Abstract
Mammalian skin comprises a multi-layered epithelium, the epidermis, and an underlying connective tissue, the dermis. The epidermal extracellular matrix is a basement membrane, whereas the dermal ECM comprises fibrillar collagens and associated proteins. There is considerable heterogeneity in ECM composition within both epidermis and dermis. The functional significance of this extends beyond cell adhesion to a range of cell autonomous and nonautonomous processes, including control of epidermal stem cell fate. In skin, cell-ECM interactions influence normal homeostasis, aging, wound healing, and disease. Disturbed integrin and ECM signaling contributes to both tumor formation and fibrosis. Strategies for manipulating cell-ECM interactions to repair skin defects and intervene in a variety of skin diseases hold promise for the future.
Interactions between cell surface integrins and matrix proteins such as laminins generate signals that control skin homeostasis. These are altered in wound healing, aging, and cancer as patterns of integrin expression change.
The focus of this review is the role of cell-ECM interactions in the physiology of normal and diseased mammalian skin. The skin has epithelial and mesenchymal components and contains ECM comprising both fibrillar collagen and basement membrane. Experimentally, it is a highly tractable tissue, and a range of in vitro and in vivo approaches are available to explore cell-ECM interactions. Such studies are of medical importance because of the wide variety of benign and malignant skin diseases. Research on skin therefore provides an integrated, in vivo, context for understanding the functional significance of specific molecular interactions and signaling pathways involved in cell-ECM adhesion.
STRUCTURE OF MAMMALIAN SKIN
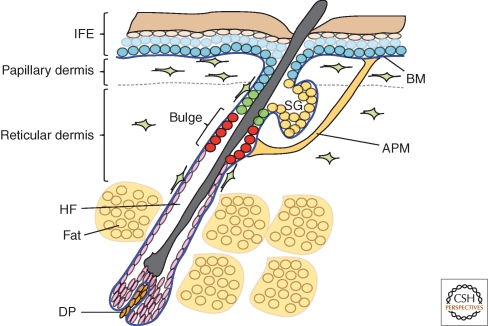
Mammalian skin comprises several distinct layers (Fig. 1). The outermost layer is the epidermis, which consists of a multilayered epithelium, the interfollicular epidermis, and associated structures that include the hair follicles and sebaceous glands (reviewed by Shimizu 2007). The epidermis is maintained by proliferation of stem cells and differentiation of their progeny (Fuchs 2008; Watt and Jensen 2009). There are multiple pools of stem cells located in different epidermal regions, including the permanent portion of the hair follicle (the bulge) and the interfollicular epidermis (reviewed by Jones et al. 2007; Watt and Jensen 2009; Jaks et al. 2010).
Figure 1.
Different layers of mammalian skin. IFE: interfollicular epidermis; HF: hair follicle; SG: sebaceous gland; BM: basement membrane; DP: dermal papilla; APM: arrector pili muscle.
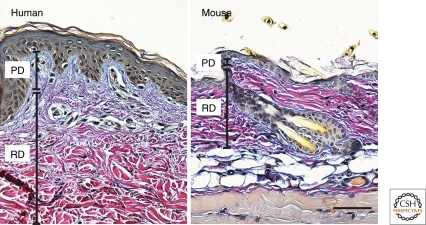
The basal layer of the epidermis is attached to a basement membrane, which overlies the connective tissue layer known as the dermis (Fig. 1). The dermis is rich in collagen fibers (mainly types I and III) and comprises distinct layers. The layer that is closest to the epidermis, known as the papillary (or upper) dermis, has thin collagen fibers. Below that lies the reticular (or deep) dermis, which has dense collagen fibers, and overlies the subcutaneous fat layer. The different types of collagenous ECM in the dermis can be visualized in a number of different ways, including classic histochemical staining (Fig. 2).
Figure 2.
Heterogeneity of dermal ECM. Adult human and mouse skin stained with Herovici’s picropolychrome stain. Highly cross-linked collagen in the reticular dermis (RD) stains purple, whereas collagen in the papillary dermis (PD) stains blue. The papillary dermis is thinner in mouse than human skin. Scale bar: 100 µm.
Fibroblasts, macrophages, mast cells, T and B cells, blood vessels, lymphatics, and nerves are the common cellular components of the dermis. The dermis also contains arrector pili muscles, which insert in the basement membrane of the hair follicle bulge and are responsible for erection of the hair follicles to conserve body heat (Fig. 1).
SKIN ECM HETEROGENEITY
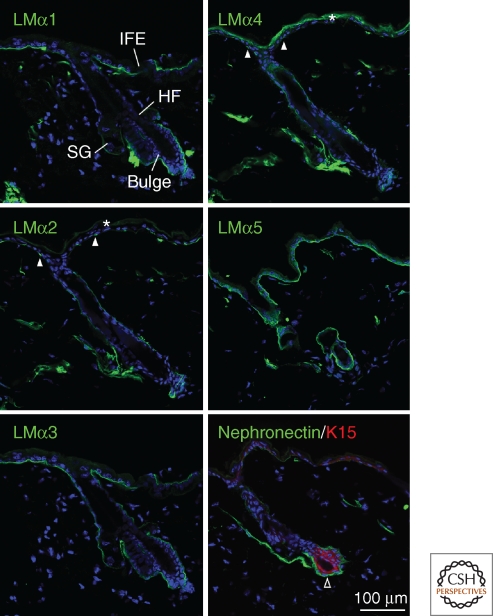
Over the past 20 years, the expression of integrins and extracellular matrix proteins in the skin has been characterized extensively (Watt 2002; Wilhelmsen et al. 2006; Sugawara et al. 2008; Breitkreutz et al. 2009; Ko and Marinkovich 2010). In addition to the differences in ECM composition of upper and deep dermis (Fig. 2), there is regional variation in the composition of the basement membrane, which can be detected both by immunolabeling of skin sections (Fig. 3) and by gene expression profiling (Table 1). The major epidermal integrins are α2β1, α3β1, and α6β4, but other integrins are expressed, albeit at lower levels, and there is variation in the level of integrin expression in different regions of the epidermis (Watt 2002).
Figure 3.
Epidermal basement membrane heterogeneity in adult mouse skin. The laminin (LM) α5 and α3 chains are expressed ubiquitously, whereas the α1 chain is most abundant in the interfollicular epidermis (IFE) and the α2 and α4 chains are most abundant in the hair follicle (HF). Note focal deposition of the α2 and α4 chains in the interfollicular epidermis (closed arrowheads). Nephronectin is specifically expressed in the bulge (open arrowhead), which also expresses keratin 15 (red), and also in the arrector pili muscle. Blue staining is DAPI nuclear counterstain. *Indicates nonspecific labeling of the outermost epidermal layers. SG: sebaceous gland. Scale bar: 100 µm.
Table 1.
