Abstract
Risk factors for grades 2-4 acute graft-versus-host disease (GVHD) and for chronic GVHD as defined by National Institutes of Health consensus criteria were evaluated and compared in 2941 recipients of first allogeneic hematopoietic cell transplantation at our center. In multivariate analyses, the profiles of risk factors for acute and chronic GVHD were similar, with some notable differences. Recipient human leukocyte antigen (HLA) mismatching and the use of unrelated donors had a greater effect on the risk of acute GVHD than on chronic GVHD, whereas the use of female donors for male recipients had a greater effect on the risk of chronic GVHD than on acute GVHD. Total body irradiation was strongly associated with acute GVHD, but had no statistically significant association with chronic GVHD, whereas grafting with mobilized blood cells was strongly associated with chronic GVHD but not with acute GVHD. Older patient age was associated with chronic GVHD, but had no effect on acute GVHD. For all risk factors associated with chronic GVHD, point estimates and confidence intervals were not significantly changed after adjustment for prior acute GVHD. These results suggest that the mechanisms involved in acute and chronic GVHD are not entirely congruent and that chronic GVHD is not simply the end stage of acute GVHD.
Introduction
During the past 3 decades, several studies have identified risk factors associated with the development of acute and chronic graft-versus-host disease (GVHD).1 In these studies, acute GVHD generally referred to disease manifestations that occurred within the first 100 days after hematopoietic cell transplantation (HCT),2–4 and chronic GVHD referred to disease manifestations that were present after day 100.5 The most consistently reported factors significantly associated with an increased risk of grades 2-4 acute GVHD were recipient human leukocyte antigen (HLA) mismatching with the donor,6–8 alloimmunization of the donor,9–12 the use of a female donor for male recipients,9,11–13 and older patient age.11,13,14 Less consistently reported risk factors have included prior cytomegalovirus infection in the recipient,14,15 higher intensity of the conditioning regimen (irradiation),12,14 donor age,16 and grafting with growth factor–mobilized blood cells.14,17 For chronic GVHD, the most consistently reported risk factors include prior acute GVHD,18–20 grafting with growth factor–mobilized blood cells,17,21,22 the use of a female donor for male recipients,19,20,23 older patient age,18–20,23 and mismatched and unrelated donors.20,24
The objective of the current study was to compare risk factor profiles for grades 2-4 acute and chronic GVHD. For this purpose, we used diagnostic criteria recommended by the National Institutes of Health (NIH) Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease.25 According to these criteria, acute and chronic GVHD are distinguished by differences in clinical manifestations and not by the time after HCT. Differences in the profile of risk factors for acute GVHD and NIH chronic GVHD would suggest that these syndromes result from distinct pathogenic pathways. Elucidation of risk factors would also help to identify subgroups of patients who might benefit from new approaches for the prevention of acute and chronic GVHD.
Methods
Patients
This retrospective study included 2941 adult and pediatric patients who received a first related or unrelated allogeneic HCT with bone marrow or growth factor–mobilized blood cells after high-intensity (ie, myeloablative) conditioning regimens for treatment of hematologic malignancies between July 1992 and December 2005 at the Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance. Patients had given written consent allowing the use of medical records for research in accordance with the Declaration of Helsinki, and the institutional review board approved the study. Follow-up clinical information was available from medical records submitted by referring physicians and from documentation generated by a dedicated long-term follow-up clinical program.
Definition
Acute GVHD was graded according to previously described criteria.26 Endoscopic biopsies were used to establish the diagnosis of grade-2 GVHD in patients with isolated upper gastrointestinal tract symptoms such as anorexia, nausea, and emesis.27 All cases of historically defined chronic GVHD requiring systemic treatment were retrospectively reclassified according to the presence or absence of features as defined in the NIH consensus criteria at onset.25,28 Patients with historically defined chronic GVHD that met NIH criteria at onset were considered thereafter as having NIH chronic GVHD. Patients with historically defined chronic GVHD that did not meet NIH criteria were classified as late-acute GVHD. Records for these patients were reviewed to ascertain the subsequent onset of any chronic GVHD according to NIH criteria.
