Abstract
Dose-intensified treatment strategies for Hodgkin lymphoma (HL) have demonstrated improvements in cure but may increase risk for acute and long-term toxicities, particularly in children. The Children's Oncology Group assessed the feasibility of a dose-intensive regimen, BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) in children with high-risk HL (stage IIB or IIIB with bulk disease, stage IV). Rapidity of response was assessed after 4 cycles of BEACOPP. Rapid responders received consolidation therapy with guidelines to reduce the risk of sex-specific long-term toxicities of therapy. Females received 4 cycles of COPP/ABV (cyclophosphamide, vincristine, procarbazine, prednisone, doxorubicin, bleomycin, vinblastine) without involved field radiation therapy (IFRT). Males received 2 cycles of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) with IFRT. Slow responders received 4 cycles of BEACOPP and IFRT. Ninety-nine patients were enrolled. Myelosuppression was frequent. Rapid response was achieved by 74% of patients. Five-year event-free-survival is 94%, IFRT with median follow-up of 6.3 years. There were no disease progressions on study therapy. Secondary leukemias occurred in 2 patients. Overall survival is 97%. Early intensification followed by less intense response-based therapy for rapidly responding patients is an effective strategy for achieving high event-free survival in children with high-risk HL. This trial is registered at http://www.clinicaltrials.gov as #NCT00004010.
Introduction
Cure rates for pediatric Hodgkin lymphoma (HL) remain among the highest in pediatric oncology, although relapse has still been a concern for patients with advanced-stage disease. Treatment strategies in both children and adults with HL using dose-intensified treatment regimens1–3 have resulted in improvements in HL-free survival for both adults and children with advanced-stage HL. However, cure often comes with a significant cost in the form of delayed effects of therapy, including an elevated risk for second malignant neoplasms, gonadal and nongonadal endocrine dysfunction, cardiotoxicity, and pulmonary toxicity, with several of these toxicities impacting long-term survival rates.4
The risk for some late toxicities varies by sex, with male patients being at higher risk for infertility associated with alkylator-based therapy5–7 and female patients at greater risk for the development of a second malignancy, especially breast cancer within the field of radiation therapy (RT).8–10 In a large retrospective cohort study of survivors of childhood cancer and sibling controls, chemotherapy with alkylating agents, with or without radiation to sites below the diaphragm, were associated with a fertility deficit of approximately 60% in men, whereas there was no apparent effect of alkylating-agent therapy, when administered in lieu of radiation below the diaphragm on fertility in women.5 Other studies have confirmed that, in females, maintenance of ovarian function can be expected in at least 80%, even after 6 cycles of alkylator-based chemotherapy.11 The risk of male infertility appears to be lowest when using ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) therapy compared with MOPP (mechlorethamine, oncovin, procarbazine, and prednisone) or MOPP/ABVD combination regimens.6,12 Females are almost twice as likely as males to develop a second malignancy after treatment for HL.8 The actuarial risk of a second malignancy at 20 years follow-up was 9.7% for males,16.8% for females, and 9.2% for breast cancer for 694 children and adolescents after therapy.8 Elsewhere, the risk of breast cancer has been reported to approach 35% by age 40 years, with the majority of the excess breast cancer risk within the field of radiation.9 The risk appears to be highest for women 19 years or younger at the time of treatment.10
Early response to chemotherapy has been associated with improvements in event-free survival (EFS) in patients with advanced-stage HL.13–15 Tailoring therapy to early response allows for limitation of cumulative therapy to those with more chemosensitive tumors, thereby limiting long-term toxicities in these patients. Further intensification of therapy for slow early responders may facilitate improvements in long-term disease control. Response-adapted therapy, in which overall treatment intensity is adjusted according to the initial response to chemotherapy, has been developed as a potential means of further reducing therapy, and especially the associated sex-specific toxicities for some while maintaining high cure rates overall.
