Abstract
Interferon-γ (IFN-γ) production by natural killer (NK) cells and cytotoxic lymphocytes is a key component of innate and adaptive immune responses. Because inhibitor of κB-ζ (IκBζ), a Toll-like receptor (TLR)/interleukin-1 receptor (IL-1R) inducible transcription factor, regulates IFN-γ production in KG-1 cells, we tested IκBζ's role in the classic lymphocyte pathway of IL-12/IL-18–induced IFN-γ. Upon stimulation with IL-12/IL-18, monocyte-depleted human peripheral blood lymphocytes expressed the 79-kDa form of IκBζ and released IFN-γ. CD56+ NK cells were shown to be the IκBζ-producing lymphocyte subpopulation, which also released abundant IFN-γ in response to IL-12/IL-18. Importantly, IκBζ was undetectable in CD56− lymphocytes where IFN-γ release was 10-fold lower. In addition, small interfering RNA knockdown of IκBζ suppressed IFN-γ expression in CD56+ cells. The association of IκBζ with the IFN-γ promoter was documented by chromatin immunoprecipitation. IFN-γ promoter activity from IκBζ overexpression was confirmed by luciferase reporter assay. Finally, IκBζ coprecipitated with p65 and p50 NF-κB in NK cells in response to IL-12/IL-18, suggesting that IκBζ's effects on IFN-γ promoter activity are coregulated by NF-κB. These results suggest that IκBζ functions as an important regulator of IFN-γ in human NK cells, further expanding the class of IκBζ-modulated genes.
Introduction
Interferon-γ (IFN-γ) is a classic TH1 cytokine whose immunomodulatory functions are critical for innate and adaptive immune responses. Its functions include: up-regulation of antigen presentation1–3; classic activation of the macrophage4–6; development of cellular immunity against viral and bacterial infections by promoting the differentiation of naive CD4 T cells into Th1 effectors3; enhancement of lymphocyte recruitment and their prolonged activation in the tissues7,8; and regulation of cell functions, such as B cell-mediated immunoglobulin production and class switching and natural killer (NK) cell activity.4,9,10 In humans, unregulated IFN-γ production and signaling are associated with increased susceptibility to mycobacterial and Salmonella infections, autoimmune and autoinflammatory diseases such as inflammatory bowel disease, multiple sclerosis, and diabetes mellitus.3 IFN-γ has also been implicated in preventing tumorigenesis.11,12 Thus, understanding the mechanisms that regulate IFN-γ production is of crucial importance for therapeutic development in various disease states.
Inhibitor of κB-ζ (IκBζ), or molecule possessing ankyrin repeats induced by lipopolysaccharide (MAIL), is a primary response gene that is strongly induced on Toll-like receptor (TLR)/interleukin-1 receptor (IL-1R) activation of monocytes and macrophages.13–16 It is a homolog of the IκB family of proteins and harbors multiple ankyrin repeat sequences at its carboxy-terminus that are responsible for binding to nuclear factor-κB (NF-κB).17 The amino-terminal end encodes a nuclear localization sequence and a transcriptional activation domain.14 Along with lipopolysaccharide and IL-1β, other TLR ligands such as peptidoglycan, bacterial and mycoplasmal lipopeptides, flagellin, CpG oligonucleotides, ligands for TLR 2, 5, 7, and 9, and cytokines such as IL-18 and IL-17 also induce the expression of IκBζ.18–20 The role of IκBζ as a transcriptional regulator of secondary response genes induced on TLR activation is well established. IκBζ mediates its transcriptional regulation by specifically interacting with NF-κB and thereby regulating NF-κB-mediated transcription of secondary response genes.14,21 IκBζ has been shown to regulate the expression of proinflammatory cytokines, such as IL-6, IL-12 (p40), IL-18, granulocyte-macrophage colony-stimulating factor, and granulocyte colony-stimulating factor as well as the antimicrobial peptides, neutrophil gelatinase-associated lipocalin, and human β-defensin 2.18,19,22,23
Recent data from our group suggest that synergy exists between the proinflammatory cytokines of the IL-1 family (IL-1β and IL-18) and tumor necrosis factor-α (TNF-α) for IFN-γ production in the human acute myeloid leukemic KG-1 cell line.24 Although the IL-1 and TNF-α receptors belong to different families, their signaling pathways use similar molecules leading to the activation of NF-κB and mitogen-activated protein kinases. IκBζ was identified as the common gene product downstream of these receptors that is crucial for this synergy.20
Of human lymphocytes, NK cells and T cells are the predominant producers of IFN-γ on stimulation with IL-12 and IL-18.3,25,26 Although IκBζ is involved in IFN-γ production in the KG-1 cell line, its function in the regulation of lymphocyte IFN-γ has not been previously evaluated. Here we dissect the role of IκBζ as a regulator of IFN-γ in response to IL-12 and IL-18 in human lymphocytes. We identify NK cells as the population of lymphocytes that express IκBζ in response to IL-12 and IL-18 and provide evidence to support the role of IκBζ as a transcriptional regulator of IFN-γ in these cells.
Methods
Reagents and antibodies
CD14 and CD56 beads were purchased from Miltenyi Biotec. The following reagents were obtained from the following sources: RPMI 1640 (Cellgro), fetal bovine serum (Atlanta Biologicals), penicillin-streptomycin (Invitrogen), and Dulbecco modified Eagle medium high glucose (Invitrogen). Recombinant human IL-12 and IL-2 were purchased from R&D Systems. Recombinant human IL-18 was purchased from MBL International. The following antibodies were obtained from the indicated sources: IκBα (Millipore), actin (monoclonal clone C4; MP Biomedicals), and p50 and p65 NF-κB (Santa Cruz Biotechnology). Rabbit antiserum against IκBζ was generated in our laboratory using recombinant protein expressed in Escherichia coli as previously described.16
Plasmids
Of the different isoforms of IκBζ, the long isoform IκBζ-L, was cloned in pCDNA 3.1 Myc/His-B (Invitrogen) as previously described.16 The 3.6-kb Ifng promoter construct was a kind gift from Dr Howard A. Young (National Cancer Institute, National Institutes of Health, Frederick, MD) and was reported previously.27
Cell culture
Human peripheral blood mononuclear cells (PBMCs) were isolated by Histopaque density gradient centrifugation from fresh source leukocytes from the American Red Cross. Lymphocytes were isolated after CD14− selection. NK cells were isolated from PBMCs by CD56+ (Miltenyi Biotec) magnetic selection as directed by the manufacturer. Lymphocytes (5 × 106/mL) and NK cells (2 × 106/mL) were plated in RPMI 1640 supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Lymphocytes and NK cells were stimulated with IL-12 (10 ng/mL), IL-18 (10 ng/mL), and IL-2 (100 U/mL) for the times indicated. HEK 293 cells stably transfected with TLR4/IL-1R/MD-2 were grown in Dulbecco modified Eagle medium (high glucose) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin.
Quantitative PCR
Lymphocytes and NK cells were stimulated for the times indicated, and total RNA was extracted using Trizol (Invitrogen) following the manufacturer's protocol. Total RNA (0.25-1 μg) was converted to cDNA using Thermoscript reverse transcriptase (Invitrogen). This cDNA was used as a template for the quantitative polymerase chain reaction (PCR) using the SYBR Green method.28 Primers specific for IκBζ, IFN-γ, IL-8, and IκBα were used for analyzing mRNA expression. Gene expression was normalized to 2 housekeeping genes: GAPDH and CAP-1. Primer sequences are available on request. Values are expressed as relative copy numbers.
