Abstract
Objective
Exaggerated inflammatory response occurs in preeclampsia. Preeclampsia is also associated with elevated endogenous digoxin-like factors (EDLFs). Clinical data suggest that Digibind (a polyclonal sheep digoxin binding Fab fragment) binds to EDLF and may have the potential to attenuate vasoconstriction and other clinical symptoms of preeclampsia. This study was undertaken to determine if Digibind could attenuate increased endothelial inflammatory response induced by tumor necrosis factor-α (TNFα).
Study Design
Confluent endothelial cells were treated with TNFα at different concentrations with or without Digibind in culture. Endothelial adhesion molecule ICAM, VCAM and E-selectin expressions were determined by an immunoassay directly detected on the endothelial surface. Effects of Digibind on TNFα-induced extracellular signal-regulated kinase and Na+/K+-ATPase expressions were also examined.
Result
(1) TNFα induced dose-dependent increases in ICAM, VCAM and E-selectin expressions in endothelial cells; (2) Digibind could attenuate and reduce TNFα-induced upregulation of endothelial E-selectin, ICAM and VCAM expressions. The blocking effect was in a concentration dependent manner; (3) Digibind had no effects on TNFα-induced upregulation of extracellular signal-regulated kinase phosphorylation, but could block TNFα-induced downregulation of Na+/K+-ATPase β1 expression.
Conclusion
Digibind may exert beneficial effects by preserving cell membrane Na+/K+-ATPase function and consequently to offset increased inflammatory response in endothelial cells.
Keywords: Digibind, endothelial cells, Na+/K+-ATPase, preeclampsia, inflammatory response
Introduction
It was reported that circulating levels of endogenous digoxin-like factor(s) such as ouabain, bufadienolide, marinobufagenin, and cardenolide were elevated in women with preeclampsia,1–3 a hypertensive and multiple system disorder unique to human pregnancy. Studies have shown that digoxin-like factors extracted from plasma from women with preeclampsia could inhibit erythrocyte Na+/K+-ATPase activity3 and Na+/K+-ATPase purified from human mesenteric arteries.2 Inhibition of the sodium pump could result in an increase in intracellular calcium levels and lead to vasoconstriction in the systemic vasculature. Therefore, the vasoconstrictive property of digoxin-like factors is believed to contribute to maternal hypertension in preeclampsia.
Endothelial activation/dysfunction is a central pathophysiological feature in the maternal vascular system in preeclampsia.4 Pregnancy is also an inflammatory state and preeclampsia is considered to be an exaggerated inflammatory response during pregnancy.5 It is believed that altered endothelial function constitutes the exaggerated inflammatory response in this pregnancy disorder,5 which includes activation of leukocytes and platelets, increased circulating cytokine levels of tumor necrosis factor-α (TNFα), interferon-γ and interleukin-66,7 and increased endothelial adhesion molecule levels such as intercellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM).8,9
Digibind is a polyclonal-fragmented Digoxin-immune Fab antibody raised in sheep. Previous published work has shown that in vitro treatment of erythrocytes from preeclamptic patients with Digibind could restore the cell Na+/K+-ATPase activity.3 Furthermore, administration of Digibind to both antepartum and postpartum women with preeclampsia could improve maternal symptoms and increase fetoplacental perfusion10,11 (Dr Adair’s unpublished data), which suggest that Digibind could be a potential therapy for preeclampsia. To study if Digibind exerts beneficial effects on endothelial cells, we examined the role of Digibind in TNFα-induced inflammatory response in endothelial cells. Endothelial surface adhesion molecule expressions ICAM, VCAM and E-selectin were used as the endpoint readout. Effects of Digibind on endothelial extracellular signal-regulated kinases (ERKs) and Na+/K+-ATPase expressions affected by cytokine TNFα were also examined.
Methods
Endothelial isolation and culture
Human umbilical vein endothelial cells were isolated by collagenase digestion as previously described.12 Umbilical cords were collected from normal pregnant women after delivery at Louisiana State University Health Sciences Center in Shreveport hospital. Normal pregnancy was defined as a pregnancy in which the mother had normal blood pressure (≤140/90 mm Hg), absence of medical and obstetrical complications. This study was approved by the Institutional Review Board for Human Research at LSUHSC-Sh, LA.
