Abstract
The extent to which repetition suppression is modulated by expertise is currently unknown. We used event-related fMRI to test whether architecture students would respond faster to buildings and would exhibit stronger repetition suppression in the fusiform gyrus (FG) and parahippocampa cortex (PHC) than students from other disciplines. Behaviorally, we found shorter response latencies with target repetition in all subjects. Moreover, the repetition of targets and distracters was associated with decreased neural responses in the FG and PHC in all subjects. In control, but not in architecture students, reaction times during the first repetition of the target were correlated with activation in the cuneus, lingual gyrus, inferior parietal lobule, insula, and anterior cingulate cortex, indicating that the non-experts had to recruit additional regions in order to perform the task. Our findings suggest that due to their expertise, architects were able to encode and detect building stimuli at a lower neural cost.
Keywords: Architecture, Cognition, Expertise, fMRI, Object recognition, Parahippocampal cortex, Working memory
Introduction
The neural signature of stimulus repetition is decreased activation in the cortex, known as repetition suppression. Single-unit recordings in nonhuman primates have shown reduced neural responses to repeated visual stimuli in extrastriate cortex (e.g., Miller, Li, & Desimone, 1991b). Functional brain imaging studies in humans, using various techniques—positron emission tomography (PET), functional magnetic resonance imaging (fMRI), and event-related potentials (ERPs)—and various classes of visual stimuli (e.g., words, objects, and faces), have also shown that stimulus repetition results in decreased cortical activation (Buckner et al., 1995; Rugg, Soardi, & Doyle, 1995; Vuilleumier, Henson, Driver, & Dolan, 2002). Repetition suppression is stimulus-specific, size- and location-invariant, and observed under anesthesia (Miller, Gochin, & Gross, 1991a). These properties have suggested that repetition suppression is an automatic, intrinsic response of cortical neurons (Desimone, 1996; Wiggs & Martin, 1998). The neural mechanisms that mediate repetition suppression are currently unknown. Three models have been proposed to account for the main empirical findings: the fatigue model, according to which the amplitude of the firing of neurons decreases with stimulus repetition; the sharpening model, which posits that fewer neurons respond to repeated stimuli; and the facilitation model, according to which the latency or duration of neural activity is shortened with stimulus repetition (Grill-Spector, Henson, & Martin, 2006). It has also been suggested that in contrast with reduced responses to repeated stimuli in ventral temporal regions, which reflect perceptual processing, the repetition suppression effects observed in prefrontal regions reflect the retrieval of learned stimulus-response associations (Dobbins, Schnyer, Verfaellie, & Schacter, 2004; Horner & Henson, 2008; Race, Shanker, & Wagner, 2009). In some repetition suppression studies, the first repetition of a target evoked enhanced neural responses relative to the responses during target encoding. These “match enhancement” effects were found in inferior temporal and prefrontal neurons in both monkeys (Miller & Desimone, 1994) and humans (Ishai, Pessoa, Bikle, & Ungerleider, 2004) performing delayed matching tasks.
Repetition suppression is modulated by various cognitive factors, such as task demands and attention (Eger, Henson, Driver, & Dolan, 2004; Yi & Chun, 2005; Xu, Turk-Browne, & Chun, 2007; Soto, Humphreys, & Rotshtein, 2007). We have previously shown that repetition suppression is also modulated by emotion. Within a distributed network of face-responsive regions, repetition of fearful faces was associated with stronger suppression than the repetition of neutral faces. We interpreted these results to suggest that emotional faces become more sharply tuned than neutral faces, presumably due to their biological significance (Ishai, Bikle, & Ungerleider, 2006; Ishai et al., 2004). It is currently unknown, however, whether these findings can be generalized. Specifically, it is unknown how expertise and experience modulate the decrease in neural response to repeated stimuli. The goal of our study was to test whether architecture students would exhibit differential behavioral and neural responses while performing a working memory task in which an encoded building target was repeated twice among distracter buildings. It has been consistently shown that visual perception of houses and buildings evokes activation in medial fusiform gyrus (Aguirre, Zarahn, & D'Esposito, 1998; Ishai, Ungerleider, Martin, & Haxby, 2000; Ishai, Ungerleider, Martin, Schouten, & Haxby, 1999), as well as the parahippocampal cortex (PHC), a region that mediates the encoding of local environments and extraction of spatial information for navigation, which was termed the “parahippocampal place area” (Epstein & Kanwisher, 1998). Moreover, the repetition of scenes that include buildings has been associated with decreased neural responses in the PHC (e.g., Epstein, Graham, & Downing, 2003). Based on our previous findings of stronger repetition suppression for emotional than neutral faces (Ishai et al., 2004, 2006), we hypothesized that if experience-dependent changes are manifested by sharp-tuning of the neural response to visual stimuli, then compared with students from other disciplines, architecture students would respond faster to building stimuli and would exhibit stronger repetition suppression and/or match enhancement effects in building-responsive regions, namely the FG and the PHC. Thus, faster behavioral responses and stronger repetition suppression would reflect the sharp tuning of the response to buildings in architects.