Preparation of cell lysates and immunoblotting
Cells were lysed using lysis buffer (50mM Tris-HCl, pH 8.0, 125mM NaCl, 10mM ethylenediaminetetraacetic acid, 10mM NaF, 10mM sodium pyrophosphate, 1% Triton X-100) in the presence of a protease inhibitor cocktail mixture (Sigma-Aldrich), 10mM methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone, 1mM phenylmethylsulfonyl fluoride (Sigma-Aldrich), and 3mM sodium orthovanadate. Cell extracts were incubated on ice for 15 minutes and then centrifuged at 16 000g for 10 minutes. Total protein was estimated using Lowry assay (Bio-Rad), and an equal protein (10-20 μg) was loaded per lane of NuPAGE 7% Tris-acetate gel (Invitrogen). The separated proteins were transferred onto polyvinylidene difluoride membranes, which were blocked with 10% nonfat milk. Blocked membranes were blotted overnight at 4°C with appropriate primary antibody followed by secondary antibody and visualization by enhanced chemiluminescence (GE Healthcare).
Nuclear cytosol isolation
The nuclear cytosol isolation for cellular localization assay was performed as described in the Supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The nuclear cytosol isolation for the immunoprecipitation was performed using the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Electron) according to the manufacturer's protocol.
siRNA
NK cells (3 × 106) were nucleofected with 30nM control or IκBζ small interfering RNA (siRNA) (sequence UGAUGGACCUGCUUGCAAA; Dharmacon RNA Technologies, Thermo Electron) using a nucleofector kit (Lonza Switzerland). After nucleofection, the cells were resuspended in RPMI 1640 supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. The nucleofected cells were plated in 12-well plates at a density of 1.5 × 106/mL. After 18 hours, the cells were stimulated with IL-12 (10 ng/mL) and IL-18 (10 ng/mL) for an additional 6 hours and 24 hours. Cell supernatants and lysates were harvested and used for immunoblotting, quantitative PCR, and enzyme-linked immunosorbent assay (ELISA).
ChIP
Chromatin immunoprecipitation (ChIP) was performed as described in the Supplemental data. The following primers spanning the different NF-κB sites on the IFN-γ promoter were designed: 5′-CCCCTGGGAATATTCTCTACACTGTATTTCAAGG-3′ and 5′-GCCCAGTAGAAATACAATACAAGCCACACAGG-3′ for the κB and C3-1P sites in the −786 to −763-bp region, 5′-AGAGTCAACATTTTACCAGGGCGAAGTGGG-3′ and 5′-GTTTCCTTTAGACTCCTTGGGTCCTTTGACG-3′ for the C3-3P site in the −278 to −268-bp region, 5′-GCATCTCTGATAGGCTGTCATCTCTTGTAGGC-3′ and 5′-CCTTGACTATCACAACTGAATGAGTTCCCACC-3′ for the C3 1st intron site in the 459- to 470-bp region.
Luciferase reporter assay
HEK293 TLR4/IL-1R/MD-2 cells (2.5 × 106) were resuspended in 500 μL Dulbecco modified Eagle medium (high glucose) media. Ifng promoter (with the κB, C3-1P, C3-3P sites; see Figure 5B) in a pGL3 vector (Promega) and IκBζ-L in a pCDNA3.1 vector (Invitrogen) or the pCDNA3.1 empty vector were cotransfected into HEK293 TLR4/IL-1R/MD-2 cells by Lipofectamine 2000 with Plus Reagent (Invitrogen) according to the manufacturer's protocol. A total of 1 μg of each plasmid was used for transfection. The pGL3 basic reporter vector was used as a control for basal promoter activity. The cotransfected Renilla luciferase vector pRL-TK (Promega) served as an internal control for transfection efficiency. Cells were harvested 48 hours after transfection and assessed for luciferase activity using the dual-luciferase reporter assay system (Promega). All assays were done in triplicate. The activity of the pGL3 basic reporter vector alone was subtracted from that of the vector with the Ifng promoter. All values were normalized for transfection efficiency against Renilla luciferase expression.
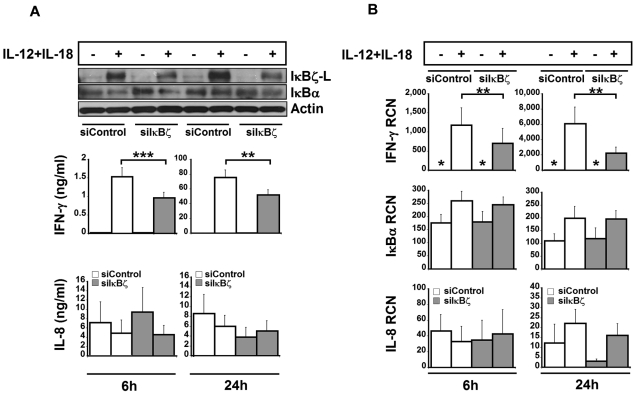
Figure 5.
Effect of siRNA-mediated knockdown of IκBζ in human NK cells. To assess the role of IκBζ in IFN-γ production, human NK cells were nucleofected with siRNA targeting IκBζ (30nM) or siControl (30nM). After nucleofection, the cells were stimulated with recombinant IL-12 and IL-18 (10 ng/mL each). (A) Protein expression for IκBζ and IFN-γ. IκBα, actin, and IL-8 are also shown as controls. (B) Gene expression profiles for IFN-γ, IκBα, and IL-8 in IκBζ knockdown cells. The immunoblot is representative of 7 independent experiments. Bar values represent the mean ± SEM for 5 to 7 experiments. *Values in the nondetectable range. **P < .05 vs controls. ***P < .005 vs controls. RCN indicates relative copy number.
Immunoprecipitation
NK cells (20 × 106) were stimulated with IL-12 (10 ng/mL) and IL-18 (10 ng/mL) for 6 hours. Cells were lysed in RIPA lysis buffer (50mM Tris-HCl, pH 7.5, 1% sodium deoxycholate, 1mM ethylenediaminetetraacetic acid, 1mM NaF, 150mM NaCl, 1% Nonidet P-40) in the presence of a protease inhibitor cocktail mixture (Sigma-Aldrich), 1mM phenylmethylsulfonyl fluoride, 10mM methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone, and 3mM sodium orthovanadate. Protein normalized cell lysates were immunoprecipitated using anti-IκBζ or anti-p65 (NF-κB) antibody overnight at 4°C. The immunoprecipitated samples were incubated with protein A-Sepharose (Invitrogen) beads for 2 hours at 4°C. The immunoprecipitates were recovered after centrifugation 16 000g for 5 minutes and were washed 3 times with RIPA buffer. The proteins were eluted with 2 times sodium dodecyl sulfate–polyacrylamide gel electrophoresis sample buffer at 95°C for 10 minutes, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblotted with the respective antibodies. The immunoprecipitation from the nuclear-cytosol isolates was performed using the Pierce crosslink IP kit (Thermo Electron) according to the manufacturer's protocol.
