Abstract
Problem
To evaluate whether impaired endothelial function and endothelial inflammatory response occur in parallel in the women with preeclampsia.
Method of Study
Venous blood was drawn from normal (n = 40) and severe preeclamptic (sPE) (n = 40) pregnant women when they were admitted to the L&D Unit and 24 hrs after delivery. Plasma and serum samples were extracted and measured for 6-keto PGF1α and TXB2 (stable metabolites of PGI2 and TXA2), and intercellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM) by ELISA. Data are analyzed by Mann–Whitney test and paired t-test. The statistical significance is set as P < 0.05.
Results
Plasma 6-keto PGF1α levels were significantly reduced at admission and 24 hr after delivery in sPE compared to normal pregnant controls, P < 0.01. The ratio of 6-keto PGF1α and TXB2 was significant less in sPE than that in normal pregnant controls before delivery. There was no significant difference for ICAM and VCAM levels between normal and patients with sPE before and after delivery.
Conclusion
Maternal 6-keto PGF1α levels and the ratio of 6-keto PGF1α and TXB2 were decreased in patients with sPE compared to normal pregnant controls. In contrast, maternal ICAM and VCAM levels were not different between the two groups. These data suggest that serum ICAM and VCAM levels may not be sensitive inflammatory biomarkers for preeclampsia.
Keywords: Intercellular adhesion molecule, preeclampsia, Prostacyclin, thromboxane, vascular cell adhesion molecule
Introduction
Increased vasoconstriction and exaggerated inflammatory response are the two major underlying pathophysiological characteristics in the maternal systemic vasculature in preeclampsia.1,2 The marked increase in the vascular peripheral resistance contributes to the maternal hypertension. In terms of vasoactivators, it is well known that vascular endothelium is the main source of prostacyclin (PGI2) and nitric oxide (NO), which play an important role in vasculature relaxation. PGI2, especially, is a potent vasodilator and an inhibitor of platelet aggregation. In contrast, thromboxane A2 (TXA2), which results from the degranulation of platelets upon activation, is a potent vasoconstrictor as well as a platelet aggregator. In normal pregnancy, delicate balance exists between vasodilators and vasoconstrictors to maintain a normal vasomotor tone and vasoactivity. Whereas during preeclampsia, decreased PGI2 levels and/or increased TX levels or the imbalance of PGI2 to TX contribute significantly to the increased vasoconstriction in both the maternal systemic and the placental vasculatures. In preeclampsia, maternal vessels also display structural changes.3,4 For example, Svedas et al.3 observed that endothelial cells were shrunken with blebbing, and thin plasma membranes detached from basal lamina in myometrial artery vessels from preeclampsia. These arteries were noted to have reduced bradykinin-mediated dilatory response, confirming impaired vasodilatation in the vessels from women with preeclampsia.
Exaggerated inflammatory responses are also believed to be a significant pathophysiological event during preeclampsia.5 Several biochemical parameters indicate that endothelial dysfunction is related to the inflammatory response in preeclampsia, such as elevated serum cellular fibronectin levels6,7 and increased endothelial adhesion molecules P-selectin, intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), and platelet endothelial cell adhesion molecule (PECAM).8–11 These measurable soluble molecules have been considered as biomarkers to monitor endothelial function in preeclampsia.5,8,9
Both decreased vasodilator PGI2 levels and increased soluble endothelial adhesion molecule levels are linked to endothelial dysfunction during preeclampsia. Although many studies have shown decreased PGI2 with or without increased TX levels, as well as altered endothelial adhesion molecule levels in women with preeclampsia compared to those from normal pregnant women, it is not known whether the two events, impaired endothelial vasodilator production and endothelial activation resulting in increased endothelial adhesion molecule expression/release, occur in parallel in the maternal system in women with preeclampsia. To test this, in this study, we measured plasma vasodilator PGI2 levels as an indicator of endothelial ability to respond vasoconstriction and serum soluble ICAM and VCAM levels as indicators of endothelial inflammatory response in women with preeclampsia. We then compared the levels with those levels in women with normal pregnancy. We also measured vasoconstrictor TX levels as a comparison to PGI2. We hypothesized that the two events may occur simultaneously in the maternal system during preeclampsia.
