Abstract
The reticulon (Rtn) family of proteins are localized primarily to the endoplasmic reticulum (ER) of most cells. The Rtn-4 family, (aka Nogo) consists of 3 splice variants of a common gene called Rtn-4A, Rtn-4B, and Rtn-4C. Recently, we identified the Rtn-4B (Nogo-B) protein in endothelial and smooth muscle cells of the vessel wall, and showed that Nogo-B is a regulator of cell migration in vitro and vascular remodeling and angiogenesis in vivo. However, the role of Nogo-B in inflammation is still largely unknown. In the present study, we use 2 models of inflammation to show that endothelial Nogo-B regulates leukocyte transmigration and intercellular adhesion molecule-1 (ICAM-1)–dependent signaling. Mice lacking Nogo-A/B have a marked reduction in neutrophil and monocyte recruitment to sites of inflammation, while Nogo-A/B−/− mice engrafted with wild-type (WT) bone marrow still exhibit impaired inflammation compared with WT mice engrafted with Nogo-A/B−/− bone marrow, arguing for a critical role of host Nogo in this response. Using human leukocytes and endothelial cells, we show mechanistically that the silencing of Nogo-B with small interfering RNA (siRNA) impairs the transmigration of neutrophils and reduces ICAM-1–stimulated phosphorylation of vascular endothelial-cell cadherin (VE-cadherin). Our results reveal a novel role of endothelial Nogo-B in basic immune functions and provide a key link in the molecular network governing endothelial-cell regulation of diapedesis.
Introduction
The reticulon (Rtn) family of proteins are localized primarily to the endoplasmic reticulum (ER) of most cells.1,2 In mammals, there are 4 family members, Rtn-1, Rtn-2, Rtn-3, and Rtn-4, with each gene giving rise to multiple isoforms. Insights into Rtn functions have been dissected using overexpression, knock-down, or knockout strategies, and a clear role for these proteins in tubulogenesis of peripheral ER and membrane curvature has emerged.3–5 However, despite the similarities of these proteins, there is evidence that different isoforms of each Rtn subclass may exert additional roles in mammalian cell function other than establishing the ER membrane curvature.
The Rtn-4 family (aka Nogo) consists of 3 splice variants of a common gene called Rtn-4A, Rtn-4B, and Rtn-4C. Nogo-A and Nogo-C are highly expressed in the central nervous system,6,7 with Nogo-C uniquely found in skeletal muscle and Nogo-B found in most tissues.8 We have previously reported that Nogo-B, but not Nogo-A, is expressed in mouse blood vessels and in human endothelial cells, with the majority found in the ER and a lower amount on the cell surface.8 Nogo-A has been characterized as a myelin-associated inhibitor of axonal sprouting9 and Nogo-B regulates aspects of blood vessel and tissue remodeling; however, the role of Nogo-B in the inflammatory processes is virtually unknown.
Leukocyte accumulation into sites of injury is a key feature of inflammatory diseases such as rheumatoid arthritis, autoimmune diseases, atherosclerosis, and sepsis. This process involves a sequence of adhesive events between leukocytes and endothelial receptors that culminates in leukocyte extravasation across the endothelium.10 The first interaction between leukocytes and the endothelium involves the endothelial adhesion molecules E- and P-selectin, followed by intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), leading to firm adhesion and subsequent transmigration, while other receptors such as platelet endothelial cell adhesion molecule 1 (PECAM-1),11 junctional adhesion molecule 1 (JAM1),12 and CD9913 contribute to facilitate diapedesis.14 However, endothelial VCAM-1, as well as VCAM, are not only docking receptors for leukocyte adhesion and migration, but their engagement by leukocyte integrins α4β1 and αL/Mβ2, respectively, triggers “outside-in signaling” toward the endothelium. This event contributes to leukocyte diapedesis and the endothelial inflammatory response.15–17 ICAM-1 is essential for stable adhesion and transmigration of leukocytes in most types of inflammatory processes.18 Further, blocking antibodies against ICAM-1 inhibit leukocyte adhesion, while the deletion of the cytoplasmic domain of ICAM-1 completely blocks neutrophil transmigration but not the adhesion, demonstrating the importance of ICAM-1–dependent signaling in mediating neutrophil transmigration.19 ICAM-1 engagement or antibody-mediated cross-linking of ICAM-1 induces endothelial signaling, including elevation of intracellular Ca2+, changes in the cytoskeleton, and activation of the small GTPase Rho, mitogen-activated protein kinase (MAPK), Pyk2, c-src, and protein kinase C.20–22 It has been reported that ICAM-1–mediated tyrosine phosphorylation of vascular endothelial cadherin (VE-cadherin),23,24 a major component of adherens junctions, is a critical event regulating leukocyte transendothelial migration (TEM) and is dependent on the tyrosine kinases src and Pyk2.21,22,25
In the present study, we show that endothelial Nogo regulates leukocyte TEM by modulating ICAM-1–dependent signaling. Mice lacking Nogo-A/B have a marked reduction in neutrophil and monocyte recruitment to sites of inflammation. Despite the presence of Nogo-B in neutrophils and monocytes, its loss does not affect neutrophil migration and monocyte activation in vitro; in addition, bone marrow transplantation experiments clearly support a role of host Nogo-B in driving the inflammatory response. The role of endothelial Nogo-B is bolstered by reduced neutrophil and monocyte transmigration in human dermal microvascular endothelial cells (HDMECs) deficient in Nogo-B. Mechanistically, the loss of Nogo-B impairs ICAM-1–mediated signaling to src and Pyk2, thereby reducing the phosphorylation of VE-cadherin, which can explain reduced leukocyte transmigration. These data identify Nogo-B as a new component of the adhesion-induced signaling leading to inflammation.
Methods
Animals
Nogo-A/B–deficient mice were generated as described previously.8 Congenic male, 8- to 14-week-old Nogo-A/B−/− mice and their wild-type (WT) littermates were used. All experiments were approved by the Institutional Animal Care and Use Committee of Yale University.
Induction of edema in the mouse paw
Male Nogo-A/B−/− mice and their WT littermates were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). Each group of animals received a subplantar injection of 50 μL of saline or λ-carrageenan (Sigma-Aldrich) 2% wt/vol in saline into the left footpad, and the paw volume was measured as described previously.26,27 In some experiments, paws were fixed in paraformaldehyde, and frozen sections (6 μm) were stained with the monocyte differentiation marker CD68.
Carrageenan air pouch
Air pouches were generated as described previously.27 On day 6, 0.5 mL of carrageenan suspension (1% wt/vol) in sterile saline was injected into the pouch. Exudates and leukocytes were recovered from the pouches 1, 4, or 24 hours after carrageenan injection. Migrated leukocytes (mainly neutrophils) were counted using trypan blue staining. Western blot analysis for inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), and caspase-3 was performed on air pouch–recruited leukocytes 24 hours after carrageenan injection.
HDMEC culture and treatment
HDMECs were cultured in EGM2-MV growth medium (Cambrex), as described previously,17 and were used between passages 4 and 8 for the experiments described. Cells were stimulated for 20 hours with tumor necrosis factor-α (TNF-α; 10 ng/mL), serum-starved for 3 hours, and incubated for 1 hour at 37°C with a monoclonal antibody against human ICAM-1 (clone 15.2; Serotec), followed by cross-linking with GAM immunoglobulin G (IgG) for 5, 10, and 15 minutes.
Isolation of murine endothelial cells from lung tissue
Primary endothelial cells from WT and Nogo-A/B−/− adult mice lungs (MLECs) were isolated as described previously.28 The bead-bound cells were plated in gelatin-coated dishes in complete culture medium (Dulbecco modified Eagle medium containing 20% fetal calf serum, 100 μg/mL of heparin, and 100 μg/mL of οϕ endothelial cell growth factor growth supplement). After TNF-α administration (10 ng/mL for 24 hours), MLECs were serum starved for 2 hours, incubated with a rat anti–ICAM-1 antibody (BioLegend) for 30 minutes at 37°C, followed by ICAM-1 cross-linking with a secondary antibody donkey anti–rat (Jackson Immunoresearch Laboratories) for 5, 10, and 15 minutes.