ELISA
ELISA for IFN-γ was purchased from eBioscience. ELISA for IL-8 and TNF-α, which have been previously described,29 was developed in our laboratory.
Statistical analysis
Values are expressed either as mean ± SEM or mean ± SD. Standard t test was used for all comparisons. Significance was defined as P < .05.
Results
IκBζ expression in human lymphocytes
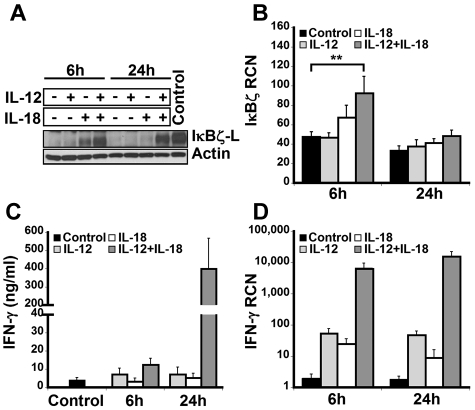
To examine the expression of IκBζ in human lymphocytes, CD14-depleted peripheral blood mononuclear cells were treated with IL-12 and/or IL-18 for 6 hours and 24 hours. On cytokine stimulation, freshly isolated lymphocytes predominantly expressed the IκBζ-L protein isoform (79 kDa) as assessed by immunoblotting with the IκBζ specific antibody (Figure 1A). Although IκBζ-L protein expression was induced at 6 hours in response to IL-18, expression of IκBζ-L was further increased with the combination of IL-12 and IL-18. However, at 24 hours, sustained expression of IκBζ-L protein required the IL-12/IL-18 combination.
Figure 1.
IκBζ and IFN-γ expression in human lymphocytes. Human lymphocytes were assessed for the expression of IκBζ and IFN-γ in response to recombinant IL-12 and IL-18 (10 ng/mL each) at 6 and 24 hours. (A) Time course of IκBζ protein expression in human lymphocytes by immunoblot compared with lipopolysaccharide-stimulated monocyte lysate (Control). (B) IκBζ mRNA expression in human lymphocytes assessed by quantitative PCR. (C) IFN-γ release from human lymphocytes in response to IL-12 and IL-18 was determined by ELISA. (D) IFN-γ mRNA expression in human lymphocytes. The immunoblot is representative of 3 independent experiments. Bar values represent mean ± SEM for 3 to 5 experiments. **P < .05 vs controls. RCN indicates relative copy number.
Unlike IκBζ protein, IκBζ mRNA was expressed in the unstimulated cells (Figure 1B). Moreover, stimulation with IL-12 did not change the IκBζ mRNA expression in these cells over 24 hours. In contrast to IL-12, 6 hours of IL-18 stimulation resulted in a small increase in the expression of IκBζ mRNA. The combination of IL-12/IL-18 enhanced IκBζ mRNA expression almost 2-fold compared with the control (P < .05). In agreement with the literature documenting the short half-life of IκBζ mRNA,30 expression levels were back to basal at 24 hours, regardless of the stimulus.
IFN-γ expression in human lymphocytes
The expression of IFN-γ in human lymphocytes paralleled the IκBζ response but in a delayed fashion (Figure 1C-D). Although IFN-γ mRNA was increased at 6 hours, the major protein response was most notable at 24 hours. Taken together, these results demonstrate that IκBζ mRNA and IκBζ-L protein are expressed in human lymphocytes in response to IL-12 and IL-18, a potent costimulus for IFN-γ expression in these cells.3 Thus, we hypothesized that IκBζ may function as a regulator of IFN-γ expression in response to IL-12 and IL-18.
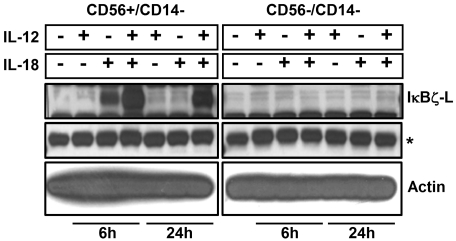
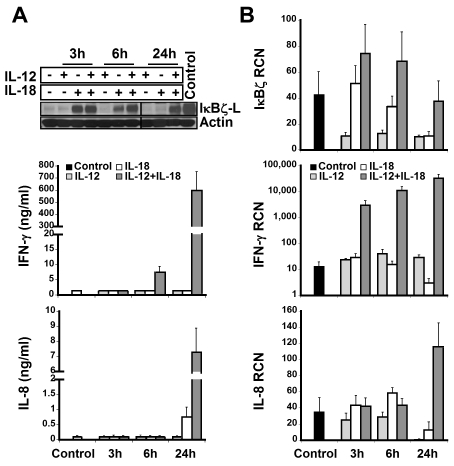
IκBζ expression and cellular localization in CD56+ human NK cells
To identify the subpopulation of lymphocytes that express IκBζ in response to IL-12 and IL-18, CD56+ NK cells and CD56− lymphocytes were analyzed for IκBζ expression. We identified CD56+ NK cells as the predominant IκBζ-L protein expressing population in response to IL-12 and IL-18 (Figure 2; Table 1). CD56+ NK cells were stimulated with IL-12 and/or IL-18 for 3 hours, 6 hours, and 24 hours. By 3 hours, freshly isolated CD56+ NK cells expressed IκBζ-L protein (79 kDa) in response to IL-18 alone, but only the combined IL-12/IL-18 stimulus maintained stable IκBζ-L protein at 24 hours (Figure 3A top panel). Importantly, IFN-γ release by CD56+ NK cells correlated with IκBζ-L protein expression induced by the combination of IL-12 and IL-18 (Figure 3A middle panel). Gene expression analysis suggested that, unlike the IκBζ protein, IκBζ mRNA is expressed in freshly isolated CD56+ NK cells (Figure 3B top panel). This baseline expression was enhanced in response to IL-18 and the combination stimulus of IL-12/IL-18, with highest expression at 3 hours and 6 hours. IFN-γ mRNA expression steadily increased over time (3-24 hours; Figure 3B middle panel) after the induction of IκBζ mRNA and protein. This observation is supportive of the role of IκBζ as a regulator of IFN-γ in human NK cells.
Figure 2.
IκBζ expression in CD56+ and CD56− cells. Human lymphocytes were isolated from PBMCs after CD14 depletion followed by positive selection for CD56+ cells. Cells were stimulated (5 × 106 cells) with recombinant IL-12 and IL-18 (10 ng/mL each) for 6 hours and 24 hours. Protein normalized cell lysates were immunoblotted for IκBζ and actin. Results are representative of 2 independent experiments. *Nonspecific band (IκBζ blot), which confirms equal protein loading as the actin expression is very high.
Table 1.