Isolated cells were incubated with endothelial cell growth medium (BioWhittaker Inc., Walkersville, MD, USA). Only the first-passage (P1) endothelial cells were used in this study. Cells used for adhesion molecule expression experiments were grown in 48 wells per plate and cells used for protein extraction were grown in 25 cm2 culture flasks. Confluent endothelial cells were treated with TNFα (Sigma, St Louis, MO, USA) or combined with Digibind (GlaxoSmithKline, Research Triangle Park, NC, USA).
Endothelial surface molecule expression assay
Cellular surface molecule expressions for ICAM, VCAM and E-selectin were determined as we previously described.12 Briefly, after endothelial cells were treated with TNFα or combined with Digibind in culture, cells were fixed with 1% paraformaldehyde and then incubated with a primary antibody (mouse anti-human) to ICAM-1 (CD54), VCAM-1 (CD106) or E-selectin (CD62E), respectively. Horseradish peroxidase-goat anti-mouse immunoglobulin G (Sigma) was used as the secondary antibody. Hydrogen peroxide (0.003%) and 3,3′,5,5′-tetramethybenzidine (TMB) (0.1 mg ml−1) were used as substrate and color generation. The reaction was terminated by 8 n H2SO4. Cells that reacted with secondary antibody only were used as background. After reaction, plates were read at 450 nm by an autoplate reader (Molecular Devices, Sunnyvale, CA, USA). All samples were tested in triplicate.
Western blot analysis
At the end of each experiment, total cellular protein was extracted with an ice-cold lysis buffer that contained 50 mmol l−1 Tris-HCl (pH7.6), 1% Triton X-100, 0.5% NP-40, 1 mmol l−1 phenylmethylsufonyl fluoride and 0.5%mmol l−1 dithiotheritol. The lysate was centrifuged at 14 000 r.p.m. at 4 °C for 15 min to remove insoluble materials. All samples were stored at −70 °C. The total endothelial cell protein extract (10 µg per sample) was subjected to electrophoresis on 12% polyacrylamide gels by using the Mini-protein 3 gel running system (Bio-Rad, Hercules, CA, USA) and then transferred to nitrocellulose membrane. The membranes were probed with a primary monoclonal antibody against ERK (Santa Cruz, San Diego, CA, USA), pERK (Santa Cruz), Na+/K+-ATPase β1 (Santa Cruz) or β-actin (Sigma). The secondary antibody was horseradish-linked anti-mouse antibody. The bound antibodies were visualized with an enhanced chemiluminescent deletion Kit (Amersham Corp., Arlington Heights, IL, USA). Nitrocellulose membranes were stripped and blocked before they were probed again with different primary antibodies.
Statistical analysis
Data are presented as mean ± s.e. Statistical analysis was performed with analysis of variance by a computer software program StatView (SAS Institute Inc., Cary, NC, USA). A probability level less than 0.05 was considered statistically significant.
Results
Digibind attenuates TNFα-induced endothelial surface adhesion molecule expressions
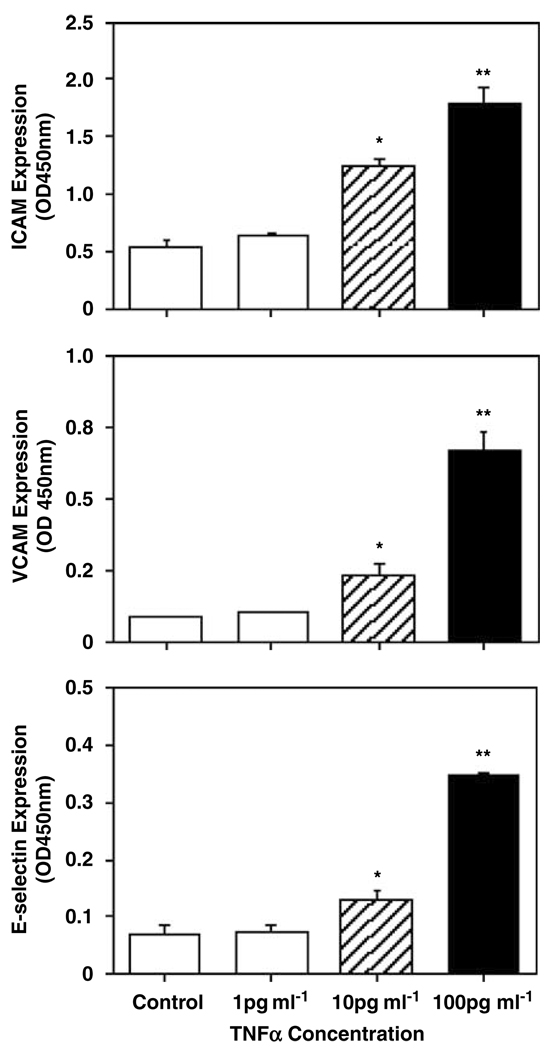
Endothelial inflammatory response was induced by cytokine TNFα. Confluent endothelial cells were treated with TNFα at concentrations of 1, 10 and 100 pg ml−1 for 2 h, then endothelial adhesion molecule ICAM, VCAM and E-selectin expressions were determined. TNFα at a concentration of 100 pg ml−1 was relative compatible with the TNFα levels in the maternal plasma in women with preeclampsia.13 Figure 1 shows dose-dependent increase in endothelial ICAM, VCAM and E-selectin expressions induced by TNFα.