Statistical analysis
We evaluated differences in risks for acute GVHD that occurred at any time after HCT and for NIH chronic GVHD requiring systemic treatment. Patients with de novo NIH “overlap syndrome” were considered to have both acute and chronic GVHD. Patients with de novo NIH “classic” chronic GVHD who later developed overlap syndrome were considered to have acute GVHD at the onset of overlap syndrome. Cox regression analyses were used to evaluate risk factors for both types of GVHD, treating death or recurrent malignancy without GVHD as competing events. Risk factors evaluated for association with grades 2-4 acute GVHD and for NIH chronic GVHD included patient and donor ages at transplantation, donor type, type of graft (marrow or growth factor-mobilized blood cells), recipient/donor sex combination, use of total body irradiation (TBI) or rabbit anti-thymocyte globulin (ATG) in the conditioning regimen, prior infection of patient or donor with cytomegalovirus, a diagnosis of chronic myeloid leukemia (CML), and myeloid malignancy. HLA matching of donor and recipient pairs was based on the best available typing results at the time of the analysis, including typing provided by the National Marrow Donor Program (NMDP) for some unrelated donor and recipient pairs.29 Multivariate models were also adjusted for year of transplantation. A separate analysis of risk factors for NIH chronic GVHD included adjustment for prior acute GVHD. Factors having a P value of less than .1 for association with acute GVHD or with NIH chronic GVHD by univariate testing were added sequentially to a multivariate Cox regression model using a step-up procedure until the P value for the likelihood ratio test was more than .05. Cumulative incidence estimates of acute and NIH chronic GVHD and the associated 95% confidence intervals (CIs) were derived according to methods described previously.30 The analysis was carried out as of July 2009.
Results
The median age of the study cohort at the time of HCT was 40 years (range, 0.6-71 years). Of the 2941 patients, 1927 (66%) received bone marrow, 1284 (44%) had HLA-matched related donors, 780 (26%) had HLA-matched unrelated donors, and 877 (30%) had HLA-mismatched related or unrelated donors. Other demographic characteristics of the cohort are summarized in Table 1.
Table 1.
Characteristics of the study cohort
| Characteristic | Value |
|---|---|
| No. of patients | 2941 |
| Median age (range), y | 40.3 (0.6-71.6) |
| Diagnosis at transplantation, no. (%) | |
| Acute myeloid leukemia | 859 (29) |
| Acute lymphoid leukemia | 419 (14) |
| Chronic myeloid leukemia | 895 (30) |
| Myelodysplastic syndromes including myeloproliferative diseases | 504 (17) |
| Chronic lymphocytic leukemia and lymphomas | 191 (7) |
| Multiple myeloma | 73 (2) |
| Disease at transplantation, no. (%)* | |
| Low risk | 646 (22) |
| Standard risk | 1419 (48) |
| High risk | 877 (30) |
| Donor age, median (range), y | 38.6 (0.9-81.7) |
| Donor/patient sex, no. (%) | |
| Female/male | 722 (25) |
| Other | 2219 (75) |
| High-intensity conditioning regimens, no. (%) | |
| With TBI | 1730 (59) |
| Without TBI | 1137 (39) |
| With rabbit ATG | 74 (3) |
| GVHD prophylaxis, no. (%) | |
| Methotrexate with calcineurin inhibitors | 2394 (81) |
| Other | 547 (19) |
| Graft source, no. (%) | |
| Mobilized blood hematopoietic stem cells | 1014 (34) |
| Bone marrow | 1927 (66) |
| Donor type, no. (%) | |
| HLA-identical related | 1284 (44) |
| HLA-matched unrelated | 780 (26) |
| HLA-mismatched related | 270 (9) |
| HLA-mismatched unrelated | 607 (21) |
Low-risk diseases included CML in chronic phase; high-risk diseases included acute leukemia not in remission, CML in blast crisis, refractory anemia with excess blasts (RAEB), or RAEB in transformation and myeloma; all other diseases and stages were categorized as standard risk.
