Abstract
AML1-ETO (AE) is a fusion product of t(8;21) observed in 40% French-American-British M2 type of acute myeloid leukemia (AML). Clinical data suggest that Ras mutation is a frequent cooperating event in t(8;21) AML. Whether constitutively active Ras promotes leukemogenesis on the t(8;21) background has not been demonstrated experimentally. Here, we retrovirally expressed N-RasG12D in AE-expressing human hematopoieticcells to investigate cooperativity. The AE/N-RasG12D cultures were cytokine-independent, enriched for CD34 positivity, and possessed increased colony-forming and replating abilities. N-RasG12D expression led to Bcl-2 up-regulation and reduced apoptosis. Ectopic Bcl-2 expression also resulted in enhanced colony-forming and replating abilities but was insufficient to sustain cytokine independence. AE/N-RasG12D cells were more sensitive to Bcl-2 inhibition with ABT-737 than parent AE cells. Enhanced engraftment of AE/N-RasG12D cells was observed on intrafemoral injection into immunodeficient mice, presumably because of improved survival in the bone marrow microenvironment. N-RasG12D promotes progression toward transfor-mation in AE-expressing cells, partially through up-regulating Bcl-2.
Introduction
The classic “2-hit” model proposes that a class I mutation, which confers proliferative and/or survival advantage, and a class II mutation, which impairs hematopoietic differentiation, are both required for leukemic transformation in acute myeloid leukemia (AML).1 AML1-ETO (AE) is a fusion product of translocation (8;21)(q22;q22), one of the recurrent balanced translocations in AML. AE is generally recognized as a class II mutation, as both murine and human studies indicate that AE promotes self-renewal but does not by itself cause leukemia.2–6 A clinical karyotype analysis suggested that AE may be present for as long as 10 years before disease onset, implying the necessity of a cooperating event for blast transformation.7 Indeed, murine models using mutagen treatment or activated receptor tyrosine kinases have provided evidence demonstrating fulfillment of the “2-hit” model in this type of AML.8–10
Ras is a family of small GTPase proteins that are involved in cell growth, survival, and differentiation. Aberrant Ras activation is frequently detected in human malignancies, including AML.11–13 It is not known whether Ras mutation plays a role in the initiation or progression in AML development.14 N-Ras is one of the most frequently mutated genes found in t(8;21) AML (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).9,15–18 In the current study, we delivered N-RasG12D retrovirally to AE-expressing human hematopoietic cells to analyze cooperativity.
Methods
Complete methods are provided in the Supplemental data. All experiments have been approved by the Institutional Review Board and the Institutional Animal Care and Use Committee of Cincinnati's Children's Hospital Medical Center.
Retroviral production, cell culture, and methylcellulose assay
AE cells and AE/N-RasG12D cells were cultured in media containing 20% BIT9500 with 10 ng/mL recombinant human cytokines, including stem cell factors, thrombopoietin, FMS-like tyrosine kinase 3 ligand, interleukin-3, and interleukin-6, as described.22 Protocols for magnetic selection of CD34+ cells, retroviral transduction, and methylcellulose assay have been described.8
Flow cytometric analysis
Human antibodies for flow cytometric analysis were all purchased from BD Biosciences. Cells were incubated with FcBlock (BD Biosciences) and antibodies (BD Biosciences) for 30 minutes at 4°C, washed with phosphate-buffered saline/2% fetal bovine serum, and analyzed on a FACSCantoII flow cytometer (BD Biosciences).
Antibodies and Western blotting
Anti–N-Ras, –H-Ras, and –K-Ras antibodies (Santa Cruz Biotechnology) were a kind gift from Dr Nancy Ratner (Cincinnati Children's Hospital Medical Center, Cincinnati, OH). Anti–Flt-3 antibody was from Santa Cruz Biotechnology. Western blotting was performed as described.21 Anti–Bcl-2 antibody was from Cell Signaling. Anti-β-actin antibody was from Sigma-Aldrich.
