Abstract
A testicular mass in an elderly man has a high probability of being a lymphoma. However, when the pathologist is faced with a neoplastic lymphoid infiltrate in the testes, one rarely considers Hodgkin's lymphoma as a possibility, as a vast majority turn out to be diffuse large B cell lymphomas. The authors discuss the second case of Hodgkin's lymphoma, nodular sclerosis subtype, presenting as a painless enlarged testicle and associated symptoms similar to irreducible hernia in a 73-year-old gentleman. The patient was later found to have subdiaphragmatic lymph node disease. Hodgkin's lymphoma should therefore be given due consideration in the differential diagnosis of a testicular tumour with a predominantly lymphoid infiltrate.
Background
This is the second case of diffuse nodular sclerosis Hodgkin's lymphoma presenting primarily as a unilateral testicular mass. This case gives evidence that Hodgkin's lymphoma should be given more consideration in the differential diagnosis of a testicular tumour with a predominant atypical lymphoid infiltrate. Extensive Hodgkin's lymphoma with secondary testicular involvement should be considered, as primary testicular Hodgkin's lymphoma has not yet been reported.
Case presentation
A 73-year-old gentleman presented with a painless right groin mass that had gradually increased in size over 6 months. Clinical examination showed a smoothly enlarged right testicle and a clinical suspicion of an irreducible hernia. The patient was otherwise asymptomatic at the time of presentation. The only relevant history of this patient was receiving mitomycin chemotherapy for a grade II transitional cell carcinoma of the bladder 11 years preceding this presentation.
Investigations
A staging CT scan of the chest and abdomen showed grossly enlarged retroperitoneal, pelvic and right groin lymph nodes, together with some fluid collection in the right inguinal canal. There was no axillary or mediastinal lymphadenopathy. Liver and spleen did not show any abnormalities.
All routine laboratory investigations including erythrocyte sedimentation rate and lactate dehydrogenase were within normal limits in the preoperative period. Surgical exploration showed an enlarged right testicle and a single enlarged right femoral canal lymph node. A right radical orchiectomy and excision of femoral lymph node was performed.
Sections taken for histology were fixed in 10% neutral buffered formalin. Immunohistochemical analyses were performed on paraffin-embedded tissue. All tissue blocks were cut at 3 µm, mounted on glass slides coated with silane (3-aminopropyltriethoxysilane) and dried for 30 min at 60ºC. The sections were deparaffinised in xylene and rehydrated through different grades of alcohols. Endogenous peroxidase activity was blocked by incubating the sections in 0.3% hydrogen peroxide for 30 min, followed by washing in distilled water. The sections were subjected to heat-mediated antigen retrieval using citrate buffer pH 6.0. A standard avidin biotin peroxidase method (Vector, Burlingame, California, USA) was used with diaminobenzidine as the chromogen. Positivity was indicated by the presence of brown cytoplasmic or membrane staining. Antibodies, clones, dilutions and sources are shown in table 1.
Table 1.
Antibodies, clones, dilutions and sources
| Names | Clones | Dilutions | Sources |
|---|---|---|---|
| LCA | 2B11 + PD7/26 | 1/50 | Dako (Glostrup, Denmark) |
| CD20 | L26 | 1/400 | Dako |
| CD3 | A0452 (Code) | 1/100 | Dako |
| CD15 | C3D-1 | 1/35 | Dako |
| CD30 | BerH2 | 1/30 | Dako |
| CD56 | 1B6 | 1/50 | Vector (Burlingame, California) |
| EBNA 2 | PEZ | 1/100 | Dako |
| EBV LMP | CS1-4 | 1/100 | Dako |
The testicle measured 60 mm×50 mm and on sectioning had a very soft white consistency. The tunica albuginea was intact. The tunica vaginalis was connected by thick connective tissue bundles to a 30 mm encapsulated mass submitted with and in close vicinity to the testicle. This smaller mass was also found to have a soft white homogenous cut surface. The femoral lymph node was submitted separately and measured 40 mm in diameter. Although it presented a clearly demarcated cortical zone, the consistency was also very soft, and the specimen had an overall pale colour.
Both the testicle and the separate adjacent nodule were diffusely infiltrated by a polymorphous infiltrate composed of lymphocytes, plasma cells and eosinophils, with numerous scattered atypical cells. Several broad vascularised collagen bands divided the infiltrate into ill-defined nodules. There was total effacement of the native testicular parenchyma, even when multiple repeat sections were examined (figure 1A,B). The large atypical cells were evenly dispersed and conformed to the description of mononuclear and lacunar Reed-Sternberg (RS) cells. Occasional diagnostic RS cells and a few mummified cells were seen (figure 1C,D). The background lymphoid population was composed of small lymphocytes and a few centrocytes, but no centroblasts or immunoblasts. Focal areas where the atypical cells formed a syncytium and others, which showed a spindle fibriohistiocytic proliferation, were noted, although these occupied less than 25% of the area examined. A few atypical mitotic figures were also seen.