Cumulative incidence estimates of acute grades 2-4 GVHD and NIH chronic GVHD
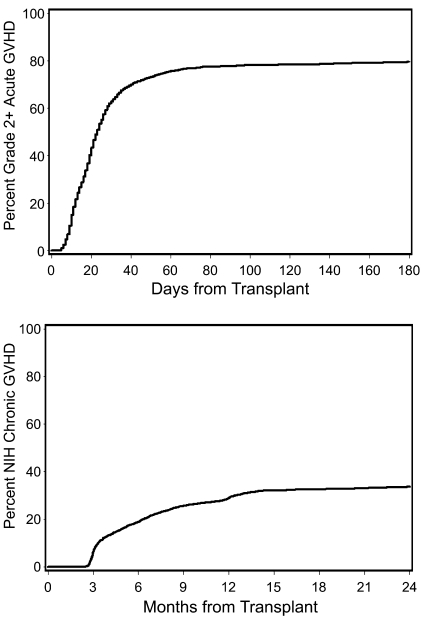
The cumulative incidence of grades 2-4 acute GVHD at 6 months was 80% (95% CI, 78%-81%), including patients with isolated upper gastrointestinal GVHD27 (Figure 1 top panel). The 2-year cumulative incidence of NIH chronic GVHD treated with systemic immunosuppression was 34% (95% CI, 32%-35%; Figure 1 bottom panel). Of the 2941 patients in our study, 461 (16%) did not develop either acute GVHD or NIH chronic GVHD. In the entire cohort, 1433 (48.7%) patients had acute GVHD without NIH chronic GVHD, 100 (3.4%) had NIH chronic GVHD without acute GVHD, and 922 (31.3%) had both acute GVHD and NIH chronic GVHD. Among these 922 patients, 840 (91%) had acute GVHD before NIH chronic GVHD, 77 (8%) developed de novo NIH overlap syndrome, and 5 (0.5%) patients with de novo NIH classic chronic GVHD subsequently developed NIH overlap syndrome. The median time from HCT to onset of acute grades 2-4 GVHD was 20 (range, 3-711) days. The median time from HCT to onset of NIH chronic GVHD was 162 (range, 66-2805) days.
Figure 1.
Cumulative incidence of grades 2-4 acute GVHD (top panel) and NIH chronic GVHD (bottom panel).
Risk factors for the development of grades 2-4 acute GVHD
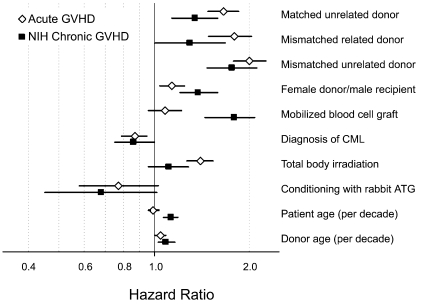
As shown in Figure 2, the multivariate analysis identified 4 factors strongly associated with an increased risk of grades 2-4 acute GVHD with P < .001. These risk factors included HCT with HLA-matched unrelated donors (HR, 1.66; 95% CI, 1.48-1.85), HCT with HLA-mismatched related donors (HR 1.74; 95% CI, 1.49-2.03), HCT with HLA-mismatched unrelated donors (HR, 2.00; 95% CI, 1.78-2.25), all compared with HCT with HLA-matched related donors, and the use of TBI in the conditioning regimen (HR 1.49; 95% CI, 1.27-1.54) compared with no TBI in the conditioning regimen. The use of a female donor for a male recipient was also associated with an increased risk of grades 2-4 acute GVHD, (HR, 1.14; 95% CI, 1.04-1.25; P = .006). The 2 factors associated with a decreased risk of grades 2-4 acute GVHD were the use of rabbit ATG in the pretransplantation conditioning regimen (HR 0.77; 95% CI, 0.58-1.03; P = .08) and diagnosis of CML (HR, 0.87; 95% CI, 0.79-0.95; P = .003). Grafting with growth factor–mobilized blood cells and patient and donor age showed no statistically significant associations with risk of grades 2-4 acute GVHD (Figure 2).