The results of the pilot study CCG-59704 in pediatric patients with advanced-stage HL evaluating the feasibility of a risk-adapted approach assessing the use of escalated dose BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) for initial dose intensification is reported here. Early responders received reduced intensity consolidation therapy with sex-specific regimens, as an approach to minimize risk of late toxicities. Slow responders continued with dose-intensified therapy with escalated dose BEACOPP.
Methods
Patients
From October 1999 to May 2002, a prospective multicenter study was initiated for children and adolescents younger than 21 years, with advanced-stage HL. Stages, based on clinical evaluation, were defined by the Ann Arbor staging system.16 Patients were eligible for the study if they had HL stage IV, or stage II/III with B symptoms and bulky disease. These subsets were classified as high risk based on the finding of a significantly poorer 3-year EFS (< 80%) on the prior Children's Oncology Group (COG) trial, C5942.1 B symptoms were defined as the presence of at least one of the following: unexplained recurrent fever greater than 39°C, unexplained loss of more than 10% of body weight, and drenching night sweats. Bulky disease was defined as either a mediastinal mass with a diameter at least one-third of the intrathoracic diameter measured at the level of T5 on an upright chest radiograph or an extrathoracic nodal aggregate with a diameter greater than 10 cm on physical examination, computed tomography (CT), or magnetic resonance imaging scan. Involved regions were classified as any node more than 2 cm3 (cervical or axillary node > 3 cm3) on physical examination, ultrasound, CT, or magnetic resonance imaging scan unless pathologically proven to be negative; any cluster of matted or adherent nodes; any enlarged supraclavicular nodes; any mediastinal adenopathy; any Gallium-positive nodes, liver, or spleen; and a spleen or liver that has focal defects on CT or ultrasound or magnetic resonance imaging. Pathologic material was reviewed centrally and classified based on the revised European-American classification of lymphoid neoplasm criteria, as these were the criteria in use at the time of the study conduct.17 For this report, the pathologic material was reclassified according to the World Health Organization classification.18
Treatment protocol
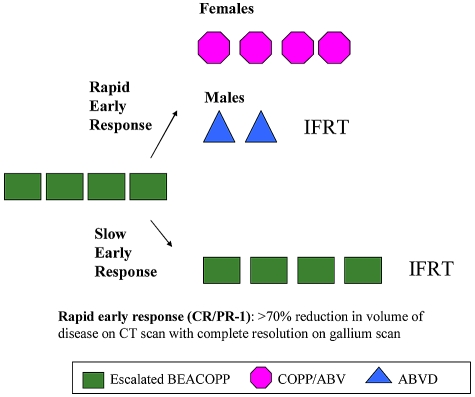
The study schema is illustrated in Figure 1. Details of the treatment regimens are listed in Table 1. All patients received 4 courses of induction chemotherapy with escalated-dose BEACOPP2 administered every 21 days. After induction, response assessment by physical examination and imaging studies (CT of areas initially involved and gallium 67–single-photon emission computed tomography) results determined the subsequent therapeutic regimen. Fluorodeoxyglucose positron emission tomography (FDG-PET) imaging was not widely available at the time of this study. Patients with a rapid response to therapy received consolidation therapy according to sex-specific guidelines. Females received 4 courses of COPP/ABV hybrid chemotherapy and no radiotherapy. Males received 2 courses of ABVD chemotherapy followed by radiotherapy. Female and male patients with a slow response to induction chemotherapy were administered 4 more courses of escalated-dose BEACOPP followed by radiotherapy. Radiotherapy was scheduled to begin 3 weeks after completion of chemotherapy. A total dose of 21 Gy was given in 12 fractions of 1.75 Gy to all involved fields. Patients with residual disease identified on imaging studies at the completion of chemotherapy received boost doses of 14 Gy (1.75 Gy × 8 fractions) to a total dose of 35 Gy. Treatment for lung involvement for stage IV patients was 1.05 Gy in 12 fractions using partial transmission lung blocks. Involved fields were defined by nodal sites of involvement and reflected volumes that had historically been treated. The involved field techniques used were typical for the time period and have been previously described.19 Because these patients had advanced disease, and involved fields were treated, often all treatment regions could not be encompassed in a single field. In that case, it was recommended that the area of initial bulkiest involvement be treated first with a 3-week interval between fields (ie, chest, abdomen, and pelvis). At the time the protocol was designed, data from the previous Children's Cancer Group Hodgkin study1 were not available to modify our fields.