Figure 1.
Tumor necrosis factor-α (TNFα) dose-dependently increased in endothelial ICAM, VCAM and E-selectin expressions. Data are means from six independent experiments, each in triplicate. *P<0.05, **P<0.01, respectively.
TNFα at lower dose (1 pg ml−1) had no effects on endothelial ICAM, VCAM and E-selectin expressions compared to the controls, ICAM: 0.645 ± 0.028 vs 0.564 ± 0.036; VCAM: 0.101 ± 0.005 vs 0.090 ± 0.002; and E-selectin: 0.073 ± 0.011 vs 0.070 ± 0.014, respectively. Endothelial ICAM, VCAM and E-selectin expressions were significantly increased when TNFα concentrations were used at 10 pg ml−1 (P<0.05) and 100 pg ml−1 (P<0.01)—ICAM: 1.261 ± 0.067 and 1.789 ± 0.143; VCAM: 0.236 ± 0.032 and 0.663 ± 0.072; and E-selectin: 0.128 ± 0.020 and 0.345 ± 0.007, respectively.
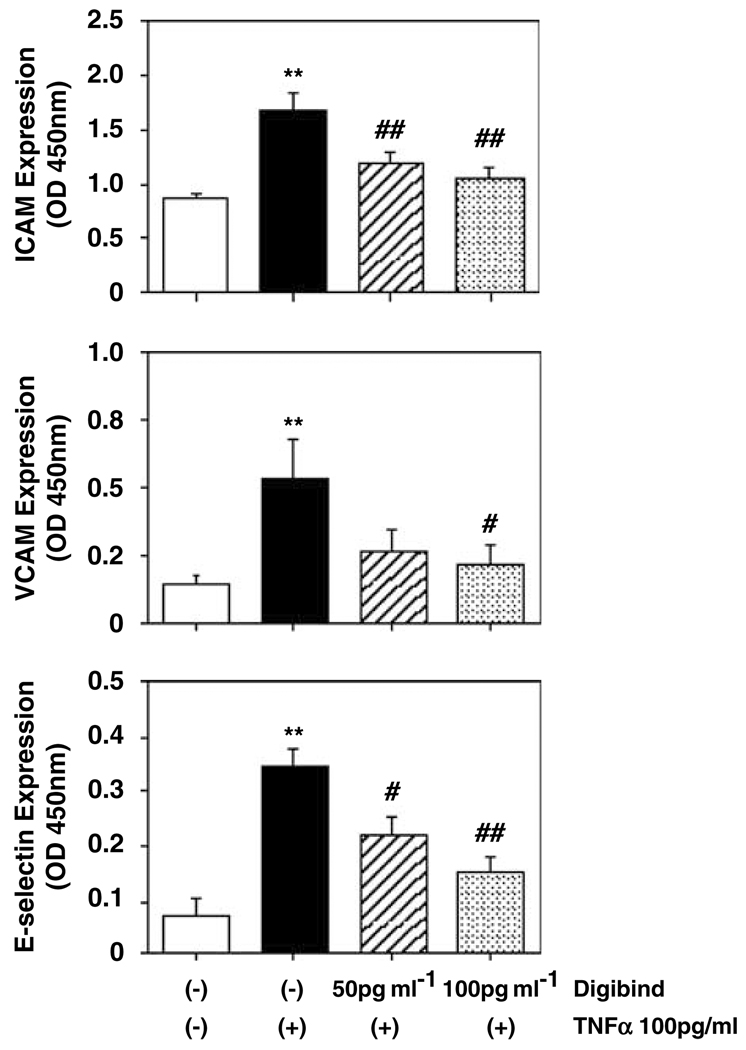
To determine if Digibind could attenuate TNFα-induced ICAM, VCAM and E-selectin expressions in endothelial cells, cells were pretreated with Digibind for 1 h and then TNFα at a concentration of 100 pg ml−1 for 2 h. Two concentrations of Digibind (50 and 100 µg ml−1) were used. Figure 2 shows that Digibind could dose-dependently attenuate TNFα-induced upregulation of endothelial ICAM, VCAM and E-selectin expressions, Digibind at 50 pg ml−1 + TNFα and at 100 pg ml−1 + TNFα vs TNFα alone—ICAM: 1.214 ± 0.103 and 1.074 ± 0.086 vs 1.704 ± 0.147, P<0.01; VCAM: 0.271 ± 0.080 and 0.226 ± 0.069 vs 0.531 ± 0.147, P<0.05; E-selectin: 0.220 ± 0.034, P<0.05 and 0.150 ± 0.028, P<0.01 vs 0.342 ± 0.034, respectively.
