Abstract
Objective: This study aimed to examine (1) whether polarity protein partitioning defective-3 (PARD-3) was expressed in endothelial cells (ECs) and contributed to endothelial barrier integrity and (2) whether altered PARD-3 expression and distribution were associated with disturbed endothelial junction protein VE-cadherin expression induced by factors derived from preeclamptic (PE) placentas. Methods: PARD-3 and VE-cadherin expressions were examined by immunofluorescent staining and Western blot in confluent ECs and in ECs treated with normal and PE placental conditioned medium (CM). Protein-protein interactions between PARD-3/VE-cadherin, PARD-3/ atypical protein kinase C (aPKCλ), and VE-cadherin/aPKCλ were examined by immuno-precipitation and immunobloting. Results: Similar to VE-cadherin, PARD-3 is localized at the cell contacts in control ECs. Both PARD-3 and VE-cadherin expressions were markedly reduced in cells treated with PE-CM for 2h, but not in cells treated with normal-CM compared to non-treated controls. Cytosol staining of VE-cadherin and PARD-3 was pronounced in cells after 24h treatment with PE-CM. PARD-3/VE-cadherin and PARD-3/aPKCλ complexes were detected in PE-CM treated cells, but not in untreated control cells and in cells after recovery. In contrast, VE-cadherin/aPKCλ complex was detected in control cells and in cells after recovery, but not in PE-CM treated cells. Conclusions: Polarity protein PARD-3 is localized at cell contacts. Factors-derived from PE placentas not only interrupt junction protein VE-cadherin distribution, but also perturb polarity protein PARD-3 expression and distribution in ECs. The results of PARD-3/VE-cadherin and PARD-3/aPKCλ complexes formation in cells treated with placental CM suggest that factors-derived from placenta could interfere both junction protein and polarity protein functions in ECs.
Keywords: endothelium, PARD-3, aPKCλ, VE-cadherin, preeclampsia
Introduction
Increased vascular permeability is an underlying pathophysiology in the vascular system in preeclampsia. In preeclampsia especially in severe cases, extracellular fluid volume is markedly expanded due to endothelial injury with subsequent capillary leakage into the extracellular space. Previously published works by our laboratory showed that altered endothelial-specific junction adhesion protein vascular endothelial (VE)-cadherin expression and distribution is very likely responsible for the impaired endothelial junction barrier in preeclampsia.1 We and others have also demonstrated that maternal plasma or serum from women with preeclampsia or factors derived from preeclamptic (PE) placentas could alter both endothelial adhesion junction protein VE-cadherin and tight junction protein occludin expression and distribution and result in an increase in endothelial permeability.2–4
Resent studies suggest that altered partitioning defective (PARD) protein expressions may associate with disturbed epithelial junction protein expressions. For example, PARD-3 is essential not only for asymmetric cell division and polarized growth but also for proper cellular protein distribution at different stages during cell development.5,6 By recruiting signaling molecules, including atypical protein kinase C (aPKC) and PARD-6, into multiprotein complexes, PARD-3 regulates apical/basal polarity and cell migration.7–9 PARD-3/aPKC and PARD-6/aPKC protein-protein interactions are known as core ternaries that regulate protein distribution (trafficking) between cytoplasm and plasma membranes.10–12 In epithelial cells, PARD-3/aPKC complex is tethered to tight junctions via its association with junction adhesion molecule (JAM), indicating a potential role for JAM in the generation of cell polarity in epithelial cells.13 However, little information is available as to whether PARD is present in endothelial cells and whether it is responsible for endothelial integrity. Polarity protein has never been studied in association with endothelial barrier function in preeclampsia.
This study was undertaken to investigate whether polarity proteins play a role in maintaining endothelial integrity and involving in barrier structure regulation. We examined polarity protein PADR-3 expression and its localization in endothelial cells and we tested whether placental-derived factors could alter PARD-3 expression and cellular distribution. We then determined whether PARD-3 expression and distribution were associated with junction protein VE-cadherin expression in endothelial cells. Protein complex formation with VE-cadherin and signaling regulatory molecule aPKCλ was also examined.