Figure 1.
Study schema for CCG-59704. Consolidation therapy was stratified by response after 4 cycles of induction therapy, and for patients meeting the criteria for a rapid early response, also by sex.
Table 1.
Details of chemotherapy
| BEACOPP (repeat course every 21 days) |
| (B) Bleomycin 10 U/m2 IV, day 7 |
| (E) Etoposide 200 mg/m2 IV, days 0, 1, 2 |
| (A) Doxorubicin 35 mg/m2 IV, day 0 |
| (C) Cyclophosphamide 1200 mg/m2 IV, day 0 |
| (O) Vincristine 2 mg/m2 (maximum 2 mg) IV push, day 7 |
| (P) Prednisone 40 mg/m2 per day PO, divided into 2 doses, days 0-13 |
| (P) Procarbazine 100 mg/m2 per day, days 0-6 |
| G-CSF 5 μg/kg per day SC, starting day 8 and continuing until absolute neutrophil count was > 1000/μL |
| COPP/ABV (female rapid early responders, repeat course every 28 days) |
| (C) Cyclophosphamide 600 mg/m2 IV, day 0 |
| (O) Vincristine 1.4 mg/m2 (no maximum dose) IV push, day 0 |
| (P) Prednisone 40 mg/m2 per day PO, divided into 2 doses, days 0-13 |
| (P) Procarbazine 100 mg/m2 per day, days 0-6 |
| (A) Doxorubicin 35 mg/m2 IV, day 7 |
| (B) Bleomycin 10 U/m2 IV, day 7 |
| (V) Vinblastine 6 mg/m2 IV, day 7 |
| ABVD (male rapid early responders, repeat course every 28 days) |
| (A) Doxorubicin 25 mg/m2 IV, days 0, 14 |
| (B) Bleomycin 10 U/m2 IV, days 0, 14 |
| (V) Vinblastine 6 mg/m2 IV, days 0, 14 |
| (D) Dacarbazine 375 mg/m2 IV, days 0, 14 |
IV indicates intravenously; PO, orally; G-CSF, granulocyte colony-stimulating factor; and SC, subcutaneously.
The protocol was reviewed and approved by the National Cancer Institute and the institutional review boards of the participating institutions. Written informed consent was obtained from patients, parents, and/or guardians in accordance with the Declaration of Helsinki as required by government regulations.
Response criteria
Response to chemotherapy was defined on the basis of changes from diagnosis in clinical, radiologic, and radioisotopic evidence of disease.20 Complete response (CR) was defined as complete resolution of all areas of disease and resolution of B symptoms. A favorable partial response (PR-1) was classified as resolution of B symptoms, resolution of palpable adenopathy, at least a 70% reduction in tumor volume of a bulky mediastinal mass, or nodal aggregate, complete resolution on gallium scan of any postchemotherapy residual masses that had initial uptake on gallium scan or biopsy confirmation that persistent gallium positive areas do not contain tumor, resolution of extranodal soft tissue and marrow disease, at least partial healing of bone lesions, and resolution of effusions and ascites. Patients with resolution of B symptoms but only 50% to 70% reduction in tumor volume or persistent gallium uptake were considered partial responders (PR-2). Stable disease was defined as a less than 50% tumor reduction after initial chemotherapy. Progressive disease was classified as clinical or radiographic evidence of increased tumor volume in a previously involved site or as involvement of a new site while the patient received chemotherapy or radiotherapy. Early response was assessed at the treating institution after 4 courses of induction chemotherapy. Patients meeting criteria for CR or PR-1 were classified as rapid early responders (RERs). Patients classified PR-2 or SD were considered slow early responders (SERs). Relapse was defined as the recurrence of HL, pathologically confirmed, after complete or partial response to initial therapy. Imaging studies and radiation plans were reviewed centrally by the study radiologist and radiation oncologist at the completion of the trial.