Figure 2.
Multivariate risk factor profiles for grades 2-4 acute GVHD and NIH chronic GVHD. Hazard ratio and 95% CI for each risk factor are shown. The analysis included 2355 grades 2-4 acute GVHD events and 1022 NIH chronic GVHD events. Hazard ratios are relative to patients without the risk factor.
Risk factors for development of NIH chronic GVHD treated with systemic immunosuppression
Seven factors showed statistically significant associations with an increased risk of NIH chronic GVHD (Figure 2 and Table 2): HCT with HLA-matched unrelated donors (P < .001), HCT with HLA-mismatched related donors (P = .05), HCT with HLA-mismatched unrelated donors (P < .001), all compared with HCT with HLA-matched related donors, the use of a female donor for a male recipient (P < .001), grafting with mobilized blood cells (P < .001), and older donor and recipient age (P = .006 and < .001, respectively). As shown in Figure 2, 2 factors associated with a decreased risk of NIH chronic GVHD were the use of rabbit ATG in the pretransplant conditioning regimen (P = .06) and a diagnosis of CML (P = .04).
Table 2.
Multivariate analysis of risk factors for NIH chronic GVHD before and after adjustment for acute GVHD
| Risk factor | Adjustment for acute GVHD,* HR (95% CI) |
|
|---|---|---|
| No | Yes | |
| Unrelated donor | 1.35 (1.14-1.59) | 1.30 (1.10-1.54) |
| HLA-mismatched related donor | 1.30 (1.01-1.68) | 1.24 (0.95-1.60) |
| HLA-mismatched unrelated donor | 1.76 (1.47-2.11) | 1.67 (1.39-2.00) |
| Female donor for male patient | 1.38 (1.21-1.59) | 1.37 (1.19-1.57) |
| Mobilized blood cell grafts | 1.74 (1.45-2.08) | 1.74 (1.46-2.08) |
| Diagnosis of CML | 0.86 (0.75-1.00) | 0.88 (0.77-1.02) |
| Conditioning with TBI | 1.11 (0.96-1.28) | 1.07 (0.92-1.34) |
| Conditioning with ATG | 0.68 (0.45-1.02) | 0.70 (0.47-1.06) |
| Patient age per decade | 1.13 (1.07-1.19) | 1.13 (1.07-1.19) |
| Donor age per decade | 1.09 (1.03-1.16) | 1.09 (1.02-1.15) |
| Acute GVHD grade† | ||
| 1 | n/a | 0.90 (0.62-1.30) |
| 2 | n/a | 1.14 (0.95-1.37) |
| 3-4 | n/a | 1.42 (1.14-1.77) |
HR indicates hazard ratio; and n/a, not applicable.
HRs are relative to patients without the risk factor.
Time-dependent covariate.
A separate analysis was carried out to assess the association of acute GVHD with risk of NIH chronic GVHD. When acute GVHD was evaluated as a time-dependent covariate with no adjustment for the risk factors listed in the left column of Table 2, results showed no statistically significant association of grade 1 (HR, 0.90; 95% CI, 0.62-1.30) or grade 2 (HR, 1.14; 95% CI, 0.95-1.37) acute GVHD with risk of NIH chronic GVHD. Grades 3-4 acute GVHD, however, showed a statistically significant association with an increased risk of NIH chronic GVHD (HR, 1.42; 95% CI, 1.14-1.77).
Table 2 compares risk factors for NIH chronic GVHD before and after adjustment for prior acute GVHD. Adjustment for prior acute GVHD produced little change in the hazard ratio point estimates and 95% confidence intervals for the association of other risk factors with NIH chronic GVHD. The absence of major change in the model indicates that the association of risk factors with NIH chronic GVHD is independent of their association with acute GVHD.