Murine neutrophil isolation
Mice were euthanized and femurs and tibias removed. The ends of the bones were resected and the bone marrow was removed by perfusion of 5 mL of ice-cold phosphate-buffered saline (PBS). The bone marrow was then suspended by drawing it through a 20-gauge needle and the cell solution obtained over a discontinuous Percoll gradient consisting of Histopaque-1119 and Histopaque-1077. The cell solution was centrifuged for 30 minutes at room temperature according to the manufacturer's instructions. Purified murine neutrophils were washed in RPMI buffer and suspended in RPMI medium containing 2% fetal bovine serum at 2.0 × 106 cells/mL.
In vitro migration assay
Chemotaxis of bone marrow neutrophils in response to N-formyl-Met-Leu-Phe (fMLP) and human interleukin-8 (IL-8) was assayed using the ChemoTx system (Neuro Probe) according to the manufacturer's protocol described previously.27
Primary culture of BMDMs
Bone marrow–derived monocytes (BMDMs) were obtained as reported previously,27 seeded in 6-well plates at a density of 1 × 106 cells per well, and cultured overnight at 37°C before being stimulated by medium supplemented by exudates (diluted 1 to 10) from a 24-hour carrageenan air pouch.
Bone marrow transplantation
Eight-week-old male Nogo-A/B−/− and WT littermate mice were subjected to bone marrow transplantation.27 Five weeks after transplantation, peripheral blood was collected by tail vein for polymerase chain reaction (PCR) analysis of bone marrow reconstitution. Carrageenan-induced paw edema was induced in fully chimeric mice as described above.
TEM assays
Nogo-B siRNA and control treated HDMECs were grown to confluence on 35-mm fibronectin-coated cover glasses, incubated with 10 ng/mL of TNF-α for 4 hours (24 hours for monocyte TEM) before the flow assays, and assembled with a parallel-flow–chamber apparatus (GlycoTech), as described previously.29 Discarded anonymized human leukapheresis collections from adult blood were obtained from the Yale New Haven Hospital Blood Bank under a protocol approved by the Yale Human Investigation Committee. The isolation of human neutrophils was performed using the gradient density centrifugation method with Histopaque-1077 solution (Sigma-Aldrich) according to the manufacturer's instructions. Human neutrophils (106 cells/500 μL) suspended in the same medium were loaded onto the endothelial cell monolayer at 1 dyne/cm2 for 2 minutes, followed by medium only at 1 dyne/cm2 for 10 minutes. Samples were then fixed with 3.7% formaldehyde in PBS, samples were stained with fluorescein isothiocyanate (FITC)–conjugated anti-CD11b (Immunotech) for neutrophils or FITC-conjugated anti-CD45 for monocyte THP-1, followed by Alexa Fluor 488–conjugated rabbit anti-FITC and Alexa Fluor 488–conjugated goat anti–rabbit IgG to increase the signal, and examined by microscopy. Using a 40×/0.60 Korr Ph2 objective, phase-contrast optics were used to determine whether neutrophils or monocyte cells were on top of, in between, or underneath the endothelial cell monolayer. Neutrophils or monocytes that were captured, not spread, were round and bright when viewed under phase-contrast microscopy. Cells considered transmigrated were either spread with a cellular protrusion between endothelial junctions or completely transmigrated under the endothelial monolayer, with a darker contrast to adherent cells on top of the endothelial cell monolayer. The percentage of transmigrated cells was calculated as reported previously.29 For cell-capture experiments, samples were stained as aforementioned. The number of CD11b+ (or CD45+) cells in 10 fields (1000 × 1000 pixels) viewed with a 10× objective were counted to determine the number of cells per field.
Western blot analysis and immunoprecipitation
Tissues were snap-frozen in liquid nitrogen, pulverized, resuspended in lysis buffer, and proteins were analyzed with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting, as described previously.27 Primary antibodies included the following: against Nogo (1-18) and VE-cadherin (Santa Cruz Biotechnology); heat-shock protein 90 (Hsp90), caveolin-1 (Cav-1), anti-phosphotyrosine PY20, and focal adhesion kinase (FAK; BD Transduction Laboratories); β-actin and β-catenin (Sigma-Aldrich); F4/80 (Serotec); COX-2 and iNOS (Cayman-Chemical); ICAM-1 (R&D Systems); PECAM-1 (Dako); src (Millipore); src-pY418 and FAK-pY861 (Invitrogen); and pyk2, pyk2-pY402, myosin light chain (MLC), and pMLC (Cell Signaling Technology). Secondary antibodies were fluorescence labeled (LI-COR Biosciences). Bands were visualized with the Odyssey Infrared Imaging System (LI-COR Biosciences). For immunoprecipitation, cells were lysed in a buffer containing 10mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-NaOH, pH 7.4, 100mM NaCl, 50mM β-glycerophosphate, 2mM mgCl2, 5mM EGTA (ethyleneglycoltetraacetic acid), 5mM EDTA (ethylenediaminetetraacetic acid), 1% IGEPAL (Sigma-Aldrich), 1mM orthovanadate, 1mM NaF, and protease inhibitors (Roche).27 Clarified cell extracts were incubated with 2 μg of antibody against phosphotyrosine (PY20) or against VE-cadherin (Santa Cruz Biotechnology). Antigen-antibody complexes were collected using protein G-Sepharose beads (Sigma-Aldrich). SDS-PAGE and immunoblotting were performed as described above, and used as the antibody against VE-cadherin (Santa Cruz Biotechnology) or phophotyrosine (PY20, 4G10).
Sucrose gradient centrifugation
HDMECs treated with control or Nogo-B siRNA and stimulated with TNF-α were lysed with 1 mL of 500mM Na2CO3, pH 11, supplemented with a protease-inhibitor cocktail (Roche Diagnostics). Solubilized material was resolved on top of a 5%-42% linear sucrose gradient (12 mL), as described previously.30 Equal volumes of each fraction were loaded and run on the SDS/12% PAGE, and analyzed by Western blotting for ICAM-1, VE-cadherin, Nogo-B, and Cav-1 with the antibodies.
Flow cytometric analysis
Analysis for lineage and adhesion molecules expressed by WT and Nogo-A/B−/− BMDMs was performed as reported previously.27 Briefly, BMDMs were washed and incubated with the fluorochrome-conjugated monoclonal antibodies FITC-conjugated rat anti-CD11a (Pharmingen), FITC-conjugated rat anti-CD11b (BioLegend), phycoerythrin-Cy5–conjugated rat anti-F4/80 (eBioscience), and phycoerythrin-conjugated mouse anti–PGSL-1 (BD Biosciences), and analyzed with an FACSCalibur (BD Biosciences).
HDMECs were incubated with 5 μg of E-selectin, ICAM-1, and VECAM-1 (R&D Systems), or with isotype-matched, FITC-labeled control antibody.
Immunofluorescent staining in cultured cells.
