, Affourtit J, Eppig JJ 2007. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev Biol 302: 104–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez SS 2008a. Control of hyperactivation in sperm. Hum Reprod Update 14: 647–657 [DOI] [PubMed] [Google Scholar]
- Suarez SS 2008b. Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol 52: 455–462 [DOI] [PubMed] [Google Scholar]
- Sun L, Haun S, Jones RC, Edmondson RD, Machaca K 2009. Kinase-dependent regulation of IP3-dependent Ca2+ release during oocyte maturation. J Biol Chem 284: 20184–20196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FZ, Hoyland J, Huang X, Mason W, Moor RM 1992. A comparison of intracellular changes in porcine eggs after fertilization and electroactivation. Development 115: 947–956 [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Manandhar G, Wu A, Oko R 2003. Interactions of sperm perinuclear theca with the oocyte: implications for oocyte activation, anti-polyspermy defense, and assisted reproduction. Microsc Res Tech 61: 362–378 [DOI] [PubMed] [Google Scholar]
- Swann K 1990. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development 110: 1295–1302 [DOI] [PubMed] [Google Scholar]
- Swann K, Lai FA 1997. A novel signalling mechanism for generating Ca2+ oscillations at fertilization in mammals. Bioessays 19: 371–378 [DOI] [PubMed] [Google Scholar]
- Swann K, Igusa Y, Miyazaki S 1989. Evidence for an inhibitory effect of protein kinase C on G-protein-mediated repetitive calcium transients in hamster eggs. EMBO J 8: 3711–3718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann K, Saunders CM, Rogers NT, Lai FA 2006. PLCzeta (zeta): a sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin Cell Dev Biol 17: 264–273 [DOI] [PubMed] [Google Scholar]
- Swenson KI, Farrell KM, Ruderman JV 1986. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell 47: 861–870 [DOI] [PubMed] [Google Scholar]
- Szado T, Vanderheyden V, Parys J, De Smedt H, Rietdorf K, Kotelevets L, Chastre E, Khan F, Landegren U, Soderberg OL, et al. 2008. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Natl Acad Sci 105: 2427–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SL, Yoon SY, Morshedi MS, Lacey DR, Jellerette T, Fissore RA, Oehninger S 2010. Complete globozoospermia associated with PLCzeta deficiency treated with calcium ionophore and ICSI results in pregnancy. Reprod Biomed Online 20: 559–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Tovey SC 2010. IP3 receptors: toward understanding their activation. Cold Spring Harb Perspect 10.1101/cshperspect.a004010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesarik J, Testart J 1994. Treatment of sperm-injected human oocytes with Ca2+ ionophore supports the development of Ca2+ oscillations. Biol Reprod 51: 385–391 [DOI] [PubMed] [Google Scholar]
- Thomas TW, Eckberg WR, Dube F, Galione A 1998. Mechanisms of calcium release and sequestration in eggs of Chaetopterus pergamentaceus. Cell Calcium 24: 285–292 [DOI] [PubMed] [Google Scholar]
- Tosti E, Boni R 2004. Electrical events during gamete maturation and fertilization in animals and humans. Hum Reprod Update 10: 53–65 [DOI] [PubMed] [Google Scholar]
- Tung JJ, Hansen DV, Ban KH, Loktev AV, Summers MK, Adler JR 3rd, Jackson PK 2005. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc Natl Acad Sci 102: 4318–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunquist BJ, Maller JL 2003. Under arrest: cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Genes Dev 17: 683–710 [DOI] [PubMed] [Google Scholar]
- Turner PR, Jaffe LA, Fein A 1986. Regulation of cortical vesicle exocytosis in sea urchin eggs by inositol 1,4,5-trisphosphate and GTP-binding protein. J Cell Biol 102: 70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PR, Sheetz MP, Jaffe LA 1984. Fertilization increases the polyphosphoinositide content of sea urchin eggs. Nature 310: 414–415 [DOI] [PubMed] [Google Scholar]
- Vanderheyden V, Wakai T, Bultynck G, De Smedt H, Parys JB, Fissore RA 2009. Regulation of inositol 1,4,5-trisphosphate receptor type 1 function during oocyte maturation by MPM-2 phosphorylation. Cell Calcium 46: 56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C 2007. TRP channels. Annu Rev Biochem 76: 387–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa D, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner RP, et al. 2006. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312: 1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveiros MM, Hirao Y, Eppig JJ 2001. Evidence that protein kinase C (PKC) participates in the meiosis I to meiosis II transition in mouse oocytes. Dev Biol 235: 330–342 [DOI] [PubMed] [Google Scholar]
- Westendorf JM, Rao PN, Gerace L 1994. Cloning of cDNAs for M-phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope. Proc Natl Acad Sci 91: 714–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M 2006. Calcium at fertilization and in early development. Physiol Rev 86: 25–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston NJ, McGuinness O, Johnson MH, Maro B 1995. The exit of mouse oocytes from meiotic M-phase requires an intact spindle during intracellular calcium release. J Cell Sci 108 ( Pt 1): 143–151 [DOI] [PubMed] [Google Scholar]
- Wu H, He CL, Fissore RA 1997. Injection of a porcine sperm factor triggers calcium oscillations in mouse oocytes and bovine eggs. Mol Reprod Dev 46: 176–189 [DOI] [PubMed] [Google Scholar]
- Xu Z, Kopf GS, Schultz RM 1994. Involvement of inositol 1,4,5-trisphosphate-mediated Ca2+ release in early and late events of mouse egg activation. Development 120: 1851–1859 [DOI] [PubMed] [Google Scholar]
- Yoda A, Oda S, Shikano T, Kouchi Z, Awaji T, Shirakawa H, Kinoshita K, Miyazaki S 2004. Ca2+ oscillation-inducing phospholipase C zeta expressed in mouse eggs is accumulated to the pronucleus during egg activation. Dev Biol 268: 245–257 [DOI] [PubMed] [Google Scholar]
- Yoneda A, Kashima M, Yoshida S, Terada K, Nakagawa S, Sakamoto A, Hayakawa K, Suzuki K, Ueda J, Watanabe T 2006. Molecular cloning, testicular postnatal expression, and oocyte-activating potential of porcine phospholipase Czeta. Reproduction 132: 393–401 [DOI] [PubMed] [Google Scholar]
- Yoon SY, Fissore RA 2007. Release of phospholipase C zeta and [Ca2+]i oscillation-inducing activity during mammalian fertilization. Reproduction 134: 695–704 [DOI] [PubMed] [Google Scholar]
- Yoon S, Jellerette T, Salicioni A, Lee H, Yoo M, Coward K, Parrington J, Grow D, Cibelli J, Visconti PA, et al. 2008. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J Clin Invest 118: 3671–3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Sensui N, Inoue T, Morisawa M, Mikoshiba K 1998. Role of two series of Ca2+ oscillations in activation of ascidian eggs. Dev Biol 203: 122–133 [DOI] [PubMed] [Google Scholar]
- Yu F, Sun L, Machaca K 2009. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc Natl Acad Sci 106: 17401–17406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernicka-Goetz M 1991. Spontaneous and induced activation of rat oocytes. Mol Reprod Dev 28: 169–176 [DOI] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS 1993. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci 90: 6295–6299 [DOI] [PMC free article] [PubMed] [Google Scholar]