Comparison of risk factors for acute GVHD and NIH chronic GVHD
Whereas the overall profiles of risk factors for grades 2-4 acute GVHD and NIH chronic GVHD were similar, we found some notable differences (Figure 2). Recipient HLA mismatching and grafts from unrelated donors had a greater effect on the risk of acute GVHD compared with the effect on NIH chronic GVHD, whereas the use of a female donor for a male recipient had a greater effect on the risk of NIH chronic GVHD compared with the effect on the risk of acute GVHD. Our analysis identified 3 discordant associations with acute GVHD and NIH chronic GVHD: (1) the use of a mobilized blood cell graft was associated with an increased risk of NIH chronic GVHD but had no statistically significant association with the risk of acute GVHD; (2) the use of TBI in the conditioning regimen was associated with an increased risk of acute GVHD but had no statistically significant association with the risk of NIH chronic GVHD; and (3) older patient age was associated with an increased risk of NIH chronic GVHD and with a trend suggesting a slightly decreased risk of acute GVHD.
Discussion
Previous studies have identified a variety of factors associated with risks of acute7,9–14,17,31 and historically defined chronic GVHD (Table 3).20,23 In the current study, all of these risk factors showed statistically significant association with chronic GVHD defined by NIH criteria. To the best of our knowledge, no previous study has conducted a side-by-side analysis of risk factors for both acute and chronic GVHD in the same cohort using either historical diagnostic criteria for chronic GVHD or the NIH consensus definition.
Table 3.
Summary of factors associated with increased risk of grades 2-4 acute and chronic GVHD
| Factor | Acute GVHD |
Chronic GVHD |
||
|---|---|---|---|---|
| Previously reported* | Current study | Previously reported† | Current study‡ | |
| HLA mismatch or unrelated donor | yes | yes | yes | yes |
| Older patient age | yes | no | yes | yes |
| Older donor age | yes | yes | yes | yes |
| Female donor for male recipient | yes | yes | yes | yes |
| Parity of female donor (allosensitization) | yes | § | yes | § |
| Intensity of conditioning regimen | yes | ‖ | no | ‖ |
| Mobilized blood cell graft | no/yes | no | yes | yes |
| Donor lymphocyte infusion | yes | ¶ | yes | ¶ |
| Prior acute GVHD | n/a | n/a | yes | yes |
n/a indicates not applicable.
Results from selected large studies.7-10,12,14,16,21,22
Historically defined chronic GVHD.
By NIH chronic GVHD criteria.
Not consistently reported or data not available for analysis.
DLI was treated as a competing risk in our analysis for risk factors for GVHD.
All patients received a high-intensity conditioning regimen.
Our observation that grafting with mobilized blood cells was associated with an increased risk of NIH chronic GVHD is consistent with results of previous studies evaluating risk factors for historically defined chronic GVHD.17,21,22 Mobilized blood cell grafts have been consistently associated with increased risk of historically defined chronic GVHD but not with an increased risk of acute GVHD.14,17,32 The strong association of mobilized blood cells grafts with an increased risk of NIH chronic GVHD but not acute GVHD—and, again, the association of older age with an increased risk of NIH chronic GVHD but not acute GVHD—may suggest differences in the pathogenesis of these GVHD syndromes.
The use of rabbit ATG in the conditioning regimen was the only risk factor strongly associated with a decreased risk of both acute and NIH chronic GVHD, a finding that is in agreement with other reports using historical criteria for GVHD.33 The use of rabbit ATG may decrease the risk of acute GVHD by depleting donor T cells or by interfering with their activation by recipient alloantigens. Expansion of activated donor T cells results in the development of “cytokine storm,” resulting in tissue damage and causing the acute GVHD inflammatory phenotype in the skin, liver, and gastrointestinal tract. Chronic GVHD has discrete clinical features resembling a variety of collagen vascular disorders, including Sjogren syndrome and scleroderma. The decreased risk of chronic GVHD associated with the use of rabbit ATG is not completely understood, but may result from a reduction in thymic damage during clinically acute GVHD, thereby allowing appropriate negative selection of undesirable T cells that recognize donor or recipient alloantigens.34,35
We found that the risks of acute and NIH chronic GVHD were decreased in patients with CML compared with other diagnoses, but some studies have shown an increased risk of acute GVHD14,36 and chronic GVHD19,20 in patients with CML. The previously reported point estimates for the hazard ratio varied from 1.35 (95% CI, 1.15-1.59)14 to 1.6 (95% CI, 1.05-2.43)36 for acute GVHD, and was 1.52 (95% CI, 1.09-2.12)19 for chronic GVHD. The explanation for this discrepancy is not clear. The association of CML with reduced risks of acute GVHD and NIH chronic GVHD in our study was not strong.