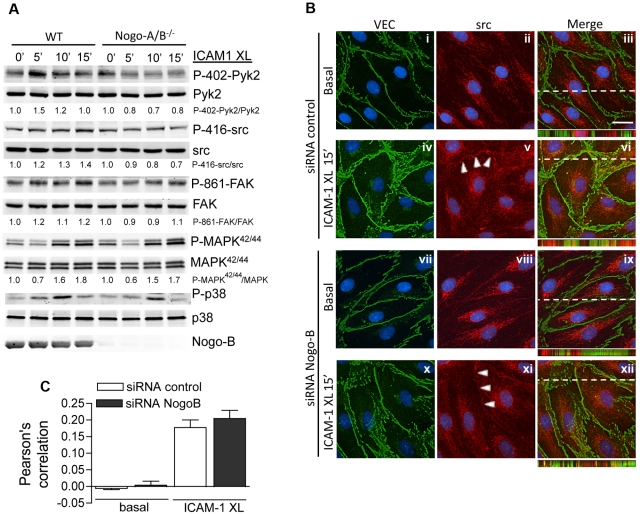
After siRNA-treated HDMECs were grown to confluence and stimulated with TNF-α (10 ng/mL) overnight, cells were serum starved with serum-free endothelial cell basal medium-2 for 2 hours before incubation with anti–ICAM-1 antibody (1 μg/mL; Serotech) for 45 minutes at 37°C. Cells were rinsed and 1 μg/mL of fluorophore-coupled secondary antibodies was added for 0 and 60 minutes. Cells were washed with PBS and fixed in 3.7% paraformaldehyde for 10 minutes, blocked with 5% bovine serum albumin in PBS for 10 minutes, permeabilized with 0.2% Triton X-100 in PBS for 5 minutes, and incubated overnight with α-Nogo-B (1:200; Santa Cruz Biotechnology) at 4°C. Alexa Fluor 488–labeled phalloidin (1:50; Molecular Probes). Images were captured with a Zeiss Axiovert epifluorescence microscope, followed by deconvolution using Openlab software (Improvision). Images shown in Figure 7B were captured at a 0.3-μm slice thickness (z-stack) using a Zeiss Axiovert epifluorescence microscope and a 63× oil-immersion objective, after deconvolution with Openlab software.
Figure 7.
Nogo-B mediates ICAM-1–induced src and Pyk2 activation in MLECs, but does not affect VE-cadherin or src localization. (A) Nogo-A/B−/− and WT MLECs were treated with TNF-α as above, followed by ICAM-1 cross-linking for 5 minutes, 10 minutes and 15 minutes. Lysates were analyzed by immunoblotting with antibodies and the phospho/total ratio determined for each pathway. (B) Immunofluorescence microscopy of VE-cadherin (green) and src (red) in TNF-α–stimulated HDMECs in the absence or presence of Nogo-B before (basal) and after ICAM-1 cross-linking (ICAM1-XL, 15 minutes). Blue reflects 4,6-diamidino-2-phenylindole, dihydrochloride staining of nuclei. Bar = 20 μm. The images in panel B were captured at a 0.3-μm slice thickness (z-stack) using a Zeiss Axiovert epifluorescence microscope and a 63× oil-immersion objective, followed by deconvolution using Openlab software. In panel C, the colocalization of src and VE-cadherin was quantified using the Pearson correlation. Data are representative of at least 3 experiments. XL, cross-linking.
Statistical analysis
Data are expressed as means ± standard error of the mean (SEM). The level of statistical significance was determined by analysis of variance, followed by the Bonferroni t test for multiple comparisons or Student t test using GraphPad Prism software Version 4.
Results
Vascular Nogo-A/B drives the influx of neutrophils at the site of inflammation
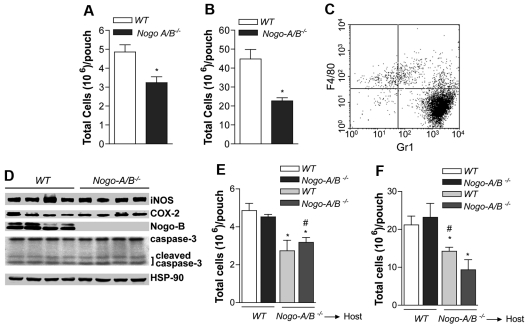
To investigate the role of Nogo-A/B in acute inflammation, particularly in neutrophil recruitment, carrageenan and zymosan air-pouch models were created. The advantage of the pouch model is the ability to recover, quantify, and analyze leukocytes (mainly neutrophils) from the pouch after instillation of carrageenan or zymosan. Carrageenan is thought to induce nonimmune-mediated inflammation,31 while zymosan generates immune-mediated responses.32,33 As seen in Figure 1A-B, the number of cells that emigrated from blood into tissue was drastically reduced in Nogo-A/B−/− mice (using carrageenan or zymosan, respectively). In the early phase of inflammation (4 hours), recruited leukocytes were largely Gr-1 positive and F4/80 depleted, which is consistent with a neutrophil-rich infiltrate (Figure 1C). Given that the number of neutrophils regress in the later/resolution phase of inflammation via neutrophil apoptosis and phagocytosis by inflammatory macrophages,34 inflammatory cells (mainly neutrophils) were recovered from the pouches (24 hours after carrageenan) and examined for activation and levels of apoptosis. WT and Nogo-A/B−/− neutrophils showed induction of iNOS and COX-2, as well as increased levels of cleaved caspase-3, an index of apoptosis (Figure 1D), suggesting that the loss of Nogo-A/B did not influence neutrophil activation or apoptosis.
Figure 1.
Vascular Nogo-A/B regulates neutrophil infiltration into air pouches. Nogo-A/B−/− mice displayed a significant reduction of (A) carrageenan and (B) zymosan (1% or each, wt/vol)-induced neutrophil recruitment into the air pouches 4 hours after instillation compared with WT mice (n = 5 per group). (C) Four hours after intrapouch injection of carrageenan, leukocytes were recovered and evaluated for lineage molecule expression Gr-1 (neutrophil marker) and CD68 (monocyte/macrophage marker) on leukocytes isolated from the pouch or (D) 24 hours for evaluation of protein expression by Western blot analysis (n = 4 per group). (E) Carrageenan and (F) zymosan air-pouch models were created in chimeric mice WT → Nogo-A/B−/−, Nogo-A/B−/− → WT and control WT → WT, Nogo-A/B−/− → Nogo-A/B−/− (n = 5 per group). Mice were killed 4 hours after instillation, and viable cells recovered from the pouches were counted using trypan blue. The data shown represent the means ± SEM. *P < .05; **P < .01 compared with WT group; #P < .05 compared with Nogo-A/B−/− → WT group.
In another set of experiments, we evaluated whether the loss of Nogo-A/B could affect the release of chemokines during the initial phase of the inflammatory response. WT and Nogo-A/B−/− mice were injected with carrageenan, and 1 hour later the exudates were recovered from the pouches and tested for CXCL-1 chemokines, the neutrophil chemoattractant KC, and macrophage inflammatory protein-2 (MIP-2). The levels of chemokines in WT and Nogo-A/B−/− exudates were, respectively, 92.7 ± 31.1 and 90.2 ± 20.1 ng/mL for KC, and 124.3 ± 20.0 and 131.7 ± 15.6 pg/mL, respectively, for MIP-2 (n = 4 per group). These data suggested that the defect of inflammatory cell recruitment in Nogo-A/B−/− mice was not because of an impairment in chemokine production.
We performed bone marrow transplantation experiments to investigate the role of Nogo-A/B in leukocytes versus host vasculature. Nogo-A/B−/− and WT mice were lethally irradiated, engrafted with bone marrow from WT mice (WT → WT; WT → Nogo-A/B−/−) or Nogo-A/B−/− mice (Nogo-A/B−/− → WT; Nogo-A/B−/− → Nogo-A/B−/−) and left to reconstitute for 6 weeks. After this time, mice were subjected to carrageenan and zymosan air-pouch models. In both models of inflammation, the number of neutrophils recovered 4 hours later was significantly reduced in Nogo-A/B−/− mice engrafted with WT or Nogo-A/B−/− bone marrow compared with WT mice engrafted with WT or Nogo-A/B−/− bone marrow (Figure 1E-F, respectively). These provocative data suggest that host Nogo-A/B, presumably endothelial Nogo-B, is necessary for neutrophil extravasation from the bloodstream to the site of inflammation.