Materials and Methods
Endothelial Cell Culture
Human umbilical vein endothelial cells (HUVECs) were isolated as previously described.14 Isolated endothelial cells were cultured with Medium199 (Sigma, St. Louis, Missouri) supplemented with 20% fetal bovine serum (FBS), 2 mmol/L l-glutamine, streptomycin, and penicillin. Confluent endothelial cells were treated with placental conditioned medium (CM) as previously described.15 Cells grown on 12-mm glass coverslips were used for immunoflurorescent staining, cells grown in 6-well/cluster plates or 25 cm2 flasks were used for extraction of total protein, and cells grown in 75 cm2 flasks were used for immunoprecipitation and immunobloting.
Placental CM Preparation
Placentas from normal and PE pregnant women were collected immediately after delivery at Labor/Delivery of the main hospital at Louisiana State University Health Sciences Center in Shreveport (LSUHSC-S). Collection of placenta was approved by the Institutional Review Board (IRB) for Human Research at LSUHSC-Shreveport. Normal pregnancy was defined as pregnancy with normal blood pressure (<140/90 mm Hg), negative proteinuria, and absence of obstetrical and medical complications. Preeclampsia was defined as follows: sustained systolic blood pressure of ≥140 mm Hg or a sustained diastolic blood pressure of ≥90 mm Hg on 2 separate readings, proteinuria measurement of 1+ or more on dipstick, or 24-hour urine protein collection with ≥300 mg in the specimen. To avoid clinical phenotypic differences, patients complicated with hemolysis, elevated liver enzyme, and low platelet count (HELLP) syndrome, diabetes, and/or renal disease were excluded. No patient had a sign of infection and smokers were excluded. Freshly obtained placentas were processed and placental CM was prepared as previously described.15 Placental villous tissues were incubated with serum-free Dulbecco’s Modified Eagles Medium (DMEM; Sigma) containing streptomycin and penicillin for 48 hours. Conditioned medium was then stored at −20°C and pooled CM from at least 2 to 3 placental cultures were used to treat endothelial cells and different CM was used in each experiment.
Immunofluorescent Staining
Cells grown on coverslips were fixed with 2% formaldehyde, permeabilized with 0.5% Triton X-100 (v/v)/0.3 mol/L glycine, blocked with 1% bovine serum albumin (BSA) in phosphate buffered-saline, and probed with primary monoclonal antibody specific against VE-cadherin (Beckman Coulter Technology, Inc, Fullerton, California) and PARD-3 (Santa Cruz Technology, Inc, San Diego, California). Secondary antibody was either Cy3- or fluorescein isothyocyanate (FITC)-conjugated anti-mouse or anti-rabbit antibody (Jackson Immunotech Lab, Westgrove, Pennsylvania). Coverslips were mounted with Vectashield (Vector Lab Inc, Burlingame, California) and revealed by fluorescent microscope (Olympus IX71, Tokyo, Japan). Images were captured by a digital camera with PictureFrame computer software (Uptronics Inc, Sunnyvale, California) and recorded to a microscope-linked PC.
Protein Expression
Protein expressions for VE-cadherin, PARD-3, PARD-6, and aPKCλ were examined by Western blot. An aliquot of total cellular protein, 30 µg per sample, was subject to electrophoresis using a Mini-cell protein-3 gel running system (Bio-Rad, Hercules, California) and transferred to Hybond-protein transfer membrane (Amersham Corp, Arlington Heights, Illinois). The membrane was probed with antibodies against VE-cadherin (Beckman Coulter), PARD-3 (Santa Cruz), PARD-6 (Santa Cruz), or aPKCλ (Santa Cruz). Bands were visualized with an enhanced chemiluminescent detection kit (Amersham). The membranes were stripped, blocked, and then reprobed with different primary and secondary antibodies, followed by detecting with an enhanced chemiluminescent detection kit and exposing on the films. β-actin expression was determined as an indicator of equal loading for each sample.
Immunoprecipitation and Immunoblotting
After cells were treated with placental CM, total cellular protein was extracted by lysis buffer and then immunoprecipitated with Protein-A immunoprecipitation kit (Sigma). An aliquot of 500 mg of total cellular protein per sample was used for immunoprecipitation with antibody of either PARD-3 or VE-cadherin. The precipitated protein was then run on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Hybond-protein transfer membrane (Amersham Corp) and probed with antibodies against VE-cadherin, PARD-3, PARD-6, or aPKCλ. Samples were visualized with an enhanced chemiluminescent detection kit (Amersham).