ABT-737 treatment and cell viability assay
ABT-737 compound was a kind gift from Abbott Laboratories. AE or AE/N-RasG12D cells were treated with ABT-737 at indicated concentrations for 72 hours, followed by cell viability measurement using WST-1 Cell Proliferation Assay Kit (Takara Mirus Bio).
Xenotransplantation study
To test engraftment, cells were injected into the bone marrow cavity of the left femur of irradiated mice. Alternatively, the cell suspension was mixed with Matrigel (BD Biosciences) at equal volume for subcutaneous injection. Statistical analysis was performed using t test assuming equal variance.
Results and discussion
It has been suggested that different downstream pathways are required for Ras-mediated oncogenesis in human and mouse cells.19 Therefore, we investigated the cooperativity between AE and N-RasG12D using human cells.6 After transducing AE-expressing CD34+ cord blood cells with N-RasG12D or control vector, cytokines were withdrawn to minimize wild-type Ras activation. All cells in the control cultures died within 7 days after cytokine withdrawal. Interestingly, although most AE/N-RasG12D cells underwent fibroblast-like differentiation or cell death after cytokine removal, the remaining viable cells repopulated the culture within 4 to 5 weeks in the absence of cytokines (Figure 1A). N-Ras was readily detectable using a monoclonal antibody (Figure 1B). For comparison, we also expressed FMS-like tyrosine kinase 3-internal tandem duplicate, another common class I mutation in AML, in AE cells, but did not observe cytokine-independent growth (supplemental Figure 1). Because the vector-transduced cells could not survive cytokine depletion, we performed the following experiments using nontransduced parent AE cells as control. Experiments have been repeated with multiple independently generated AE/N-RasG12D clones.
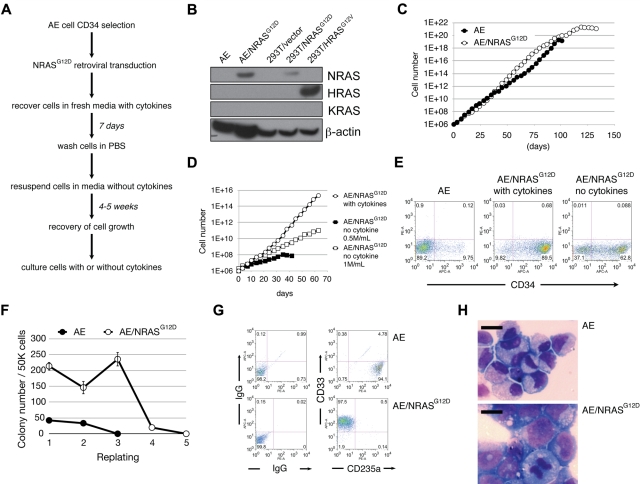
Figure 1.
AE/N-RasG12D cells exhibit various features of oncogenic transformation. (A) The “cytokine depletion protocol” for establishing AE/N-RasG12D cells. (B) Western blotting for the indicated proteins. (C) AE and AE/N-RasG12D cells were plated at 0.5 × 106/mL. Cells were counted twice a week, followed by replating at the initial density. (D) AE/N-RasG12D cells grown with cytokine supplementation were plated at 0.5 × 106/mL. AE/N-RasG12D cells grown in the absence of cytokines were plated at 0.5 × 106/mL or 1 × 106/mL. Cells were counted twice a week, followed by replating at the initial density. (E) AE and AE/N-RasG12D cells were stained with allophycocyanin-conjugated anti-CD34 antibody and analyzed by flow cytometry. (F) One representative experiment showing enhanced colony-forming and replating abilities of AE/N-RasG12D cells. CD34+ AE and AE/N-RasG12D cells were plated in methylcellulose media at 5 × 104 cells/mL per plate in triplicate. The number of colonies was scored after 14 days. Cells were collected, washed with phosphate-buffered saline, and replated at 5 × 104 cells/mL per plate in triplicate. (G) Cells collected from methylcellulose media were immunostained with antibodies against the indicated surface markers, followed by flow cytometric analysis. (H) A total of 1 × 105 cells were cytocentrifuged onto glass slides and Wright-Giemsa stained. Pictures were photographed using Leica LAS software with LeicaDMI6000B Inverted Research Microscpoe, 40× dry objective and Leica DFC290HD camera. Bars represent 10 μm.