Vascular Nogo-A/B drives monocyte/macrophage recruitment in response to carrageenan
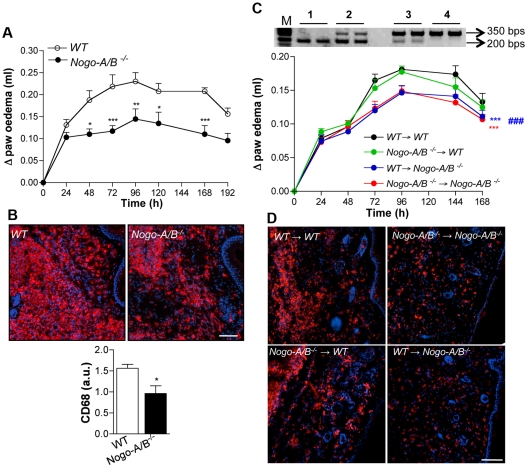
To provide additional support for the idea that vascular Nogo-B is important for inflammatory cell recruitment in vivo, we induced carrageenan-induced paw edema as a model of subchronic inflammation. WT and Nogo-A/B−/− mice were intraplantar injected with carrageenan (2%) and the time course of paw edema assessed starting at 24 hours until 196 hours after injection. In this phase of paw edema (> 24 hours), macrophages constituted the main cell population at the inflamed site. As seen in Figure 2A, WT mice developed sustained paw swelling, whereas Nogo-A/B–deficient mice displayed a marked reduction in the edema formation. Immunofluorescent staining for CD68, a monocyte/macrophage marker, in paw sections after 72 hours, clearly showed a dramatic reduction of infiltrating cells in Nogo-A/B−/− mice (Figure 2B and quantified in the graph below). These data support an important role of Nogo-A/B in the development of an acute and subchronic inflammatory response in vivo. To confirm the role of the host, presumably endothelial Nogo-A/B, in leukocyte emigration into the inflamed paw, we created a carrageenan-induced paw edema model in bone-marrow–transplanted mice. Nogo-A/B−/− and WT mice were lethally irradiated, engrafted with bone marrow from WT mice (WT → WT; WT → Nogo-A/B−/−) or Nogo-A/B−/− mice (Nogo-A/B−/− → WT; Nogo-A/B−/− → Nogo-A/B−/−), and left to reconstitute for 6 weeks. After this time, the level of donor bone marrow engraftment was estimated by PCR on blood cells (Figure 2C), mice were injected with carrageenan, and paw edema was assessed. As seen in Figure 2C, transfer of Nogo-A/B−/− bone marrow into WT hosts had no effect on the magnitude or duration of edema compared with WT mice engrafted with WT bone marrow (Figure 2C), suggesting that Nogo-A/B in bone marrow cells does not play a significant role in the acute inflammatory response. On the contrary, Nogo-A/B−/− mice engrafted with WT bone marrow and Nogo-A/B−/− mice engrafted with Nogo-A/B−/− bone marrow displayed a reduced edema formation compared with the WT → WT group, indicating a clear role for host Nogo-A/B in driving leukocyte recruitment into inflamed tissue. This was confirmed by immunofluorescent staining for CD68+ macrophages of histologic sections 72 hours after carrageenan injection (Figure 2D). Although the influx of CD68+ cells was qualitatively similar in nonirradiated versus irradiated mice, the magnitude of the inflammatory response was reduced in all groups after bone marrow transplantation. These in vivo data provide evidence for the important role of vascular Nogo-A/B in the development of inflammation.
Figure 2.
Paw edema and monocyte infiltration are markedly reduced in Nogo-A/B−/− mice. (A) Nogo-A/B−/− mice displayed reduced paw edema formation (n = 6 per group) after intraplantar injection of carrageenan. PCR on mouse blood cells 6 weeks after bone marrow transplantation confirmed the engraftment of donor bone marrow. (B) Immunostaining for CD68, a monocyte/macrophage marker, in sections from WT and Nogo-A/B−/− mice 72 hours after carrageenan injection. Nogo-A/B−/− mice showed a reduction of CD68+ monocytes/macrophages in the paw compared with WT mice (as quantified in the graph). Bar = 100 μM. (C) Carrageenan-induced paw edema was induced in lethally irradiated WT and Nogo-A/B−/− mice transplanted with bone marrow from WT mice (WT → WT; WT → Nogo-A/B−/−) or Nogo-A/B−/− mice (Nogo-A/B−/− → WT; Nogo-A/B−/− → Nogo-A/B−/−) (n = 12 per group). (D) CD68 immunostaining of paw sections 72 hours after carrageenan injection in bone marrow–transplanted mice. Bar = 100 μM. The data shown represent the means ± SEM *P < .05; **P < .01, ***P < .001. Bar = 100 μm.
Nogo-A/B is not required for neutrophil migration or monocyte activation
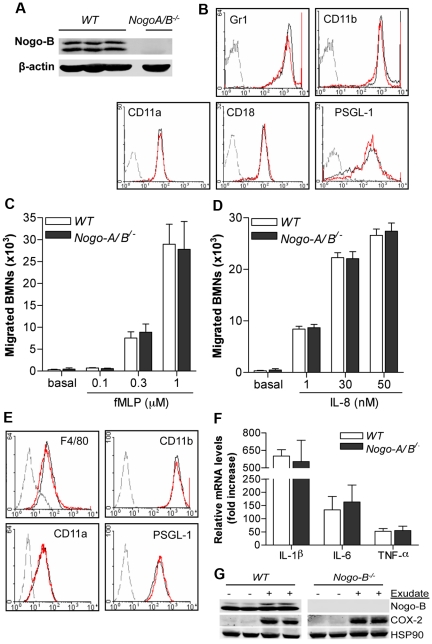
To determine the importance of Nogo-A/B in leukocyte versus vascular function during inflammation, we studied the role of Nogo-A/B in chemotaxis of bone marrow–derived neutrophils and activation of monocytes (BMDMs) (Figure 3). Bone marrow–derived neutrophils expressed Nogo-B (Figure 3A), and its loss did not affect lineage markers or adhesion molecule expression, as shown by fluorescence-activated cell sorting (FACS) analysis (Figure 3B). Bone marrow–derived neutrophil migration in vitro toward fMLP and IL-8 was not affected by the loss of Nogo-A/B (Figure 3C-D, respectively).
Figure 3.
The absence of Nogo-B does not influence fMLP- or IL-8–induced bone marrow–derived neutrophil migration or BMDM activation in vitro. (A) Bone marrow–derived neutrophils were isolated from the bone marrow of 8- to 12-week-old mice. Western blot analysis for Nogo-B in bone marrow–derived neutrophils is shown. (B) Representative histograms of adhesion and lineage molecules (Gr-1, CD11b, CD18, CD11a, and P-selectin glycoprotein ligand-1 [PSGL-1]) in WT (black line) and Nogo-A/B−/− (red line) bone marrow–derived neutrophils with isotype-matched control labeling (gray dotted line). Chemotaxis toward (C) fMLP (0.1, 0.3, and 1 μM) and (D) IL-8 (1, 30, and 50 nM) was performed in bone marrow–derived neutrophils from WT and Nogo-A/B−/− mice, as described in “Methods.” (E) Adhesion and lineage molecules in WT (black line) and Nogo-A/B−/− (red line) BMDMs and isotype-matched control antibody (gray dotted line). (F) Relative expression of cytokines by quantitative PCR in exudate-stimulated WT and Nogo-A/B−/− BMDMs. (G) Western blot analysis of WT and Nogo-A/B−/− BMDMs stimulated for 24 hours with exudates. The data shown represent the means ± SEM from 3 individual experiments.
The absence of Nogo-A/B did not affect the expression pattern of adhesion- or lineage-specific molecules on BMDMs (Figure 3E), or the response to exudates recovered from inflamed air pouches of WT mice (exudates collected 24 hours after carrageenan injection) monitored by cytokine mRNA levels (IL-1β, IL-6, and TNFα at 4 hours) or COX-2 (at 24 hours) expression (Figures 3F-G). The exudates from the inflamed pouch have a high content of inflammatory mediators such as cytokines, chemokines, prostaglandins, and leukotrienes, and “mimic” the microenvironment that monocytes/macrophages are exposed to during inflammation. The above in vitro data, together with in vivo data, argue against the functional role of Nogo-A/B in leukocytes and suggest a key role for vascular Nogo-A/B in the development of inflammation.