METHODS
Subjects
Twenty-four healthy, right-handed subjects (13 males, 11 females, mean age 26 years) with normal vision participated in the study. All subjects gave informed written consent for the procedure in accordance with protocols approved by the University Hospital of Zurich. Twelve subjects (7 males, 5 females) were architecture students from the Swiss Federal Institute of Technology (ETH) in their last year of the Master's program. During the 4 years of studies toward their bachelor degree, the architecture students took courses in design, technology, humanities, and social and natural sciences, and worked 6 months on practical assignments that involved drawing and constructing three-dimensional models of buildings. During their first year of the Master's degree the architecture students took additional courses in creative design and completed more practical assignments. The other 12 subjects were students from the University and ETH Zurich, who study biology, neurobiology, chemistry, pharmacy, veterinary medicine, and computer science.
Stimuli and tasks
Stimuli were displayed using Presentation (www.neurobs.com, version 12.2) and were projected with a magnetically shielded LCD video projector onto a translucent screen placed at the feet of the subject. We used 320 new monochrome photographs of buildings from Barcelona and Tel Aviv taken by the photographer Ilan Nachum (see http://ilannachum.com/galleries.html). Except for a few photographs of famous Gaudi buildings, which two architecture students and two control subjects recognized, all images were of unfamiliar buildings (see Figure 1). Scrambled images of these buildings, which were created by phase scrambling luminance and color information from these pictures, were used as control stimuli. Subjects performed a working memory task. In each trial, a target building was presented for 4 s (encoding), followed by 10 buildings, each presented for 2 s. The target and one of the distracters were repeated twice, intermixed with 6 nonrepeated distracters. Stimuli were presented successively, without an interstimulus interval. Subjects were instructed to memorize the target building and press a button when detecting it, thereby making the target the behaviorally relevant stimulus. Each time series included 8 trials with buildings and 2 trials with scrambled buildings. Five times series were collected for each subject and the order of trial type was randomized.
Figure 1.

Stimuli and task. In each trial, a target building was presented for 4 s (ENC), followed by 10 buildings, each presented for 2 s. The target (T1, T2) and one of the distracters (D1, D2) were repeated twice, intermixed with 6 nonrepeated distracters. Subjects were instructed to memorize the target and press a button when detecting it.
Data acquisition
Data were collected using a 3 T Philips Intera whole body MR scanner (Philips Medical Systems, Best, The Netherlands). Changes in blood-oxygenation level-dependent MRI signal were measured by using sensitivity encoded gradient-echo echoplanar sequence (SENSE, Pruessmann, Weiger, Scheidegger, & Boesiger, 1999) with 33 axial slices, TR = 2 s, TE = 35 ms, flip angle = 80°, field of view = 220 mm, acquisition matrix = 128 × 128, reconstructed voxel size = 1.72 × 1.72 × 4 mm, and SENSE acceleration factor R = 2.
High-resolution spoiled gradient recalled echo structural images were collected in the same session for all the subjects (160 sagittal slices, TR = 8.27 ms, TE = 3.9 ms, field of view = 240 mm, acquisition matrix = 256 × 256, reconstructed voxel size = 1 × 0.9 × 0.9 mm). These high-resolution structural images provided detailed anatomical information for the region-of-interest (ROI) analysis and for 3D normalization to the Talairach and Tournoux atlas (1988).
Data analysis
For each subject, the behavioral data, namely responses and reaction times, were computed for the first and the second repetition of the target buildings. ANOVA was used to compare the various conditions.