AE/N-RasG12D cells have an extended life span compared with parent AE cells (Figure 1C). AE/N-RasG12D cells grow significantly slower and are strictly density-dependent when cultured in the absence of cytokines (Figure 1D). The majority of AE/N-RasG12D cells express the CD34 marker (Figure 1E). AE/N-RasG12D cells formed significantly more colonies and displayed improved replating ability relative to parent AE cells (Figure 1F). Surprisingly, despite the ability of AE/N-RasG12D cells to sustain cytokine-independent growth in liquid media, we observed no colony formation in methylcellulose in the absence of cytokines, suggesting a non–cell-autonomous effect possibly involving cell-cell contact or paracrine growth-promoting signals. Notably, most of the AE/N-RasG12D progeny cells were CD33+, as opposed to CD235a+ in the parent AE cell group in methylcellulose assays, suggesting a shift from erythroid-biased to myeloid-biased growth (Figure 1G). Moreover, a Wright-Giemsa staining showed that AE/N-RasG12D cells exhibited a blast-like morphology, with larger cell size, higher nuclear-to-cytoplasmic ratio, and less condensed chromatin structure (Figure 1H). Taken together, AE/N-RasG12D cells recapitulate several features consistent with progression toward AML, including cytokine-independent growth, increased myeloid clonogenicity, and blast-like morphology. However, the lack of immortalization suggests that transformation has not occurred.
We and others previously reported that AE expression is associated with repression of genes involved in DNA repair and an increase in baseline apoptosis.20,21 Interestingly, although AE expression in CD34+ cells led to Bcl-2 down-regulation, we found that N-RasG12D expression in AE cells resulted in Bcl-2 restoration, which was accompanied by a decrease in baseline apoptosis (Figure 2A-B). To determine whether Bcl-2 plays a role in the N-RasG12D phenotype, we ectopically expressed Bcl-2 in AE cells. We observed reduced apoptosis, increased colony-forming and replating abilities, and increased long-term culture-initiating cell frequency (Figure 2B-D). These findings suggest that Bcl-2 expression may provide survival benefit to the primitive AE/N-RasG12D cells. Treatment with ABT-737, an inhibitor of the Bcl-2 family antiapoptotic proteins, led to significantly reduced growth in AE/N-RasG12D cells compared with parent AE cells (Figure 2E). Moreover, the AE/N-RasG12D cells cultured without cytokines were even more sensitive to ABT-737 treatment than those cultured with cytokines, further suggesting that Bcl-2 plays a significant role in maintaining cellular viability in the absence of cytokines (Figure 2E). Nonetheless, Bcl-2 expression alone in AE cells was not sufficient to induce cytokine-independent growth, suggesting that additional N-RasG12D-induced signals are required for cytokine-independent growth.
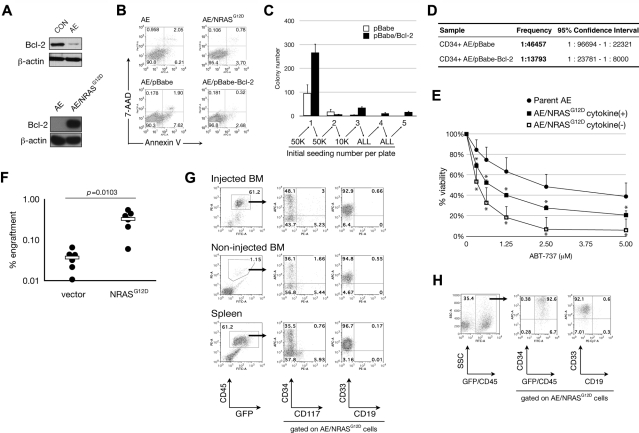
Figure 2.