Our results confirm earlier studies showing that older patient age is associated with an increased risk of chronic GVHD but not acute GVHD.18,37 The lack of association between older patient age and acute GVHD was independent of whether the conditioning regimen included TBI. The higher risk of NIH chronic GVHD in older patients might reflect a role for thymic dysfunction in the pathogenesis of chronic GVHD. Studies in mice have shown that thymic damage and defective negative selection of T cells generated from the marrow progenitors after HCT may be implicated in the pathogenesis of chronic GVHD.38,39 Contrary to our results, some studies have suggested that older patient age is associated with an increased risk of acute GVHD.11
Our study has several limitations. First, our results might not be applicable for patients treated at other centers. The incidence of acute GVHD at our center is much higher than that reported at other centers. We have previously reported a very high frequency of stage I gut GVHD presenting as anorexia, nausea, and vomiting, with little or no rash or diarrhea and no liver involvement.27,40 Endoscopic biopsy was used to confirm most cases of upper gastrointestinal GVHD, and symptoms typically resolved after treatment with prednisone at 1 mg/kg/d. Second, our observations might not apply with reduced-intensity conditioning regimens or with other approaches for preventing GVHD. For example, depletion of T cells from the graft can have a more striking effect on acute GVHD and more limited effects on chronic GVHD, whereas treatment with high-dose cyclophosphamide appears to have a more striking effect on chronic GVHD and more limited effects on acute GVHD. Third, the small number of informative patients who had had NIH chronic GVHD without prior acute GVHD provided limited statistical power to identify risk factors for NIH chronic GVHD as distinct from acute GVHD.
Despite the similarity of risk factors for acute GVHD and NIH chronic GVHD, our results indicate that the association of risk factors with acute GVHD does not explain the association of these same risk factors with NIH chronic GVHD. If the risk factors for NIH chronic GVHD were dependent on acute GVHD, we would have expected large differences between the unadjusted and adjusted analyses shown in Table 2. Our understanding of the pathogenesis of GVHD in humans remains incomplete, but recent advances in genetic profiling have begun to provide some insights. For example, Baron et al41 showed that increased or decreased expression of certain genes in CD4 and CD8 T cells of the donor was associated with the development of GVHD after HLA-identical HCT with a high-dose conditioning regimen. In that study, 46% of the involved genes in CD4 T cells were associated with both acute and chronic GVHD, whereas 54% were associated with one but not the other. Likewise, 38% of the involved genes in CD8 T cells were associated with both acute and chronic GVHD, whereas 62% were associated with one but not the other. The authors highlighted a subset of the genes associated specifically with chronic GVHD. Members of this subset included genes that regulate transforming growth factor-β signaling and cell proliferation. Although Baron et al41 did not analyze chronic GVHD according to NIH criteria, their results and our observations in the current study indicate that the mechanisms involved in acute and chronic GVHD are not entirely congruent, suggesting that chronic GVHD as defined by NIH criteria is a distinct entity and not simply an end-stage complication of acute GVHD.
Acknowledgments
We thank Drs Afonso Vigorito and Paulo Campregher for assisting with data collection, and the staff of the Long-Term Follow-Up (LTFU) program of the Fred Hutchinson Cancer Research Center for invaluable assistance with data recording, including Carina Moravec, Judy Campbell, Colleen McKinnon, Catherine Baker, Anne Chaffe, and Gina Brooks. We are also very grateful to our patients for their participation in clinical trials and to their referring physicians for their support in keeping our LTFU data updated.