Figure 2.
Digibind-attenuated tumor necrosis factor-α (TNFα)-induced ICAM, VCAM and E-selectin expressions in endothelial cells (ECs). ECs were pretreated with Digibind (50 and 100 µg ml−1) for 1 h and then TNFα at a concentration of 100 pg ml−1 for 2 h. Digibind could dose-dependently attenuate TNFα-induced upregulation of ICAM, VCAM and E-selectin expression. Data are means from six independent experiments, each in triplicate. **P<0.01: TNFα-treated only vs control; #P<0.05 and ##P<0.01: cells treated with Digibind + TNFα vs TNFα-treated only, respectively.
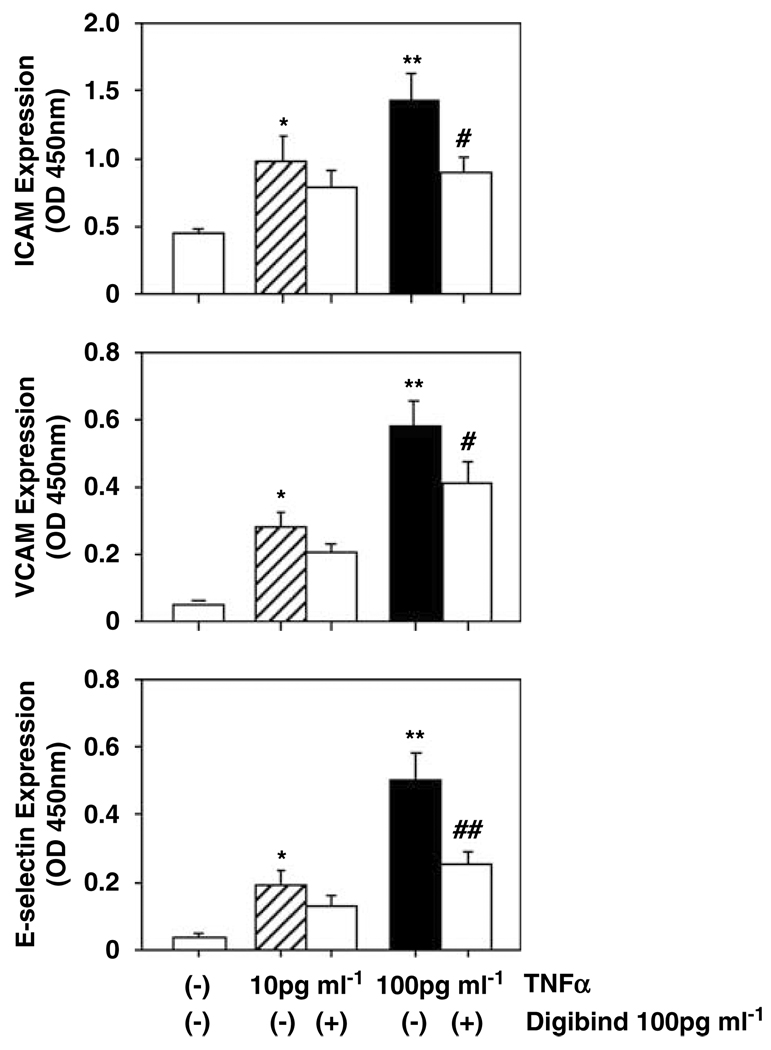
Digibind reduces TNFα-induced endothelial surface adhesion molecule expressions
To further determine if Digibind could reduce cytokine TNFα induced endothelial activation, ECs were first treated with TNFα for 1 h and then Digibind was added to the cell culture. The cells were continuously cultured for 1 h and ICAM and VCAM and E-selectin expressions were determined. In this experiment, two concentrations of TNFα (10 and 100 pg ml−1) were used. Interestingly, endothelial ICAM, VCAM and E-selectin expressions were downregulated in cells even when Digibind was added to the cell culture after TNFα treatment (Figure 3), addition of Digibind 100 pg ml−1 vs TNFα at 100 pg ml−1 alone—ICAM: 0.896 ± 0.12 vs 1.435 ± 0.192, P<0.05; VCAM: 0.413 ± 0.062 vs 0.584 ± 0.071, P<0.05; and E-selectin: 0.256 ± 0.034 vs 0.498 ± 0.086, P<0.01, respectively. These data suggest that Digibind exerts protective effects on endothelial cells against cytokine TNFα induced endothelial activation.