IFN-γ and IL-8 release by the CD56+ and CD56− lymphocytes
| Stimulation | CD56+/CD14− |
CD56−/CD14− |
||||||
|---|---|---|---|---|---|---|---|---|
| 6 hours |
24 hours |
6 hours |
24 hours |
|||||
| IFN-γ | IL-8 | IFN-γ | IL-8 | IFN-γ | IL-8 | IFN-γ | IL-8 | |
| IL-12 | 0.94 ± 0.81 | 0.14 ± 0.02 | 0.07 ± 0.04 | 0.13 ± 0.01 | 1.52 ± 0.49 | 0.34 ± 0.02 | 0.21 ± 0.09 | 0.34 ± 0.05 |
| IL-18 | 0.01 ± 0.02 | 0.14 ± 0.03 | ND | 0.52 ± 0.47 | 0.44 ± 0.08 | 0.36 ± 0.07 | 0.15 ± 0.06 | 0.47 ± 0.09 |
| IL-12 + IL-18 | 3.29 ± 3.04 | 0.12 ± 0.01 | 126 ± 58.4 | 7.92 ± 5.38 | 0.33 ± 0.09 | 0.30 ± 0.11 | 11.3 ± 3.82 | 0.46 ± 0.07 |
The relative ability of the CD56+/CD14− and the CD56−/CD14− subpopulations of lymphocytes to express IFN-γ and IL-8 was measured by ELISA. Human lymphocytes were isolated from PBMCs after CD14 depletion, and 5 × 106 cells were stimulated with rIL-12 (10 ng/mL) and rIL-18 (10 ng/mL) for 6 hours and 24 hours. The cell supernatants were analyzed for IFN-γ and IL-8 release. Values are mean ± SD for 2 experiments.
ND indicates not detected.
Figure 3.
IκBζ and IFN-γ expression in human NK cells. IκBζ and IFN-γ expression was evaluated in CD56+ selected human NK cells over time in response to recombinant IL-12 and IL-18 (10 ng/mL each). (A) Time course of IκBζ, IFN-γ, and IL-8 protein expression for IL-12 and IL-18 stimuli in human NK cells (2 × 106 cells/mL) as assessed by immunoblot of cell lysates for IκBζ and ELISA of supernatants for IFN-γ and IL-8. (B) Time course of IκBζ, IFN-γ, and IL-8 mRNA expression in human NK cell by quantitative PCR. Immunoblot is representative of 3 independent experiments and bar graphs represent the mean ± SEM for 3 experiments. Values in the undetectable range have been assigned a random insignificant number and plotted in the graph. Vertical lines have been inserted to indicate a repositioned gel lane. RCN indicates relative copy number.
In an attempt to evaluate the possible mechanisms of synergy, we tested the roles of TNF-α and IL-2, which have been previously studied for mediating IFN-γ production.3,24,31,32 We tested the possibility of self-mediated synergy by endogenously produced TNF-α for IκBζ and IFN-γ production in human NK cells on IL-12 and IL-18 stimulation. TNF-α release by NK cells (supplemental Figure 1) in response to the different stimuli was insignificant at 3 hours and 6 hours. However, at 24 hours, there was a 3-fold change in the TNF-α production in response to the combination stimulus of IL-12 and IL-18, thereby suggesting that autocrine TNF-α production might further enhance the expression of IκBζ-L and IFN-γ in human NK cells at 24 hours.
We also analyzed the effect of IL-2 on IκBζ expression and thus IFN-γ production in response to stimulation by IL-12 and IL-18 (supplemental Figure 2). The IL-2-treated cells express higher levels of IκBζ-L in response to IL-18 or the combined stimulus of IL-12/IL-18, compared with the control group of cells. However, the stimulation with IL-2 plus IL-12 has no effect on IκBζ-L expression. In addition, the IL-2-treated cells express 2.5-fold higher levels of IFN-γ in response to the combination stimulus of IL-12 and IL-18, compared with the control group of cells. These observations support a role for IκBζ in IFN-γ production by human NK cells.
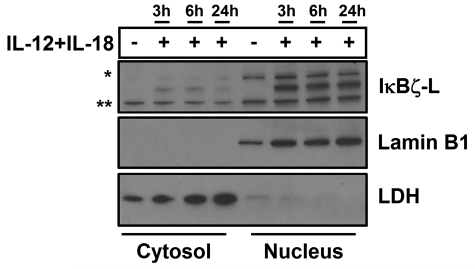
To determine the cellular localization of IκBζ, we fractionated human NK cells into cytosolic and nuclear fractions. On stimulation with the combination stimulus of IL-12 and IL-18, we observe that IκBζ-L protein is predominantly nuclear (Figure 4). The nuclear localization corroborates previous finding in other human and mouse models.16,33 Over time, there is no significant change in the nuclear expression of IκBζ-L, which is consistent with gene and protein expression in whole cell lysates in response to the combination stimulus of IL-12 and IL-18. Surprisingly, low levels of IκBζ-L are also found in the cytosol of human NK cells. Cytosolic IκBζ has been previously reported in B cells and macrophages.34
Figure 4.
Cellular localization of IκBζ in human NK cells. The cellular localization of IκBζ expression was evaluated in CD56+ human NK cells stimulated with recombinant IL-12 and IL-18 (10 ng/mL each) over time. The cellular lysates were fractionated into the nucleus and the cytosol separately. The cell lysates were immunoblotted for IκBζ, lamin B1 (nuclear marker), and LDH (cytosolic marker–lactate dehydrogenase). The immunoblot is representative of 3 independent experiments. *Nonspecific band. **Nonspecific band.
Down-regulation of IκBζ-L impairs IFN-γ expression in human NK cells
To evaluate the significance of IκBζ as a regulator of IFN-γ, IκBζ expression was suppressed by nucleofection of freshly isolated CD56+ NK cells with small interfering RNA targeting IκBζ (siIκBζ). The siIκBζ-treated NK cells expressed lower levels of IκBζ-L protein compared with the siControl-treated cells after stimulation with IL-12 and IL-18 (Figure 5A). Of note, the mRNA expression of IκBζ was not affected by the siRNA treatment (data not shown), suggesting that the siRNA suppressed IκBζ-L protein translation. Importantly, the siIκBζ-treated cells released significantly lower levels of IFN-γ in response to treatment with IL-12 and IL-18 at 6 hours (P < .005) and 24 hours (P < .05) compared with controls. Expression of IκBα and IL-8 protein was not affected by the siRNA treatment, which demonstrated specificity of the siIκBζ effect.
To evaluate the effect of IκBζ knockdown on IFN-γ gene expression, IFN-γ, IL-8, and IκBα mRNA levels were measured by quantitative PCR (Figure 5B). Knockdown of IκBζ induced a 1.5-fold and 2.5-fold reduction in the expression of IFN-γ mRNA at 6 hours and 24 hours, respectively (P < .05). In contrast to IFN-γ, IκBα and IL-8 mRNA expression was not affected by siIκBζ. Together, these results suggest that IκBζ regulates IFN-γ expression in human NK cells.