Nogo-B silencing in HDMECs reduces neutrophil and monocyte TEM
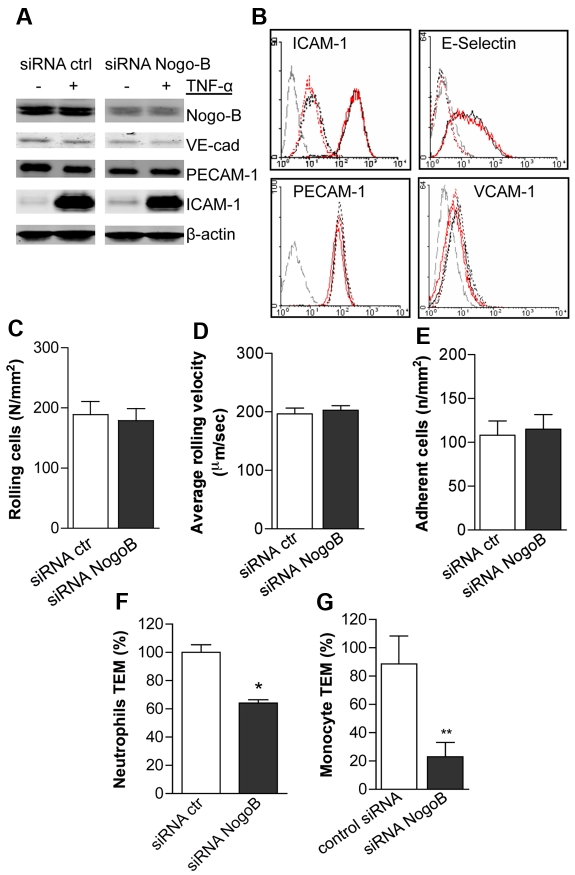
The process of leukocyte recruitment is a multistep event in which leukocyte capture and rolling are necessary to initiate firm adhesion and TEM.35 To address whether the loss of Nogo-B could affect leukocyte–endothelial cell interactions, we investigated neutrophil adhesion to TNF-α–stimulated HDMECs transfected with control or Nogo-B siRNA using a hydrodynamic parallel-flow chamber.36,37 siRNA against Nogo-B resulted in a > 80% reduction in Nogo-B protein levels compared with control siRNA–treated cells. Moreover, knockdown of Nogo-B did not alter basal and TNF-α–stimulated adhesion molecule expression, as shown by Western blotting and FACS analysis (Figure 4A-B). Hydrodynamic parallel-flow–chamber experiments using activated endothelial cells and human neutrophils did not reveal any differences in average rolling velocity, number of rolling, or firmly adherent neutrophils on HDMECs (Figure 4C-E) between the 2 groups. However, the loss of Nogo-B reduced the number of neutrophils (Figure 5F) and THP-1 monocytes (Figure 5G) able to TEM. These data strongly suggest that endogenous Nogo-B regulates aspects of leukocyte TEM, which is consistent with in vivo data in Nogo-A/B–deficient mice.
Figure 4.
The absence of Nogo-B reduces the transmigration of neutrophils and monocytes across endothelial cell monolayers. (A) Western blot analysis for Nogo-B and the adhesion molecules VE-cadherin, PECAM-1, and ICAM-1, on HDMECs transfected for 48 hours with siRNA Nogo-B and control, and stimulated with TNF-α (10 ng/mL) for 24 hours. (B) FACS analysis for E-selectin, ICAM-1, VCAM, and PECAM-1 on HDMECs transfected for 48 hours with siRNA Nogo-B and control. Transfected cells were stimulated with TNF-α (10 ng/mL) for 4 or 24 hours, and surface expression of E-selectin or ICAM-1, VCAM, and PECAM-1 expression was analyzed by FACS. TNF-α–stimulated siRNA Nogo-B or control treated HDMEC monolayers were used in flow-chamber experiments, as described in “Methods.” The number of rolling neutrophils per square millimeter (C), the average rolling velocity of neutrophils (D), and firmly adherent neutrophils (E) were not different. However, knock-down of Nogo-B in HDMECs reduced the transmigration of (F) neutrophils and (G) monocytes (THP-1) compared with control, siRNA-treated HDMECs.
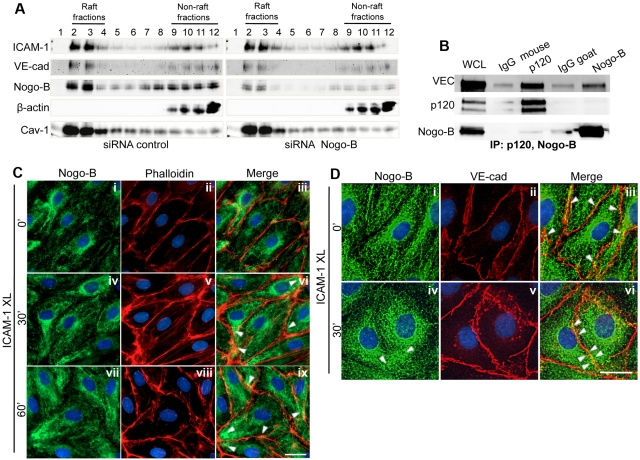
Figure 5.
Nogo-B cofractionates, interacts with VE-cadherin, and translocates toward the junction on ICAM-1 cross-linking. (A) ICAM-1, VE-cadherin, and Nogo-B sedimentation in raft membrane fractions analyzed by sucrose gradient fractionation. Nogo-B siRNA–treated or control treated HDMECs were stimulated with TNF-α (10 ng/mL for 24 hours) and then fractionated. β-Actin was used as a marker for non-raft fractions and Cav-1 for rafts and caveolae. (B) TNF-α–treated HDMECs were lysed and p120 or Nogo-B coimmunocoprecipitated. Samples were then analyzed by Western blotting using anti–VE-cadherin, anti-p120, and anti–Nogo-B antibodies, respectively. Isotype-matched, nonimmune antibody served as the control for immunoprecipitation experiments. The immunoblot shown is representative of 3 independent experiments. (C) Immunofluorescence labeling of Nogo-B (green) and F-actin–rich stress fibers (phalloidin, red) before and after ICAM-1 cross-linking for 30 and 60 minutes. Cells were stimulated with TNF-α (10 ng/mL) before ICAM-1 cross-linking. Nuclei were stained with 4,6-diamidino-2-phenylindole, dihydrochloride (blue). Bar = 20 μm. (D) Immunofluorescence colabeling of Nogo-B (green) and VE-cadherin (red) before (i-iii) and after ICAM-1 cross-linking (iv-vi). Nuclei were stained with 4,6-diamidino-2-phenylindole, dihydrochloride (blue). Bar = 20 μm. Images were captured with a Zeiss Axiovert epifluorescence microscope and a 63× oil-immersion objective. XL, cross-linking.
Nogo-B interacts with VE-cadherin and redistributes on ICAM-1 cross-linking in HDMECs
To evaluate the subcellular distribution of Nogo-B in TNF-α–stimulated HDMECs, activated cells were lysed in a sodium carbonate buffer, and lysates fractionated using a discontinuous sucrose gradient. ICAM-1 and VE-cadherin were present in Cav-1–enriched raft (fractions 2-3) and non-raft fractions,8–12 and codistributed with Nogo-B (Figure 5A left panel). However, the loss of Nogo-B did not affect the distribution of ICAM-1, VE-cadherin, or Cav-1 (Figure 5A right panel). Next, we examined whether Nogo-B biochemically interacts with ICAM-1 or VE-cadherin via coimmunoprecipitation experiments. As a positive control for a VE-cadherin–interacting protein, p120, a well-known binding partner of VE-cadherin38 was used. As seen in Figure 5B, immunoprecipitation of p120 resulted in the coassociation of VE-cadherin compared with a nonimmune IgG as a control, precipitating antibody (lanes 2 and 3). Under these conditions, immunoprecipitation of Nogo-B resulted in the coassociation of with a fraction of VE-cadherin not bound to p120 (lanes 4 and 5).