Figure 3.
Digibind reduced tumor necrosis factor-α (TNFα)-induced ICAM, VCAM and E-selectin expressions in ECs. In this experiment, Digibind was added to the cell culture 1 h after TNFα treatment. Data are means from six independent experiments, each in triplicate. *P<0.05 and **P<0.01: TNFα-treated vs control cells; #P<0.05 and ##P<0.01: cells treated with TNFα + Digibind vs TNFα-treated only, respectively.
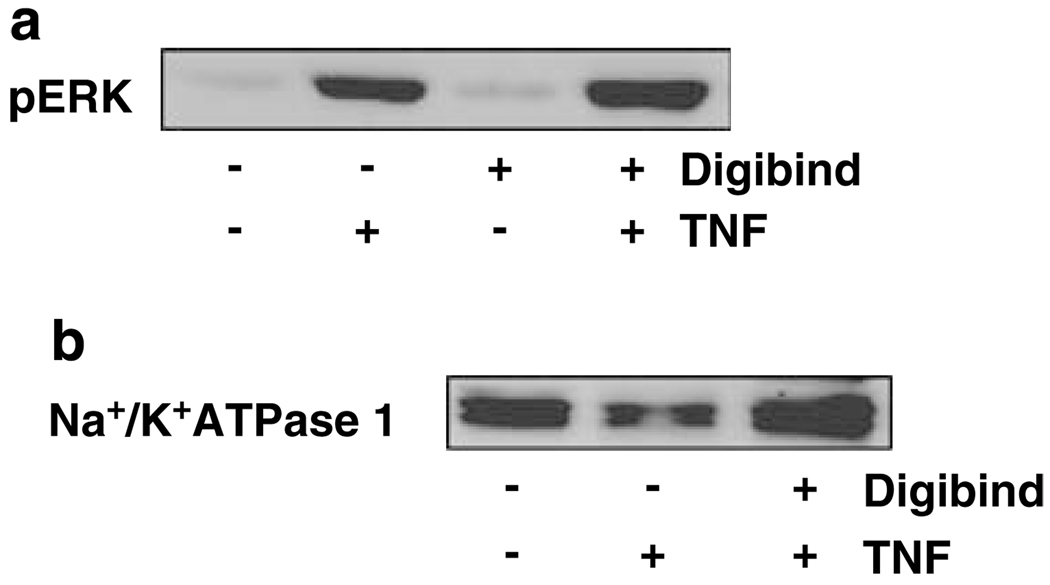
Digibind attenuates TNFα-induced downregulation of Na+/K+-ATPase β1 expression, but has no effect on TNFα-induced ERK phosphorylation in endothelial cells
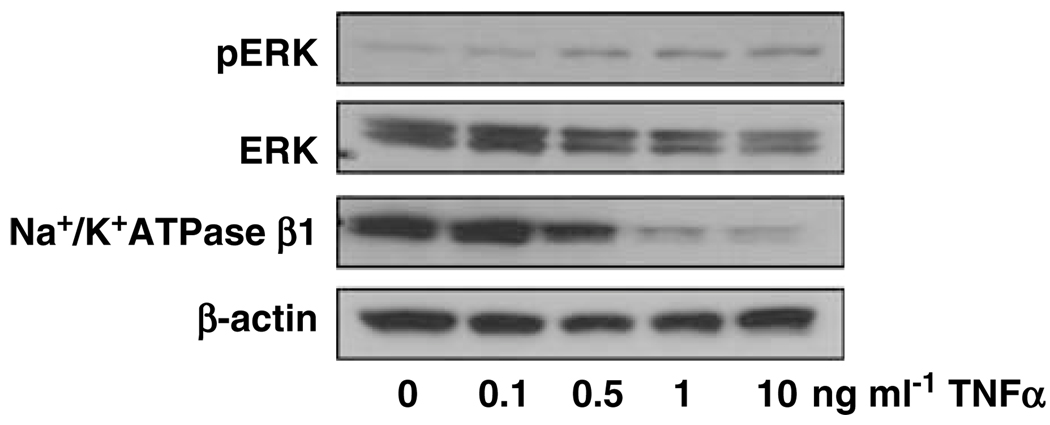
It is known that cytokine induced inflammatory response is involved in extracellular signal-regulated kinases (ERKs) activation. Using ERK expression as a comparison, we examined if increased endothelial adhesion molecule expression induced by TNFα is associated with alteration of Na+/K+-ATPase expression in endothelial cells. Endothelial cells were treated with TNFα at different concentrations and then ERK, pERK and Na+/K+-ATPase expression were examined by western blot analysis.