Study design and statistical methods
The primary objective of the statistical analysis was to estimate the rate of BEACOPP-specific toxicity and to assess whether this was in an acceptable range. The secondary objectives included obtaining preliminary estimates of response rate after 2 and 4 courses of BEACOPP, and assessment of EFS and overall survival (OS) probability estimates.
Course-specific and cumulative toxicity rates were estimated for all grade 3 or 4 toxicities (National Cancer Institute Common Toxicity Criteria, Version 2.0, http://ctep.info.nih.gov). Life-table methods21 were used to compute the cumulative toxicity rate allowing patients to be removed from a risk set after experiencing a particular toxicity. Data shown represent the course-specific toxicity rates.
The Kaplan-Meier method was used to estimate EFS and OS probabilities with Greenwood SEs.21 EFS was measured from the date of enrollment until date of relapse, progressive disease, second malignancy, death from any cause, or date of last contact (if no event occurred). OS was measured from the date of registration until the date of death from any cause or date of last contact.
Results
Patients
Ninety-nine patients were enrolled in this study between October 1999 and May 2001. One patient was declared ineligible because of failure to obtain a proper consent. The characteristics of the remaining 98 patients are summarized in Table 2. Patients ranged in age from 4 to 20 years (median, 14 years). The numbers of males and females entered in this study were identical.
Table 2.
Presenting features
| Characteristic | N (%) |
|---|---|
| Age at on study, y | |
| 0-4 | 2 (2) |
| 5-9 | 8 (8) |
| 10-14 | 41 (42) |
| 15+ | 47 (48) |
| Sex | |
| Male | 49 (50) |
| Female | 49 (50) |
| Race | |
| White | 67 (68) |
| Hispanic | 12 (12) |
| Black | 9 (9) |
| Other | 7 (7) |
| Not reported | 3 (3) |
| Clinical stage | |
| II | 23 (23) |
| III | 17 (17) |
| IV | 58 (59) |
| Hodgkin lymphoma subtype | |
| Nodular sclerosis, classic | 86 (87) |
| Mixed cellularity, classic | 7 (7) |
| Nodular lymphocyte predominant | 3 (3) |
| Lymphocyte-rich, classic | 1 (1) |
| Not classifiable into one of the above | 1 (1) |
| Presence of B symptoms | |
| No | 28 (29) |
| Yes | 69 (70) |
| Not reported | 1 (1) |
| Hilar adenopathy | |
| No | 24 (24) |
| Yes | 72 (73) |
| Not reported | 2 (2) |
| Node/aggregate > 10 cm | |
| No | 60 (61) |
| Yes | 33 (34) |
| No. of nodal regions | |
| 1 | 6 (6) |
| 2 | 20 (20) |
| 3 | 23 (23) |
| 4 | 13 (13) |
| 5+ | 36 (37) |
| Anterior mediastinal mass | |
| No | 14 (14) |
| Yes < one-third diameter | 29 (30) |
| Yes > one-third diameter | 51 (52) |
| Not reported | 4 (4) |
| Bulk disease | |
| No | 33 (34) |
| Yes | 62 (63) |
| Not reported | 3 (3) |
There were 23 stage II (23%), 17 stage III (17%), and 58 stage IV (59%) patients. Eighty (82%) patients presented with an anterior mediastinal mass at study entry (51 > one-third the intrathoracic diameter). The majority of patients presented with high-risk features, including B-symptoms (70%), hilar adenopathy (73%), nodal aggregate more than 10 cm (61%), and 3 or more nodal regions involved (74%). Sixty-four (63%) patients were classified as having bulk disease at diagnosis.