Statistical Analysis
For the protein expression results (Figures 1C, 2A, and D), band density was scanned and analyzed by NIH Image 1.16. Relative densities for PARD-3 and VE-cadherin expressions were normalized by β-actin expression for each sample. Data were analyzed by analysis of variance (ANOVA) with Student-Newman-Keuls test as a post hoc test. A computer software program Statview (Cary, North Carolina) was used. Data were expressed as mean ± SE and a probability level of P < .05 was considered statistically significant.
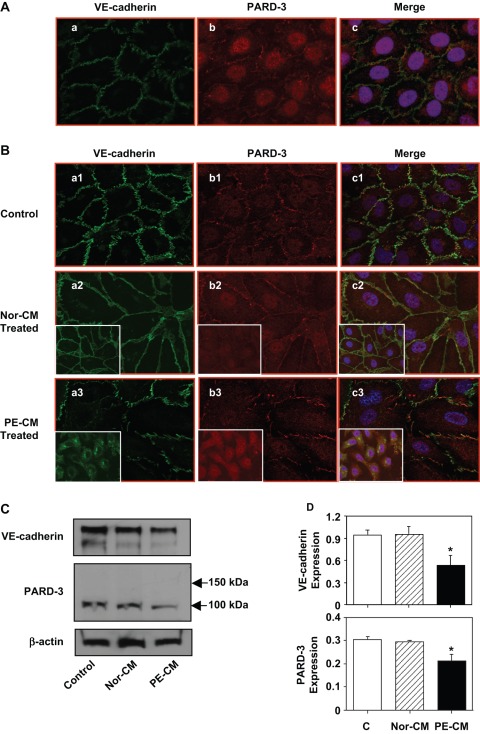
Figure 1.
Expression and distribution of VE-cadherin and partitioning defective-3 (PARD-3) in endothelial cells with or without exposure to placental conditioned medium. A, Immunofluorescent staining of VE-cadherin and PARD-3 in confluent endothelial cells (ECs); (a) VE-cadherin; (b) PARD-3; and (c) a and b merged with DAPI (nucleus) staining. Both VE-cadherin and PARD-3 locate at cell contact regions in confluent ECs. B, Immunofluorescent staining of VE-cadherin (a) and PARD-3 (b) in control cells (a1, b1, and c1) and in cells treated with normal (Nor-CM: a2, b2, and c2) and preeclamptic (PE-CM: a3, b3, and c3) placental conditioned medium (CM) for 2 hours. The inserts show cells treated with normal or PE-CM for 24 hours. No significant changes for VE-cadherin and PARD-3 expressions in cells treated with normal CM at 2 and 24 hours. Reduced and disorganized VE-cadherin and PARD-3 were seen in cells treated with PE-CM. C, Protein expressions for VE-cadherin and PARD-3 in cells treated with normal and PE placental CM by Western blot. A 100-kDa band was detected for PARD-3. Consistent with immunostaining results, both VE-cadherin and PARD-3 expressions were down-regulated in cells treated with PE-CM compared to the control cells or cells treated with normal-CM. D, Relative protein expressions for VE-cadherin and PARD-3 after normalized with actin, *P < .05. At least 3 independent assays were performed for these experiments and results are consistent.