Figure 1.
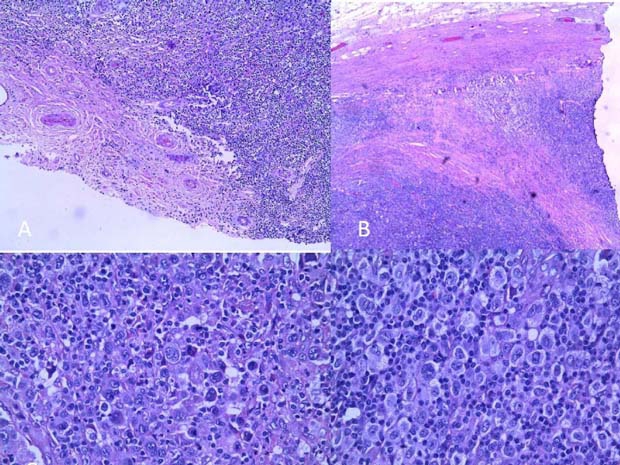
(A) Diffuse destruction of testicular tissue sparing only part of capsule with adventitial vessels. (B) Broad collagenised fibrous bands. (C) Rare diagnostic Reed Sternberg cell and a few mummified cells. (D) Cellular area showing numerous mononuclear and lacunar atypical RS cells, with a scanty background lymphocyte population.
All atypical cells were found to be positive for CD30 in a membrane and Golgi pattern while half were positive for CD15 with paranuclear intense globule staining. All atypical cells were negative for LCA, CD56, EBNA and LMP-1 staining. Staining for CD3 and CD20 was negative for the atypical cells, but the great majority of the background small lymphocyte population stained positively for CD3. Lymphocytes intimately adjacent to atypical cells were exclusively CD3 positive (figure 2).
Figure 2.
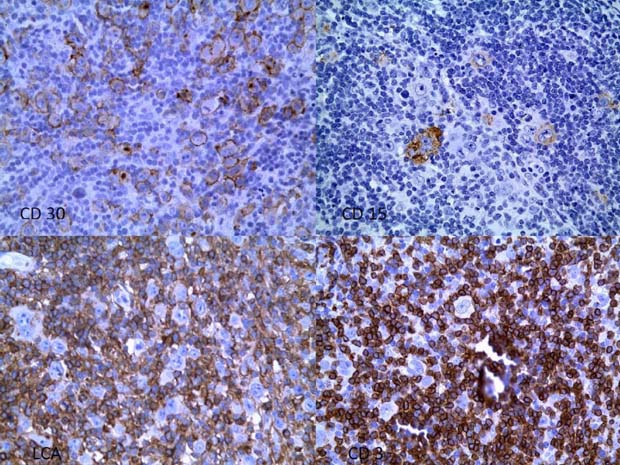
Top left: CD30; top right: CD15; bottom left: LCA; bottom right: CD3.
The separately submitted femoral lymph node showed identical histology and immunohistochemical properties as described above. In view of the diffuse involvement of the testicle, the patient was thought to be stage IVA instead of IIEA. A staging bone marrow biopsy was not considered necessary at that point.
Outcome and follow-up
The patient was referred to a tertiary oncology centre for further management. On referral to the clinic, 44 days after surgery, the patient was lethargic and was noted to lose weight, but did not complain of pruritus or night sweats. The patient unfortunately passed away a few days later while at home, without receiving chemotherapy. A postmortem examination was not performed.