Functional MRI data were analyzed in Brain Voyager QX Version 1.10 (Brain Innovation, Maastricht, The Netherlands). All volumes were realigned to the first volume, corrected for motion artefacts and spatially smoothed using a 5-mm full-width-at-half-maximum Gaussian filter. Stimulus events were modeled using a delta function, which was convolved with a canonical hemodynamic response function to yield a regressor for each condition. The main effects of interest (buildings vs. scrambled images; first repetition of a target vs. second repetition of a target; encoding vs. first repetition of a target; first repetition of a distracter vs. second repetition of a distracter) were analyzed using the general linear model (Friston et al., 1995). Based on the main effect (buildings vs. scrambled buildings), a set of ROIs was anatomically defined for each subject with clusters that showed a significant effect (p < .01, uncorrected). These regions included the inferior occipital gyrus (IOG), fusiform gyrus (FG), and parahippocampal cortex (PHC). The specification of ROIs was therefore orthogonal to the subsequent tests that were addressed at the second-level analysis. For each subject and in each ROI, the mean parameter estimates were calculated separately for each experimental condition (i.e., encoding and first and second repetitions of targets and distracters) and were used for between-subjects random-effects analyses.
To test for task-related effects, additional ROIs were defined based on the contrast of repeated targets vs. repeated distracters (T > D) with clusters that showed a significant effect (p < .005, uncorrected). These regions included the intraparietal sulcus (IPS), insula (INS), anterior cingulate cortex (ACC), and orbitofrontal cortex (OFC). However, we did not find any differences between the two groups. To test for match enhancement effects, we localized all regions that responded more to the first repetition of the target than to target encoding (T1 > ENC). These regions included the IOG, FG, PHC, INS, and ACC.
Finally, we tested whether reaction times were correlated with brain activation by including the response latencies as a covariate in the GLM analysis. For each subject, the reaction times of detecting the first and second repetitions of the targets were normalized by z-transformation, and the standard hemodynamic response function (HRF) was then multiplied with the new z-values for each trial, thus creating a latency-correlated design-matrix.
RESULTS
Behavioral data
The mean accuracies and standard error of the mean (SEM) for detecting the first (T1) and second (T2) building targets were 97 ± 1% and 99 ±1%, respectively, for the control students, and 97 ± 1% and 98 ± 2% for the architecture students. The differences between the groups were not statistically significant. In all subjects, the proportion of correct responses was significantly higher for T2 than for T1, 97 ± 1% and 98 ± 1%, respectively, t(46) = 2.51, p < .05.
Averaged across all 24 subjects, detecting the second presentation of the target was significantly faster than detecting its first presentation, 615 ± 24 ms for T1 and 588 ± 23 for T2; t(46) = 3.41, p < .01. Relative to the control group, the architecture students responded faster to both targets, but this difference did not reach statistical significance, T1: 649 ± 33 ms, T2: 616 ± 34 for the control group; T1: 581 ± 34 ms, T2: 561 ± 30 for the architecture students, F(1, 47) = 3.49, p > .05.
The mean accuracies for detecting the scrambled targets were also similar, T1: 93 ± 3 %, T2: 93 ± 2 % for the control group; T1: 98 ± 2 %, T2: 98 ± 1 % for the architecture students, F(1, 47) = 1.21, p > .05. The architecture students responded faster to the first repetition of the scrambled targets (687 ± 34 ms) than the control group, 817 ± 52 ms, t(22) = 2.40, p < .05. The response latencies to the second presentation of the scrambled targets were very similar in the two groups, controls: 782 ± 50 ms; architects: 689 ± 42 ms, t(22) = 1.81, p > .05. A two-way ANOVA revealed a significant difference in the reaction times averaged across both scrambled targets between the two groups, F(1, 47) = 6.15, p < .05.
Imaging data
The main effect, namely responses evoked by all buildings as compared with the scrambled buildings, revealed activation within a distributed cortical network that included multiple, bilateral regions. Consistent with previous findings, significant activation was found in extrastriate regions that respond more to buildings, including the FG and PHC (Figure 2). The contrast of repeated targets vs. repeated distracters revealed activation in attention- and task-related areas, namely the IPS, IFG, INS, and ACC (see Table 1 for cluster size and Talairach coordinates). We then conducted an ROI analysis to test for differences between T1 and T2, first (D1) and second (D2) repetitions of the distracters, and encoding of the target (ENC) vs. T1.
Figure 2.
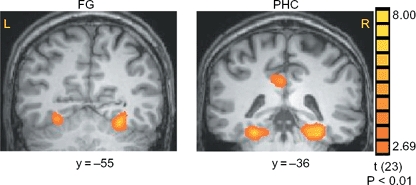
Activation evoked by buildings as compared with scrambled buildings. Group statistical maps, averaged across all subjects, illustrating significant activation in the FG and PHC.