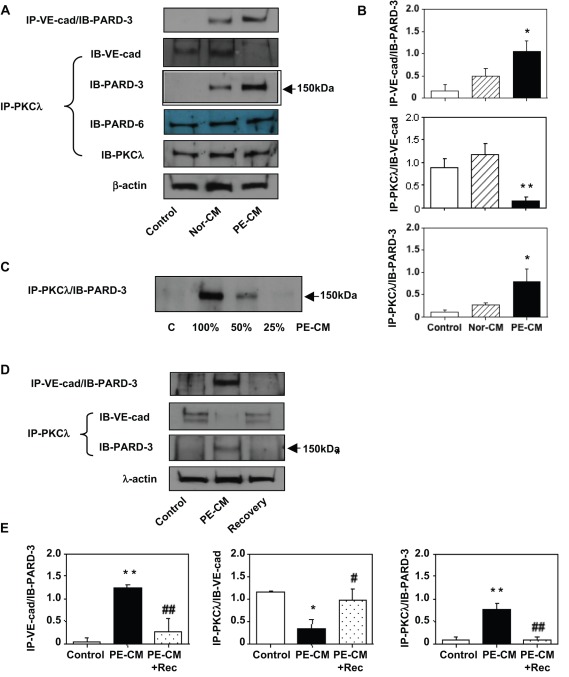
Figure 2.
Protein-protein interactions between VE-cadherin and atypical protein kinase C (aPKC)λ with partitioning defective-3 (PARD-3). A, Protein-protein interaction was evaluated in control cells and in cells that were treated with normal and preeclamptic (PE) placental conditioned medium (CM). Total cellular protein was immunoprecipitated with VE-cadherin antibody and immunoblotted with PARD-3 (IP-VE-cad/IB-PARD-3) or immunoprecipitated with aPKCλ antibody and immunobloted with VE-cad (IP-PKCλ/IB-VE-cad), PARD-3 (IP-PKCλ/IB-PARD-3), or PARD-6 (IP-PKCλ/IB-PARD-6) and aPKCλ (IP-PKCλ/IB-PKCλ) respectively. At least 3 independent assays were performed for these experiments. These results suggest that formations of VE-cad/PARD-3 and aPKCλ/PARD-3 complexes were present in cells treated with PE-CM. In contrast, PKCλ/VE-cadherin complex was dissociated in cells treated with PE-CM. B, Bar graphs represent protein expression for IP-VE-cad/IB-PARD-3, IP-PKCλ/IB-VE-cad, and IP-PKCλ/IB-PARD-3 shown in A, *P < .05; and **P < .01 in cells treated with PE-CM vs control cells and cells treated with Nor-CM, respectively. C, Protein-protein interaction was examined in cells treated with different concentrations of PE-CM (C-0%, 100%, 50%, and 25%). Cell lysate was immunoprecipitated with aPKCλ and immunobloted with PARD-3. Formation of PKCλ/PARD-3 complex seems associated with the potency of stimuli present in the CM. D, Protein-protein interaction was determined in control cells, in cells treated with PE-CM, and after recovery. Cell lysate was immunoprecipitated with VE-cadherin or aPKCλ and immunobloted with PARD-3 or VE-cadherin, respectively. At least 3 independent assays were performed for these experiments. These data indicate that PE-CM induced VE-cad/PARD-3 and aPKCλ/PARD-3 complexes formation are no longer present after PE-CM was removed. E, Bar graphs present relatively quantitative protein expression for IP-VE-cad/IB-PARD-3, IP-PKCλ/IB-VE-cad, and IP-PKCλ/IB-PARD-3 shown in D, *P < .05 and **P < .01 for cells treated with PE-CM vs control cells; # P < .05 and ## P < .01 for cells after recovery vs cells treated with PE-CM, respectively. IP indicates immunoprecipitation; and IB = immunoblotting.
Results
Endothelial Cells Express PARD-3
Since PARD-3 expression was not described in endothelial cells, we first examined whether PARD-3 was expressed in endothelial cells and where it was located. Confluent endothelial cells were dual immunostained with fluorescent-labeled antibodies against VE-cadherin and PARD-3. VE-cadherin is a specific endothelial junction adhesion molecule and plays a critical role in maintaining endothelial barrier integrity. We found PARD-3 is expressed in endothelial cells. Similar to VE-cadherin, PARD-3 is also localized at the cell border and co-localized with VE-cadherin at cell contact regions (Figure 1A).