ECM genes up-regulated or down-regulated in Keratin-15-positive mouse hair follicle stem cells (Morris et al. 2004).
| Fold change value (log2) | Regulation | Gene symbol | Gene title |
|---|---|---|---|
| 7.42 | Up | Igfbp5 | Insulin-like growth factor binding protein 5 |
| 6.33 | Up | Ctgf* | Connective tissue growth factor |
| 6.07 | Up | Postn* | Periostin |
| 5.65 | Up | Sparc | Secreted acidic cysteine rich glycoprotein |
| 4.88 | Up | Ltbp2* | Latent transforming growth factor β-binding protein 2 |
| 4.20 | Up | Col6a1* | Collagen type-VI α1 |
| 3.82 | Up | Npnt* | Nephronectin |
| 3.39 | Up | Col18a1* | Collagen type-XVIII α1 |
| 2.98 | Up | Col5a2* | Collagen type-V α2 |
| 2.53 | Up | Tnc* | Tenascin C |
| 2.52 | Up | Fbln1* | Fibulin 1 |
| 2.27 | Up | Col4a1 | Collagen type-IV α1 |
| 2.13 | Up | Col6a2 | Collagen type-VI α2 |
| 2.09 | Up | Timp2 | Tissue inhibitor of metalloproteinase 2 |
| 1.66 | Up | Igfbp6 | Insulin-like growth factor binding protein 6 |
| 1.63 | Up | Col1a2 | Collagen type-I α2 |
| 3.57 | Down | Smoc2* | SPARC related modular calcium binding 2 |
| 2.47 | Down | Thbs2 | Thrombospondin 2 |
Asterisks indicate the genes that are also up-regulated or down-regulated in mouse epidermal label retaining cells (Tumbar et al. 2004).
The marked variation in ECM and integrin expression within different regions of the skin and at different developmental stages creates many distinct adhesive environments that can potentially recruit and modulate the properties of different cell populations. We believe that this is highly important in establishing and maintaining tissue organization and function. An excellent resource for finding where specific basement membrane proteins are expressed is the Mouse Basement Membrane Bodymap, part of the MATRIXOME project that is under the direction of Kiyotoshi Sekiguchi (Manabe et al. 2008). The database contains high-resolution virtual slides of immunohistochemically stained sections from different embryonic stages and tissues, including skin from different body sites (http://www.matrixome.com/bm/Home/home/home.asp). Another valuable resource for skin researchers is the human protein atlas (http://www.proteinatlas.org/), which shows the expression patterns of many proteins, including ECM components and integrins, in a wide range of human tissues.
INTEGRINS AND ECM PROTEINS AS EPIDERMAL STEM CELL MARKERS
The behavior of virtually all stem cells, whether pluripotent or tissue-specific, embryonic or adult, is controlled by the interplay between intrinsic transcriptional programs and extrinsic signals (Watt and Driskell 2010). The extrinsic signals are provided by the local microenvironment, or niche, in which the stem cell resides. There is a growing appreciation that ECM is an important niche component for stem cells in a wide range of tissues and organisms (Hall and Watt 1989; Scadden 2006; Spradling et al. 2001; Watt and Hogan 2000).
High levels of integrin expression were reported long ago to be a marker of epidermal stem cells (Jones and Watt 1993; Jones et al. 1995) and now integrins are used to enrich for stem cells in a variety of different cell types (see, for example, Wagers and Weissman 2006; Stingl et al. 2006; Shackleton et al. 2006). Although the difference in integrin levels between stem cells and their more differentiated progeny within the basal layer of the interfollicular epidermis is only two- to threefold, it is sufficient to ensure that stem cells are more adhesive to ECM proteins (Jones et al. 1995). Microarray analysis has revealed more marked differences in expression of ECM genes between hair follicle bulge stem cells (defined on the basis of Keratin-15 expression or DNA label retention) and other epidermal cell populations (Table 1). The functional significance of the unique ECM of the bulge is incompletely understood. It may serve to anchor stem cells in the niche, and may also play a role in communication between bulge cells and adjacent dermal cell populations, such as the arrector pili muscle.
There have been relatively few attempts to understand how epidermal integrin and ECM gene expression is regulated at the transcriptional level (Corbi et al. 2000; Turner et al. 2006; Takaoka et al. 1998). However, one aspect that has been explored is negative regulation of integrin, cytoskeleton, and ECM genes by Myc (Watt et al. 2008; Berta et al. 2010). The negative effects of Myc on epidermal adhesion and stem cell maintenance are counteracted by the Rho GTPase Rac1 (Benitah et al. 2005). Rac1 negatively regulates c-Myc through p21-activated kinase 2 (PAK2) phosphorylation, which decreases Myc stability and DNA binding (Benitah et al. 2005; Watt et al. 2008; Berta et al. 2010).
Myc negatively regulates integrin gene expression in other cell types (Inghirami et al. 1990), but the consequences are different. In the epidermis, high levels of Myc induce stem cell differentiation and this is proposed to be a fail-safe mechanism to protect against uncontrolled proliferation. In contrast, down-regulation of integrins in B lymphoid cells is linked to uncontrolled growth (Inghirami et al. 1990). Nevertheless, in both epidermis and the hemopoietic system, Myc activation drives changes in stem cell anchorage to the niche (Murphy et al. 2005; Watt et al. 2008).
INTEGRIN-ECM INTERACTIONS REGULATE STEM CELL FATE
Integrin and ECM expression not only provide epidermal stem cell markers, but also regulate stem cell fate. Loss of contact with the ECM or reduced integrin expression in adherent cells triggers terminal differentiation of cultured epidermal cells (Watt 2002; Grose et al. 2002). In culture, β1 integrin activation suppresses terminal differentiation (Evans et al. 2003). Studies with cultured human epidermal cells have delineated some of the signaling pathways involved (Watt 2002), revealing a key role for Erk MAPK (MAP kinase) signaling downstream from β1 integrins in providing a differentiation-inhibitory signal (Zhu et al. 1999; Levy et al. 2000; Haase et al. 2001; Evans et al. 2003).
Consistent with the differentiation of epidermal cells in suspension, restricting the spreading of single cells on micropatterned substrates stimulates terminal differentiation (Watt et al. 1988; Gautrot et al. 2010). However, in this case the signal is not dependent on the composition or concentration of the ECM coating, or on focal adhesion formation. Instead, differentiation is regulated by the state of assembly of the actin cytoskeleton: initiation of differentiation is correlated with a low level of G actin (Connelly et al. 2010). These observations have led to the identification of the SRF/MAL transcription factor complex in regulating differentiation via AP1 transcription factors (Connelly et al. 2010). E-cadherin signaling also regulates SRF/MAL in epithelial cells (Busche et al. 2008), which increases the intriguing possibility that these transcription factors integrate signals from both cell-ECM and cell-cell interactions.