TABLE 1.
Regions activated during presentation of buildings
|
Control group |
Architects group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Coordinates |
Coordinates |
|||||||||
| Region | N | Mean cluster size [mm3] | X | Y | Z | N | Mean cluster size [mm3] | X | Y | Z |
| Main effect (buildings vs. scrambled) | ||||||||||
| L. IOG | 10 | 826(64) | −29(2) | −81(1) | −14(1) | 7 | 886(68) | −26(2) | −82(2) | −12(2) |
| R. IOG | 9 | 845(51) | 27(3) | −79(2) | −11(1) | 9 | 939(50) | 24(1) | −79(1) | −10(2) |
| L. FG | 12 | 832(42) | −26(1) | −55(1) | −11(2) | 12 | 809(54) | −27(1) | −54(1) | −11(1) |
| R. FG | 12 | 794(57) | 26(1) | −54(1) | −9(1) | 12 | 856(61) | 25(1) | −56(1) | −10(1) |
| L. PHC | 11 | 860(46) | −25(1) | 43(1) | −11(1) | 9 | 877(43) | −25(1) | −13(1) | −13(1) |
| R. PHC | 11 | 838(32) | 25(1) | −42(1) | −11(1) | 11 | 893(29) | 25(1) | −43(1) | −12(1) |
| OFC | 6 | 788(82) | −1(1) | 52(2) | −4(2) | 5 | 793(73) | −3(1) | 49(3) | −8(2) |
| Targets vs. distracters | ||||||||||
| L. IPS | 5 | 848(41) | −43(3) | −43(2) | 38(3) | 7 | 917(23) | −38(2) | −34(1) | 47(2) |
| R. IPS | 5 | 856(85) | 39(5) | −43(3) | 36(3) | 3 | 808(135) | 40(6) | −35(2) | 46(2) |
| L. INS | 8 | 854(66) | −43(2) | −2(1) | 9(1) | 9 | 832(40) | −42(1) | −4(1) | 10(1) |
| R. INS | 6 | 571(116) | 42(2) | −1(1) | 9(2) | 8 | 570(53) | 40(1) | −1(1) | 9(1) |
| ACC | 11 | 803(55) | −4(1) | 0(1) | 45(2) | 8 | 875(27) | −3(1) | −1(2) | 46(2) |
| L. IFG | 4 | 729(60) | −38(6) | 16(3) | 13(5) | 3 | 881(44) | −45(7) | 10(5) | 8(6) |
| R. IFG | 4 | 788(82) | 41(2) | 15(4) | 15(8) | 3 | 593(158) | 43(7) | 11(6) | 14(6) |
Notes: N indicates number of subjects that showed activation in a region. Coordinates are in the normalized space of the Talairach and Tournoux brain atlas. Numbers in parentheses indicate SEM.
Repetition suppression
Having localized the visual activation evoked by buildings, we analyzed the amplitude of the response associated with specific events, namely, encoding of targets, repetition of targets, and repetition of distracters. We first provide the results for activation evoked by the tar gets. Figure 3 shows the amplitude of the response evoked by the first and second repetitions of the targets in the FG and PHC. As bilateral activation was found in both regions, we averaged the data across the two hemispheres. In both the FG and PHC, T1 evoked stronger activation than T2, and the differences were statistically significant in both control, t(46) = 3.27, p < .01 in the FG; t(42) = 3.22, p < .01 in the PHC, and architecture students, t(46) = 3.79, p < .001 in the FG; t(40) = 3.96, p < .001 in the PHC.
Figure 3.
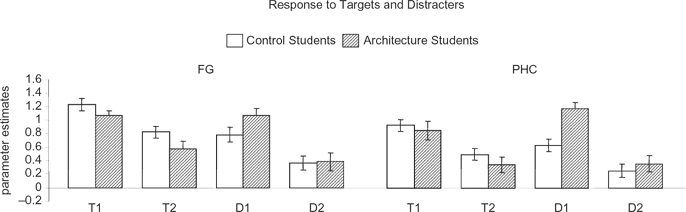
Repetition suppression. Responses evoked by repeated targets and repeated distracters within the FG and PHC. T1 = first repetition of the target; T2 = second repetition of the target; Dl = first repetition of the distracter; D2 = second repetition of the distracter. Data were averaged across the two hemispheres and all subjects that showed activation in the region. Error bars indicate SEM.