Redistribution and Downregulation of VE-Cadherin and PARD-3 Expressions in Cells Treated With PE Placental CM
We previously reported that factors derived from PE placentas could disturb VE-cadherin expression and disorganized VE-cadherin at cell junction was associated with increased endothelial permeability.2 Because PARD-3 was also expressed at cell junctions, we then examined whether factors derived from placentas could also interrupt PARD-3 expression and distribution. This was examined by both dual fluorescent immunostaining and Western blot in endothelial cells treated with normal and PE placental CM. As shown in Figure 1B, compared to control cells, cells treated with normal placental CM were elongated, but VE-cadherin and PARD-3 were intact along the cell border. In contrast, when cells were treated with PE placental CM (2 hours), both VE-cadherin and PARD-3 expressions were reduced at cell contact region and showed positive staining in cytosol. However, after 24-hours (prolonged) treatment, staining of VE-cadherin and PARD-3 was mainly localized in cytosol (Figure 1B inserts). These results suggest that both VE-cadherin and PARD-3 were internalized in cells after prolonged stimulation with factors derived from PE placentas. De novo synthesis of PARD-3 may also contribute to the strong cytosol staining in cells treated with PE placental CM (Figure 1B: a3, b3, and c3). Decreased VE-cadherin and PARD-3 expressions were confirmed by Western blot examination (Figure 1C). These images and blots were representative from at least 3 repeated experiments. Results were consistent.
Protein-Protein Interactions Between PARD-3 and VE-Cadherin and PARD-3 and aPKCλ in Cells Respond to Stimuli Derived From the Placenta
Because VE-cadherin and PARD-3 were localized at cell junction in control cells (Figure 1B, a1, and b1) and stained in cytosol in cells after challenged with PE placental CM (Figure 1B a3 and b3), we then examined whether the 2 molecules interact to each other at cell junction and cytosol. PARD-3 and aPKCλ protein-protein interaction was also determined since PARD-3 and aPKCλ complex was found to be associated with tight junction in epithelial cells.13 Endothelial cells were treated with normal and PE placental CM and an aliquot of total cellular protein was immunoprecipitated with antibody against VE-cadherin or aPKCλ. Expressions for PARD-3, PARD-6, and aPKCλ were then determined. Interestingly, PARD-3 expression was detected in cells treated with normal and PE placental CM when samples were precipitated with VE-cadherin or aPKCλ, but PARD-3 expression was not detected in control untreated cells (Figure 2A), indicating PARD-3/VE-cadherin and PARD-3/aPKCλ interactions were only present when cells were under challenge with factors derived from the placenta but not in untreated cells. In comparison, PARD-6/aPKCλ complex was present in control cells and cells treated with normal and PE placental CM and the level was not different between control and treated cells (Figure 2A). In addition, there was no difference for aPKCλ and β-actin expressions between the control cells and cells treated with placental CM (Figure 2A).
Although normal placental CM also induced protein-protein interaction between PARD-3 and VE-cadherin or aPKCλ (Figure 2A), the protein expression level was much less in cells treated with normal placental CM than in cells treated with PE placental CM. This phenomenon suggests that the stimuli derived from PE placentas are stronger than that from normal placentas. To test this, endothelial cells were treated with different concentrations of PE placental CM, in which undiluted CM (100%), and CM diluted with endothelial cell growth medium in ratios of 1:2 (50%) and 1:4 (25%) were used, and then cellular protein was precipitated with aPKCλ and immunoblotted with PARD-3. Our results showed that PARD-3 expression was intensively expressed in cells treated with undiluted CM, but the level of PARD-3 expression was reduced and the decreased PARD-3 expression was correlated with less concentration of PE CM in culture (Figure 2C). These data demonstrate that factors released or produced by PE placentas are more potent to induce protein-protein interaction between PARD-3 and aPKCλ than that from normal placentas.
To determine whether PARD-3/VE-cadherin and PARD-3/aPKCλ protein-protein interactions only occur when cells were exposed to placenta derived stimuli, we further evaluated PARD-3 and VE-cadherin expressions in cells treated with PE placental CM and in cells after recovery, in which cells were continually cultured with fresh endothelial growth medium for 24 hours after PE CM was removed. Cells without treatment were used as control. We found that PARD-3/VE-cadherin and PARD-3/aPKCλ complexes were only present when cells were treated with CM but not in control cells and in cells after recovery (Figure 2D). In contrast, VE-cadherin/aPKCλ complex was present in control cells and cells after recovery but not in cells treated with placental CM (Figure 2D).