The initial evidence that integrin-ECM adhesion regulates the epidermal stem cell compartment was obtained with cultured cells. When the consequences of genetic deletion of integrins in transgenic mice were evaluated, it was suggested that integrins did not regulate differentiation (Raghavan et al. 2000; Watt 2002). This may reflect, at least in part, compensatory up-regulation of αvβ6 (Raghavan et al. 2003; cf. Grose et al. 2002). Notwithstanding the obvious differences between the in vivo and in vitro cellular environment, there is now strong evidence that integrins do regulate epidermal stem cells in vivo.
Piwko-Czuchra et al. (2009) recently generated hypomorphic mice in which epidermal β1 integrins are expressed at reduced levels, rather than being completely deleted. These studies showed that keratinocytes expressing β1 integrins in vivo undergo considerable expansion relative to β1-null or low β1 expressing epidermal cells. This shows that β1 integrin expression is essential for expansion of epidermal stem cells and tissue homeostasis. Deletion of β1 integrins in the basal layer of adult mouse epidermis reveals a correlation between the level of β1 integrins and proliferation in the interfollicular epidermis that is remarkably consistent with human epidermis (López-Róvira et al. 2005). Although β1 integrin expression is required for stem cell maintenance, β1 integrin activation via mutation of the I-like domain is not sufficient to expand the stem cell compartment in vivo (Ferreira et al. 2009).
Although one mechanism of stem cell maintenance via β1 integrins involves Erk MAPK (Zhu et al. 1999), another is by controlling the orientation of the mitotic spindle (Lechler and Fuchs 2005). Asymmetric cell divisions are important in stem cell fate selection in a wide range of tissues (Hall and Watt 1989; Watt and Hogan 2000; Spradling et al. 2001), and in developing epidermis the transition from a single to a multilayered epithelium is achieved by vertically oriented mitoses. β1 integrins are essential for the apical localization of a protein complex comprising atypical protein kinase C, Par3-LGN-Inscuteable, and NuMA-dynactin that aligns the spindle (Lechler and Fuchs 2005).
Different integrins and ECM proteins play different roles in regulating epidermal homeostasis, and those roles are not obligatorily linked to physical anchorage to the basement membrane. For example, the α6β4 integrin primarily mediates anchorage of the interfollicular epidermis, and the β1 integrins are required for hair follicle maintenance (Watt 2002; Conti et al. 2003), whereas spindle orientation depends on β1, but not β4 integrins (Lechler and Fuchs 2005). Laminin-511 (laminin-10; α5β1γ1) is up-regulated in growing hair follicles and Lama5–/– skin shows a failure of hair germ elongation followed by complete hair follicle regression, defects that are not caused by cell detachment from the basement membrane (Li et al. 2003). Whereas laminin-511 is primarily of importance in the hair follicle, knockout of laminin-α3, β3, and γ2 chains of laminin-332 results in severe skin-blistering (Sugawara et al. 2008). Laminin-332 is thus essential for adhesion of the epidermis to the basement membrane, consistent with human patients with laminin-332 mutations having junctional Epidermolysis bullosa.
The effects of deleting integrin-associated proteins resemble, as anticipated, those of integrin loss, and also reveal differences in the extent to which basement membrane adhesion is compromised (see also Wickstrom et al. 2011). Thus, Kindlin-1–deficient mice show skin atrophy and reduced keratinocyte proliferation, consistent with an effect on the epidermal stem cell compartment, but in the absence of epidermal detachment from the basement membrane (Ussar et al. 2008). Epidermal deletion of focal adhesion kinase (FAK) results in hair follicle defects (Essayem et al. 2006; Schober et al. 2007), and epidermal and sebaceous gland hypoplasia, again pointing to stem cell defects; however, cell migration is unaffected by loss of FAK (Essayem et al. 2006). Conversely, epidermal deletion of ILK leads to epidermal defects and hair loss, but in this case, the phenotypes are attributable to epidermal detachment from the basement membrane, rather than to stem cell depletion (Lorenz et al. 2007).
The conclusion from these studies is that epidermal interactions with the ECM are crucial for stem cell anchorage and self-renewal. There is specificity in the nature of those interactions, and stem cell defects are manifest even when basement membrane adhesion is preserved.
NON-CELL-AUTONOMOUS FUNCTIONS OF EPIDERMAL INTEGRINS AND ECM
Reciprocal signaling between epidermal stem cells and cells of the dermis is essential for normal skin function, and there is clear evidence that epidermal integrins and basement membrane proteins are involved in communication with other cell types. The best-characterized interaction involves the dermal papilla (DP) (Fig. 1), a specialized population of fibroblasts at the base of the hair follicle, which is required for hair follicle formation during development and for the postnatal hair growth cycle (Millar 2002). In recent years it has emerged that laminin-511 regulates dermal papilla function (Li et al. 2003; Gao et al. 2008). At E16.5, dermal papillae from mice lacking laminin-511 fail to maintain expression of the morphogen noggin. Introduction of noggin or sonic hedgehog (Shh), which is produced downstream from noggin, is sufficient to restore hair follicle development in Lama5–/– skin. Shh signaling requires primary cilia, and laminin-511 mutant dermal papillae have defects in primary cilia. Thus, the role of laminin-511 in hair follicle morphogenesis and maintenance is not to anchor epithelial cells to the basement membranes, but to maintain the primary cilium via Noggin and Shh signaling.
Examples of how epidermal integrin expression regulates the properties of other cell types include effects on melanocytes and endothelial cells. Epidermal cells control proliferation of melanocytes via both secreted factors and intercellular adhesion (Haass et al. 2005). When the β1 integrin is deleted in adult mouse epidermis, these signaling events are disturbed and there is an increase in epidermal melanocytes (López-Róvira et al. 2005). Conditional epidermal deletion of the α3 integrin subunit leads to impaired wound angiogenesis, and this correlates with decreased expression of the proangiogenic factors mitogen-related protein 3 (MRP3) and MMP9 (Mitchell et al. 2009; Iyer et al. 2005).
CHANGES IN ECM AND INTEGRIN EXPRESSION ASSOCIATED WITH AGING
During skin aging there are marked changes in ECM and integrin expression. Although the epidermal stem cell compartment does not show evidence of major depletion, there is a reduction in β1 integrin expression and, in human skin, a flattening of the boundary between epidermis and dermis (Bosset et al. 2003; Makrantonaki and Zouboulis 2007; Giangreco et al. 2008, 2010; Racila and Bickenbach 2009). Given the non-cell-autonomous roles of integrins, one can speculate that reduced epidermal integrin levels may affect other cell compartments, such as skin vasculature and pigmentation. Laminin-511 levels decline during aging and it has been suggested that this may contribute to age-related hair loss (Sugawara et al. 2008).
The dermis and underlying fat layer change in thickness and cellularity with age. With age, there is a decrease in cell density within the dermis and an increase in subcutaneous fat (Giangreco et al. 2008). UV-induced changes in human skin (photo-aging) include depletion of fibrillin and type VII collagen in the upper dermis and accumulation of abnormal elastic fibers in the mid and deep dermis, with increased dermal levels of versican (Makrantonaki and Zouboulis 2007; Farage et al. 2008). Abnormal accumulation of elastic fibers correlates with decreased deposition of collagen I fibers in photo-aged skin, resulting from increased collagen degradation. In sun-protected aging skin both collagen and elastic fibers show decreased levels (Makrantonaki and Zouboulis 2007; Farage et al. 2008).