Materials and methods
Sample Collection and Patient Information
This study was approved by the Institutional Review Board (IRB) for Human Research at Louisiana State University Health Sciences Center – Shreveport (LSUHSC-S), LA. Patients, women diagnosed with normal pregnancy and severe preeclampsia, were recruited when they were admitted to the Labor and Delivery Unit at LSUHSC-S from January 2003 to December 2005. Written consent was obtained. Normal pregnancy is defined as pregnancy with normal blood pressure (<140/90 mmHg), no proteinuria, and absence of obstetrical and medical complications. Diagnosis of severe preeclampsia is defined as ACOG Practice Bulletin12: sustained systolic blood pressure of ≥160 mmHg or a sustained diastolic blood pressure of ≥110 mmHg; proteinuria measurement of 3+ or more on dipstick, or 24-hr urine protein collection with ≥5 g in specimen; urine output <30 mL per hour for 2 hr unresponsive to 500 mL intravenous fluid challenge; presence of persistent headache, and visual disturbances, or epigastric pain; patients with one or more abnormal laboratory values reflecting thrombocytopenia, elevated LDH or increased aspartate aminotransferase; pulmonary edema or evidence of intrauterine growth restriction. Each patient had two venous blood draws, one at admission before medication and one at 24 hr post-partum. Aliquot plasma and serum samples were stored in −70°C.
Measurement of Prostacyclin and Thromboxane
Plasma levels of PGI2 and TXA2 were measured by their stable metabolites of 6-keto-PGF1α and TXB2 by enzyme-linked immunosorbent assay (ELISA). Both 6-keto-PGF1α and TXB2 ELISA kits were purchased from Oxford Biomedical Research, Ins. (Oxford, MI, USA). Plasma samples for measurements of 6-keto-PGF1α and TXB2 were extracted by C18 Sep-Pak column (Waters Corporation, Milford, MA, USA) following the standard manufacture instruction before assay. Briefly, 0.2 mL of methanol was added to 1.0 mL of plasma sample and mixed well, and then the mixture was applied into a C18 Sep-Pak column with flow rate to 1.0 mL per minute. The C18 Sep-Pak column was preconditioned with 2.0 mL of 100% methanol and 2.0 mL of double distilled H2O. After the plasma/methanol mixture passed the column, the column was washed with 2.0 mL of 15% methanol followed by 2.0 mL of petroleum ether. The 6-keto-PGF1α and TXB2 sample was eluted by 2.0 mL of methyl formate, which was evaporated by nitrogen gas. The residue was diluted with 1.0 mL of extraction buffer provided by the ELISA kit.
The assay was performed in a 96-well ELISA plate. The ELISA kit includes ELISA buffer, washing buffer, extraction buffer, substrate, standard enzyme conjugate, and antibody-coated plate. The standard curve for 6-keto-PGF1α had a range of 20–200 pg/mL. The standard curve for TXB2 had a range of 4–400 pg/mL. An aliquot of 50 µL per sample was assayed in duplicate for both 6-keto-PGF1α and TXB2 ELISA assays. Within- and between-assay variations were less than 8%.
Measurement of ICAM and VCAM
Maternal serum levels for ICAM and VCAM were measured by enzyme-linked immunosorbent assay (ELISA). The DuoSet ELISA Development kit for both ICAM and VCAM kits were purchased from R&D systems (R&D systems, Minneapolis, MN, USA). The range of the standard curve was 31.25–2000 pg/mL for both ICAM and VCAM assays. All samples were measured in duplicate. Within- and between-assay variations were less than 7%. Assays were preformed according to the manufacturers’ instruction.
Statistical Analysis
Demographic and clinical data are presented as mean ± S.D. (Tables I and II). Data for 6-keto-PGF1α, TXB2, ICAM, and VCAM are presented as mean ± S.E. and analyzed by Mann–Whitney test or student t-test for comparison between normal and preeclamptic groups. Paired t-test was used for analysis of 6-keto-PGF1α, TXB2, ICAM, and VCAM data in normal and preeclamptic groups before and after delivery. Simple regression was used to analyze regression coefficients between platelet counts and maternal TXB2 levels at admission in women with preeclampsia. Computer software StatView (SAS Institute, Inc. Cary NC, USA) was used. The statistical significance is defined as P < 0.05. Power analysis was performed by a computer program Power and Precision (Biostat, Englewood, NJ, USA). Based on admission 6-keto-PGF1α levels, it needs 32 patients in each arm to achieve 80% power level, i.e., alpha = 0.05, 2-tailed, and power = 0.80. In this study, we included 40 patients (25% more patients) in each arm to minimize patient selection bias.