Discussion
We made several observations in the current study: (1) polarity protein PARD-3 is expressed in endothelial cells and co-localized with adhesion protein VE-cadherin at cell contact regions; (2) factors derived from PE placentas disturb PARD-3 and VE-cadherin distributions at intercellular junction; (3) dynamic protein-protein interactions occur between PARD-3 and VE-cadherin and PARD-3 and aPKCλ in cells that respond to placenta-derived stimuli; (4) protein-protein interaction between PARD-6 and aPKCλ is detected in control cells and in cells that respond to placenta-derived stimuli, but the expression level is not different; (5) disturbed VE-cadherin distribution at cell junction is associated with its dissociation with aPKCλ.
In mammalian epithelial cells, polarity protein complex PARD-3/aPKC/PARD-6 asymmetrically locates at apical-lateral cell membrane and plays indispensable roles in the regulation of various cell polarization events.12 In this study, we found PARD-3 is present in vascular endothelial cells. The localization of PARD-3 at intercellular junction and disturbed PARD-3 distribution after cells challenged with placental CM indicates that polarity PARD-3 is involved in maintaining the barrier integrity in endothelial cells and sensitive to factors derived from PE placentas. Previously, we found that altered VE-cadherin at endothelial junction is associated with increased endothelial permeability in women with preeclampsia1 and factors derived from PE placentas interfere in the homophilic binding of VE-cadherin between adjacent endothelial cells and dissociate VE-cadherin from intracellular proteins β-catenin and p120.2,16 Therefore, we believe that cell polarity alteration may associate with disturbed endothelial barrier function in endothelial cells.
Since both VE-cadherin and PARD-3 locate at intercellular junctions and are expressed in cytosol after PE placental factor stimulation, we then determined whether PARD-3 interacts with VE-cadherin in this transition event. Our results showed that VE-cadherin/aPKCλ complex was detected in control ECs and in cells treated with normal CM but not/or very little in cells treated with PE-CM. VE-cadherin/aPKCλ complex was also detected in cells after recovery. These observations suggest that factors derived from PE placentas could induce VE-cadherin/aPKCλ complex dissociation. In contrast, PARD-3 and VE-cadherin interaction was detected in cells treated with placental CM but not in untreated control cells. Similar phenomenon was also seen for protein-protein interaction between PARD-3 and aPKCλ. These observations indicate that there are no protein-protein interactions between PARD-3 and VE-cadherin and between PARD-3 and aPKCλ in quiescent control cells, but they do form complex or bind to each other when cells are under stress. In contrast, protein-protein interaction between PARD-6 and aPKCλ is present in control cells, which is not affected when cells are under stimulation, suggesting that the interaction between PARD-6 and aPKCλ is relatively stable in endothelial cells. Therefore, PARD-3, but not PARD-6, seems actively involved in the structural alterations that associate with barrier function changes in endothelial cells.
Suzuki et al reported that in MDCK cells, aPKC is required for the stable localization of PARD-3 at cell-cell contact regions and phosphorylation of PARD-3 by aPKC allows PARD-3 to be incorporated tightly into the junction structures.12,17 However, the case seems different in endothelial cells. We found that instead of PARD-3 and aPKC complex, the protein-protein interaction between VE-cadherin and aPKC is detected in unchallenged cells and in cells after recovery. We further found that destabilization of VE-cadherin and aPKC interaction is associated with protein-protein complex formation between PARD-3 and aPKC and between PARD-3 and VE-cadherin when cells were challenged with factors derived from PE placentas. It is very likely that aPKC is necessary to maintain endothelial junction structure and barrier integrity, and dissociation of VE-cadherin from aPKC allows aPKC and VE-cadherin to interact with PARD-3 and form PARD-3/aPKC and PARD-3/VE-cadherin complexes. These observations provide an evidence of aPKC as a scaffold protein to regulate the rapport between adhesion junction protein and polarity protein in endothelial cells.