EPIDERMAL HYPERPROLIFERATION AND WOUND HEALING
During skin wound healing there is extensive interaction between cells of the epidermis, dermis and bone marrow, and ECM proteins and integrins play key roles in those interactions (Werner et al. 2007). The earliest phase in wound repair is inflammation. This is followed by deposition of new connective tissue, known as granulation tissue, and migration of keratinocytes over the granulation tissue to repair the epidermis. Fibroblast activation, which results in α-SMA (α-smooth muscle actin) expression, exerts mechanical tension and contributes to normal wound repair; in addition, DP fibroblasts are recruited to the wound site (Gharzi et al. 2003; Biernaskie et al. 2009). Activated fibroblasts synthesize ECM proteins, a process that is controlled by TGF-β. Keratinocytes and fibroblasts also collaborate to form the new basement membrane.
In vivo, the loss of epidermal β1 integrins causes a severe defect in wound healing, which correlates with an increased inflammatory response (Grose et al. 2002). Wound healing is also compromised when the α3 integrin subunit is deleted in epidermal cells and this is because of a failure to up-regulate Smad7, an inhibitor of TGF-β1 signaling (Reynolds et al. 2008).
In hyperproliferative epidermis, integrin expression is no longer confined to the basal layer, but instead integrins are expressed, in the absence of ECM ligands, in the suprabasal layers of cells undergoing terminal differentiation (Watt 2002). Suprabasal integrin expression is also observed during skin wound healing, in psoriasis and in some cancers. When integrins are overexpressed in the suprabasal layers of transgenic mouse epidermis this results, in the case of β1 integrins, but not α6β4, in sporadic epidermal hyperproliferation and skin inflammation associated with elevated epidermal cytokine production (Watt 2002; Owens and Watt 2003; Teige et al. 2010).
When β1 integrins are expressed in the suprabasal layers of hyperproliferative human and mouse epidermis there is elevated Erk MAPK signaling in those layers (Haase et al. 2001). When activated MEK1 is expressed suprabasally in transgenic mice, there is constitutive epidermal hyperproliferation and inflammation (Hobbs et al. 2004). One of the cytokines that is up-regulated on suprabasal epidermal integrin expression is IL1-α (Hobbs and Watt 2003; Hobbs et al. 2004). The effect of suprabasal integrin expression on cytokine production is another example of non-cell-autonomous integrin signaling. During skin inflammation, cells of both epidermis and dermis communicate with inflammatory cells via cytokine release (Szabowski et al. 2000), which can result in loss of the subdermal fat layer (Guinea-Viniegra et al. 2009).
In summary, the dramatic changes in ECM and integrin expression during wound healing are not only required for re-epithelialization and regeneration of the basement membrane and dermal ECM, but are also involved in intercellular communication. The role of ECM signaling in regulating inflammation is incompletely understood and warrants further investigation.
FIBROSIS
Although fibroblast activation during wound healing is beneficial in rebuilding the dermal ECM, excess collagen deposition by fibroblasts results in fibrosis and scar formation (Gurtner et al. 2008). Fibrosis is a hallmark of many connective tissue diseases, including scleroderma and systemic sclerosis (reviewed by Ihn 2005; Krieg et al. 2007; Olson and Soriano 2009). One of the factors expressed by keratinocytes that promotes fibroblast activation in systemic sclerosis is IL1-α (Aden et al. 2010).
TGF-β is a key mediator of fibrosis and acts by stimulating fibroblast proliferation and ECM synthesis (Ihn 2005; Trojanowska 2002). TGF-β also inhibits ECM degradation by down-regulating MMP-1, which mediates collagen degradation, and up-regulating tissue inhibitors of matrix metalloproteinases (TIMPs) (Ihn 2005). Activation of the TGF-β pathway in postnatal fibroblasts is sufficient to induce skin fibrosis in mice, including epidermal thinning, loss of hair follicles and dermal fibrosis (Sonnylal et al. 2007). Dermal accumulation of collagen type I and III and up-regulation of other ECM proteins, including fibronectin, osteopontin, and SPARC, are observed. The correlation of fibrosis with epidermal hypoplasia may be because of the growth inhibitory effect of TGF-β on epidermal cells.
Two other growth factors that mediate fibrosis are PDGF and connective tissue growth factor (CTGF). Increased PDGFRα signaling is sufficient to drive excessive fibroblast proliferation and ECM deposition, and acts either independently or downstream from TGF-β (Olson and Soriano 2009). CTGF, which also modulates fibroblast growth and ECM synthesis, is induced by TGF-β (Ihn 2005).
Integrins are also implicated in fibrosis. Expression of the vitronectin receptor, αvβ5, is up-regulated by fibroblasts in systemic sclerosis. αvβ5 activates latent TGF-β (Munger et al. 1999). In addition, ECM-bound vitronectin stabilizes the active form of plasminogen activator inhibitor-1, resulting in inhibition of the plasmin-mediated pericellular proteolytic cascade, and contributing to autocrine TGF-β signaling (Asano et al. 2006a, b).
Studies of fibrosis thus indicate that, as in normal skin homeostasis and wound healing, ECM and integrins play an important role.
ECM AND INTEGRINS IN CANCER
Integrin expression is disrupted in tumors of multi-layered epithelia, including benign (papillomas) and malignant (squamous cell carcinomas; SCCs) tumors (Watt 2002). Changes include suprabasal integrin expression, loss of expression and changes in the types of integrins that are expressed (Owens and Watt 2003; Janes and Watt 2006).
The positive role of β1 integrins in epidermal tumor development is well established (Watt 2002; Janes and Watt 2006) and recent studies suggest that high β1 integrin levels may not only enrich for normal stem cells, but also for cancer stem cells (Jensen et al. 2008). When tumors are induced by application of the tumor initiator DMBA and the tumor promoter TPA, epidermal deletion of FAK results in both a reduction in papillomas and in SCCs (McLean et al. 2004). Conversely, epidermal deletion of the TGF-β type II receptor increases susceptibility to squamous cell carcinoma development and this is associated with enhanced integrin signaling via FAK (Guasch et al. 2007).
One particularly powerful approach to studying human SCC formation is to express cancer-associated genes in cultured human epidermal cells grown on normal human dermis and to graft the reconstituted tissue onto immunocompromised mice. Sequential gene expression profiling has led to the identification of a core cancer progression signature that has been subjected to network modeling. Network topology predicts that tumor formation depends on specific extracellular matrix-interacting network hubs (Reuter et al. 2009). The center of the gene network comprises genes that encode ECM-interacting proteins, mediators of inflammation and integrin subunits. One of the hubs is β1 integrin and, consistent with this, anti-β1 treatment inhibits the growth of developing and established tumors. What is particularly intriguing about these observations is that they suggest, once again, that integrin-ECM interactions not only have cell autonomous effects within the epidermis, but also mediate communication with the dermis and inflammatory cells.