AE/N-RasG12D cells have enhanced self-renewal and improved in vivo engraftment partly as a result of Bcl-2 up-regulation. (A) Top: CD34+ umbilical cord blood cells were retrovirally transduced with vector control (CON) or AE and expanded for 2 weeks. Cells were then collected for blotting of indicated proteins. Bottom: Protein lysates from AE and AE/N-RasG12D cells were blotted for the indicated proteins. (B) Cells were analyzed by flow cytometry for expression of annexin V and uptake of 7-amino-actinomycin D (7-AAD). (C) Colony formation assay was performed as described in Figure 1F with indicated cell numbers. (D) Long-term culture-initiating cells assay comparing AE/pBabe and AE/Bcl-2 cells. (E) Cells were treated with ABT-737 compound at indicated concentrations for 72 hours in triplicate, followed by WST-1 cell proliferation assay. Optical density (OD) values obtained from each experiment were normalized to vehicle control (0μM group). Averages from multiple experiments are shown: n = 5 in parent AE and AE/N-Ras cytokine(−); n = 3 in AE/N-Ras cytokine(+). Bars represent SD. *P < .05. (F) AE cells were transduced with viruses containing an empty vector or the N-RasG12D gene, followed by injection of 5 × 105 cells into the left femur of NSG mice. Initial transduction was approximately 20% in both groups. Six weeks after injection, bone marrow aspiration was performed in the injected bone to determine engraftment; n = 6 in each group. (●) represents one mouse. White bars represent average. (G-H) AE/N-RasG12D cells were injected intrafemorally into NSS mice (G) or NSG mice (H). Total bone marrow cells from injected and noninjected bones as well as total spleen cells were obtained by crushing the organs, followed by immunostaining with antibodies against indicated surface markers and flow cytometric analysis.
It has been demonstrated that N-RasG12D expression in cord blood did not improve in vivo engraftment in immunodeficient mice.22 To determine whether N-RasG12D promotes engraftment of AE cells, we transduced AE cells with empty vector or N-RasG12D, followed by direct injection into femurs of NOD/SCID/IL2rg−/− (NSG) mice. We found significantly higher engraftment (by one log) in the N-RasG12D group (Figure 2F). Similarly, established AE/N-RasG12D clones engrafted more efficiently than parent AE cells into NOD/SCID mice transgenic for stem cell factor, granulocyte-macrophage colony-stimulating factor, and interleukin-3 (NSS) and NSG mice (Figure 2G-H; and data not shown). Human cells (hCD45+) were detected in the noninjected bones and spleen, suggesting hematogenous spreading. The engrafted AE/N-RasG12D cells all expressed CD33. Notably, despite significantly increased engraftment, we did not detect overt leukemia development.
We also injected cells subcutaneously and found that AE/N-RasG12D cells, but not parent AE cells, formed a well-demarcated tumor (supplemental Figure 2A). Most cells derived from the tumor mass were of human origin with characteristics of myeloid differentiation (supplemental Figure 2B).
Our data support the idea that N-Ras activation cooperates with AE fusion protein in the stepwise leukemic transformation. The cooperativity illustrated in this report may further implicate the critical role of the hyperactivation of the N-Ras signaling pathway during t(8;21) leukemia formation, making this particular pathway a potential target for therapy development. The present model is different from the published AE9a/N-RasG12D murine model, where the role of N-RasG12D was in shortening disease latency rather than contributing to tumorigenesis.23 Furthermore, FMS-like tyrosine kinase 3–internal tandem duplicate, another frequently observed class I mutation in AML, was insufficient to induce cytokine-independent growth of AE cells but was sufficient in MLL-AF9–expressing cells (M.W. and J.C.M., unpublished observations, August 2006), indicating the unique role of N-RasG12D in substituting for cytokine signals in AE cells. The findings of sufficient Bcl-2 restoration on N-RasG12D expression to account for the increased frequency of primitive cells and reduced baseline apoptosis recapitulate our earlier findings that AE expression impairs cell survival (because of defective DNA repair mechanisms and subsequent increased p53 activity).21 It is possible that improved survival may also account for improved engraftment of AE/N-RasG12D cells in immunodeficient mice. Indeed, a recent study in colon cancer suggests that the primary role of a constitutively active N-Ras mutant is to promote cell survival.24 In conclusion, we propose that N-RasG12D cooperates with AE in stepwise AML formation in humans partly by improving survival of the primitive AE-expressing cells and by promoting cytokine-independent growth. The model may facilitate investigation of novel therapy against a hyperactive N-Ras pathway in t(8;21) AML treatment.