In MDCK cells, aPKC negatively autoregulates its binding activity to PARD-3 and aPKC-induced PARD-3 phosphorylation impairs aPKC and PARD-3 binding.12 We did not specifically test whether PARD-3 phosphorylation occurs in the event of aPKC and PARD-3 interaction, but our immunoprecipitation and immunobloting data support the possibility, because a 100-kDa band was detected for PARD-3 by Western blot (Figure 1C), but a 150-kDa band was detected for PARD-3 when protein samples were immunoprecipitated with aPKC (Figure 2A, C, and D). PARD-3 has 3 splice isoforms at 100 kDa, 150 kDa, and 180 kDa.18 The 150 kDa isoform has an aPKC-binding site. A 150-kDa bind was detected in cells treated with placental CM. This phenomenon suggests that PARD-3 phosphorylation likely occurs in cells treated with CM. Cells treated with CM induced dissociation of VE-cadherin and aPKC and PARD-3 phosphorylation. Phosphorylated PARD-3 then recruited aPKC to PARD-3 and formed aPKC/PARD-3 and VE-cadherin/PARD-3 complexes. It is likely that protein-protein interactions between PARD-3 or VE-cadherin and aPKC are associated with disrupted intercellular adhesion and impaired barrier integrity in endothelial cells when cells were exposed to factors derived from PE placentas. This explanation is supported by the data shown in Figure 1. Another explanation for not seeing 150 kDa PARD-3 in Western blot but in immunoprecipitation samples is that conformational changes of the 150 kDa isoform may occur in the form of aPKCλ/PARD-3 and VE-cadherin/PARD-3 complexes in cells after being treated with placental CM. In fact, our results support the possibility since no 150-kDa bands were detected in control cells (Figure 2).
In summary, we found PARD-3-aPKC system may play an important role in the endothelial junction and polarity axis regulation in vascular endothelial cells. Factors derived from PE placentas not only interrupt junction molecule VE-cadherin distribution but also interfere in polarity protein associations. A hypothesis and schematic drawing of interactions between VE-cadherin, PARD-3, and aPKC in control cells and in cells under stress are presented in Figure 3 . We believe that altered PARD-3 and aPKC interactions with VE-cadherin are associated with disturbed barrier integrity in endothelial cells. Further studies are needed to decipher the molecular mechanisms of polarity proteins in regulation of adhesion junction and tight junction and barrier function in endothelial cells.
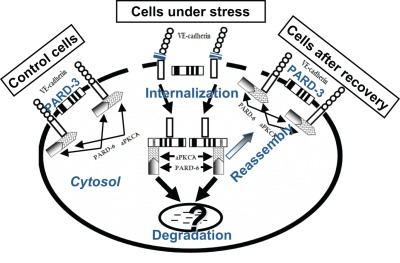
Figure 3.
Proposed hypothesis that illustrate interactions between VE-cadherin, partitioning defective-3 (PARD-3), and atypical protein kinase C (aPKC) in control cells and cells under stress. In quiescent control cells, endothelial junction structure is intact. VE-cadherin forms a complex with aPKCλ, which interacts with PARD-6. Although both VE-cadherin and PARD-3 are located at cell junction, the 2 molecules have no interaction. However, when cells are under stress conditions, such as when cells were treated with preeclamptic conditioned medium (PE-CM), expression and distribution of VE-cadherin and PARD-3 are reduced at cell junction, and internalized in cytosol. The event is accompanied with dissociation of VE-cadherin and aPKCλ interactions. Dissociated VE-cadherin and aPKCλ then interact with PARD-3 and form VE-cadherin/PARD-3 and aPKCλ/PARD-3 complexes. At present it is not known whether VE-cadherin/PARD-3 and aPKCλ/PARD-3 complex formations are associated with cell polarity changes or this process is a mechanism that protects VE-cadherin or PARD-3 from degradation by proteolysis. However, it is clear that the changes of the protein-protein interactions between VE-cadherin and PARD-3 and aPKCλ and PARD-3 are transient and only occur when cells are under stress challenge. Because after eliminating the stimuli (CM), interactions between VE-cadherin and PARD-3 and aPKCλ and PARD-3 no longer exist, that is, after recovery, expression and distribution of VE-cadherin and PARD-3 at intercellular junction and the interaction between VE-cadherin and aPKC and PARD-3 return to the control cell levels.
Footnotes
The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: supported in part by grants from National Institute of Health, NHLBI (HL65997) and NICHD (HD36822).