The amplitude of the responses during the first and second repetition of the repeated distracters is also shown in Figure 3. Although the repeated distracters were task-irrelevant, we found that the neural response to D1 was stronger than activation evoked by D2. In both groups, the difference between D1 and D2 was significant in the FG, controls: t(46) = 3.04, p < .01; architects: t(46) = 6.14, p < .0001, and in the PHC, controls: t(42) = 2.72, p < .01; architects: t(40) = 6.73, p < .0001. Within the PHC we also found significant interaction between group and distracters, F(1, 85) = 4.75, p < .05.
Within the PHC, activation evoked by D1 was significantly higher in the architects than in the control group, t(41) = 4.15, p < .0001. The nonrepeated distracters evoked similar activation and the differences between the two groups were not significant in the FG, t(46) = 0.28, p > .05, and the PHC, t(41) = 0.03, p > .05.
Match enhancement
We then compared activation evoked by encoding of the target buildings and the first repetition of these targets. Within the IOG, FG, PHC, INS, and ACC, the first repetition of the target evoked higher activation than the encoding of the target (Figure 4). This “match enhancement” effect was significant in the IOG, controls: t(36) = 3.24, p < .01; architects: t(30) = 2.40, p < .05; FG, controls: t(46) = 6.08, p < .0001; architects: t(46) = 5.67, p < .0001; PHC, controls: t(42) = 4.01, p < .001; architects: t(40) = 2.94, p < .01; INS, controls: t(26) = 6.87, p < .0001; architects: t(32) = 5.85, p < 0.0001; and ACC, controls: t(20) = 4.77, p < .001; architects: t(14) = 4.11, p < .01.
Figure 4.
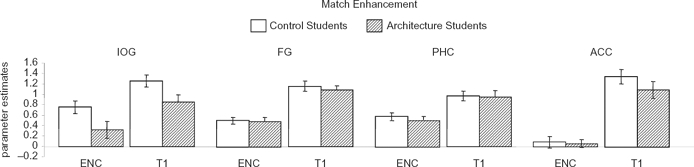
Match enhancement. Responses evoked during encoding and first repetition of the targets in the IOG, FG, PHC, and ACC. ENC = encoding of the target; T1 = first repetition of the target. Data were averaged across the two hemispheres and all subjects that showed activation in the region. Error bars indicate SEM.
Correlations between reaction time and brain activation
Finally, we tested whether the reaction times of detecting the first and second repetitions of the targets were correlated with brain activation. Interestingly, we found that in control but not in architecture students, detecting the first repetition of a target was correlated with activation in multiple regions (Figure 5), which included the cuneus (mean Talairach coordinates: −2, −80, 20); lingual gyrus (14, −64, 0); inferior parietal lobule (58, −28, 26); insula (−49, −20, 11; 45, −14, 13) and ACC (5, −17, 43).
Figure 5.
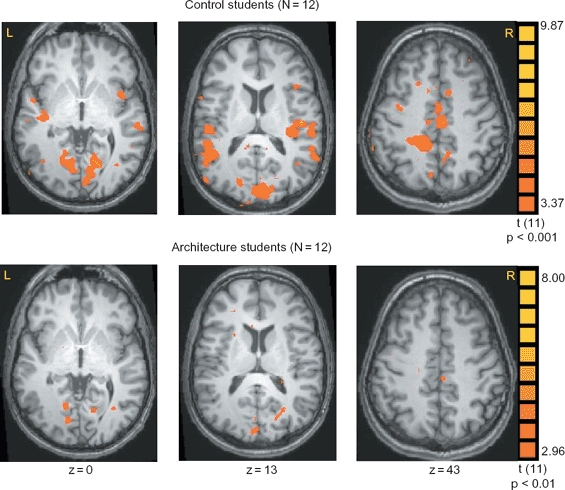
Correlations between response latencies and brain activation. In control subjects, reaction times recorded during the first repetition of the targets were correlated with activation in a network of regions that included the cuneus, lingual gyrus, inferior parietal lobule, insula and ACC.
DISCUSSION
We investigated, using event-related fMRI, the neural responses evoked by repetition of buildings, which were either task-relevant (targets) or -irrelevant (distracters), in architecture and non-architecture students. We found in all subjects shorter response latencies with target repetition, as well as reduced neural responses to repeated stimuli within the FG and PHC, and stronger activation to the first repetition of the encoded target in the IOG, FG, PHC, and ACC. Contrary to our hypothesis, we did not find evidence for sharper tuning of the response to buildings as manifested by greater neural decreases to repeated targets in architecture students. Nevertheless, regression analysis of the reaction time with cortical activity revealed in control, but not in architecture students, activation in the cuneus, lingual gyrus, inferior parietal lobule, insula, and anterior cingulate cortex.