Recent studies have identified ICAM-1 on endothelial cells as a key regulator of leukocyte TEM at both junctional (paracellular) and nonjunctional (transcellular) locations.39–41 Antibody-mediated cross-linking of ICAM-1 can mimic endothelial cell activation induced by ICAM-1 engagement via leukocytes, resulting in stress fiber formation, activation of src and Pyk2, and ultimately VE-cadherin phosphorylation, a molecular end point of an endothelial signaling cascade facilitating paracellular TEM.15,42 In endothelial cells, Nogo-B is mainly localized in the tubular ER, with a small fraction in the plasma membrane.8,9 Therefore, because the ER is a dynamic structure that is able to closely align with microdomains of the plasma membrane,43,44 we examined whether the activation of endothelial cells by ICAM-1 cross-linking could affect the distribution of Nogo-B in relationship to endothelial cell junctions. To induce ICAM-1, HDMECs were stimulated with TNF-α, followed by ICAM-1 cross-linking for different time points. ICAM-1 cross-linking (30 minutes) induced the assembly of F-actin–rich stress fibers across the endothelial cell body (Figure 5Cv), as reported previously,45 and induced the redistribution of Nogo-B toward endothelial junctions. This effect was particularly pronounced at the 60-minute time point (as shown by arrowheads in 5Cix). As depicted in Figure 5Di-iii, there were few points of contact between Nogo-B and VE-cadherin+ junctions in TNFα-activated HDMECs; however, on ICAM-1 cross-linking, Nogo-B redistributed to points of colocalization with VE-cadherin at the junctions (Figure 5Dvi). These data strongly suggest the reorganization of Nogo-B, and likely the tubular ER, during ICAM-1 engagement.
The silencing or loss of Nogo-B impairs ICAM-1 cross-linking–induced VE-cadherin phosphorylation by reducing src and Pyk2 activation
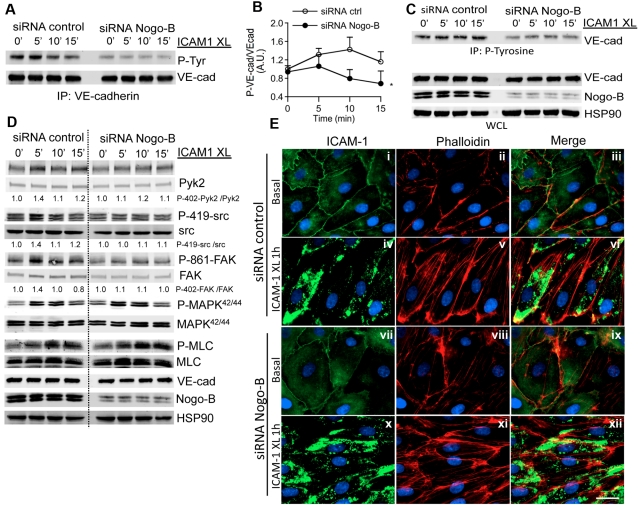
The data shown in Figures 4F-G and 5 prompted us to examine whether Nogo-B influenced the signaling cascade activated in endothelial cells on ICAM-1 cross-linking. ICAM-1 cross-linking induced time-dependent tyrosine phosphorylation of VE-cadherin in control siRNA-treated HDMEC, whereas this effect was significantly reduced in Nogo-B siRNA–treated HDMECs (Figure 6A and quantified in B). Similarly, immunoprecipitation of total phosphotyrosine (with the antibody PY20) after ICAM-1 cross-linking in Nogo-B siRNA–treated HDMECs resulted in reduced VE-cadherin levels (Figure 6C).
Figure 6.
Nogo-B deficiency impairs ICAM-1 cross-linking–induced VE-cadherin phosphorylation but not ICAM-1 clustering, stress fiber formation, and MLC phosphorylation. (A) Tyrosine phosphorylation of VE-cadherin after ICAM-1 cross-linking. HDMECs were transfected with Nogo-B siRNA or control siRNA were stimulated with TNF-α (10 ng/mL) for 24 hours, serum-starved, and incubated with ICAM-1 antibody 15.2 (5 μg/mL) for 30 minutes, followed by cross-linking for the indicated times. VE-cadherin immunoprecipitates were then analyzed by immunoblotting against phosphorylated tyrosine (4G10, PY20 clone). (B) The amount of tyrosine-phosphorylated VE-cadherin was quantified by densitometry from 4 independent experiments and is expressed as the fold increase of untreated controls. (C) Whole-cell lysates (WCLs) from HDMECs treated as described were immunoprecipitated for phosphotyrosine using the antibody PY20 or the 4G10 clone, and immunoprecipitates were analyzed by immunoblotting against VE-cadherin. The phosphorylation of VE-cadherin on ICAM-1 cross-linking was reduced in Nogo-B siRNA compared with control siRNA–treated HDMECs. (D) The activation of additional pathways (P-410 Pyk2, Pyk2, P-419-src, src, P-861-FAK, FAK, P-MAPK42/44, MAPK, P-MLC, MLC) were examined after ICAM-1cross-linking. Densitometric quantification of phospho/total for each pathway is below. (E) The loss of Nogo-B did not impair ICAM-1 clustering or stress-fiber formation. HDMECs transfected with Nogo-B siRNA or control were stimulated with TNF-α (10 ng/mL) for 24 hours, and surface ICAM-1 (green) and F-actin (red) examined after ICAM-1 cross-linking for 1 hour. Bar = 20 μm. Images were captured using a Zeiss Axiovert epifluorescence microscope and a 63× oil-immersion objective. XL, cross-linking.
Activation of c-src and Pyk2 plays an important role in regulating the phosphorylation of cortactin46 and VE-cadherin,22 and may contribute to paracellular leukocyte TEM. Therefore, we investigated whether the knock-down of Nogo-B affects src/Pyk2 activation. ICAM-1 cross-linking induced phosphorylation of src (Y419) and Pyk2 (Y402) in control siRNA–treated HDMECs, and this effect was blunted in Nogo-B siRNA–treated HDMECs (Figure 6D, with quantification of phospho/total underneath the blots). As a downstream readout of src activation,47 we also examined the phosphorylation of FAK. ICAM-1 cross-linking induced time-dependent phosphorylation of FAK at Y861, and this effect was similarly diminished in absence of Nogo-B (Figure 6D). However, the phosphorylation of MAPK42/44 and MLC on ICAM-1 cross-linking was not affected by a reduction of Nogo-B (Figure 6D). Moreover, the loss of Nogo-B did not affect the clustering of ICAM-1 or the formation of stress fibers (Figure 6E).
To confirm our data in human endothelial cells, MLECs were isolated from WT and Nogo-A/B−/− mice, as described previously.28 These endothelial cells were then treated with TNF-α, followed by ICAM-1 cross-linking. The phosphorylation of src, Pyk2, and FAK were reduced after ICAM-1 cross-linking in Nogo-A/B−/− compared with WT MLECs, but MAPK42/44 and p38 activation were not affected. Finally, we investigated whether the loss of Nogo-B could affect the localization of src, and consequently its activation. As shown in Figure 7B, src (middle panels, red) was predominately localized in the cytosol under basal conditions (Figure 7Bii,viii). After ICAM-1 cross-linking, src was recruited into junctions, as evidenced by the partial colocalization of src with VE-cadherin (Figure 7Bvi,xii and quantified in Figure 7C). Nogo-B silencing did not affect the distribution of src or VE-cadherin under basal conditions or after ICAM-1 cross-linking (Figure 7Bxii vs 7Biii) using the Pearson correlation (Figure 7C) to quantify the extent of src and VE-cadherin colocalization. These data support the concept that Nogo-B regulates ICAM-1–mediated signaling, leading to VE-cadherin phosphorylation and ultimately TEM.