The α6β4 integrin and laminin also play a role in SCC development, and this has been revealed in part through the use of epidermal cells from patients with skin blistering disorders resulting from mutation of integrin or ECM genes. Such studies have shown that the ability of oncogenic Ras and NF-κB blockade to convert human epidermis into squamous cell carcinoma is dependent on laminin-332 (laminin-5) and the α6β4 integrin (Dajee et al. 2003).
Patients with Recessive Dystrophic Epidermolysis Bullosa (RDEB) have mutations in the COL7A1 gene encoding the epidermal basement membrane protein type VII collagen. Cells from RDEB patients that retain expression of the amino terminus of collagen VII, which binds laminin-332 (laminin-5), are susceptible to Ras-induced SCC formation, whereas cells that completely lack collagen VII are not (Ortiz-Urda et al. 2005). It turns out that individual domains on the laminin-332 β3 chain have distinct roles in tumor formation (Waterman et al. 2007). Domain VI is essential for adhesion but is not required in SCC. In contrast, domain V-III, which binds type VII collagen, is essential for carcinogenesis and invasion. Thus, distinct adhesive and signaling functions reside in different domains of laminin-332. It appears that the protumorigenic effect of the laminin-332/type VII collagen complex is via activation of phosphoinositol-3-kinase signaling rather than adhesion (Waterman et al. 2007).
A different mechanism by which cell-ECM interactions contribute to cancer is via a switch in integrin expression from αvβ5 to αvβ6 in SCCs (Janes and Watt 2004, 2006). αvβ5-expressing SCC cells undergo suspension-induced apoptosis (anoikis), whereas αv-negative cells and cells expressing αvβ6 do not. Resistance to anoikis depends on the β6 cytoplasmic domain and correlates with PKB/Akt activation. It is proposed that by replacing αvβ5 with αvβ6 on the cell surface SCCs can activate an Akt survival signal that protects them from anoikis.
Intriguingly, a further mechanism by which integrins can contribute to SCC is via integrin mutation (Evans et al. 2003, 2004; Ferreira et al. 2009). One mutation, T188I, found in a human SCC, maps to the I-like domain of the β1 integrin. It results in constitutive activation of ligand binding and sustained activation of Erk MAPK (Evans et al. 2003). Mutations in the β1 I-like domain in human SCCs are rare, and most likely correspond to germline polymorphisms rather than tumor-acquired mutations (Evans et al. 2004). Larger, population-based, studies are required to determine whether β1 polymorphisms have any association with SCC development or prognosis. Nevertheless, when the T188I mutant integrin is expressed in the epidermis of transgenic mice it increases DMBA/TPA induced papilloma formation and results in development of poorly differentiated SCCs (Ferreira et al. 2009).
The role of suprabasal integrin expression in tumor formation has been studied in transgenic mice expressing integrin subunits under the control of the involucrin promoter. The mice do not develop spontaneous tumors. However, when subjected to a chemical carcinogenesis protocol involving a tumor initiator and tumor promoter, different integrins have different effects: α3β1 decreases conversion of benign to malignant tumors, whereas both α5β1 and α6β4 stimulate formation of primary tumors and metastases (Owens and Watt 2003; Janes and Watt 2006).
The mechanisms by which the suprabasal integrins exert their effects are non-cell-autonomous, because the transgene-positive cells do not themselves divide. In the case of α6β4, the mechanism is dependent on E-cadherin-mediated adhesion of the transgene-positive cells to the underlying basal layer and renders the basal cells relatively unresponsive to TGF-β-mediated growth inhibition. In the case of suprabasal β1 integrin expression, epidermal secretion of cytokines such as IL-1α is potentially involved (Janes and Watt 2006; Tiege et al. 2010; Arwert et al. 2010).
WOUND HEALING, FIBROSIS, AND CANCER
Cancer can be viewed from a number of different perspectives, ranging from step-wise accumulation of genetic alterations to the tumor as an organ, comprising multiple cell types (Egeblad et al. 2010). A key tenet of the latter perspective is that to understand how tumors form and progress it is necessary to consider the mutant cells in the context of all the different cell types with which they interact. This is particularly appropriate when considering how ECM signaling contributes to skin tumors.
Over the years, many researchers have noted the similarities between wound repair and cancer, and tumors are sometimes referred to as wounds that never heal (Dvorak 1986). Tumors can develop at sites of chronic injury, and chronic inflammation is a known risk factor in cancer. Gene expression profiling of skin wounds and tumors has revealed many similarities between wound healing and cancer (Schäfer and Werner 2008). There are also striking similarities between fibrosis and carcinogenesis, leading to speculation that blocking tissue fibrosis might inhibit cancer formation (Cutroneo et al. 2006).
Aberrant fibroblast behavior also contributes to cancer initiation and progression (Tlsty and Coussens 2006; Kalluri and Zeisberg 2006; Erez et al. 2010). Fibroblasts in the tumor stroma have a modified phenotype resembling activated fibroblasts in healing wounds and secrete increased levels of ECM-degrading enzymes (Sneddon et al. 2006). For example, studies of mice lacking MMP13 have shown that it is essential for maintenance of angiogenesis and for tumor-cell invasion. In wild-type mice, MMP13 is induced in fibroblasts at the onset of tumor invasion, resulting in release of VEGF from the ECM (Lederle et al. 2010).
LOOKING TO THE FUTURE
Although much has been learned from studying the interaction of individual cell types with specific ECM proteins, recent studies in the skin highlight the importance of ECM proteins and integrins in communication between different cell types, particularly epidermal cells, fibroblasts, and inflammatory cells. The concept that ECM signaling extends beyond simple anchorage is no longer in doubt, and it is exciting to think that, as the different types of communication are elucidated, new opportunities for intervening in disease states will appear.
There are, undoubtedly, major obstacles to translating proof-of-concept findings in the lab into real clinical advances. However, it is exciting that gene therapy has already been used to repair a laminin-332 (laminin-5) defect in a patient with junctional epidermolysis bullosa (Mavilio et al. 2006). Furthermore, a potential alternative to gene therapy may be to restore a functional ECM in blistering disorders by injection of nonmutant fibroblasts (Fritsch et al. 2008). Another advance that is highly appealing is to use extracellular matrix biomaterials for skin repair (Cornwell et al. 2009; Wolf et al. 2009). Finally, the insights obtained into how changes in the dermis impact on the epidermis and vice versa are likely to offer new, and overlapping, approaches to the treatment of cancer and fibrosis.
ACKNOWLEDGMENTS
The work of our laboratory is funded by Cancer Research UK, the MRC, Wellcome Trust and European Union, and supported by the University of Cambridge and Hutchison Whampoa Ltd. H.F. gratefully acknowledges financial support from the Uehara Memorial Foundation.