Pathology
Eighty-seven tumors were reviewed centrally, and all were confirmed HL. Of 80 that were subclassified by both institution and reviewer, 73 (91%) were concordant.
Table 2 shows the aggregate of the review diagnosis supplemented by institutional diagnosis when the former was not available. Eighty-six were nodular sclerosis classic HL, 7 mixed cellularity classic HL, 3 nodular lymphocyte predominant HL, one lymphocyte-rich classic HL, and one was not classifiable into a specific HL subtype.
Toxicity
Grade 3 and 4 acute toxicities by phase of treatment are summarized in Table 3. The majority of toxicities occurred during the induction phase of therapy (cycles 1-4 of BEACOPP). As expected, hematologic toxicity was common. This was followed by febrile neutropenia at 34% and infection at 35%. The most common nonhematologic toxicities experienced with BEACOPP include stomatitis, dysphagia, esophagitis, and odynophagia. Allergic reactions/hypersensitivity reactions presumably related to etoposide were more prevalent during BEACOPP induction and nearly all during cycle 1. One patient electively changed therapy to COPP/ABV secondary to a grade 4 anaphylactic reaction with the first cycle of BEACOPP. The main toxicities observed among both the male and female RERs were hematologic (leukocytopenia, neutropenia, anemia requiring transfusions). However, the female RERs who were administered 4 cycles of COPP/ABV experienced slightly more febrile neutropenia and infection with grade 3 or 4 neutropenia.
Table 3.
Common grade 3/4 toxicities
| Toxicity | BEACOPP 1-4, % (N = 98) | BEACOPP 5-8, % (N = 24) | COPP/ABV 1-2, % (N = 38) | ABVD 1-2, % (N = 34) |
|---|---|---|---|---|
| Neutropenia | 83 | 96 | 95 | 88 |
| Anemia | 79 | 92 | 39 | 32 |
| Thrombocytopenia | 62 | 83 | 18 | 6 |
| Febrile neutropenia (fever of unknown origin) | 34 | 25 | 42 | 18 |
| Infection | 35 | 25 | 37 | 15 |
| Stomatitis/pharyngitis | 15 | 4 | 8 | 0 |
| Pulmonary (dyspnea, DLCO, hypoxia) | 8 | 8 | 3 | 6 |
| Peripheral neuropathy | 4 | 4 | 8 | 0 |
| Nausea/vomiting | 3 | 4 | 3 | 3 |
| Allergic reaction/hypersensitivity | 7 | 0 | 0 | 0 |
DLCO indicates diffusing capacity of the lung for carbon monoxide.
An unexpected toxicity was the development of typhlitis in 4 patients during the BEACOPP induction. One case was fatal, with death from sepsis secondary to Clostridium septicum during the neutropenic phase after the fourth cycle of BEACOPP. Three additional patients recovered completely and went on to receive chemotherapy (2 patients received BEACOPP at reduced doses) without any further complication and remain in long-term CR. Chemotherapy doses were not modified for hematologic toxicity. There were no significant delays (> 2 weeks) in commencing the next course of BEACOPP.
Response
Seventy-two of 97 (74%) patients with institutional response evaluation achieved a CR or PR1 after 4 cycles of BEACOPP and were thus classified as having an RER (Table 4). These RERs included 34 of 49 males and 38 of 48 females. The RER rate did not differ significantly by sex (P = .27). One patient was not assessable for response secondary to treatment toxicity. There were no cases of refractory disease or disease progression on therapy.
Table 4.
Response to 4 cycles of BEACOPP
| Response | Institution, N (%) | Central review, N (%) |
|---|---|---|
| Complete response (CR) | 12 (12) | 3 (5) |
| Partial response 1 (PR1) | 60 (62) | 52 (81) |
| Partial response 2 (PR2) | 24 (25)* | 9 (14) |
| Stable disease (SD) | 1 (1) | 0 (0) |
| Progressive disease (PD) | 0 (0) | 0 (0) |
| Not evaluable/unknown | 1 | 34 |
Two patients included here only completed cycles 1 and 2 of BEACOPP.