As shown in Figure 4, TNFα induced upregulation of ERK phosphorylation (pERK) was in a dose-dependent manner. In contrast, Na+/K+-ATPase β1 expression was downregulated in endothelial cells treated with TNFα. The TNFα induced inhibitory effect on Na+/K+-ATPase β1 expression was also in a dose-dependent manner. We further determined if Digibind-exerted protective effects against TNFα-induced downregulation of Na+/K+-ATPase β1 expression in endothelial cells. As shown in Figure 5a, pretreatment of Digibind did not affect pERK expression in endothelial cells stimulated with TNFα. In contrast, pretreatment of Digibind could attenuate TNFα-induced down-regulation of Na+/K+-ATPase β1 expression (Figure 5b).
Figure 4.
Effects of tumor necrosis factor-α (TNFα) on extracellular signal-regulated kinase (ERK), pERK and Na+/K+-ATPase β1 expressions in ECs. β-Actin expression was used as control. TNFα dose-dependently induced pERK upregulation and Na+/K+-ATPase β1 downregulation in ECs. The blots are representative from three independent experiments.
Figure 5.
Effects of Digibind on pERK and Na+/K+-ATPase β1 expression in ECs. Digibind had no effect on tumor necrosis factor-α (TNFα)-induced upregulation of pERK (a), but could block TNFα-induced downregulation of Na+/K+-ATPase β1 (b) in ECs. The blots are representative from three independent experiments.
Discussion
In this study, using TNFα as an inflammatory stimulator we examined potential beneficial effects of Digibind on vascular endothelial cells. We found that TNFα could not only induce an endothelial inflammatory response as evidenced by upregulation of endothelial surface adhesion molecule expression and induction of ERK phosphorylation, but also downregulate Na+/K+-ATPase expression in endothelial cells. Na+/K+-ATPase is important cell membrane ionic machinery, which keeps membrane potential in a proper order and maintains intracellular and extracellular ionic balance. TNFα induced upregulation of endothelial adhesion molecule ICAM, VCAM and E-selectin expressions, accompanied by downregulation of Na+/K+-ATPase expression, suggest that enhanced endothelial inflammatory response may associate with altered Na+/K+-ATPase activity or function in endothelial cells.
One of the most important findings of our study is that Digibind may have protective effects to offset endothelial inflammatory response as demonstrated not only by attenuating but also by reducing TNFα-induced increased endothelial inflammatory responses determined by E-selectin, ICAM and VCAM expressions on the endothelial surface. Both in vivo and in vitro studies have shown that enhanced adhesion molecule expression in endothelial cells is directly related to activation of leukocytes, increased leukocyte–endothelial adhesion and leukocyte extravasation.14,15 In preeclampsia, maternal TNFα levels, as well as soluble levels of E-selectin, ICAM and VCAM are elevated compared to normal pregnant controls.9,16 As maternal circulating levels of endogenous digoxin-like factor(s) are also increased in women with preeclampsia,1,2 Digibind may not only be able to neutralize endogenous digoxin-like factor(s) in the circulation but also exert protective effects on vasculature by reducing endothelial inflammatory response in preeclampsia.