This work was supported in part by grants CA18029, HL36444, CA15704, and CA 118953 from the National Institutes of Health, Department of Health and Human Services. Y.I. is a recipient of a Banyu Fellowship from Banyu Life Science Foundation International.
HLA typing for some donor and recipient pairs was provided by the National Marrow Donor Program (NMDP) and validated through their ongoing retrospective high-resolution typing program.29 The NMDP is supported by grant N00014-06-1-0704 through the Office of Naval Research. The views expressed in this article do not reflect the official policy or position of the Department of the Navy, the Department of Defense, or any other agency of the US Government.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.E.D.F. designed the study, collected and analyzed data, and wrote the paper; P.J.M. designed the study, analyzed data, and wrote the paper; B.E.S. performed the statistical analysis and reviewed the paper; Y.I., S.J.L., P.A.C., H.-P.K., R.A.N., M.M., M.L.F., E.H.W., J.E.S., R.F.S., and F.R.A. wrote the paper; and E.W.P. and S.E.P. updated the better HLA typing data and reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary E. D. Flowers, MD, Fred Hutchinson Cancer Research Center, D5-290, PO Box 19024, Seattle, WA 98109-1024; e-mail: mflowers@fhcrc.org.
References
- 1.Sullivan KM. Graft-versus-host disease. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. Malden, MA: Blackwell Sciences; 1999. pp. 515–536. [Google Scholar]
- 2.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 4.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97(4):855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 5.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 6.Anasetti C, Beatty PG, Storb R, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990;29(2):79–91. doi: 10.1016/0198-8859(90)90071-v. [DOI] [PubMed] [Google Scholar]
- 7.Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313(13):765–771. doi: 10.1056/NEJM198509263131301. [DOI] [PubMed] [Google Scholar]
- 8.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 9.Gale RP, Bortin MM, van Bekkum DW, et al. Risk factors for acute graft-versus-host disease. Br J Haematol. 1987;67(4):397–406. doi: 10.1111/j.1365-2141.1987.tb06160.x. [DOI] [PubMed] [Google Scholar]
- 10.Flowers ME, Pepe MS, Longton G, et al. Previous donor pregnancy as a risk factor for acute graft-versus-host disease in patients with aplastic anaemia treated by allogeneic marrow transplantation. Br J Haematol. 1990;74(4):492–496. doi: 10.1111/j.1365-2141.1990.tb06340.x. [DOI] [PubMed] [Google Scholar]
- 11.Weisdorf D, Hakke R, Blazar B, et al. Risk factors for acute graft-versus-host disease in histocompatible donor bone marrow transplantation. Transplantation. 1991;51(6):1197–1203. doi: 10.1097/00007890-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Nash RA, Pepe MS, Storb R, et al. Acute graft-versus-host disease: analysis of risk factors after allogeneic marrow transplantation and prophylaxis with cyclosporine and methotrexate. Blood. 1992;80(7):1838–1845. [PubMed] [Google Scholar]
- 13.Eisner MD, August CS. Impact of donor and recipient characteristics on the development of acute and chronic graft-versus-host disease following pediatric bone marrow transplantation. Bone Marrow Transplant. 1995;15(5):663–668. [PubMed] [Google Scholar]
- 14.Hahn T, McCarthy PL, Jr., Zhang MJ, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;26(35):5728–5734. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobsen N, Badsberg JH, Lonnqvist B, et al. Graft-versus-leukaemia activity associated with CMV-seropositive donor, post-transplant CMV infection, young donor age and chronic graft-versus-host disease in bone marrow allograft recipients. The Nordic Bone Marrow Transplantation Group. Bone Marrow Transplant. 1990;5(6):413–418. [PubMed] [Google Scholar]
- 16.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 17.Cutler C, Giri S, Jeyapalan S, Paniagua D, Viswanathan A, Antin JH. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol. 2001;19(16):3685–3691. doi: 10.1200/JCO.2001.19.16.3685. [DOI] [PubMed] [Google Scholar]