Table I.
Demographic Data for Study Subjects
| Normal Pregnancy (n = 40) |
Preeclampsia (n = 40) |
P value | |
|---|---|---|---|
| Maternal agea | 22 ± 3 | 24 ± 6 | >0.5 |
| Racial status | |||
| Black | 35 | 24 | – |
| White | 4 | 15 | – |
| Other | 1 | 1 | – |
| Gestational agea | 40 ± 1 | 34 ± 4 | <0.0001 |
| Primagravida (%) | 45% | 51% | – |
| Blood pressurea | |||
| Systolic | 125 ± 10 | 166 ± 17 | <0.0001 |
| Diastolic | 67 ± 12 | 102 ± 13 | <0.0001 |
| Mode of delivery | |||
| Vaginal delivery | 28 | 15 | – |
| Cesarean section | 12 | 25 | – |
Data are presented as mean ± S.D.
Table II.
Maternal Blood Pressures, Platelet Counts, Hematocrits, and MgSO4 Concentrations at Admission and 24 hr Post-partum in Women Complicated by Preeclampsia
| Admission | 24 hr post-partum |
P value | |
|---|---|---|---|
| Blood pressure (mmHg) | |||
| Systolic BP | 166 ± 17 | 144 ± 15 | <0.0001 |
| Diastolic BP | 102 ± 13 | 84 ± 10 | <0.0001 |
| Platelets (103/µL) | 224 ± 57 | 216 ± 60 | 0.270 |
| Hematocrit (%) | 34.03 ± 4.08 | 29.55 ± 4.97 | <0.0001 |
| MgSO4 concentration (mg/dL) | 3.64 ± 1.16a | 4.13 ± 1.70 | 0.245 |
Data are presented as mean ± S.D.
2 hr after the bolus infusion.
Results
Patient Clinical Data
A total of eighty pregnant patients were included in this study, forty with normal pregnancy and forty with severe preeclampsia. The clinical characteristics of the study population for normal and preeclamptic pregnancies are shown in Table I. There are no statistical differences for maternal age and primagravida between normal and preeclamptic groups. Gestational age was significantly less, and systolic/diastolic blood pressure was significantly higher in the preeclamptic group than in the normal pregnant group. Table II presents maternal blood pressures, platelet counts and hematocrits at admission, and MgSO4 concentrations 2 hr after initial bolus infusion and 24 hr post-partum for preeclamptic patients. Maternal blood pressure and hematocrit were significantly reduced 24 hr after delivery. There was no difference for platelet counts at admission and blood MgSO4 levels 2 hr after initial bolus infusion and 24 hr after delivery. None of patients with preeclamptic has HELLP syndrome.
Maternal Levels for 6-keto PGF1α and TXB2
The maternal 6-keto PGF1α and TXB2 levels at admission and 24 hr post-partum are presented in Table III. Maternal 6-keto PGF1α levels are significantly reduced in women with preeclampsia compared to those in women with normal pregnancies at admission and at 24 hr after delivery; admission: 158.01 ± 15.20 versus 254.51 ± 27.31 pg/mL, P < 0.01; 24 hr post-partum: 144.37 ± 21.90 pg/mL versus 225.41 ± 24.25 pg/mL, P < 0.01. In contrast, there is no difference for TXB2 levels at admission between preeclamptic and normal pregnant women, 41.28 ± 8.42 pg/mL versus 46.36 ± 4.23 pg/mL. The TXB2 levels are reduced in patients with preeclampsia 24 hr after delivery compared to those in normal pregnant controls, 31.74 ± 6.16 versus 49.10 ± 7.15 pg/mL, P < 0.05, respectively. Maternal levels of 6-keto PGF1α and TXB2 are not different between admission and 24 hr after delivery in normal pregnant controls (Table III).
Table III.