Diagnostic and end of induction imaging studies were available for central review by the study radiologist after completion of the trial in 64 of the 98 patients. The lack of requirement for “real time” assessment of response impacted compliance with submission of radiographic studies for central review. Institutional and central reviews were concordant for RER in 46 and concordant for SER (PR/SD) in 5. Four patients were downgraded from RER to SER on review, and 9 were upgraded from SER to RER. Overall RER/SER concordance rate was 51 of 64 (80%).
RT
Compliance with specified RT was investigated in 46 patients. Overall compliance was appropriate in 18 (39%). Major deviations were observed in 13 (28%), whereas only minor dose deviations related to uniformity deviations were noted in 5 cases (11%). In 10 (22%) patients, the radiation compliance was not evaluable. Some patients were not able to receive RT to all sites of initial involvement resulting from toxicities or anticipated toxicities. In some instances, if it appeared the patient had responded to chemotherapy, when pulmonary function tests or liver function tests were abnormal, the treating physician made the decision to exclude these areas from RT. This was counted as a major deviation on the protocol. The sites of failure did not correlate with the areas that were not treated.
EFS and survival
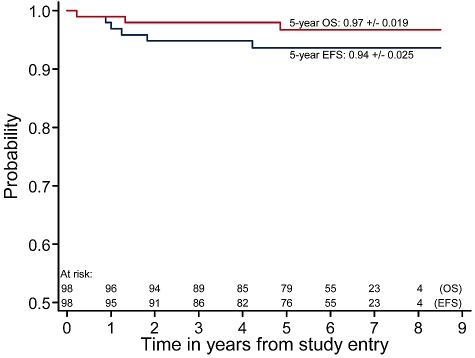
The 5-year EFS is 94% ± 2.4%, whereas the OS is 97% ± 1.9% (Figure 2). Median follow-up among patients with no reported event is 6.3 years, with 91% of patients followed a minimum of 3 years.
Figure 2.
EFS and survival from study entry.
Six treatment failures have occurred to date, and 3 of these patients have died. Three patients experienced disease relapse 10 to 21 months after the beginning of therapy; one female stage IVA RER patient relapsed 10 months after study entry, one male stage IVB RER at 12 months after study entry, and one male stage IVA SER 21 months after study entry. One death occurred secondary to sepsis, as noted in “Toxicity.” Two second malignant neoplasms have occurred to date. One case of chromosome 11q23-associated AML developed in a female stage IVA RER patient 14 months after entering the study, and she died one month later. A case of monosomy 7-associated myelodysplastic syndrome developed in a male stage IIIB SER patient at 50 months from study entry, and he died 7 months later.
Long-term toxicity
Long-term toxicities were not uniformly reported in the post-therapy surveillance period. Specifically, among 93 patients for whom at least one follow-up data form was submitted, 13 had echocardiographic evidence of reduced left ventricular shortening fraction (as defined as shortening fraction < 30%). Only one patient was reported to have been prescribed cardiac medications (digoxin, enalapril). Fertility data were not uniformly collected, although there were 5 reported pregnancies and 2 reports of ovarian failure among the 49 females registered on this study. No cases of breast cancer have been reported; however, median follow-up is only 6 years. Only 11 females received RT in this study, and one received it at time of relapse.