Discussion
Extranodal presentation of classical Hodgkin's lymphoma is well documented, most likely in advanced disease, the commonest sites involved being liver, spleen and bone marrow. As long as large atypical cells with appropriate immunophenotype are present in an appropriate background, involvement of bone marrow and liver by Hodgkin's lymphoma can be ascertained even in the absence of RS cells.1 Diagnosis of extranodal Hodgkin's lymphoma at other sites needs the recognition of typical ‘diagnostic’ cells and appropriate phenotypic markers, especially in patients with no previous history of Hodgkin's lymphoma.1 Histological prognostic features in extranodal locations are obsolete, as stage (localised or diffuse involvement of extranodal site) is the most important prognostic marker.2 One of the notable exceptions is the spleen where the presence of five or more affected nodules imparts a worse prognosis.1 Rare cases of primary extranodal Hodgkin's disease have been reported in the gastrointestinal tract in association with inflammatory bowel disease,3 bone,4 lung5 and even skin.6
Primary Hodgkin's lymphoma of the testes is still an elusive myth. Two studies not using immunophenotyping published 30 years ago reported Hodgkin's sarcoma and nodular sclerosis Hodgkin's.7 8 In the largest and most recent review of testicular lymphoma, which included a total of 69 testicular lymphomas studied with detailed immunophenotypic analysis, three cases originally diagnosed as Hodgkin's lymphoma were later interpreted as non-Hodgkin's lymphoma.9 10 To our knowledge, only one case of diffuse classical Hodgkin's lymphoma confirmed by immunohistochemistry, which presented as a testicular mass, exists in the literature.10 The authors here described a 52-year-old man who presented with a left testicular mass and accompanying hydrocoele. Enlarged contiguous left groin and left external iliac lymph nodes were found on CT scan. Histological examination and immunophenotyping of the testicle revealed nodular sclerosis Hodgkin's lymphoma. The patient had a good response to ABVD chemotherapy.10
In the case described in our report, the histology is compatible with nodular sclerosis Hodgkin's lymphoma. The predominant forms of RS cells were the mononuclear and lacunar cell forms, with occasional diagnostic cells. Although fibrous bands were not identified in all the sections, and eosinophils dominated the background in some areas, nodular sclerosis Hodgkin's was adjudged to be the subtype in this case. A diagnosis of nodular sclerosis takes precedence over other histologic subtypes of Hodgkin's, if this is present, even focally.1 The atypical cells also showed a typical immunoprofile. The background lymphocytic population showed a predominant CD3 T cell infiltrate, especially around the atypical cells. The case described in this report was not classified as grade II (according to British National Lymphoma Investigation grading),11 as atypical mitotic figures, syncytial areas and fibrohistiocytic areas were only focal.
Although age, stage of presentation, presence of B symptoms and risk factors such as AIDS are thought to be the main prognostic factors in Hodgkin's lymphoma, mixed cellularity and lymphocyte-deplete subtypes also impart a significantly poorer prognosis to classical Hodgkin's lymphoma cases,12 hence correct identification of histological subtype should always be one of the main goals of the investigation. The encapsulated involved nodule within the tunica vaginalis but separate from the testis proper by a layer of connective tissue could theoretically be a secondarily involved appendix testes or a displaced lymph node from the deep inguinal chain of lymph nodes. This case also illustrates how although the most likely route of spread to the testis was through contiguous spread from adjacent lymph nodes, diffuse involvement of an extranodal organ is to be considered stage IV and not stage II.2 The situation is analogous to the previously mentioned case report.10
It is known that certain alkylating agents like mitomycin C, used for chemotherapy of solid cancers, increase sister chromatid expression (SCE) mean scores in lymphocytes, both in vitro and in vivo.13 Increased SCE expression is found in most untreated lymphomas. The incidence of Hodgkin's lymphoma following alkylating agent therapy can be accentuated with concomitant radiotherapy.14
Malignant lymphoma of the testes constitutes approximately 5% of all testicular neoplasms,1 and is usually a late manifestation of disseminated disease, occurring in about 20% of all systemic lymphomas.9 The commonest testicular neoplasms over the age of 50 are lymphoma, spermatocytic seminoma and Leydig cell tumours of the testis. Seminomas, malignant teratoma undifferentiated (embryonal carcinoma), malignant trophoblastic teratoma (choriocarcinoma) and mixed germ cell tumours prevail in adulthood. Differentiated teratoma and yolk sac tumours are tumours that occur in childhood.1
Primary malignant lymphoma of the testis is rare. Around 80% of primary testicular lymphomas were diffuse large B cell, making this the commonest lymphoma of the testes.9 The presence of CD15-positive, LCA-negative atypical cells were not compatible with large B cell lymphoma in our case. Other differential diagnoses are all uncommon. The T cell-rich variant of diffuse large B cell lymphoma has been described in the testis and has a characteristically aggressive course at the outset.15 Metastatic carcinoma must be excluded in syncytial variants of nodular sclerosis Hodgkin's lymphoma.1
Rare cases of Burkitt's lymphoma, follicular lymphoma in children, peripheral T cell lymphoma (type unspecified), anaplastic large cell lymphoma, precursor T lymphoblastic lymphoma and extranodal natural killer (NK) cell/T cell lymphoma have all been described to occur primarily in the testis.1 10 At least nine cases of NK cell lymphoma have been described to be arising in the testis,16 justifying in our opinion, using CD56 in the immunohistochemical panel for suspected Hodgkin's lymphoma of the testis.