- 18.Storb R, Prentice RL, Sullivan KM, et al. Predictive factors in chronic graft-versus-host disease in patients with aplastic anemia treated by marrow transplantation from HLA-identical siblings. Ann Intern Med. 1983;98(4):461–466. doi: 10.7326/0003-4819-98-4-461. [DOI] [PubMed] [Google Scholar]
- 19.Carlens S, Ringden O, Remberger M, et al. Risk factors for chronic graft-versus-host disease after bone marrow transplantation: a retrospective single centre analysis. Bone Marrow Transplant. 1998;22(8):755–761. doi: 10.1038/sj.bmt.1701423. [DOI] [PubMed] [Google Scholar]
- 20.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 21.Körbling M, Anderlini P. Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood. 2001;98(10):2900–2908. doi: 10.1182/blood.v98.10.2900. [DOI] [PubMed] [Google Scholar]
- 22.Eapen M, Logan BR, Confer DL, et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transplant. 2007;13(12):1461–1468. doi: 10.1016/j.bbmt.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atkinson K, Horowitz MM, Gale RP, et al. Risk factors for chronic graft-versus-host disease after HLA-identical sibling bone marrow transplantation. Blood. 1990;75(12):2459–2464. [PubMed] [Google Scholar]
- 24.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 25.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Leisenring WM, Martin PJ, Petersdorf EW, et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood. 2006;108(2):749–755. doi: 10.1182/blood-2006-01-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin PJ, McDonald GB, Sanders JE, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):320–327. doi: 10.1016/j.bbmt.2003.12.304. [DOI] [PubMed] [Google Scholar]
- 28.Vigorito AC, Campregher PV, Storer BE, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114(3):702–708. doi: 10.1182/blood-2009-03-208983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spellman S, Setterholm M, Maiers M, et al. Advances in the selection of HLA-compatible donors: refinements in HLA typing and matching over the first 20 years of the National Marrow Donor Program Registry. Biol Blood Marrow Transplant. 2008;14(9 suppl):37–44. doi: 10.1016/j.bbmt.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 31.Stem Cell Trialists' Collaborative Group. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23(22):5074–5087. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bensinger WI, Clift R, Martin P, et al. Allogeneic peripheral blood stem cell transplantation in patients with advanced hematologic malignancies: a retrospective comparison with marrow transplantation. Blood. 1996;88(7):2794–2800. [PubMed] [Google Scholar]
- 33.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 34.Shulman HM, Sullivan KM. Graft-versus-host disease: allo- and autoimmunity after bone marrow transplantation. Concepts Immunopathol. 1988;6:141–165. [PubMed] [Google Scholar]
- 35.Tivol E, Komorowski R, Drobyski WR. Emergent autoimmunity in graft-versus-host disease. Blood. 2005;105(12):4885–4891. doi: 10.1182/blood-2004-12-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yakoub-Agha I, Mesnil F, Kuentz M, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24(36):5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 37.Storb R, Prentice RL, Buckner CD, et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med. 1983;308(6):302–307. doi: 10.1056/NEJM198302103080602. [DOI] [PubMed] [Google Scholar]
- 38.Sakoda Y, Hashimoto D, Asakura S, et al. Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood. 2007;109(4):1756–1764. doi: 10.1182/blood-2006-08-042853. [DOI] [PubMed] [Google Scholar]
- 39.Martin PJ. Biology of chronic graft-versus-host disease: implications for a future therapeutic approach. Keio J Med. 2008;57(4):177–183. doi: 10.2302/kjm.57.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mielcarek M, Storer BE, Boeckh M, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. 2009;113(13):2888–2894. doi: 10.1182/blood-2008-07-168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baron C, Somogyi R, Greller LD, et al. Prediction of graft-versus-host disease in humans by donor gene-expression profiling. PLoS Med. 2007;4(1):e23. doi: 10.1371/journal.pmed.0040023. [DOI] [PMC free article] [PubMed] [Google Scholar]