Footnotes
Editors: Richard Hynes and Kenneth Yamada
Additional Perspectives on Extracellular Matrix Biology available at www.cshperspectives.org
REFERENCES
- Aden N, Nuttall A, Shiwen X, de Winter P, Leask A, Black CM, Denton CP, Abraham DJ, Stratton RJ 2010. Epithelial cells promote fibroblast activation via IL-1α in systemic sclerosis. J Invest Dermatol PMID: 20445556. [DOI] [PubMed] [Google Scholar]
- Arwert EN, Lal R, Quist S, Rosewell I, van Rooijen N, Watt FM 2010. Tumor formation initiated by nondividing epidermal cells via an inflammatory infiltrate. Proc Natl Acad Sci PMID: 21041641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Jinnin M, Mimura Y, Tamaki K 2006a. Involvement of αvβ5 integrin in the establishment of autocrine TGF-β signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol 126: 1761–1769 [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K 2006b. Increased expression of integrin αvβ5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol 168: 499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitah SA, Frye M, Glogauer M, Watt FM 2005. Stem cell depletion through epidermal deletion of Rac1. Science 309: 933–935 [DOI] [PubMed] [Google Scholar]
- Berta MA, Baker CM, Cottle DL, Watt FM 2010. Dose and context dependent effects of Myc on epidermal stem cell proliferation and differentiation. EMBO Mol Med 2: 16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Paris M, Morozova O, Fagan BM, Marra M, Pevny L, Miller FD 2009. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell 5: 610–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosset S, Bonnet-Duquennoy M, Barré P, Chalon A, Lazou K, Kurfurst R, Bonté F, Schnébert S, Disant F, Le Varlet B, et al. 2003. Decreased expression of keratinocyte β1 integrins in chronically sun-exposed skin in vivo. Br J Dermatol 148: 770–778 [DOI] [PubMed] [Google Scholar]
- Breitkreutz D, Mirancea N, Nischt R 2009. Basement membranes in skin: unique matrix structures with diverse functions? Histochem Cell Biol 132: 1–10 [DOI] [PubMed] [Google Scholar]
- Busche S, Descot A, Julien S, Genth H, Posern G 2008. Epithelial cell-cell contacts regulate SRF-mediated transcription via Rac-actin-MAL signalling. J Cell Sci 121: 1025–1035 [DOI] [PubMed] [Google Scholar]
- Connelly JT, Gautrot JE, Trappmann B, Tan DW-M, Donati G, Huck WTS, Watt FM 2010. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat Cell Biol 12: 711–718 [DOI] [PubMed] [Google Scholar]
- Conti FJ, Rudling RJ, Robson A, Hodivala-Dilke KM 2003. α3β1-integrin regulates hair follicle but not interfollicular morphogenesis in adult epidermis. J Cell Sci 116: 2737–2747 [DOI] [PubMed] [Google Scholar]
- Corbi AL, Jensen UB, Watt FM 2000. The α2 and α5 integrin genes: identification of transcription factors that regulate promoter activity in epidermal keratinocytes. FEBS Lett 474: 201–207 [DOI] [PubMed] [Google Scholar]
- Cornwell KG, Landsman A, James KS 2009. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg 26: 507–523 [DOI] [PubMed] [Google Scholar]
- Cutroneo KR, White SL, Chiu JF, Ehrlich HP 2006. Tissue fibrosis and carcinogenesis: Divergent or successive pathways dictate multiple molecular therapeutic targets for oligo decoy therapies. J Cell Biochem 97: 1161–1174 [DOI] [PubMed] [Google Scholar]
- Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, et al. NF-κB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. 2003. Nature 421: 639–643 [DOI] [PubMed] [Google Scholar]
- Dvorak HF 1986. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315: 1650–1659 [DOI] [PubMed] [Google Scholar]
- Egeblad M, Nakasone ES, Werb Z 2010. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell 18: 884–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez N, Truitt M, Olson P, Arron ST, Hanahan D 2010. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell 17: 135–147 [DOI] [PubMed] [Google Scholar]
- Essayem S, Kovacic-Milivojevic B, Baumbusch C, McDonagh S, Dolganov G, Howerton K, Larocque N, Mauro T, Ramirez A, Ramos DM, et al. 2006. Hair cycle and wound healing in mice with a keratinocyte-restricted deletion of FAK. Oncogene 25: 1081–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RD, Perkins VC, Henry A, Stephens PE, Robinson MK, Watt FM 2003. A tumor-associated β1 integrin mutation that abrogates epithelial differentiation control. J Cell Biol 160: 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RD, Jones J, Taylor C, Watt FM 2004. Sequence variation in the I-like domain of the β1 integrin subunit in human oral squamous cell carcinomas. Cancer Lett 213: 189–194 [DOI] [PubMed] [Google Scholar]
- Farage MA, Miller KW, Elsner P, Maibach HI 2008. Functional and physiological characteristics of the aging skin. Aging Clin Exp Res 20: 195–200 [DOI] [PubMed] [Google Scholar]
- Ferreira M, Fujiwara H, Morita K, Watt FM 2009. An activating β1 integrin mutation increases the conversion of benign to malignant skin tumors. Cancer Res 69: 1334–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch A, Loeckermann S, Kern JS, Braun A, Bösl MR, Bley TA, Schumann H, von Elverfeldt D, Paul D, Erlacher M, et al. 2008. A hypomorphic mouse model of dystrophic epidermolysis bullosa reveals mechanisms of disease and response to fibroblast therapy. J Clin Invest 118: 1669–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E 2008. Skin stem cells: rising to the surface. J Cell Biol 180: 273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, DeRouen MC, Chen CH, Nguyen M, Nguyen NT, Ido H, Harada K, Sekiguchi K, Morgan BA, Miner JH, et al. 2008. Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev 22: 2111–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautrot JE, Trappmann B, Oceguera-Yanez F, Connelly J, He X, Watt FM, Huck WT 2010. Exploiting the superior protein resistance of polymer brushes to control single cell adhesion and polarisation at the micron scale. Biomaterials 31: 5030–5041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharzi A, Reynolds AJ, Jahoda CA 2003. Plasticity of hair follicle dermal cells in wound healing and induction. Exp Dermatol 12: 126–136 [DOI] [PubMed] [Google Scholar]
- Giangreco A, Goldie SJ, Failla V, Saintigny G, Watt FM 2010. Human skin aging is associated with reduced expression of the stem cell markers β1 integrin and MCSP. J Invest Dermatol 130: 604–608 [DOI] [PubMed] [Google Scholar]
- Giangreco A, Qin M, Pintar JE, Watt FM 2008. Epidermal stem cells are retained in vivo throughout skin aging. Ageing Cell 7: 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fässler R, Brakebusch C, Werner S 2002. A crucial role of β1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129: 2303–2315 [DOI] [PubMed] [Google Scholar]
- Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, Fuchs E 2007. Loss of TGFβ signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell 12: 313–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinea-Viniegra J, Zenz R, Scheuch H, Hnisz D, Holcmann M, Bakiri L, Schonthaler HB, Sibilia M, Wagner EF 2009. TNFα shedding and epidermal inflammation are controlled by Jun proteins. Genes Dev 23: 2663–2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT 2008. Wound repair and regeneration. Nature 453: 314–321 [DOI] [PubMed] [Google Scholar]
- Haase I, Hobbs RM, Romero MR, Broad S, Watt FM 2001. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest 108: 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass NK, Smalley KS, Li L, Herlyn M 2005. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res 18: 150–159 [DOI] [PubMed] [Google Scholar]
- Hall PA, Watt FM 1989. Stem cells: The generation and maintenance of cellular diversity. Development 106: 619–633 [DOI] [PubMed] [Google Scholar]
- Hobbs RM, Watt FM 2003. Regulation of interleukin-1α expression by integrins and epidermal growth factor receptor in keratinocytes from a mouse model of inflammatory skin disease. J Biol Chem 278: 19798–19807 [DOI] [PubMed] [Google Scholar]
- Hobbs RM, Silva-Vargas V, Groves R, Watt FM 2004. Expression of activated MEK1 in differentiating epidermal cells is sufficient to generate hyperproliferative and inflammatory skin lesions. J Invest Dermatol 123: 503–515 [DOI] [PubMed] [Google Scholar]
- Ihn H 2005. Scleroderma, fibroblasts, signaling, and excessive extracellular matrix. Curr Rheumatol Rep 7: 156–162 [DOI] [PubMed] [Google Scholar]
- Inghirami G, Grignani F, Sternas L, Lombardi L, Knowles DM, Dalla-Favera R 1990. Down-regulation of LFA-1 adhesion receptors by C-myc oncogene in human B lymphoblastoid cells. Science 250: 682–686 [DOI] [PubMed] [Google Scholar]
- Iyer V, Pumiglia K, DiPersio CM 2005. α3β1 integrin regulates MMP-9 mRNA stability in immortalized keratinocytes: a novel mechanism of integrin-mediated MMP gene expression. J Cell Sci 118: 1185–1195 [DOI] [PubMed] [Google Scholar]
- Jaks V, Kasper M, Toftgård R 2010. The hair follicle-a stem cell zoo. Exp Cell Res 316: 1422–1428 [DOI] [PubMed] [Google Scholar]
- Janes SM, Watt FM 2004. Switch from αvβ5 to αvβ6 integrin expression protects squamous cell carcinomas from anoikis. J Cell Biol 166: 419–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes SM, Watt FM 2006. New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer 6: 175–183 [DOI] [PubMed] [Google Scholar]
- Jensen KB, Jones J, Watt FM 2008. A stem cell gene expression profile of human squamous cell carcinomas. Cancer Lett 272: 23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PH, Watt FM 1993. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73: 713–724 [DOI] [PubMed] [Google Scholar]
- Jones PH, Harper S, Watt FM 1995. Stem cell patterning and fate in human epidermis. Cell 80: 83–93 [DOI] [PubMed] [Google Scholar]
- Jones PH, Simons BD, Watt FM 2007. Sic transit gloria: farewell to the epidermal transit amplifying cell? Cell Stem Cell 1: 371–381 [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M 2006. Fibroblasts in cancer. Nat Rev Cancer 6: 392–401 [DOI] [PubMed] [Google Scholar]
- Ko MS, Marinkovich MP 2010. Role of dermal-epidermal basement membrane zone in skin, cancer, and developmental disorders. Dermatol Clin 28: 1–16 [DOI] [PubMed] [Google Scholar]
- Krieg T, Abraham D, Lafyatis R 2007. Fibrosis in connective tissue disease: the role of the myofibroblast and fibroblast-epithelial cell interactions. Arthritis Res Ther 9: S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E 2005. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437: 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederle W, Hartenstein B, Meides A, Kunzelmann H, Werb Z, Angel P, Mueller MM 2010. MMP13 as a stromal mediator in controlling persistent angiogenesis in skin carcinoma. Carcinogenesis 31: 1175–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L, Broad S, Diekmann D, Evans RD, Watt FM 2000. β1 integrins regulate keratinocyte adhesion and differentiation by distinct mechanisms. Mol Biol Cell 11: 453–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tzu J, Chen Y, Zhang YP, Nguyen NT, Gao J, Bradley M, Keene DR, Oro AE, Miner JH, et al. 2003. Laminin-10 is crucial for hair morphogenesis. EMBO J 22: 2400–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Rovira T, Silva-Vargas V, Watt FM 2005. Different consequences of β1 integrin deletion in neonatal and adult mouse epidermis reveal a context-dependent role of integrins in regulating proliferation, differentiation and intercellular communication. J Invest Dermatol 125: 1215–1227 [DOI] [PubMed] [Google Scholar]
- Lorenz K, Grashoff C, Torka R, Sakai T, Langbein L, Bloch W, Aumailley M, Fässler R 2007. Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J Cell Biol 177: 501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrantonaki E, Zouboulis CC 2007. Molecular mechanisms of skin aging: state of the art. Ann N Y Acad Sci 1119: 40–50 [DOI] [PubMed] [Google Scholar]
- Manabe R, Tsutsui K, Yamada T, Kimura M, Nakano I, Shimono C, Sanzen N, Furutani Y, Fukuda T, Oguri Y, et al. 2008. Transcriptome-based systematic identification of extracellular matrix proteins. Proc Natl Acad Sci 105: 12849–12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavilio F, Pellegrini G, Ferrari S, Di Nunzio F, Di Iorio E, Recchia A, Maruggi G, Ferrari G, Provasi E, Bonini C, et al. 2006. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med 12: 1397–1402 [DOI] [PubMed] [Google Scholar]
- McLean GW, Komiyama NH, Serrels B, Asano H, Reynolds L, Conti F, Hodivala-Dilke K, Metzger D, Chambon P, Grant SG, et al. 2004. Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev 18: 2998–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SE 2002. Molecular mechanisms regulating hair follicle development. J Invest Dermatol 118: 216–225 [DOI] [PubMed] [Google Scholar]
- Mitchell K, Szekeres C, Milano V, Svenson KB, Nilsen-Hamilton M, Kreidberg JA, DiPersio CM 2009. α3β1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3. J Cell Sci 122: 1778–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G 2004. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22: 411–417 [DOI] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. 1999. The integrin avb6 binds and activates latent TGFb1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328 [DOI] [PubMed] [Google Scholar]
- Murphy MJ, Wilson A, Trumpp A 2005. More than just proliferation: Myc function in stem cells. Trends Cell Biol 15: 128–137 [DOI] [PubMed] [Google Scholar]
- Olson LE, Soriano P 2009. Increased PDGFRα activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell 16: 303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Urda S, Garcia J, Green CL, Chen L, Lin Q, Veitch DP, Sakai LY, Lee H, Marinkovich MP, Khavari PA 2005. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science 307: 1773–1776 [DOI] [PubMed] [Google Scholar]