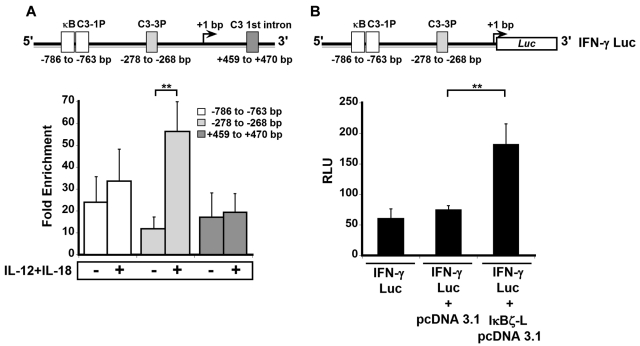
IκBζ binds to the IFN-γ promoter in response to IL-12 and IL-18
IκBζ has been shown to mediate its functions by associating with NF-κB at the NF-κB binding sites on the promoters of the secondary response target genes.21,35 Four different NF-κB binding sites have been described on the IFNG locus: κB, C3-1P, C3-3P, and C3 1st intron (Figure 6A top panel).36,37 We evaluated whether IκBζ associates with these NF-κB sites in response to IL-12 and IL-18 by ChIP (Figure 6A bottom panel). Untreated, freshly isolated NK cells display 10- to 20-fold enrichment in IκBζ association with the IFN-γ promoter. However, on treatment with IL-12 and IL-18, this fold enrichment was 5-fold higher at the C3-3P (−278 to −268 bp) site compared with the control (P < .05). In contrast, the recruitment of IκBζ to the κB and C3-1P sites (−786 to −763 bp) did not change dramatically on treatment with IL-12 and IL-18 compared with controls. In addition, the fold enrichment of IκBζ associating with the C3-1st intron site showed no difference in response to IL-12 and IL-18. This result suggests that low levels of IκBζ are initially associated with the IFN-γ promoter and that treatment with IL-12 and IL-18 markedly enhances this association at the C3-3P site. This finding suggests that the C3-3P site is responsible for the IκBζ-mediated regulation of IFN-γ production in human NK cells.
Figure 6.
IκBζ associates with the IFN-γ promoter and activates luciferase expression in human NK cells. The binding of IκBζ to the IFN-γ promoter was studied by ChIP in human NK cells. IκBζ's function in promoting IFNG gene expression was studied in a luciferase reporter assay in HEK 293 cells stably transfected with TLR4/IL-1R/MD-2. (A) Top: Diagrammatic representation of 4 NF-κB binding sites (κB, C3-1P, C3-3P, and C3 1st intron as reported37) present on the IFN-γ promoter. Bottom: The effect of IL-12/IL-18 stimulation on the relative enrichment of IκBζ association with the 4 NF-κB sites as determined by ChIP. (B) Top: Diagrammatic representation of the IFN-γ luciferase reporter construct used in the luciferase reporter assay. Bottom: The ability of IκBζ-L to induce expression of the IFN-γ luciferase reporter. Values are mean ± SEM for 3 independent experiments. **P < .05 vs controls. RLU indicates relative luciferase units.
IκBζ-L increases IFN-γ promoter activity
To understand the mechanism of IκBζ in regulating IFN-γ expression, we used an IFN-γ promoter, luciferase reporter assay (Figure 6B top panel). HEK 293/TLR4/IL-1R/MD-2 cells were transfected with a 3.6-kb IFN-γ promoter construct (IFN-γ Luc; Figure 6B bottom panel) alone or in combination with IκBζ-L pCDNA3.1 or control pCDNA3.1 vector. The IFN-γ promoter construct had a basal level of luciferase expression, and this was unaffected by the cotransfection of the control pCDNA3.1 vector. However, on coexpression of IκBζ-L, there was a 2.5-fold increase in the luciferase expression compared with the control vector (P < .05). Thus, the activating effect of IκBζ on the IFN-γ promoter in HEK 293/TLR4/IL-1R/MD-2 cells supports the role of IκBζ as a transcriptional regulator of IFN-γ expression.
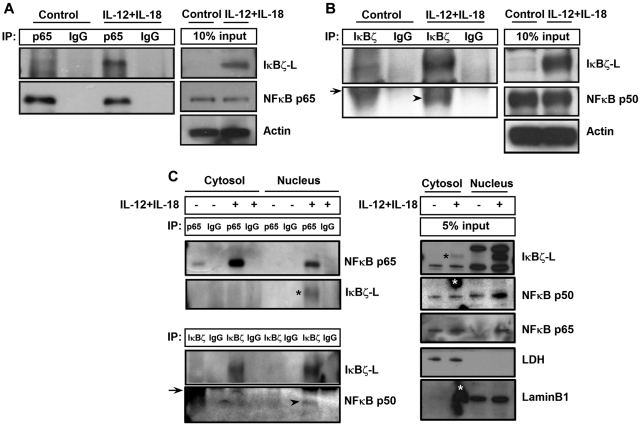
IκBζ-L regulates IFN-γ expression by binding to NF-κB
It has been previously reported that IκBζ regulates NF-κB-mediated control of gene expression for several genes.17,33 To evaluate the role of NF-κB in IκBζ-mediated regulation of IFN-γ, CD56+ NK cells were stimulated with IL-12 and IL-18 for 6 hours and analyzed for IκBζ binding to p65 and p50 subunits of NF-κB. As shown in Figure 7A, IκBζ-L coimmunoprecipitated with the p65 NF-κB subunit in human NK cells in response to IL-12 and IL-18. In addition, the p50 NF-κB subunit also coimmunoprecipitated with IκBζ-L in human NK cells on stimulation with IL-12 and IL-18 (Figure 7B).
Figure 7.
IκBζ coimmunoprecipitates with NF-κB specifically in the nucleus of human NK cells. (A) To assess the ability of IκBζ to bind to NF-κB, human NK cells were stimulated with recombinant IL-12 and IL-18 (10 ng/mL each) for 6 hours. The cell lysates were immunoprecipitated with the indicated antibodies overnight at 4°C. The immunoprecipitated lysates were immunoblotted with respective antibodies as indicated in the figure. (A) IκBζ binding to the p65 NF-κB subunit. (B) IκBζ binding to the p50 NF-κB subunit. (C) Binding of IκBζ to NF-κB specifically in the nucleus. Arrowhead represents p50 NF-κB subunit; and arrow, immunoglobulin heavy chain. *(Black) IκBζ-L band. *(White) Nonspecific smudge mark on the Western blot. Results are representative of 3 independent experiments. Lamin B1, nuclear marker. LDH indicates lactate dehydrogenase (cytosolic marker).
To evaluate whether the binding of IκBζ to NF-κB occurs in the cytosol or the nucleus, we separated the nuclear and cytosolic fractions of control and IL-12–/IL-18–stimulated human NK cells. These different fractions were analyzed for IκBζ binding to the p65 and p50 subunits of NF-κB (Figure 7C). The p65 subunit of NF-κB is expressed in the cytosol of the control untreated cells, and it translocates to the nucleus on stimulation with IL-12/IL-18. Unlike the p65 subunit of NF-κB, the p50 subunit is expressed in the cytosol and the nucleus of control as well as IL-12–/IL-18–stimulated NK cells. Although IκBζ expression is predominantly nuclear, there is a small cytosolic fraction expressed on IL-12/IL-18 stimulation in human NK cells. However, on treatment with IL-12/IL-18, the association of IκBζ with the p50 and p65 subunit of NF-κB occurs only in the nucleus, suggesting that, although both sets of proteins are expressed in the cytosol, they do not interact with each other until they translocate to the nucleus. These results suggest that the observed IκBζ-mediated regulation of IFN-γ is NF-κB dependent.