In this study, we also found that TNFα down-regulated endothelial Na+/K+-ATPase β1 expression, suggesting that increased inflammatory response is accompanied by altered sodium pump activity or function on the cell membrane. Regarding the fact of reduced Na+/K+-ATPase activity in erythrocytes from women with preeclampsia,1,2 our finding of downregulation of Na+/K+-ATPase β1 expression induced by TNFα indicated that increased endothelial inflammatory response may directly or indirectly influence endothelial Na+/K+-ATPase activity or function. This concept is supported by our data that TNFα-induced downregulation of endothelial Na+/K+-ATPase β1 expression could be blocked by pretreatment of endothelial cells with Digibind in culture.
At the present time, we do not know the direct relationship of upregulation of endothelial adhesion molecule expression to downregulation of Na+/K+-ATPase β1 expression stimulated by TNFα in endothelial cells. Our data showed that upregulation of E-selectin, ICAM and VCAM expression was related to upregulation of phosphorylated EKR expression in endothelial cells after TNFα stimulation, which indicates that TNFα-induced endothelial inflammatory response is an transcriptional factor regulated event, at least in part, through the ERK pathway regulation. However, Digibind could attenuate or reduce TNFα-induced endothelial adhesion molecule expression, but had no effects on TNFα-induced pERK upregulation. These observations suggest that attenuation of TNFα-induced endothelial adhesion molecule expression by Digibind is not mediated through the ERK pathway regulation.
This study further supports the potential function of Digibind for a possible clinical application for preeclampsia patients. The recently reported Digibind Efficacy Evaluation in Preeclampsia (DEEP) study, Gov trial no. NCT00158743, showed improved renal hemodynamic effects of Digibind on preterm severe preeclampsia, which correlated with improved erythrocyte sodium potassium ATPase pump function.17,18 As pump inhibition maintains several key cellular membrane functions, Digibind potentially may exert its observed clinical benefit by reversal of sodium pump inhibition followed by improving cell membrane function.18 These observed effects would not have limitation to preeclampsia only but could hypothetically be extrapolated to other diseases related to the increased inflammatory response and would imply a potential platform technology for Digibind.
Na+/K+-ATPase is a highly conserved ubiquitous membrane protein, which is composed of three subunits: α, β and γ. We found that TNFα had no effect on Na+/K+-ATPase α-subunit expression (data not shown), but downregulation of β-subunit was observed. Interestingly, Digibind could block the downregulation of β-subunit expression induced by TNFα in endothelial cells. Although the exact mechanism of Digibind preservation of the β-subunit is not clear, studies have shown that β-subunit may be more intimately involved in the mechanism of active transport function of Na+/K+-ATPase,19 as the cation affinity of the Na+/K+-ATPase can be affected by changes in the β-subunit,19,20 which indirectly supports our data that Digibind could preserve the β-subunit function in vascular endothelium.
In summary, in this study we found that Digibind could attenuate cytokine TNFα-induced increased endothelial surface adhesion molecule expression and decreased Na/K-ATPase β1 expression in cultured endothelial cells. As it is impossible to obtain maternal systemic vessels during pregnancy, it limits us to directly study the endothelial response to Digibind in an in vivo situation such as in preeclampsia. However, the DEEP study result that Digibind could reverse erythrocyte Na/K-ATPase pump function18 supports the idea that Digibind may exert protective effects on vascular endothelial function by restoring Na/K-ATPase pump function and increase the pump activity. Although the Digibind action on endothelial function is largely unknown, our data do suggest that Digibind may exert antiinflammatory effects on vascular endothelial cells, the mechanism of which warrants further investigation.