Maternal Plasma Levels for 6-keto PGF1α and TXB2 at Admission and 24 hr Post-Partum in Normal Pregnant Women and in Women Complicated by Preeclampsia
| 6-keto PGF1α (pg/mL) |
TXB2 (pg/mL) |
6K/TX Ratio |
||||
|---|---|---|---|---|---|---|
| Admission | 24 hr PP | Admission | 24 hr PP | Admission | 24 hr PP | |
| Nor-Pregnancies | 254.51 ± 27.31 | 225.41 ± 24.25 | 46.36 ± 4.23 | 49.10 ± 7.15 | 7.21 ± 0.91 | 7.75 ± 1.16 |
| Preeclampsia | 158.01 ± 15.20** | 144.37 ± 21.90** | 41.28 ± 8.42 | 31.74 ± 6.16* | 5.05 ± 0.55* | 6.33 ± 1.01 |
Data are presented as mean ± S.E.
Preeclampsia vs. normal pregnancies:
P < 0.05;
P < 0.01.
The ratio of 6-keto-PGF1α to TXB2 is significantly less in preeclampsia at admission, 5.05 ± 0.55, compared to that in normal pregnant controls, 7.21 ± 0.91, P < 0.05. The ratio for 6-keto-PGF1α to TXB2 is approximately 25% increased at 24 hr post-partum compared to that before delivery in women with preeclampsia. There is no statistical difference for the ratio of 6-keto-PGF1α/TXB2 at 24 hr postpartum between preeclampsia and normal pregnant controls, 6.33 ± 1.01 versus 7.75 ± 1.16 (Table III).
Maternal Levels for ICAM and VCAM
Maternal serum levels for ICAM and VCAM at admission and 24 hr post-partum in normal pregnancies and pregnancies complicated by preeclampsia are shown in Table IV. There is no significant difference for ICAM and VCAM levels both at admission and 24 hr post-partum between normal pregnancy and preeclampsia.
Table IV.
Maternal Serum Levels for sICAM and sVCAM at Admission and 24 hr Post-partum in Women with Normal Pregnancies and in Women Complicated by Preeclampsia
| ICAM (ng/mL) |
VCAM (ng/mL) |
|||
|---|---|---|---|---|
| Admission | 24 hr PP | Admission | 24 hr PP | |
| Nor-Pregnancies | 169.01 ± 6.71 | 167.10 ± 8.03 | 307.90 ± 10.15 | 307.86 ± 10.43 |
| Preeclampsia | 174.96 ± 14.35 | 174.20 ± 13.26 | 322.76 ± 12.62 | 305.09 ± 12.10 |
Data are presented as mean ± S.E.
PP, post-partum; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule.
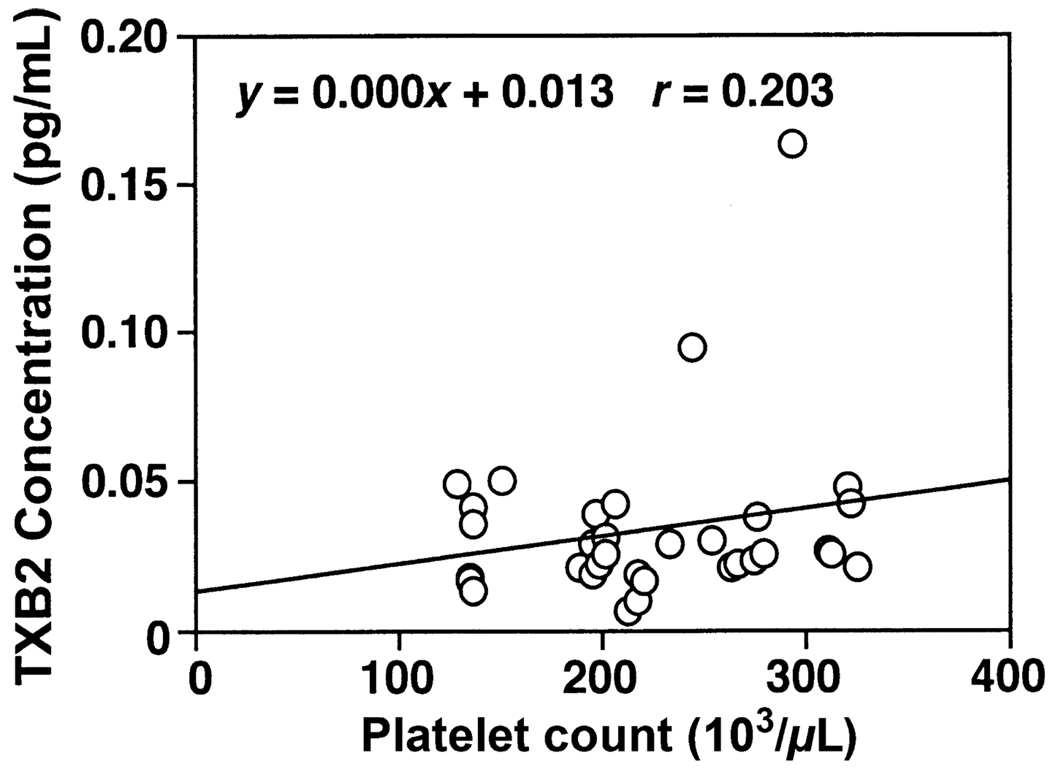
Correlation Between the Platelet Count and the TXB2 Concentration in Preeclampsia
As thromboxane is released by circulating platelets, a correlation analysis was done on the relationship between the platelet count and the maternal plasma level of TXB2 at admission in women with preeclampsia. No significant positive correlation was revealed between the maternal platelet count and the maternal plasma TXB2 as presented in Fig. 1.