Consistent with previous studies (Epstein et al., 2003; Yi & Chun, 2005), we found that the repetition of building stimuli resulted in decreased neural activation in multiple building-responsive regions along the ventral stream, namely the FG and PHC (Aguirre et al., 1998; Epstein & Kanwisher, 1998; Ishai et al., 1999, 2000). Our data are also consistent with the predictive coding model, according to which learning is reflected by reduced prediction error, as manifested by reduced activation with repetition (Friston, 2005). We also found that within the IOG, FG, PHC, and ACC the first repetition of a target evoked enhanced neural responses relative to the responses during target encoding. Similar “match enhancement” effects were previously found in inferior temporal and prefrontal neurons in monkeys performing delayed matching tasks (Miller & Desimone, 1994) and in humans (Ishai et al., 2004). It has been suggested that repetition suppression and target enhancement are two neural mechanisms with complementary functions: automatic detection of stimulus repetition and maintenance in working memory, respectively. These parallel mechanisms are required to bias the competition between multiple objects in typically crowded visual scenes, in favor of the behaviorally relevant items (Desimone, 1996). The similar patterns of repetition suppression and match enhancement observed in all subjects, regardless of their expertise, is likely due to the modern, urban environments that many of us live in. We are surrounded by buildings and houses and on a daily basis are required to remember architectural landmarks in order to navigate. Thus, due to their lifelong exposure to buildings, our control students were not completely novice subjects. When subjects were presented with eight repetitions of a stimulus, most extrastriate regions exhibited reduced response to the second, but not to subsequent presentations of the stimulus, except for the right posterior FG, which exhibited gradual reductions for all repetitions (Reber, Gitelman, Parrish, & Mesulam, 2005). As we haven't observed differential effects of expertise within this region, which according to Reber and colleagues links repetition suppression with expertise, it is likely that these effects reflect short, within-session familiarity or fluent reprocessing of the stimuli.
Within the PHC, architects showed stronger neural response to the first repetition of a distracter than control subjects. We have previously shown that distracter faces, regardless of their repetition or valence, evoked negligible activation, indicating top-down attenuation of behaviorally irrelevant stimuli in the human brain (Ishai et al., 2004). Our current findings suggest that architecture students, due to their unique training, may automatically attend not only to task-relevant stimuli, but also to irrelevant items that non-experts can filter out. The architects have probably also responded faster to the scrambled images because of their hands-on experience with geometric forms.
If architects process building stimuli in a more semantic manner, one would expect differential effects of repetition suppression within prefrontal regions (e.g., Race et al., 2009). As both control and architecture students exhibited similar behavioral and neural responses, it seems that repetition suppression is not modulated by expertise within ventral temporal and prefrontal regions. Similar findings were reported in a recent study in which aesthetic judgment of buildings and faces was investigated in architects and non-architects. Regardless of their expertise, all subjects similarly rated the aesthetic appeal of the stimuli and showed similar patterns of activation within the nucleus accumbens. However, in the orbitofrontal cortex and subcallosal cingulate gyrus, buildings evoked higher activation in architects than in non-architects, suggesting that expertise modulates the representation of stimulus value (Kirk, Skov, Christensen, & Nygaard, 2009).
We found a significant difference between the two groups when we correlated the response latencies during target detection with brain activation. This regression analysis revealed in control, but not architecture students, significant activation in a network of regions (cuneus, lingual gyrus, inferior parietal lobule, insula, and anterior cingulate cortex) that mediate various cognitive tasks that require attention and memory retrieval. It therefore seems that the control subjects performed the working memory task with a higher neural cost. The recruitment of additional regions in control subjects is reminiscent of age-related functional changes in the human brain. When compared with young adults, older adults tend to recruit additional cortical areas, especially in the prefrontal cortex, despite similar performance in terms of accuracy and speed of response (Grady, 2008). It has been suggested that cortical recruitment serves as a compensatory mechanism to counteract age-related decline in task performance. Such recruitment, however, could also reflect age-related dedifferentiation, namely difficulties in recruiting specialized neural mechanisms (Buckner, 2004; Cabeza, 2001; Gazzaley & D'Esposito, 2007). It has also been suggested that old adults are more vulnerable to environmental distractions during task performance (e.g., Stevens, Hasher, Chiew, & Grady, 2008). One could similarly argue that our control subjects were unable to suppress the neural response to the distracter buildings. However, as control and architecture students showed similar neural responses to the distracters in the FG, and architects responded more to D1 in the PHC, it seems highly unlikely that the non-architects were more distracted by these task-irrelevant stimuli.