References
- 1. Wang Y, Gu Y, Granger DN, Roberts JM, Alexander JS. Endothelial junctional protein redistribution and increased monolayer permeability in HUVECs isolated during preeclampsia. Am J Obstet Gynecol. 2002;186(2):214–220 [DOI] [PubMed] [Google Scholar]
- 2. Wang Y, Lewis DF, Gu Y, Zhang Y, Alexander JS, Granger DN. Placental trophoblast-derived factors diminish endothelial barrier function. J Clin Endocrinol Metabol. 2004;89(5):2421–2428 [DOI] [PubMed] [Google Scholar]
- 3. Groten T, Kreienberg R, Fialka I, Huber L, Wedlich D. Altered subcellular distribution of cadherin-5 in endothelial cells caused by the serum of pre-eclamptic patients. Mol Hum Reprod. 2000;6(11):1027–1032 [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Gu Y, Li H, Lucas MJ, Wang Y. Increased endothelial monolayer permeability is induced by serum from women with preeclampsia but not by serum from women with normal pregnancy or that are not pregnant. Hypertens Pregnancy. 2003;22(1):121–131 [DOI] [PubMed] [Google Scholar]
- 5. Schmoranzer J, Fawcett JP, Segura M, et al. Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr Biol. 2009;19(13):1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zen K, Yasui K, Gen Y, et al. Defective expression of polarity protein PAR-3 gene (PARD3) in esophageal squamous cell carcinoma. Oncogene. 2009;28(32):2910–2918 [DOI] [PubMed] [Google Scholar]
- 7. Pegtel DM, Ellenbroek SI, Mertens AE, van der Kammen RA, de Rooij J, Collard JG. The Par-Tiam1 complex controls persistent migration by stabilizing microtubule-dependent front-rear polarity. Curr Biol. 2007;17(19):1623–1634 [DOI] [PubMed] [Google Scholar]
- 8. Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2(8):531–539 [DOI] [PubMed] [Google Scholar]
- 9. Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2(8):540–547 [DOI] [PubMed] [Google Scholar]
- 10. Kanzaki M, Mora S, Hwang JB, Saltiel AR, Pessin JE. Atypical protein kinase C (PKCzeta/lambda) is a convergent downstream target of the insulin-stimulated phosphatidylinositol 3-kinase and TC10 signaling pathways. J Cell Biol. 2004; 164(2):279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Izumi Y, Hirose T, Tamai Y, et al. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol. 1998;143(1):95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki A, Ishiyama C, Hashiba K, Shimizu M, Ebnet K, Ohno S. aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J Cell Sci. 2002;115(pt 18):3565–3573 [DOI] [PubMed] [Google Scholar]
- 13. Ebnet K, Suzuki A, Horikoshi Y, et al. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). EMBO J. 2001;20(14):3738–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Adair CD, Coe L, Weeks JW, Lewis DF, Alexander JS. Activation of endothelial cells in preeclampsia: Increased neutrophil-endothelial adhesion correlates with up-regulation of adhesion molecule P-selectin in human umbilical vein endothelial cells isolated from preeclampsia. J Soc Gynecol Investig. 1998;5(5):237–243 [DOI] [PubMed] [Google Scholar]
- 15. Wang Y, Adair CD, Weeks JW, Lewis DF, Alexander JS. Increased neutrophil-endothelial adhesion induced by placental factors is mediated by platelet-activating factor in preeclampsia. J Soc Gynecol Investig. 1999;6(3):136–141 [DOI] [PubMed] [Google Scholar]
- 16. Gu Y, Lewis DF, Alexander JS, Wang Y. Placenta-derived chymotrypsin-like protease (CLP) disturbs endothelial junctional structure in preeclampsia. Reprod Sci. 2008;16(5):479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suzuki A, Yamanaka T, Hirose T, et al. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol. 2001; 152(6):1183–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Du D, Fang L, et al. Tyrosine phosphorylated Par3 regulates epithelial tight junction assembly promoted by EGFR signaling. EMBO J. 2006;25(21):5058–5070 [DOI] [PMC free article] [PubMed] [Google Scholar]