Fig. 1.
Correlation of plasma TXB2 concentration with platelet counts at admission in women with preeclampsia. There was no significant correlation of plasma TXB2 levels with platelet counts: y = 0.000x + 0.013, r = 0.203.
Discussion
In this study, we evaluated 6-keto-PGF1α, TXB2, ICAM, and VCAM levels in the maternal blood samples obtained from normal and preeclamptic pregnant women at admission to Labor and Delivery and 24 hr after delivery. 6-keto-PGF1α and TXB2 were used as the indicators of vasoactivity. ICAM and VCAM were used as indicators of endothelial inflammatory response. We wanted to learn whether their level changes were concomitantly or in parallel before and after delivery, and what the difference was between normal pregnancy and preeclampsia.
Our results showed that the levels for 6-keto PGF1α are significantly less in plasma samples from women with preeclampsia than those from normal pregnant controls both at the admission and 24 hr post-partum. There is no significant difference in TXB2 levels between normal and preeclamptic pregnancies at admission, but the TXB2 levels are decreased in preeclamptic pregnancies 24 hr after delivery. Although no changes were noticed for TXB2 levels in normal pregnant women between admission and 24 hr after delivery, the results show a 25% reduction in the ratio of 6-keto PGF1α to TXB2 in patients with preeclampsia at admission compared to that in normal pregnant controls. These results are consistent with previously published works,13,14 indicating the balance between PGI2 and TXA2 favors to TX2 in preeclampsia. The disproportion between PGI2 and TXA2 is believed to contribute to the increased vasoconstriction in preeclampsia.13 Furthermore, the reduced PGI2/TXA2 ratio reflects endothelial cell dysfunction, which is unable to properly generate PGI2 to compensate the increased vasoconstrictive state during preeclampsia. In fact, the reduced maternal-circulating PGI2/TXA2 ratio is in line with the imbalance of placental production between prostacyclin and thromboxane previously found in preeclampsia.15,16 It is believed that this reduced ratio represents the upcoming overt clinical symptoms characterized by vasoconstriction and portends the development of preeclampsia.17 With the imbalance between prostacyclin and thromboxane in favor of thromboxane, the biological action of thromboxane on constricting vasculature can be taken into effect in addition to the reduced vasodilatory effect by endothelial prostacyclin. The PGI2/TXA2 ratio is increased by 25% approximately 24 hr after delivery in women with preeclampsia, suggesting that there is an elevated level of PGI2 within 24 hr post-partum, which parallels a reduction in blood pressure and an improved clinical condition in preeclamptic patients. Thus, in spite of the absolute value of either PGI2 or TXA2 within the maternal circulation, the reduced PGI2/TXA2 ratio is a reliable factor for potential vasoconstriction characterizing endothelial dysfunction in preeclampsia.