- Owens DM, Watt FM 2003. Contribution of stem cells and differentiated cells to epidermal tumours. Nat Rev Cancer 3: 444–451 [DOI] [PubMed] [Google Scholar]
- Piwko-Czuchra A, Koegel H, Meyer H, Bauer M, Werner S, Brakebusch C, Fässler R 2009. β1 integrin-mediated adhesion signalling is essential for epidermal progenitor cell expansion. PLoS One 4: e5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racila D, Bickenbach JR 2009. Are epidermal stem cells unique with respect to aging? Aging 1: 746–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Vaezi A, Fuchs E 2003. A role for αβ1 integrins in focal adhesion function and polarized cytoskeletal dynamics. Dev Cell 5: 415–427 [DOI] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E 2000. Conditional ablation of β1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol 150: 1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter JA, Ortiz-Urda S, Kretz M, Garcia J, Scholl FA, Pasmooij AM, Cassarino D, Chang HY, Khavari PA 2009. Modeling inducible human tissue neoplasia identifies an extracellular matrix interaction network involved in cancer progression. Cancer Cell 15: 477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LE, Conti FJ, Silva R, Robinson SD, Iyer V, Rudling R, Cross B, Nye E, Hart IR, Dipersio CM, et al. 2008. α3β1 integrin-controlled Smad7 regulates reepithelialization during wound healing in mice. J Clin Invest 118: 965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden DT 2006. The stem-cell niche as an entity of action. Nature 441: 1075–1079 [DOI] [PubMed] [Google Scholar]
- Schäfer M, Werner S 2008. Cancer as an overhealing wound: An old hypothesis revisited. Nat Rev Mol Cell Biol 9: 628–638 [DOI] [PubMed] [Google Scholar]
- Schober M, Raghavan S, Nikolova M, Polak L, Pasolli HA, Beggs HE, Reichardt LF, Fuchs E 2007. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J Cell Biol 176: 667–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE 2006. Generation of a functional mammary gland from a single stem cell. Nature 439: 84–88 [DOI] [PubMed] [Google Scholar]
- Shimizu H 2007. Shimizu’s Textbook of Dermatology. Hokaido University Press, Japan [Google Scholar]
- Sneddon JB, Zhen HH, Montgomery K, van de Rijn M, Tward AD, West R, Gladstone H, Chang HY, Morganroth GS, Oro AE, et al. 2006. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci 103: 14842–14847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnylal S, Denton CP, Zheng B, Keene DR, He R, Adams HP, Vanpelt CS, Geng YJ, Deng JM, Behringer RR, et al. 2007. Postnatal induction of transforming growth factor β signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum 56: 334–344 [DOI] [PubMed] [Google Scholar]
- Spradling A, Drummond-Barbosa D, Kai T 2001. Stem cells find their niche. Nature 414: 98–104 [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ 2006. Purification and unique properties of mammary epithelial stem cells. Nature 439: 993–997 [DOI] [PubMed] [Google Scholar]
- Sugawara K, Tsuruta D, Ishii M, Jones JC, Kobayashi H 2008. Laminin-332 and -511 in skin. Exp Dermatol 17: 473–480 [DOI] [PubMed] [Google Scholar]
- Szabowski A, Maas-Szabowski N, Andrecht S, Kolbus A, Schorpp-Kistner M, Fusenig NE, Angel P 2000. c-Jun and JunB antagonistically control cytokine-regulated mesenchymal-epidermal interaction in skin. Cell 103: 745–755 [DOI] [PubMed] [Google Scholar]
- Takaoka AS, Yamada T, Gotoh M, Kanai Y, Imai K, Hirohashi S 1998. Cloning and characterization of the human β4-integrin gene promoter and enhancers. J Biol Chem 273: 33848–33855 [DOI] [PubMed] [Google Scholar]
- Teige I, Bäcklund A, Svensson L, Kvist PH, Petersen TK, Kemp K 2010. Induced keratinocyte hyper-proliferation in α.2β1 integrin transgenic mice results in systemic immune cell activation. Int Immunopharmacol 10: 107–114 [DOI] [PubMed] [Google Scholar]
- Tlsty TD, Coussens LM 2006. Tumor stroma and regulation of cancer development. Annu Rev Pathol 1: 119–150 [DOI] [PubMed] [Google Scholar]
- Trojanowska M 2002. Molecular aspects of scleroderma. Front Biosci 7: d608–d618 [DOI] [PubMed] [Google Scholar]
- Turner FE, Broad S, Khanim FL, Jeanes A, Talma S, Hughes S, Tselepis C, Hotchin NA 2006. Slug regulates integrin expression and cell proliferation in human epidermal keratinocytes. J Biol Chem 281: 21321–21331 [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E 2004. Defining the epithelial stem cell niche in skin. Science 303: 359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussar S, Moser M, Widmaier M, Rognoni E, Harrer C, Genzel-Boroviczeny O, Fässler R 2008. Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet 4: e1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Weissman IL 2006. Differential expression of α2 integrin separates long-term and short-term reconstituting Lin-/loThy1.1(lo)c-kit+ Sca-1+ hematopoietic stem cells. Stem Cells 24: 1087–1094 [DOI] [PubMed] [Google Scholar]
- Waterman EA, Sakai N, Nguyen NT, Horst BA, Veitch DP, Dey CN, Ortiz-Urda S, Khavari PA, Marinkovich MP 2007. A laminin-collagen complex drives human epidermal carcinogenesis through phosphoinositol-3-kinase activation. Cancer Res 67: 4264–4270 [DOI] [PubMed] [Google Scholar]
- Watt FM 2002. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J 21: 3919–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Driskell RR 2010. The therapeutic potential of stem cells. Philos Trans R Soc Lond B Biol Sci 365: 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Hogan BLM 2000. Out of Eden: Stem cells and their niches. Science 287: 1427–1430 [DOI] [PubMed] [Google Scholar]
- Watt FM, Jensen KB 2009. Epidermal stem cell diversity and quiescence. EMBO Mol Med 1: 260–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Frye M, Benitah SA 2008. MYC in mammalian epidermis: how can an oncogene stimulate differentiation? Nat Rev Cancer 8: 234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Jordan PW, O’Neill CH 1988. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc Natl Acad Sci 85: 5576–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Krieg T, Smola H 2007. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol 127: 998–1008 [DOI] [PubMed] [Google Scholar]
- Wickstrom SA, Radovanoc K, Faessler R 2011. Genetic analyses of integrin signaling. Cold Spring Harb Perspect Biol 10.1101/a005116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K, Litjens SH, Sonnenberg A 2006. Multiple functions of the integrin α6β4 in epidermal homeostasis and tumorigenesis. Mol Cell Biol 26: 2877–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P 2009. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol 20: 931–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AJ, Haase I, Watt FM 1999. Signaling via β1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci 96: 6728–6733 [DOI] [PMC free article] [PubMed] [Google Scholar]