Discussion
IκBζ is an inducible homolog of the IκB family of proteins, which exists as 2 major splice variants IκBζ-L and IκBζ-S, which regulate NF-κB-dependent gene expression.17 Unlike other members of the IκB family, which inhibit NF-κB activation by binding to the Rel-homology domain of NF-κB through their C-terminal ankyrin repeat sequences, IκBζ has been shown to up-regulate the expression of secondary response genes, including IL-6, neutrophil gelatinase-associated lipocalin, and human β-defensin 2.16,19,23
The ability of TLR ligands and IL-1β to induce IκBζ expression has been previously demonstrated.15,18 We recently reported the first direct evidence that IL-18 induces IκBζ expression in the acute monocytic leukemic cell KG-1.20 This effect probably results from IL-1β and IL-18 sharing similar signaling pathways downstream of the IL-1R and IL-18R, leading to the activation of NF-κB and mitogen-activated protein kinases. Although TNF-α activates both NF-κB as well as mitogen-activated protein kinases, it fails to induce the expression of IκBζ, thus emphasizing the importance of MyD88 (adaptor protein in the TLR pathway) in the induction of IκBζ.35 Recently, we have demonstrated that IL-1β and IL-18 synergize with TNF-α for IκBζ expression and that this synergy is responsible for IFN-γ production in KG-1 cells.20,24 IFN-γ is a classic TH1 cytokine, whose wide range of functions regulates both innate and adaptive immune responses. Lymphocytes, predominantly consisting of subpopulations of NK/NKT cells and T cells, are the predominant producers of IFN-γ.3 IL-12 and IL-18 classically activate IFN-γ production in NK cells and T cells.3,26 Several transcription factors have been shown to be involved in the transcription of Ifng gene in NK cells and T cells. These include members of the NF-κB, AP-1, and the STAT family.3,38
In this report, we demonstrate that IκBζ is expressed in human peripheral blood-derived NK cells in response to the combination stimulus of IL-12 and IL-18. Of note, at the protein level, IκBζ-L is the predominantly expressed isoform in NK cells. This preferential expression of the IκBζ-L over IκBζ-S is consistent with previous publications in murine and human cells and our own findings in human monocytes.16,34 To our knowledge, this is the first evidence of IκBζ expression in human NK cells. However, after our manuscript received its initial review, a similar finding in murine NK cells was submitted and published.39 As described here in humans, this work demonstrates the role of IκBζ in IFN-γ production in murine NK cells in response to IL-12 and IL-18. Importantly, the murine work documents the significant role that IκBζ plays in host defense against murine cytomegalovirus infections.39
Surprisingly, we noted that freshly isolated NK cells expressed IκBζ mRNA but not IκBζ protein. This absence of transcription to translation coupling was different from the direct coupling that we and others have previously noted between IκBζ mRNA and protein in monocytes.16,34 This NK cell discrepancy between mRNA and IκBζ protein translation suggested that, at least in NK cells, a second stimulus is needed to stabilize the mRNA, promote IκBζ translation, or stabilize the protein product. Because the addition of IL-18 alone was able to induce IκBζ protein, it is reasonable to suggest that IL-18 may stabilize the message of IκBζ, as has been shown for lipopolysaccharide and IL-1β.30 However, because there was baseline IκBζ mRNA in NK cells without protein, additional functions of IL-18, such as effects on translation and protein stability, should also be considered. We are in the process of investigating these questions.
Having identified CD56+ NK cells as the major population of nonmonocytic peripheral blood cells that express IκBζ, it is important to put our observation into context with what has been noted in this regard. For example, we failed to observe IκBζ protein expression in the CD56− human lymphocytes. However, Okamoto et al40 observed significant IκBζ protein expression in murine helper T-cell subset TH17 cells, which should be present in our CD56− population. We also failed to observe IκBζ-L expression in the CD3+ lymphocytes, which include the NKT as well as the T-cell subtypes (data not shown). This discrepancy may result from the CD56−/CD3+ cells being a mixed population of cells with limited numbers of subsets, such as the TH17 cells. In addition, our focused attention on the IL-12/IL-18 pathway as inducers of IκBζ expression might also be a reason for this discrepancy. It is well established that the CD56− and CD3+ lymphocytes express IFN-γ in response to IL-12 and IL-18.3,41 However, the expression of IFN-γ was 10-fold lower in the CD56− lymphocytes compared with the CD56+ lymphocytes (Table 1). Thus, future studies are aimed at understanding the role of IκBζ as the key mediator for the differential IFN-γ production between these subgroups of lymphocytes.
In the CD56+ NK cells, IL-12 and IL-18 synergize for IκBζ-L protein expression at 6 hours and 24 hours, and the protein translocates to the nucleus. This mechanism of synergy could be explained by the observation that IL-12 up-regulates the expression of IL-18R subunits, thereby leading to enhanced signaling by the IL-18R.42,43 Alternatively, the microenvironment of inflammation is a mix of cytokines secreted by the different cells. Thus we tested other possible factors, such as IFN-γ, TNF-α, and IL-2, which could explain the synergy. Positive feedback signaling mediated by IFN-γ could explain the observed synergy between IL-12 and IL-18 because IFN-γ has been shown to function through the adaptor molecule MyD88 leading to the up-regulation of proinflammatory genes.44 However, we did not detect IκBζ-L protein expression in NK cells in the presence of IFN-γ stimulation alone (data not shown). NK cells are known producers of immunoregulatory cytokines as TNF-α, which have been studied for synergy with IL-12 toward IFN-γ production.31,32 Analysis of IL-12–/IL-18–stimulated human NK cells suggests a possible role for TNF-α in the persistent expression of IκBζ at 24 hours. This persistent expression of IκBζ-L in human NK cells is unlike its expression in CD14+ monocytes. This later kinetic supports IκBζ's function as a regulator of the secondary response gene IFN-γ, whose expression in NK cells is best observed at later time points (> 6 hours). This is in contrast to the IκBζ-mediated expression of IL-6, which is expressed earlier than IFN-γ. Similarly, IL-2 has also been long known to promote NK-cell growth, differentiation, cytolytic activity, and IFN-γ production.3 IL-2 treatment in conjunction with IL-12/IL-18 suggests that IL-2 may have a role in regulating IκBζ expression either by increasing the expression of IL-12R subunits or STAT4, thereby leading to increased signaling by IL-12 and IL-18 and thus increased IFN-γ production in human NK cells as suggested previously.45
In human NK cells, IκBζ expression precedes the expression and production of IFN-γ. This relationship suggests that IκBζ regulates IFN-γ in human NK cells. Importantly, this connection was further supported by the siRNA-mediated knockdown of IκBζ in human NK cells. Knockdown of IκBζ decreased expression and production of IFN-γ in response to IL-12 and IL-18, compared with cells treated with siControl. Furthermore, ChIP experiments demonstrated that IκBζ associates with the IFN-γ promoter. This finding strongly supports the role of IκBζ as a regulator of IFN-γ production in human NK cells and concurs with IκBζ's recognized role as a transcriptional regulator of secondary response genes.35 Of note, our ChIP studies suggest that a small amount of IκBζ may be associated with the IFN-γ promoter in resting NK cells. Although this baseline IκBζ protein was not confirmed by immunoblots, it cannot be ruled out because of the higher sensitivity of the ChIP analysis compared with immunoblotting. Of course, stimulation with IL-12 and IL-18 may increase IκBζ expression and hence its increased recruitment to the IFN-γ promoter. Nevertheless, the baseline presence of IκBζ protein on the IFN-γ promoter raises the distinct possibility that IκBζ's function may be additionally controlled posttranslationally as is well known for IκBα.46 Future studies are also directed at understanding the possibility of posttranslational regulation of IκBζ.