Previously published works showed that ICAM and VCAM levels were either elevated8,9 or no difference 18 in patients with preeclampsia compared to normal pregnant controls. In this study, we found no difference for ICAM and VCAM levels at admission and 24 hr after delivery between normal pregnancy and preeclampsia in our patient population. The exact mechanism for the regulation of CAM within the circulation in preeclampsia is not clear. Marks et al.19 measured ICAM, VCAM, and E-selectin levels in pregnant women living high altitude. They found that serum levels of these adhesion molecules were not significantly different from those living at the sea level. Their data suggest that maternal systemic hypoxia is not responsible for this pathway of endothelial cell activation in preeclampsia.19 Alternatively, it was suggested that the plasma level of TX might be a regulatory/contributing factor in the expression of cell adhesion molecules. For example, an animal study showed that TX generated from the activated platelets promotes ICAM and VCAM upregulation on endothelial cells.20 Cell culture experiments also found that TXA2 inhibitor suppressed ICAM expression on activated endothelial cells while TXA2 agonists augmented the endothelial VCAM expression.21 Thus, plasma TX level might be causally linked to the circulating ICAM and VCAM levels. This might explain the results in our study with the TX level in preeclampsia that was compatible with that of the normal pregnancy, in which the soluble ICAM and VCAM also showed no significant difference before delivery and 24 hr post-partum. Another explanation could be because of the labor-induced effects. It was reported that the ripening of the cervix during labor is a pro-inflammatory reaction, leading to the upregulation of certain cell adhesion molecules within the cervix and the lower uterine segment,22,23 but it is not known whether the local upregulated cell adhesion molecules in the cervix and the lower uterine segment could contribute to the elevated circulating soluble cell adhesion molecule levels. In our patient setting, although there were about 2/3 patients in the normal pregnant group and 1/3 in the preeclamptic group who had vaginal delivery, we did not notice any difference in ICAM and VCAM levels associated with delivery mode. Thus, it is unlikely that the difference in sICAM and sVCAM levels between preeclampsia and normal pregnancy could be masked by the intra-partum elevation of these molecules during active labor in some of normal controls.
It has been shown that with the absence of upregulated endothelial expression of ICAM and VCAM, the circulating pro-inflammatory cytokines such as TNFα are unable to trigger the aggregation of leukocytes and platelets, which is the pre-requisite for the endothelial phenotypic transformation to the proinflammatory and pro-thrombotic one.24 Thus, elevated levels of these soluble cell adhesion molecules might be the more specific indicator for the endothelial inflammatory response per se. Once initiated, activated endothelium activates leukocytes and/or vice versa in the process of inflammation within the circulation.25 In the case of preeclampsia, both the endothelial activation/dysfunction and amplified activation of the circulating components have been demonstrated.5,26 However, it is not known whether the earlier changes in the preeclamptic state are leukocytic or endothelial. It is very likely that the altered endothelial function is an earlier event. Furthermore, results from this study suggest that among the consequences of endothelial activation/dysfunction responses, reduced vasodilator production of PGI2, by vascular endothelium or failure of adaptation to increased vasoconstrictive activity might occur prior to the enhanced endothelial inflammatory response during preeclampsia.
All the patients in preeclamptic group were administrated with magnesium sulfate. However, the 6-keto PGF1α data suggest that magnesium sulfate do not affect maternal PGI2 levels (Table II). We believe that the changes in PGI2 levels before and after delivery further indicate the important compensatory effects of this vasodilation agent on the physiological adaptation to the increased blood volume and reduced vascular resistance during pregnancy. Increased TX levels in the maternal circulation have been reported in women with preeclampsia compared to that in women with normal pregnancies.13 Platelets are a major source of thromboxane in the systemic circulation, however, this study did not detect difference in TX levels at admission between preeclamptic pregnancies and their normal controls. The relatively stable platelet count in the study patients (Table II) could, at least in part, explain the phenomena of compatible TX levels between normal and preeclamptic pregnancies. In comparison, we did observe a reduction in TX levels 24 hr after delivery in women with preeclampsia. In this study, most patients with preeclampsia were delivered before 34 weeks of gestation, and they usually received steroids 48 hr before termination of pregnancy to promote fetal lung maturity. Both magnesium and steroids exert platelet inhibitory function.27,28 Therefore, inhibition of platelet activation and aggregation by magnesium and steroids could protect platelets from degranulation and TX release. This could be a reason for reduced TXB2 levels seen in post-partum samples from patients with preeclampsia.