Discussion
Results from this pilot study demonstrate that early dose intensification with escalated-dose BEACOPP chemotherapy is not only feasible but highly effective in children and adolescents with advanced-stage HL. This regimen is associated with one of the highest reported EFS rates for a high-risk population of children and adolescents with HL. As with other pediatric HL trials, this study demonstrates that stratification by response is associated with equivalent outcome across risk groups as defined by front-end prognostic features.14,15 In this trial, induction therapy with BEACOPP chemotherapy is associated with a 74% early response rate. Continued-dose intensification with 8 cycles of escalated-dose BEACOPP, as administered in the adult German Hodgkin Study Group (GHSG) HD9 trial, is associated with acute and especially long-term toxicities, of which second malignant neoplasms have been a particular concern.2,22 With the high early response rate observed in this study, the majority of patients were able to avoid the substantial acute and potentially long-term complications of continued-dose intensification with BEACOPP chemotherapy and in the case of the female RER patients, with RT. This strategy of less intensified therapy for RER may limit the development of late toxicities for the majority of patients. Comparable with the experience with BEACOPP in adults with HL, early tumor control is excellent, as no cases of progressive disease were observed in this study.
Overall, the acute toxicity rates were acceptable. As expected, hematologic toxicity was frequent. These rates were higher than observed in adults in the GHSG HD9 trial, which included toxicity-adaptation for predefined toxic effects: World Health Organization grade 4 leukopenia more than 4 days, thrombocytopenia, infection, or mucositis; or an adverse effect that required a 2-week delay in treatment.2 Stepwise dose reductions for hematologic toxicity were not incorporated into this pediatric trial, as similarly this has not been the practice in prior Children's Oncology Group trials.1,15 Although the grade 3 or 4 infection rate was much higher in the pediatric population, the incidence of severe infections was low and safety monitoring rules for BEACOPP-specific acute toxicity were not met. The observation of neutropenic typhlitis in 4 patients does warrant caution. Typhlitis may result from the combination of doxorubicin-induced mucositis and delayed wound healing from prednisone, particularly in association with severe neutropenia. Although 3 patients recovered and subsequently received chemotherapy without complication, in one patient the typhlitis was associated with fatal sepsis. The potential for typhlitis during the neutropenic phase after BEACOPP chemotherapy must be considered so that symptoms of abdominal pain should be evaluated closely, especially as prednisone may mask the presence of fever.
The efficacy of this approach in reducing sex-specific long-term toxicities is not yet known. Long-term follow-up is necessary to evaluate the effectiveness of sex stratification of consolidation therapy in reducing the long-term toxicity. Despite attempts to obtain long-term toxicity data, incomplete participant ascertainment is commonplace as the primary aim of the trial was therapeutic intent for HL. RT was completely avoided in 38 of 49 females; thus, the number at risk for breast cancer is small. Cardiac toxicity is a particular concern for the males, given the concomitant use of mediastinal RT and doxorubicin. Contemporary radiation volumes/doses and anthracycline cumulative doses may mitigate some of this risk compared with older trials, but strategies aimed at further reduction of these risks are needed.23 Equivalent excellent outcomes in female and male RER patients suggest that omission of RT in males may also be considered, yet the efficacy of this approach in females was achieved with additional alkylator exposure, an approach that must be evaluated for its impact on male fertility. Continued administration of alkylating agents during consolidation was avoided in 34 of 49 males. However, whether this reduction is sufficient to avoid long-term infertility in males needs further study.7,24 Additional investigations are needed to determine the incidence of other late effects in children and adolescents treated with BEACOPP chemotherapy, which may not be sex-specific. In addition, many of these treatment sequelae may not become clinically apparent until the survivor attains maturity or is affected by the aging process.
With clinical trials using radiographic assessment of response for treatment determination, evaluation of any discrepancies between institutional and central review of response and the impact on study outcomes is essential. In this study, the treatment determinations were made at the institutional level. At time of retrospective review, 80% concordance between institution and central review of early response assessment was obtained. The discordance may have occurred for several reasons. Some variation in the method of measurement by different persons is to be expected. Another factor that may have had impact is that, in this study, tumor volume rather than area was assessed requiring craniocaudal length in addition to axial dimensions. During the period of time of the study, CT scanners acquired linear data such that the third dimension was estimated by counting the number of axial slices on which the tumor was visualized, adding some inherent variability. On central review, all masses were measured in a consistent fashion by the same person. Overall, this had no major impact on EFS, with the possible exception of a relapse in one RER female who was assessed as a SER on central review. The excellent outcomes observed in all groups suggest that the RER therapy was possibly more than was needed. Although the International Harmonization Project has developed standardized criteria for assessment of FDG-PET response in patients with lymphoma,25 central review of images with consistent criteria may be necessary in the pediatric HL setting until more standardized definitions of assessment are more broadly adopted.