The specific training and experience our architecture students had during the course of their studies likely resulted in more efficient cognitive performance that required fewer neural resources, whereas the control subjects had to recruit additional cortical regions. Consistently, several studies have shown that perceptual learning and skill acquisition result in decreased activation in sensory and motor regions (e.g. Kassubek, Schmidtke, Kimmig, Lücking, & Greenlee, 2001; Mukai et al., 2007; Poldrack et al., 2005). The additional recruitment of regions could be due to a different cognitive strategy employed by the control subjects. However, the virtually identical behavioral performance and patterns of neural activation observed in the two groups suggest that this is highly unlikely. According to the expertise framework (Bukach, Gauthier, & Tarr, 2006; Raichle, 1998), perceptual learning and category specialization result in differential cortical responses. Our surprising findings provide a new perspective on expertise, namely reduced neural cost. Future studies might determine the neural mechanisms of the lower neural cost associated with expertise and the extent to which expertise modulates the patterns of cortical connectivity among extrastriate, parietal, and prefrontal regions.
Footnotes
We thank Ilan Nachum for providing us with photographs of buildings from Barcelona and Tel Aviv, and Robert Pepperell for reading the manuscript. This study was supported by the Swiss National Science Foundation grant 3200B0-105278 and by the Swiss National Center for Competence in Research: Neural Plasticity and Repair.
References
- Aguirre G. K., Zarahn E., D'Esposito M. An area within human ventral cortex sensitive to “building” stimuli: Evidence and implications. Neuron. 1998;21:373–383. doi: 10.1016/s0896-6273(00)80546-2. [DOI] [PubMed] [Google Scholar]
- Bukach C. M., Gauthier I., Tarr M.J. Beyond faces and modularity: The power of an expertise framework. Trends in Cognitive Sciences. 2006;10:159–166. doi: 10.1016/j.tics.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Buckner R. L. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buckner R. L., Petersen S. E., Ojemann J. G., Miezin F. M., Squire L. R., Raichle M. E. Functional anatomical studies of explicit and implicit memory retrieval tasks. Journal of Neuroscience. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Cognitive neuroscience of aging: Contributions of functional neuroimaging. Scandinavian Journal of Psychology. 2001;42:277–286. doi: 10.1111/1467-9450.00237. [DOI] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins I. G., Schnyer D. M., Verfaellie M., Schacter D. L. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428:316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- Eger E., Henson R. N., Driver J., Dolan R. J. BOLD repetition decreases in object-responsive ventral visual areas depend on spatial attention. Journal of Neurophysiology. 2004;92:1241–1247. doi: 10.1152/jn.00206.2004. [DOI] [PubMed] [Google Scholar]
- Epstein R., Graham K. S., Downing P. E. Viewpoint-specific scene representations in human parahippocampal cortex. Neuron. 2003;37:865–876. doi: 10.1016/s0896-6273(03)00117-x. [DOI] [PubMed] [Google Scholar]
- Epstein R., Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Friston K. J., Holmes A. P., Poline J. B., Grasby P. J., Williams S. C., Frackowiak R. S., et al. Analysis of fMRI time-series revisited. NeuroImage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Friston K. A theory of cortical responses. Philosophical Transactions of the Royal Society, Series B: Biological Sciences. 2005;360:815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A., D'Esposito M. Top-down modulation and normal aging. Annals of the New York Academy of Sciences. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Grady C.L. Cognitive neuroscience of aging. Annals of the New York Academy of Sciences. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K., Henson R, Martin A. Repetition and the brain: Neural models of stimulus-specific effects. Trends in Cognitive Sciences. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Horner A. J., Henson R. N. Priming, response learning and repetition suppression. Neuropsychologia. 2008;46:1979–1991. doi: 10.1016/j.neuropsychologia.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A., Ungerleider L. G., Martin A., Haxby J. V. The representation of objects in the human occipital and temporal cortex. Journal of Cognitive Neuroscience. 2000;12(Suppl. 2):35–51. doi: 10.1162/089892900564055. [DOI] [PubMed] [Google Scholar]