In conclusion, we found that maternal 6-keto-PGF1α levels and the ratio of 6-keto-PGF1α/TXB2 were reduced in patients with preeclampsia compared to normal pregnant patients. We did not detect any difference for ICAM and VCAM levels between normal and preeclamptic pregnancies in our study population. This data suggest that serum ICAM and VCAM levels may not be sensitive inflammatory biomarkers for preeclampsia. It is likely that diverse regulatory mechanisms exist for prostacyclin production and ICAM and VCAM shedding from vascular endothelium, although altered vasoactivators and increased adhesion molecule levels may have the common “read out” once terminal stage has been reached.26
Acknowledgment
This study was supported in part by grants from National Institute of Health, National Institute of Child Health Development (HD36822), and National Heart Blood Lung Institute (HL65997).
References
- 1.Lindheimer MD, Roberts JM, Cunningham FC. Chesley’s Hypertensive Disorders in Pregnancy. 2nd edn. Stamford, Connecticut: Appleton & Lange; 1999. [Google Scholar]
- 2.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 3.Svedas E, Nisell H, Vanwijk MJ, Nikas Y, Kublickiene KR. Endothelial dysfunction in uterine circulation in preeclampsia: Can estrogens improve it? Am J Obstet Gynecol. 2002;187:1608–1616. doi: 10.1067/mob.2002.127378. [DOI] [PubMed] [Google Scholar]
- 4.Svedas E, Islam KB, Nisell H, Kublickiene KR. Vascular endothelial growth factor induced functional and morphologic signs of endothelial dysfunction in isolated arteries from normal pregnant women. Am J Obstet Gynecol. 2003;188:168–176. doi: 10.1067/mob.2003.110. [DOI] [PubMed] [Google Scholar]
- 5.Redman CWG, Sacks GP, Sargent IL. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 6.Friedman SA, de Groot CJM, Taylor RN, Golditch BD, Roberts JM. Plasma cellular fibronectin as a measure of endothelial involvement in preeclampsia and intrauterine growth retardation. Am J Obstet Gynecol. 1994;170:838–841. doi: 10.1016/s0002-9378(94)70295-0. [DOI] [PubMed] [Google Scholar]
- 7.Taylor RN, Crombleholme WR, Friedman SA, Jones LA, Casal DC, Roberts JM. High plasma cellular fibronectin levels correlate with biochemical and clinical features of preeclampsia but cannot be attributed to hypertension alone. Am J Obstet Gynecol. 1991;165:895–901. doi: 10.1016/0002-9378(91)90435-t. [DOI] [PubMed] [Google Scholar]
- 8.Krauss T, Kuhn W, Lakoma C, Augustin HG. Circulating endothelial cell adhesion molecules as diagnostic markers for the early identification of pregnant women at risk for development of preeclampsia. Am J Obstet Gynecol. 1997;177:443–449. doi: 10.1016/s0002-9378(97)70213-8. [DOI] [PubMed] [Google Scholar]
- 9.Lyall F, Greer IA, Boswell F, Macara LM, Walker JJ, Kingdom JCP. The cell adhesion molecule, VCAM-1, is selectively elevated in serum in pre-eclampsia: does this indicate the mechanism of leucocyte activation? Br J Obstet Gynaecol. 1994;101:485–487. doi: 10.1111/j.1471-0528.1994.tb13146.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Lewis DF, Groome L, Zhang Y, Gu Y, Philibert L. Enhanced P-selectin expression in the maternal vessel endothelium and elevated soluble P-selectin levels in the maternal plasma in women with preeclampsia. J Soc Gynecol Investig. 2005;12:276A. [Google Scholar]
- 11.Austgulen R, Lien E, Vince G, Redman CWG. Increased maternal plasma levels of soluble adhesion molecules (ICAM-1, VCAM-1, E-selectin) in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 1997;71:53–58. doi: 10.1016/s0301-2115(96)02647-4. [DOI] [PubMed] [Google Scholar]
- 12.ACOG Practice Bulletin: Diagnosis and management of preeclampsia and eclampsia. Number 33. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Walsh SW, Guo J, Zhang J. The imbalance between thromboxane and prostacyclin in preeclampsia is associated with an imbalance between lipid peroxides and vitamin E in maternal blood. Am J Obstet Gynecol. 1991;165:1695–1700. doi: 10.1016/0002-9378(91)90017-l. [DOI] [PubMed] [Google Scholar]