Supplementary Material
Acknowledgments
The authors thank the Cincinnati Children's Hospital Medical Center Flow Cytometry Core for flow cytometry help, and the Cincinnati Children's Hospital Medical Center Comprehensive Mouse and Cancer Core for irradiation of mice.
This work was supported by the Translational Trials Development and Support Laboratory for umbilical cord blood (USPHS grant MO1 RR 08084), the CancerFreeKids Association (F.-S.C.), and the National Institutes of Health (grant CA118319) (J.C.M.)
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: F.-S.C. designed and performed experiments and prepared the manuscript; M.W. performed experiments and prepared the manuscript; A.G. performed experiments; and J.C.M. designed experiments and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James C. Mulloy, Division of Experimental Hematology and Cancer Biology, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Mail Location 7013, TCHRF 7529, Cincinnati, OH 45229; e-mail: James.Mulloy@cchmc.org.
References
- 1.Kelly LM, Yu JC, Boulton CL, et al. CT53518, a novel selective FLT3 antagonist for the treatment of acute myelogenous leukemia (AML). Cancer Cell. 2002;1(5):421–432. doi: 10.1016/s1535-6108(02)00070-3. [DOI] [PubMed] [Google Scholar]
- 2.Yergeau DA, Hetherington CJ, Wang Q, et al. Embryonic lethality and impairment of haematopoiesis in mice heterozygous for an AML1-ETO fusion gene. Nat Genet. 1997;15(3):303–306. doi: 10.1038/ng0397-303. [DOI] [PubMed] [Google Scholar]
- 3.Okuda T, Cai Z, Yang S, et al. Expression of a knocked-in AML1-ETO leukemia gene inhibits the establishment of normal definitive hematopoiesis and directly generates dysplastic hematopoietic progenitors. Blood. 1998;91(9):3134–3143. [PubMed] [Google Scholar]
- 4.Mulloy JC, Cammenga J, Berguido FJ, et al. Maintaining the self-renewal and differentiation potential of human CD34+ hematopoietic cells using a single genetic element. Blood. 2003;102(13):4369–4376. doi: 10.1182/blood-2003-05-1762. [DOI] [PubMed] [Google Scholar]
- 5.Basecke J, Schwieger M, Griesinger F, et al. AML1/ETO promotes the maintenance of early hematopoietic progenitors in NOD/SCID mice but does not abrogate their lineage specific differentiation. Leuk Lymphoma. 2005;46(2):265–272. doi: 10.1080/10428190400010767. [DOI] [PubMed] [Google Scholar]
- 6.Mulloy JC, Cammenga J, MacKenzie KL, Berguido FJ, Moore MA, Nimer SD. The AML1-ETO fusion protein promotes the expansion of human hematopoietic stem cells. Blood. 2002;99(1):15–23. doi: 10.1182/blood.v99.1.15. [DOI] [PubMed] [Google Scholar]
- 7.Wiemels JL, Xiao Z, Buffler PA, et al. In utero origin of t(8;21) AML1-ETO translocations in childhood acute myeloid leukemia. Blood. 2002;99(10):3801–3805. doi: 10.1182/blood.v99.10.3801. [DOI] [PubMed] [Google Scholar]
- 8.Grisolano JL, O'Neal J, Cain J, Tomasson MH. An activated receptor tyrosine kinase, TEL/PDGFbetaR, cooperates with AML1/ETO to induce acute myeloid leukemia in mice. Proc Natl Acad Sci U S A. 2003;100(16):9506–9511. doi: 10.1073/pnas.1531730100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schessl C, Rawat VP, Cusan M, et al. The AML1-ETO fusion gene and the FLT3 length mutation collaborate in inducing acute leukemia in mice. J Clin Invest. 