- Ishai A., Ungerleider L.G., Martin A., Schouten J. L., Haxby J. V. Distributed representation of objects in the human ventral visual pathway. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A., Bikle P.C., Ungerleider L. G. Temporal dynamics of face repetition suppression. Brain Research Bulletin. 2006;70:289–295. doi: 10.1016/j.brainresbull.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Ishai A., Pessoa L., Bikle P. C., Ungerleider L. G. Repetition suppression of faces is modulated by emotion. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9827–9832. doi: 10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassubek J., Schmidtke K., Kimmig H., Lücking C. H., Greenlee M. W. Changes in cortical activation during mirror reading before and after training: An fMRI study of procedural learning. Brain Research: Cognitive Brain Research. 2001;10:207–217. doi: 10.1016/s0926-6410(00)00037-9. [DOI] [PubMed] [Google Scholar]
- Kirk U., Skov M., Christensen M. S., Nygaard N. Brain correlates of aesthetic expertise: A parametric fMRI study. Brain and Cognition. 2009;69:306–315. doi: 10.1016/j.bandc.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Miller E. K., Gochin P. M., Gross C. G. Habituation-like decrease in the responses of neurons in inferior temporal cortex of the macaque. Visual Neuroscience. 1991a;7:357–362. doi: 10.1017/s0952523800004843. [DOI] [PubMed] [Google Scholar]
- Miller E. K., Li L., Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991b;254:1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- Miller E. K., Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263:520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- Mukai I., Kim D., Fukunaga M., Japee S., Marrett S., Ungerleider L. G. Activations in visual and attention-related areas predict and correlate with the degree of perceptual learning. Journal of Neuroscience. 2007;27:11401–11411. doi: 10.1523/JNEUROSCI.3002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R. A., Sabb F. W., Foerde K., Tom S. M., Asarnow R. F., Bookheimer S. Y., et al. The neural correlates of motor skill automaticity. Journal of Neuroscience. 2005;25:5356–5364. doi: 10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessmann K. P., Weiger M., Scheidegger M. B., Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magnetic Resonance in Medicine. 1999;42:952–962. [PubMed] [Google Scholar]
- Race E. A., Shanker S., Wagner A. D. Neural priming in human frontal cortex: Multiple forms of learning reduce demands on the prefrontal executive system. Journal of Cognitive Neuroscience. 2009;21:1766–1781. doi: 10.1162/jocn.2009.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E. The neural correlates of consciousness: An analysis of cognitive skill learning. Philosophical Transactions of the Royal Society, B: Biological Sciences. 1998;353:1889–1901. doi: 10.1098/rstb.1998.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber P. J., Gitelman D. R., Parrish T. B., Mesulam M. M. Priming effects in the fusiform gyrus: Changes in neural activity beyond the second presentation. Cerebral Cortex. 2005;15:787–795. doi: 10.1093/cercor/bhh179. [DOI] [PubMed] [Google Scholar]
- Rugg M. D., Soardi M., Doyle M. C. Modulation of event-related potentials by the repetition of drawings of novel objects. Brain Research: Cognitive Brain Research. 1995;3:17–24. doi: 10.1016/0926-6410(95)00014-3. [DOI] [PubMed] [Google Scholar]
- Soto D., Humphreys G. W., Rotshtein P. Dissociating the neural mechanisms of memory-based guidance of visual selection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17186–17191. doi: 10.1073/pnas.0703706104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens W.D., Hasher L., Chiew K.S., Grady C.L. A neural mechanism underlying memory failure in older adults. Journal of Neuroscience. 2008;28:12820–12824. doi: 10.1523/JNEUROSCI.2622-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical; 1988. [Google Scholar]
- Vuilleumier P., Henson R. N., Driver J., Dolan R. J. Multiple levels of visual object constancy revealed by event-related fMRI of repetition priming. Nature Neuroscience. 2002;5:491–499. doi: 10.1038/nn839. [DOI] [PubMed] [Google Scholar]
- Wiggs C. L., Martin A. Properties and mechanisms of perceptual priming. Current Opinion in Neurobiology. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Xu Y., Turk-Browne N. B., Chun M. M. Dissociating task performance from fMRI repetition attenuation in ventral visual cortex. Journal of Neuroscience. 2007;27:5981–5985. doi: 10.1523/JNEUROSCI.5527-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi D. J., Chun M. M. Attentional modulation of learning-related repetition attenuation effects in human parahippocampal cortex. Journal of Neuroscience. 2005;25:3593–3600. doi: 10.1523/JNEUROSCI.4677-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