Our reporter assays demonstrated that IκBζ-L is an activator of luciferase expression under the IFN-γ promoter in HEK 293/TLR4/IL-1R/MD-2 cells. Because IκBζ-L is the predominantly expressed isoform in human NK cells, this suggests that the long form of IκBζ is the transcriptional regulator of IFN-γ expression in human NK cells in response to IL-12 and IL-18. Together with the ChIP experiments, these findings suggest that IκBζ associates with the IFN-γ promoter and activates transcription. The absence of IFN-γ expression in IκBζ bound resting NK cells suggests that in the absence of stimulation, IκBζ fails to undergo an essential posttranslational modification that enables it to function as a transcriptional regulator. Alternatively, IL-12/IL-18 stimulation may induce cofactors that cooperate with IκBζ to regulate transcription at the IFN-γ promoter in human NK cells.
IκBζ has been extensively investigated as a regulator of NF-κB-mediated responses.19,47–49 Of the 4 NF-κB sites (κB, C3-1P, C3-3P, and C3 1st intron) identified by Sica et al36 on the IFN-γ promoter, IκBζ association is maximized at the C3-3P site (−278 to −268 bp). This site was identified as a binding site for the Rel-homology domain-containing proteins and has been shown to be composed of the p50 and p65 subunits of NF-κB.36 It has also been demonstrated to be a site for interaction between NF-κB and members of the NFAT family of proteins.36 The possible role of IκBζ interacting with NFAT proteins is yet to be investigated.
Immunoprecipitation experiments demonstrated the binding of IκBζ to the p50 and p65 NF-κB subunits in human NK cells in response to IL-12 and IL-18. We have also evidenced this binding to be strictly nuclear. This supports the role of an IκBζ-p50-p65 complex in binding to the IFN-γ promoter, thus regulating transcription. It is thought that IκBζ facilitates the recruitment of p50-p65 heterodimers, thereby activating gene transcription on the secondary response genes.21,50 This is in agreement with our observation of increased IκBζ association with the IFN-γ promoter and its physical interaction with both p50 and p65, on IL-12 and IL-18 stimulation in human NK cells.
In conclusion, our findings indicate that IκBζ positively regulates IFN-γ expression in human NK cells in response to IL-12 and IL-18. Furthermore, this study documents the role of IκBζ as an important component of NF-κB-mediated IFN-γ expression, highlighting the role of IκBζ as global regulator of NF-κB-mediated responses.
Supplementary Material
Acknowledgments
The authors thank Dr Anasuya Sarkar for her valuable guidance through the course of the study, and Freweine Berhe and Jennifer Hollyfield for their technical assistance during the course of the study.
This work was supported by the National Institutes of Health (R01 grant HL089440) awarded to M.D.W. and the American Heart Association Great Rivers Affiliate (predoctoral fellowship) awarded to Y.K.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.K. conceived and designed the experiments with the help of M.D.W.; Y.K., J.Y., and M.W. performed the experiments; Y.K., J.Y., and M.D.W. analyzed the data; J.Y., M.A.C., and M.D.W. contributed reagents, materials, and analysis tools; and Y.K. and M.D.W. wrote the manuscript with the help of J.Y., R.M.R., S.S., and M.A.C.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark D. Wewers, Pulmonary, Allergy, Critical Care and Sleep Medicine, Davis Heart and Lung Research Institute, The Ohio State University, 473 W 12th Ave, Columbus, OH 43210; e-mail: wewers.2@osu.edu.
References
- 1.Steeg PS, Moore RN, Johnson HM, Oppenheim JJ. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 3.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 4.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 5.Collart MA, Belin D, Vassalli JD, de Kossodo S, Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986;164(6):2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill N, Sarvetnick N. Cytokines: promoters and dampeners of autoimmunity. Curr Opin Immunol. 2002;14(6):791–797. doi: 10.1016/s0952-7915(02)00403-x. [DOI] [PubMed] [Google Scholar]
- 8.Savinov AY, Wong FS, Chervonsky AV. IFN-gamma affects homing of diabetogenic T cells. J Immunol. 2001;167(11):6637–6643. doi: 10.4049/jimmunol.167.11.6637. [DOI] [PubMed] [Google Scholar]
- 9.Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140(4):1022–1027. [PubMed] [Google Scholar]
- 10.Carnaud C, Lee D, Donnars O, et al. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163(9):4647–4650. [PubMed] [Google Scholar]
- 11.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13(2):95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 12.Rosenzweig SD, Holland SM. Defects in the interferon-gamma and interleukin-12 pathways. Immunol Rev. 2005;203:38–47. doi: 10.1111/j.0105-2896.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura H, Kanehira K, Okita K, Morimatsu M, Saito M. MAIL, a novel nuclear I kappa B protein that potentiates LPS-induced IL-6 production. FEBS Lett. 2000;485(1):53–56. doi: 10.1016/s0014-5793(00)02185-2. [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki S, Muta T, Takeshige K. A novel IkappaB protein, IkappaB-zeta, induced by proinflammatory stimuli, negatively regulates nuclear factor-kappaB in the nuclei. J Biol Chem. 2001;276(29):27657–27662. doi: 10.1074/jbc.M103426200. [DOI] [PubMed] [Google Scholar]
- 15.Haruta H, Kato A, Todokoro K. Isolation of a novel interleukin-1-inducible nuclear protein bearing ankyrin-repeat motifs. J Biol Chem. 2001;276(16):12485–12488. doi: 10.1074/jbc.C100075200. [DOI] [PubMed] [Google Scholar]
- 16.Seshadri S, Kannan Y, Mitra S, Parker-Barnes J, Wewers MD. MAIL regulates human monocyte IL-6 production. J Immunol. 2009;183(8):5358–5368. doi: 10.4049/jimmunol.0802736. [DOI] [PubMed] [Google Scholar]
- 17.Muta T. IkappaB-zeta: an inducible regulator of nuclear factor-kappaB. Vitam Horm. 2006;74:301–316. doi: 10.1016/S0083-6729(06)74012-2. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, Yamazaki S, Uematsu S, et al. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IkappaBzeta. Nature. 2004;430(6996):218–222. doi: 10.1038/nature02738. [DOI] [PubMed] [Google Scholar]
- 19.Kao CY, Kim C, Huang F, Wu R. Requirements for two proximal NF-kappaB binding sites and IkappaB-zeta in IL-17A-induced human beta-defensin 2 expression by conducting airway epithelium. J Biol Chem. 2008;283(22):15309–15318. doi: 10.1074/jbc.M708289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raices RM, Kannan Y, Bellamkonda-Athmaram V, et al. A novel role for IkappaBzeta in the regulation of IFNgamma production. PLoS One. 2009;4(8):e6776. doi: 10.1371/journal.pone.0006776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trinh DV, Zhu N, Farhang G, Kim BJ, Huxford T. The nuclear I kappaB protein I kappaB zeta specifically binds NF-kappaB p50 homodimers and forms a ternary complex on kappaB DNA. J Mol Biol. 2008;379(1):122–135. doi: 10.1016/j.jmb.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 22.Fujino RS, Tanaka K, Morimatsu M, et al. Spermatogonial cell-mediated activation of an IkappaBzeta-independent nuclear factor-kappaB pathway in Sertoli cells induces transcription of the lipocalin-2 gene. Mol Endocrinol. 2006;20(4):904–915. doi: 10.1210/me.2005-0423. [DOI] [PubMed] [Google Scholar]