2005;115(8):2159–2168. doi: 10.1172/JCI24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higuchi M, O'Brien D, Kumaravelu P, Lenny N, Yeoh EJ, Downing JR. Expression of a conditional AML1-ETO oncogene bypasses embryonic lethality and establishes a murine model of human t(8;21) acute myeloid leukemia. Cancer Cell. 2002;1(1):63–74. doi: 10.1016/s1535-6108(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 11.Farr CJ, Saiki RK, Erlich HA, McCormick F, Marshall CJ. Analysis of RAS gene mutations in acute myeloid leukemia by polymerase chain reaction and oligonucleotide probes. Proc Natl Acad Sci U S A. 1988;85(5):1629–1633. doi: 10.1073/pnas.85.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartram CR, Ludwig WD, Hiddemann W, et al. Acute myeloid leukemia: analysis of ras gene mutations and clonality defined by polymorphic X-linked loci. Leukemia. 1989;3(4):247–256. [PubMed] [Google Scholar]
- 13.Ahuja HG, Foti A, Bar-Eli M, Cline MJ. The pattern of mutational involvement of RAS genes in human hematologic malignancies determined by DNA amplification and direct sequencing. Blood. 1990;75(8):1684–1690. [PubMed] [Google Scholar]
- 14.MacKenzie KL, Dolnikov A, Millington M, Shounan Y, Symonds G. Mutant N-Ras induces myeloproliferative disorders and apoptosis in bone marrow repopulated mice. Blood. 1999;93(6):2043–2056. [PubMed] [Google Scholar]
- 15.Kiyoi H, Naoe T, Nakano Y, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93(9):3074–3080. [PubMed] [Google Scholar]
- 16.Bowen DT, Frew ME, Hills R, et al. RAS mutation in acute myeloid leukemia is associated with distinct cytogenetic subgroups but does not influence outcome in patients younger than 60 years. Blood. 2005;106(6):2113–2119. doi: 10.1182/blood-2005-03-0867. [DOI] [PubMed] [Google Scholar]
- 17.Bacher U, Haferlach T, Schoch C, Kern W, Schnittger S. Implications of N-Ras mutations in AML: a study of 2502 patients. Blood. 2006;107(10):3847–3853. doi: 10.1182/blood-2005-08-3522. [DOI] [PubMed] [Google Scholar]
- 18.Kuchenbauer F, Schnittger S, Look T, et al. Identification of additional cytogenetic and molecular genetic abnormalities in acute myeloid leukaemia with t(8;21)/AML1-ETO. Br J Haematol. 2006;134(6):616–619. doi: 10.1111/j.1365-2141.2006.06229.x. [DOI] [PubMed] [Google Scholar]
- 19.Hamad NM, Elconin JH, Karnoub AE, et al. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 2002;16(16):2045–2057. doi: 10.1101/gad.993902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcalay M, Meani N, Gelmetti V, et al. Acute myeloid leukemia fusion proteins deregulate genes involved in stem cell maintenance and DNA repair. J Clin Invest. 2003;112(11):1751–1761. doi: 10.1172/JCI17595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krejci O, Wunderlich M, Geiger H, et al. p53 signaling in response to increased DNA damage sensitizes AML1-ETO cells to stress-induced death. Blood. 2008;111(4):2190–2199. doi: 10.1182/blood-2007-06-093682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen SW, Dolnikov A, Passioura T, et al. Mutant N-Ras preferentially drives human CD34+ hematopoietic progenitor cells into myeloid differentiation and proliferation both in vitro and in the NOD/SCID mouse. Exp Hematol. 2004;32(9):852–860. doi: 10.1016/j.exphem.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Zuber J, Radtke I, Pardee TS, et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev. 2009;23(7):877–889. doi: 10.1101/gad.1771409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haigis KM, Kendall KR, Wang Y, et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40(5):600–608. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.