Discussion
Despite the presence of reticulons in a variety of cell types, little is known about their endogenous functions or mechanisms of action. In the present study, we show that endothelial Nogo-B is a novel regulator of the inflammatory response in vivo and controls ICAM-1–dependent leukocyte TEM in vitro. This conclusion is supported by in vivo data in Nogo-deficient mice and in bone marrow–transplantation experiments showing that the loss of Nogo A/B in the host, but not marrow, reduces acute inflammation. Moreover, the absence or presence of Nogo did not influence markers of leukocyte differentiation or the levels of surface adhesion molecules in TNF-α–stimulated endothelial cells; however, Nogo deficiency reduced neutrophil and monocyte TEM in vitro. A central role of Nogo in regulating ICAM-1–mediated downstream signaling was substantiated by experiments showing that ICAM-1 cross-linking activates a variety of intracellular pathways leading to TEM, and the loss of Nogo-B diminishes VE-cadherin tyrosine phosphorylation through a reduction of src and Pyk2 activation, a pathway critical for leukocyte diapedesis.15,22 These results support a role for Nogo-B in regulating the extent of neutrophil and monocyte infiltration in vivo and in vitro.
Nogo isoforms are expressed in a restricted fashion in vivo, with Nogo-A mainly present in the nervous system and Nogo-C expressed at a high level in skeletal muscle. Small amounts of Nogo-B and Nogo-C transcripts are also detected in kidney, cartilage, skin, lung, and spleen48 and in multiple cell lines in vitro.1 We originally identified Nogo-B as the dominant Nogo isoform expressed in endothelial cells and vascular smooth muscle cells,8 and suggested that endogenous Nogo-B regulates vascular remodeling after injury. Our recent work has shown that Nogo-B is also expressed in monocytes/macrophages and can regulate chemokine-mediated monocyte migration and influence the extent of ischemic injury and wound healing.49 In these models, bone marrow–transplantation experiments demonstrated that Nogo-replete bone marrow partially rescues ischemia-induced arteriogenesis in Nogo-deficient mice. In the present study, we report that the loss of Nogo-A/B in vivo markedly suppresses leukocyte recruitment to sites of acute inflammation, and that this effect is host dependent, as shown by bone marrow–transplantation experiments. Although Nogo-B is highly expressed in neutrophils, it does not exert an obvious role in the neutrophil functions examined, such as differentiation, survival, migration, and homing to sites of inflammation (via bone marrow transplantation experiments). However, we cannot rule out other potential functions of Nogo in neutrophils. Why Nogo regulates monocyte/macrophage chemotaxis, but not neutrophil functions, is not obvious but may relate to the nature and duration of the inflammatory stimulus. In addition, Nogo-deficient bone marrow is not disease-causing when transferred into WT mice, thus raising the possibility that Nogo exerts independent functions in host cells (ie, vessels, as shown in this study) and bone marrow–derived cells,49 depending on the nature of the inflammatory response. It is well accepted that the initial infiltration of neutrophils in the first phase of acute inflammation can affect the following wave of innate immune response (> 24 hours) characterized by the infiltration monocytes/macrophages into inflamed tissues. Therefore, we cannot exclude the possibility that the reduced infiltration of neutrophils in the absence of Nogo-B may affect the second wave of inflammatory cell influx. However, in hydrodynamic parallel-flow–chamber experiments, silencing of Nogo-B in endothelial cells reduced TEM of both neutrophils and monocytes, but did not alter neutrophil rolling or firm adhesion to endothelial cells. These data suggest that the loss of Nogo-B impairs the capacity of activated endothelium to sustain the transit of leukocytes. Junctional adhesion molecules in endothelial cells, such as VE-cadherin, PECAM-1, and JAM-A,50 all participate in leukocyte diapedesis. In particular, VE-cadherin is necessary for paracellular diapedesis of leukocytes41 and is regulated by ICAM-1 engagement. Leukocyte adhesion and subsequent ICAM-1 engagement induces the activation of a complex network of signaling pathways that are necessary to prime endothelial cells for leukocyte transmigration by regulating cellular contraction and endothelial barrier properties.15 ICAM-1 engagement promotes tyrosine phosphorylation of VE-cadherin, an event required for the efficient TEM of lymphocytes51 and neutrophils.22 Our data demonstrate that a reduction in Nogo-B in endothelial cells did not affect stress fiber formation or the MLC phosphorylation induced by ICAM-1 cross-linking, but specifically reduced the phosphorylation of VE-cadherin. Previous studies have shown that ICAM-1 engagement enhances src and pyk2 activation,46,52 and that inhibition of src decreases VE-cadherin phosphorylation and adhesion molecule clustering at the site of leukocyte contact.22,53 In our experiments, the loss of Nogo-B impaired c-src and pyk2 phosphorylation, but not ICAM-1-clustering, stress fiber formation, or the colocalization of src with VE-cadherin, suggesting that Nogo-B influences ICAM-1/src/pyk2 pathways to regulate VE-cadherin phosphorylation and/or dephosphorylation.
Although the precise mechanism of ICAM-1–mediated VE-cadherin phosphorylation is not fully understood, ICAM-1 activation of src likely functions upstream of VE-cadherin phosphorylation.22 Moreover, the role of the ER in regulating ICAM-1 signaling is not known, although the ER is able to establish interactions with the plasma membrane in multiple cell types.43,44 Because ICAM-1 cross-linking promotes the redistribution of Nogo-B toward the plasma membrane, and Nogo-B can interact with VE-cadherin, it is feasible that the impaired TEM in Nogo-deficient endothelial cells reflects impaired dynamics of the tubular ER with the plasma membrane, which influences the coupling of ICAM-1 signaling to the adherens junction. Future experiments will explore these interesting possibilities.
In conclusion, the loss of Nogo-B suppresses the acute inflammatory response primarily because of the absence of Nogo-B in the host but not in bone marrow cells. Mechanistically, Nogo-B promotes leukocyte TEM by regulating ICAM-1–mediated src activation and the extent of VE-cadherin phosphorylation, thus exerting a controlling influence on the molecular network governing leukocyte diapedesis. Further studies are required to elucidate the regulatory role of Nogo-B and the ER during TEM and provide deeper insights into the mechanisms of inflammation in vivo.
Acknowledgments
We thank Mr Roger Babbitt for skilled technical assistance with microscopy.
This work was supported by the National Institutes of Health grants R01 HL64793, R01 HL61371, R01 HL081190, RO1 HL096670, and P01 HL70295 to W.C.S.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A. Di Lorenzo conceived the study, performed experiments, analyzed data, and wrote the paper; T.D.M. performed experiments and analyzed data; A. Davalos and P.L.W. performed experiments and analyzed data; and W.C.S. analyzed results and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William C. Sessa, PhD, Yale University School of Medicine, Amistad Research Bldg, 10 Amistad St, New Haven, CT, 06520; e-mail: william.sessa@yale.edu.