- 23.Cowland JB, Muta T, Borregaard N. IL-1beta-specific up-regulation of neutrophil gelatinase-associated lipocalin is controlled by IkappaB-zeta. J Immunol. 2006;176(9):5559–5566. doi: 10.4049/jimmunol.176.9.5559. [DOI] [PubMed] [Google Scholar]
- 24.Raices RM, Kannan Y, Sarkar A, Bellamkonda-Athmaram V, Wewers MD. A synergistic role for IL-1beta and TNFalpha in monocyte-derived IFNgamma inducing activity. Cytokine. 2008;44(2):234–241. doi: 10.1016/j.cyto.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pien GC, Satoskar AR, Takeda K, Akira S, Biron CA. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-gamma responses during viral infection. J Immunol. 2000;165(9):4787–4791. doi: 10.4049/jimmunol.165.9.4787. [DOI] [PubMed] [Google Scholar]
- 26.Chaix J, Tessmer MS, Hoebe K, et al. Cutting edge: priming of NK cells by IL-18. J Immunol. 2008;181(3):1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J, Wei M, Becknell B, et al. Pro- and antiinflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity. 2006;24(5):575–590. doi: 10.1016/j.immuni.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Gavrilin MA, Bouakl IJ, Knatz NL, et al. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc Natl Acad Sci U S A. 2006;103(1):141–146. doi: 10.1073/pnas.0504271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh CB, Lowe MP, Rovin BH, et al. Lymphocytes produce IL-1beta in response to Fcgamma receptor cross-linking: effects on parenchymal cell IL-8 release. J Immunol. 1998;160(8):3942–3948. [PubMed] [Google Scholar]
- 30.Yamazaki S, Muta T, Matsuo S, Takeshige K. Stimulus-specific induction of a novel nuclear factor-kappaB regulator, IkappaB-zeta, via Toll/Interleukin-1 receptor is mediated by mRNA stabilization. J Biol Chem. 2005;280(2):1678–1687. doi: 10.1074/jbc.M409983200. [DOI] [PubMed] [Google Scholar]
- 31.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993;90(8):3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papamichail M, Perez SA, Gritzapis AD, Baxevanis CN. Natural killer lymphocytes: biology, development, and function. Cancer Immunol Immunother. 2004;53(3):176–186. doi: 10.1007/s00262-003-0478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muta T, Yamazaki S, Eto A, Motoyama M, Takeshige K. IkappaB-zeta, a new anti-inflammatory nuclear protein induced by lipopolysaccharide, is a negative regulator for nuclear factor-kappaB. J Endotoxin Res. 2003;9(3):187–191. doi: 10.1179/096805103125001612. [DOI] [PubMed] [Google Scholar]
- 34.Kitamura H, Matsushita Y, Iwanaga T, et al. Bacterial lipopolysaccharide-induced expression of the IkappaB protein MAIL in B-lymphocytes and macrophages. Arch Histol Cytol. 2003;66(1):53–62. doi: 10.1679/aohc.66.53. [DOI] [PubMed] [Google Scholar]
- 35.Kayama H, Ramirez-Carrozzi VR, Yamamoto M, et al. Class-specific regulation of pro-inflammatory genes by MyD88 pathways and IkappaBzeta. J Biol Chem. 2008;283(18):12468–12477. doi: 10.1074/jbc.M709965200. [DOI] [PubMed] [Google Scholar]
- 36.Sica A, Dorman L, Viggiano V, et al. Interaction of NF-kappaB and NFAT with the interferon-gamma promoter. J Biol Chem. 1997;272(48):30412–30420. doi: 10.1074/jbc.272.48.30412. [DOI] [PubMed] [Google Scholar]
- 37.Sica A, Tan TH, Rice N, et al. The c-rel protooncogene product c-Rel but not NF-kappa B binds to the intronic region of the human interferon-gamma gene at a site related to an interferon-stimulable response element. Proc Natl Acad Sci U S A. 1992;89(5):1740–1744. doi: 10.1073/pnas.89.5.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4(11):900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 39.Miyake T, Satoh T, Kato H, et al. IkappaBzeta is essential for natural killer cell activation in response to IL-12 and IL-18. Proc Natl Acad Sci U S A. 2010;107(41):17680–17685. doi: 10.1073/pnas.1012977107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto K, Iwai Y, Oh-Hora M, et al. IkappaBzeta regulates T(H)17 development by cooperating with ROR nuclear receptors. Nature. 2010;464(7293):1381–1385. doi: 10.1038/nature08922. [DOI] [PubMed] [Google Scholar]
- 41.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakahira M, Ahn HJ, Park WR, et al. Synergy of IL-12 and IL-18 for IFN-gamma gene expression: IL-12-induced STAT4 contributes to IFN-gamma promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. J Immunol. 2002;168(3):1146–1153. doi: 10.4049/jimmunol.168.3.1146. [DOI] [PubMed] [Google Scholar]
- 43.Sareneva T, Julkunen I, Matikainen S. IFN-alpha and IL-12 induce IL-18 receptor gene expression in human NK and T cells. J Immunol. 2000;165(4):1933–1938. doi: 10.4049/jimmunol.165.4.1933. [DOI] [PubMed] [Google Scholar]
- 44.Sun D, Ding A. MyD88-mediated stabilization of interferon-gamma-induced cytokine and chemokine mRNA. Nat Immunol. 2006;7(4):375–381. doi: 10.1038/ni1308. [DOI] [PubMed] [Google Scholar]
- 45.Wang KS, Frank DA, Ritz J. Interleukin-2 enhances the response of natural killer cells to interleukin-12 through up-regulation of the interleukin-12 receptor and STAT4. Blood. 2000;95(10):3183–3190. [PubMed] [Google Scholar]
- 46.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18(49):6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 47.Eto A, Muta T, Yamazaki S, Takeshige K. Essential roles for NF-kappa B and a Toll/IL-1 receptor domain-specific signal(s) in the induction of I kappa B-zeta. Biochem Biophys Res Commun. 2003;301(2):495–501. doi: 10.1016/s0006-291x(02)03082-6. [DOI] [PubMed] [Google Scholar]
- 48.Totzke G, Essmann F, Pohlmann S, et al. A novel member of the IkappaB family, human IkappaB-zeta, inhibits transactivation of p65 and its DNA binding. J Biol Chem. 2006;281(18):12645–12654. doi: 10.1074/jbc.M511956200. [DOI] [PubMed] [Google Scholar]
- 49.Matsuo S, Yamazaki S, Takeshige K, Muta T. Crucial roles of binding sites for NF-kappaB and C/EBPs in IkappaB-zeta-mediated transcriptional activation. Biochem J. 2007;405(3):605–615. doi: 10.1042/BJ20061797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamazaki S, Matsuo S, Muta T, et al. Gene-specific requirement of a nuclear protein, IkappaB-zeta, for promoter association of inflammatory transcription regulators. J Biol Chem. 2008;283(47):32404–32411. doi: 10.1074/jbc.M802148200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.