References
- 1.Graves SW, Williams GH. An endogenous ouabain-like factor associated with hypertensive pregnant women. J Clin Endocrinol Metabol. 1984;59:1070–1074. doi: 10.1210/jcem-59-6-1070. [DOI] [PubMed] [Google Scholar]
- 2.Lopatin DA, Ailamazian EK, Dmitrieva RI, Shpen VM, Fedorova OV, Doris PA, et al. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertens. 1999;17:1179–1187. doi: 10.1097/00004872-199917080-00018. [DOI] [PubMed] [Google Scholar]
- 3.Averina IV, Tapilskaya NI, Reznik VA, Frolova EV, Fedorova OV, Lakatta EG, et al. Endogenous Na/K-ATPase inhibitors in patients with preeclampsia. Cell Mol Biol (Noisy-le-grand) 2006;52:19–23. [PubMed] [Google Scholar]
- 4.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 5.Redman CWG, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 6.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-a is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 1994;170:1752–1759. [PubMed] [Google Scholar]
- 7.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CWG. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Bri J Obstet Gynecol. 1995;102:20–25. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 8.Lyall F, Greer IA, Boswell F, Macara LM, Walker JJ, Kingdom JCP. The cell adhesion molecule, VCAM-1, is selectively elevated in serum in pre-eclampsia: does this indicate the mechanism of leucocyte activation? Br J Obstet Gynaecol. 1994;101:485–487. doi: 10.1111/j.1471-0528.1994.tb13146.x. [DOI] [PubMed] [Google Scholar]
- 9.Chaiworapongsa T, Romero R, Yoshimatsu J, Espinoza J, Kim YM, Park K, et al. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J Matern Fetal Neonatal Med. 2002;12:19–27. doi: 10.1080/jmf.12.1.19.27. [DOI] [PubMed] [Google Scholar]
- 10.Adair CD, Buckalew V, Taylor K, Ernest JM, Frye AH, Evans C, et al. Elevated endoxin-like factor complicating a multifetal second trimester pregnancy: treatment with digoxin-binding immunoglobulin. Am J Nephrol. 1996;16:529–531. doi: 10.1159/000169054. [DOI] [PubMed] [Google Scholar]
- 11.Adair CD, Hinshaw H, Russell G, Rose J, Veille J, Buckalew V. Effects of digoxin specific-antibody on mean arterial pressure in severe preeclampsia. Presented at the 12th Scientific Meeting of the American Society of Hypertension; May 29, 1997; San Francisco, CA. [Google Scholar]
- 12.Wang Y, Adair CD, Coe L, Weeks JW, Lewis DF, Alexander JS. Activation of endothelial cells in preeclampsia: Increased neutrophil-endothelial adhesion correlates with up-regulation of adhesion molecule P-selectin in human umbilical vein endothelial cells isolated from preeclampsia. J Soc Gynecol Investig. 1998;5:237–243. doi: 10.1016/s1071-5576(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 13.Canzoneri BJ, Lewis DF, Zhang Y, Gu Y, Philibert L, Groome LJ, et al. Maternal circulating TNFa levels are highly correlated with IL-10 levels, but not IL-6 and IL-8 levels, in women with preeclampsia. Am J Obstet Gynecol. 2006;193(6) [Google Scholar]
- 14.Ohno N, Ichikawa H, Coe L, Kvietys PR, Granger DN, Alexander JS. Soluble selectins and ICAM-1 modulate neutrophil-endothelial adhesion and diapedesis in vitro. Inflammation. 1997;21:313–324. doi: 10.1023/a:1027349900279. [DOI] [PubMed] [Google Scholar]
- 15.Silber A, Newman W, Reimann KA, Hendricks E, Walsh D, Ringler DJ. Kinetic expression of endothelial adhesion molecules and relationship to leukocyte recruitment in two cutaneous models of inflammation. Lab Invest. 1994;70:163–175. [PubMed] [Google Scholar]
- 16.Kupferminc MJ, Peaceman AM, Aderka D, Wallach D, Socol ML. Soluble tumor necrosis factor receptors and interleukin-6 levels in patients with severe preeclampsia. Obstet Gynecol. 1996;88:420–427. doi: 10.1016/0029-7844(96)00179-2. [DOI] [PubMed] [Google Scholar]
- 17.Lam G, Johnson D, Robinson C, Saade G, Lewis DF, Porter K, et al. Antepartum administration of a Digoxin immune fab (Digibind) improves renal function in patients with severe preeclampsia. Presented at the XVI World Congress of International Society for the Study of Hypertension in Pregnancy; Sept. 20–24, 2008; Washington, DC, USA. 2008. pp. 1–2. [Google Scholar]
- 18.Hopate M, Graves S, Adair CD, Lam G, Johnson D, Saade G, et al. In-vivo reversal of functional sodium pump inhibition with Digibind Treatment. Presented at the XVI World Congress of International Society for the Study of Hypertension in Pregnancy; Sept. 20–24, 2008; Washington, DC, USA. 2008. [Google Scholar]
- 19.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 20.Eakle KA, Kabalin MA, Wang SG, Farley RA. The influence of beta subunit structure on the stability of Na+/K(+)-ATPase complexes and interaction with K+ J Biol Chem. 1994;269:6550–6557. [PubMed] [Google Scholar]