References
- 1.Oertle T, Schwab ME. Nogo and its paRTNers. Trends Cell Biol. 2003;13(4):187–194. doi: 10.1016/s0962-8924(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 2.Teng FY, Tang BL. Cell autonomous function of Nogo and reticulons: The emerging story at the endoplasmic reticulum. J Cell Physiol. 2008;216(2):303–308. doi: 10.1002/jcp.21434. [DOI] [PubMed] [Google Scholar]
- 3.Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124(3):573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 4.Shibata Y, Voss C, Rist JM, et al. The reticulon and DP1/Yop1p proteins form immobile oligomers in the tubular endoplasmic reticulum. J Biol Chem. 2008;283(27):18892–18904. doi: 10.1074/jbc.M800986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shnyrova A, Frolov VA, Zimmerberg J. ER biogenesis: self-assembly of tubular topology by protein hairpins. Curr Biol. 2008;18(11):R474–R476. doi: 10.1016/j.cub.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Huber AB, Weinmann O, Brosamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 2002;22(9):3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 8.Acevedo L, Yu J, Erdjument-Bromage H, et al. A new role for Nogo as a regulator of vascular remodeling. Nat Med. 2004;10(4):382–388. doi: 10.1038/nm1020. [DOI] [PubMed] [Google Scholar]
- 9.Oertle T, van der Haar ME, Bandtlow CE, et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci. 2003;23(13):5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 11.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178(2):449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nourshargh S, Krombach F, Dejana E. The role of JAM-A and PECAM-1 in modulating leukocyte infiltration in inflamed and ischemic tissues. J Leukoc Biol. 2006;80(4):714–718. doi: 10.1189/jlb.1105645. [DOI] [PubMed] [Google Scholar]
- 13.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3(2):143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 14.Johnson-Leger C, Aurrand-Lions M, Imhof BA. The parting of the endothelium: miracle, or simply a junctional affair? J Cell Sci. 2000;113(pt 6):921–933. doi: 10.1242/jcs.113.6.921. [DOI] [PubMed] [Google Scholar]
- 15.Turowski P, Adamson P, Greenwood J. Pharmacological targeting of ICAM-1 signaling in brain endothelial cells: potential for treating neuroinflammation. Cell Mol Neurobiol. 2005;25(1):153–170. doi: 10.1007/s10571-004-1380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. J Leukoc Biol. 2005;77(4):487–495. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kluger MS. Vascular endothelial cell adhesion and signaling during leukocyte recruitment. Adv Dermatol. 2004;20:163–201. [PubMed] [Google Scholar]
- 18.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 19.Sans E, Delachanal E, Duperray A. Analysis of the roles of ICAM-1 in neutrophil transmigration using a reconstituted mammalian cell expression model: implication of ICAM-1 cytoplasmic domain and Rho-dependent signaling pathway. J Immunol. 2001;166(1):544–551. doi: 10.4049/jimmunol.166.1.544. [DOI] [PubMed] [Google Scholar]
- 20.Durieu-Trautmann O, Chaverot N, Cazaubon S, Strosberg AD, Couraud PO. Intercellular adhesion molecule 1 activation induces tyrosine phosphorylation of the cytoskeleton-associated protein cortactin in brain microvessel endothelial cells. J Biol Chem. 1994;269(17):12536–12540. [PubMed] [Google Scholar]
- 21.Corada M, Liao F, Lindgren M, et al. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97(6):1679–1684. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- 22.Allingham MJ, van Buul JD, Burridge K. ICAM-1–mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179(6):4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 23.Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167(2):223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4(6):915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 25.Corada M, Mariotti M, Thurston G, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999;96(17):9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vinegar R, Truax JF, Selph JL, Johnston PR, Venable AL, McKenzie KK. Pathway to carrageenan-induced inflammation in the hind limb of the rat. Fed Proc. 1987;46(1):118–126. [PubMed] [Google Scholar]
- 27.Sato M, Sano H, Iwaki D, et al. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J Immunol. 2003;171(1):417–425. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- 28.Underhill DM. Macrophage recognition of zymosan particles. J Endotoxin Res. 2003;9(3):176–180. doi: 10.1179/096805103125001586. [DOI] [PubMed] [Google Scholar]
- 29.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61(4):375–380. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 30.Worthylake RA, Burridge K. Leukocyte transendothelial migration: orchestrating the underlying molecular machinery. Curr Opin Cell Biol. 2001;13(5):569–577. doi: 10.1016/s0955-0674(00)00253-2. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence MB, McIntire LV, Eskin SG. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987;70(5):1284–1290. [PubMed] [Google Scholar]
- 32.Goebel S, Huang M, Davis WC, et al. VEGF-A stimulation of leukocyte adhesion to colonic microvascular endothelium: implications for inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2006;290(4):G648–G654. doi: 10.1152/ajpgi.00466.2005. [DOI] [PubMed] [Google Scholar]
- 33.Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61(4):514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 34.Shaw SK, Ma S, Kim MB, et al. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J Exp Med. 2004;200(12):1571–1580. doi: 10.1084/jem.20040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106(2):584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167(2):377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adamson P, Etienne S, Couraud PO, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J Immunol. 1999;162(5):2964–2973. [PubMed] [Google Scholar]
- 38.Staehelin LA. The plant ER: a dynamic organelle composed of a large number of discrete functional domains. Plant J. 1997;11(6):1151–1165. doi: 10.1046/j.1365-313x.1997.11061151.x. [DOI] [PubMed] [Google Scholar]
- 39.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell. 2000;6(1):11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 40.Thompson PW, Randi AM, Ridley AJ. Intercellular adhesion molecule (ICAM)-1, but not ICAM-2, activates RhoA and stimulates c-fos and rhoA transcription in endothelial cells. J Immunol. 2002;169(2):1007–1013. doi: 10.4049/jimmunol.169.2.1007. [DOI] [PubMed] [Google Scholar]
- 41.Yang L, Kowalski JR, Zhan X, Thomas SM, Luscinskas FW. Endothelial cell cortactin phosphorylation by Src contributes to polymorphonuclear leukocyte transmigration in vitro. Circ Res. 2006;98(3):394–402. doi: 10.1161/01.RES.0000201958.59020.1a. [DOI] [PubMed] [Google Scholar]
- 42.Eliceiri BP, Puente XS, Hood JD, et al. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol. 2002;157(1):149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ackah E, Yu J, Zoellner S, et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115(8):2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen MS, Huber AB, van der Haar ME, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403(6768):434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 45.Oertle T, Huber C, van der Putten H, Schwab ME. Genomic structure and functional characterisation of the promoters of human and mouse nogo/rtn4. J Mol Biol. 2003;325(2):299–323. doi: 10.1016/s0022-2836(02)01179-8. [DOI] [PubMed] [Google Scholar]
- 46.Yu J, Fernandez-Hernando C, Suarez Y, et al. Reticulon 4B (Nogo-B) is necessary for macrophage infiltration and tissue repair. Proc Natl Acad Sci U S A. 2009;106(41):17511–17516. doi: 10.1073/pnas.0907359106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martìn-Padura I, Lostaglio S, Schneemann M, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142(1):117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turowski P, Martinelli R, Crawford R, et al. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci. 2008;121(Pt 1):29–37. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang L, Kowalski JR, Yacono P, et al. Endothelial cell cortactin coordinates intercellular adhesion molecule-1 clustering and actin cytoskeleton remodeling during polymorphonuclear leukocyte adhesion and transmigration. J Immunol. 2006;177(9):6440–6449. doi: 10.4049/jimmunol.177.9.6440. [DOI] [PubMed] [Google Scholar]
- 50.Tilghman RW, Hoover RL. The Src-cortactin pathway is required for clustering of E-selectin and ICAM-1 in endothelial cells. FASEB J. 2002;16(10):1257–1259. doi: 10.1096/fj.01-0969fje. [DOI] [PubMed] [Google Scholar]
- 51.Henriques MG, Silva PM, Martins MA, et al. Mouse paw edema. A new model for inflammation? Braz J Med Biol Res. 1987;20(2):243–249. [PubMed] [Google Scholar]
- 52.Di Lorenzo A, Fernandez-Hernando C, Cirino G, Sessa WC. Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proc Natl Acad Sci U S A. 2009;106(34):14552–14557. doi: 10.1073/pnas.0904073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manes TD, Hoer S, Muller WA, Lehner PJ, Pober JS. Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins block distinct steps in transendothelial migration of effector memory CD4+ T cells by targeting different endothelial proteins. J Immunol. 184(9):5186–5192. doi: 10.4049/jimmunol.0902938. [DOI] [PMC free article] [PubMed] [Google Scholar]