- 14.Goeschen K, Henkel E, Behrens O. Plasma prostacyclin and thromboxane concentrations in 160 normotensive, hypotensive, and preeclamptic patients during pregnancy, delivery, and the post partum period. J Perinat Med. 1993;21:481–489. doi: 10.1515/jpme.1993.21.6.481. [DOI] [PubMed] [Google Scholar]
- 15.Walsh SW. Preeclampsia: An imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol. 1985;152:335–340. doi: 10.1016/s0002-9378(85)80223-4. [DOI] [PubMed] [Google Scholar]
- 16.Walsh SW, Wang Y. Trophoblast and placental villous core production of lipid peroxides, thromboxane, and prostacyclin in preeclampsia. J Clin Endocrinol Metab. 1995;80:1888–1893. doi: 10.1210/jcem.80.6.7775637. [DOI] [PubMed] [Google Scholar]
- 17.Chavarria ME, Lara-Gonzalez L, Gonzalez-Gleason A, Garcia-Paleta Y, Vital-Reyes VS, Reyes A. Prostacyclin/thromboxane early changes in pregnancies that are complicated by preeclampsia. Am J Obstet Gynecol. 2003;188:986–992. doi: 10.1067/mob.2003.203. [DOI] [PubMed] [Google Scholar]
- 18.Lyall F, Hayman RG, Ashworth JR, Duffie E, Baker PN. Relationship of cell adhesion molecule expression to endothelium-dependent relaxation in normal pregnancy and pregnancies complicated with preeclampsia or fetal growth restriction. J Soc Gynecol Investig. 1999;6:196–201. doi: 10.1016/s1071-5576(99)00014-3. [DOI] [PubMed] [Google Scholar]
- 19.Marks L, Zamudio S, Cousins F, Duffie E, Lyall F. Endothelial activation and cell adhesion molecule concentrations in pregnant women living at high altitude. J Soc Gynecol Investig. 2006;13:399–403. doi: 10.1016/j.jsgi.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitsuhashi M, Tanaka A, Fujisawa C, Kawamoto K, Itakura A, Takaku M, Hironaka T, Sawada S, Matsuda H. Necessity of thromboxane A2 for initiation of plateletmediated contact sensitivity: dual activation of platelets and vascular endothelial cells. J Immunol. 2001;166:617–623. doi: 10.4049/jimmunol.166.1.617. [DOI] [PubMed] [Google Scholar]
- 21.Ishizuka T, Suzuki K, Kawakami M, Hidaka T, Matsuki Y, Nakamura H. Thromboxane A2 receptor blockade suppresses intercellular adhesion molecule-1 expression by stimulated vascular endothelial cells. Eur J Pharmacol. 1996;312:367–377. doi: 10.1016/0014-2999(96)00478-5. [DOI] [PubMed] [Google Scholar]
- 22.Osman I, Crawford M, Jordan F, Young A, Norman J, Thomson A. Expression and localization of cell adhesion molecules in human fetal membranes during parturition. J Reprod Immunol. 2004;63:11–21. doi: 10.1016/j.jri.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Winkler M, Kemp B, Fischer DC, Ruck P, Rath W. Expression of adhesion molecules in the lower uterine segment during term and preterm parturition. Microsc Res Tech. 2003;60:430–444. doi: 10.1002/jemt.10281. [DOI] [PubMed] [Google Scholar]
- 24.He P, Zhang H, Zhu L, Jiang Y, Zhou X. Leukocyte-platelet aggregate adhesion and vascular permeability in intact microvessels: role of activated endothelial cells. Am J Physiol Heart Circ Physiol. 2006;291:H591–H599. doi: 10.1152/ajpheart.01228.2005. [DOI] [PubMed] [Google Scholar]
- 25.Mesri M, Altieri DC. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J Biol Chem. 1999;274:23111–23118. doi: 10.1074/jbc.274.33.23111. [DOI] [PubMed] [Google Scholar]
- 26.Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23(5):359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 27.Ravn HB, Vissinger H, Kristensen SD, Husted SE. Magnesium inhibits platelet activity-an in vitro study. Thromb Haemost. 1996;76:88–93. [PubMed] [Google Scholar]
- 28.Sheu JR, Hsiao G, Shen MY, Fong TH, Chen YW, Lin CH, Chou DS. Mechanisms involved in the antiplatelet activity of magnesium in human platelets. Br J Haematol. 2002;119:1033–1041. doi: 10.1046/j.1365-2141.2002.03967.x. [DOI] [PubMed] [Google Scholar]