Also one must always exclude, although not yet reported in the testis, angioimmunoblastic T cell lymphoma, as atypical immunoblasts in a mixed inflammatory background, and occasional binucleated B cells resembling RS cells can be present, which to complicate matters can rarely be CD30 and CD15 positive and also harbour EBV, hence leading one to misdiagnose as Hodgkin's lymphoma.1 Finally, one must not forget reactive immunoblastic proliferations such as infectious mononucleosis, which can mislead even experienced pathologists without immunohistochemistry.1
Learning points.
-
▶
A testicular mass in an elderly patient composed predominantly of an atypical lymphoid infiltrate is more likely to be neoplastic than non-neoplastic.
-
▶
In the exceptional cases where one is faced with a possible diagnosis of Hodgkin's lymphoma of the testis, further investigation as to the possibility of intra-abdominal/pelvic disease must be sought.
-
▶
Hodgkin's lymphoma is a very rare cause of a testicular mass and should be considered in the differential diagnosis of a testicular tumour with a predominantly atypical lymphoid infiltrate.
-
▶
Primary Hodgkin's lymphoma of the testis with no lymph node involvement elsewhere has so far not yet been reported.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Fletcher CM. Diagnostic Histopathology of Tumours. Third edition Philadelphia, PA: Elsevier Ltd; 2007 [Google Scholar]
- 2.AJCC AJCC Cancer Staging Manual. Seventh edition New York, NY: Springer-Verlag; 2009 [Google Scholar]
- 3.Kumar S, Fend F, Quintanilla-Martinez L, et al. Epstein-Barr virus positive primary gastrointestinal Hodgkin's disease: association with inflammatory bowel disease and immunosuppression. Am J Surg Pathol 2000;24:66–73 [DOI] [PubMed] [Google Scholar]
- 4.Gebert C, Hardes J, Ahrens H, et al. Primary multifocal osseus Hodgkin's disease: a case report and review of literature. J Cancer Res Clin Oncol 2005;131:163–8 [DOI] [PubMed] [Google Scholar]
- 5.Habermann TM, Ryu JH, Inwards DJ, et al. Primary pulmonary lymphoma. Semin Oncol 1999;26:307–15 [PubMed] [Google Scholar]
- 6.Khalifeh I, Hughey LC, Huang CC, et al. Solitary plaque on the scalp as a primary manifestation of Hodgkin lymphoma: a case report and review of the literature. J Cutan Pathol 2009;36(Suppl 1):80–5 [DOI] [PubMed] [Google Scholar]
- 7.Vishniavsky S. Hodgkin's sarcoma involving the testicle and adnexa. Va Med Mon (1918) 1973;100:534–7 [PubMed] [Google Scholar]
- 8.Glaholm J, Brada M, Horwich A. Hodgkin's disease of the epididymis and testis. J R Soc Med 1989;82:558–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferry JA, Harris NL, Young RH, et al. Malignant lymphoma of the testis, epididymis, and spermatic cord. A clinicopathologic study of 69 cases with immunophenotypic analysis. Am J Surg Pathol 1994;18:376–90 [DOI] [PubMed] [Google Scholar]
- 10.Rania M S, Kudakwashe C, Swerldow S, et al. Classical Hodgkin's lymphoma presenting as a testicular mass: report of a case. Int J Surg Pathol 2007;5:207–12 [DOI] [PubMed] [Google Scholar]
- 11.MacLennan KA, Bennett MH, Tu A, et al. Relationship of histopathologic features to survival and relapse in nodular sclerosing Hodgkin's disease. A study of 1659 patients. Cancer 1989;64:1686–93 [DOI] [PubMed] [Google Scholar]
- 12.Allemani C, Sant M, De Angelis R, et al. ; EUROCARE Working Group Hodgkin disease survival in Europe and the U.S.: prognostic significance of morphologic groups. Cancer 2006;107:352–60 [DOI] [PubMed] [Google Scholar]
- 13.Kurvink K, Bloomfield CD, Keenan KM, et al. Sister chromatid exchange in lymphocytes from patients with malignant lymphoma. Hum Genet 1978;44:137–44 [DOI] [PubMed] [Google Scholar]
- 14.Reimer RR, Hoover R, Fraumeni JF, Jr., et al. Acute leukemia after alkylating-agent therapy of ovarian cancer. N Engl J Med 1977;297:177–81 [DOI] [PubMed] [Google Scholar]
- 15.Ingram RM, Williams ME, Fintel WA, et al. Testicular lymphoma: a literature review and report of a case with a zoster-like cutaneous recurrence. Clin Lymphoma 2002;2:238–41 [DOI] [PubMed] [Google Scholar]
- 16.Kim YB, Chang SK, Yang WI, et al. Primary NK/T cell lymphoma of the testis. A case report and review of the literature. Acta Haematol 2003;109:95–100 [DOI] [PubMed] [Google Scholar]