The respective roles of chemotherapy and RT in high-risk HL have yet to be defined. Recent pediatric trials have evaluated different strategies for combined modality therapy (Table 5).1,15,26–28 Risk-adapted regimens have evolved as a way to limit long-term toxicities of therapy, especially in younger patients. However, long-term results in some trials investigating reductions in chemotherapy26 or RT1,27 have demonstrated compromised disease control, which potentially may offset the potential benefits of reducing late effects.
Table 5.
Comparison of recent trials in high-risk pediatric HL
| Study group/trial | Sample size | Chemotherapy | Radiotherapy | EFS or DFS, % | OS, % | Follow-up, y |
|---|---|---|---|---|---|---|
| Children's Cancer Group CCG-59704 | 98 | 4 BEACOPP + M RER: 2 ABVD F RER: 4 COPP/ABV SER: 4 BEACOPP | 21 Gy IFRT (no RT for F RER) | 94 | 97 | 6 |
| Pediatric Oncology Group P942515 | 163 | 3 DBVE-PC ± 2 DBVE-PC | 21 Gy IFRT | 85 | NR | 5 |
| Children's Cancer Group CCG-59421 | 141 | COPP/ABV + CHOP + cytarabine/etoposide | ± 21 Gy IFRT | 82 | 93 | 3 |
| Stanford/St Jude/Dana-Farber25 | 159 | 3 VAMP/ 3 COP | 15-25.5 Gy IFRT | 75.6 | 92.7 | 5 |
| German Multicenter GPOH HD-9526 | 341 | 2 OPPA/OEPA + 4 COPP | 20-35 Gy IFRT (no RT if CR) | 83 (CR 79; PR 91) | NR | 3 |
| German Multicenter GPOH HD-200227 | 239 | 2 OPPA/OEPA + 4 COPADC | 19.8-35 Gy IFRT | 87 | 95 | 5 |
DFS indicates disease-free survival; M, male; F, female; COPP, cyclophosphamide, vincristine, procarbazine, prednisone; DBVE-PC, doxorubicin; bleomycin, vincristine, etoposide, prednisone, cyclophosphamide; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; VAMP, vinblastine, doxorubicin, methotrexate, prednisone; OPPA, vincristine, procarbazine, prednisone, doxorubicin; OEPA, vincristine, etoposide, prednisone, doxorubicin; COPDAC, cyclophosphamide, vincristine, prednisone, dacarbazine; IFRT, involved field radiation therapy; and NR, not reported.
RT has been shown to be of value in high-risk HL in terms of local control, but there is great interest in eliminating RT to reduce the risks of its long-term toxicities. As demonstrated for female RER patients in this trial, the use of intensive chemotherapy allows omission of RT. This finding was also observed in a trial of adults with HL using BEACOPP alone for patients achieving a CR.29 However, 2 studies found a reduction in EFS for those who did not receive RT. In a COG trial, study accrual was stopped early because of the reduction in EFS in patients achieving a CR to chemotherapy but randomized to not receive involved field RT.1 In the Pediatric German Multicenter HD-95 trial, RT was given only to those with a PR; this subset subsequently had higher EFS than the CR patients who did not get RT.27 Although the omission of RT has not reduced OS in the most recent updates of these trials, curative salvage therapy usually requires additional intensive chemotherapy with stem cell transplantation and/or high-dose radiation. These approaches would be expected to have greater long-term risks than low-dose limited-field radiation, such that these risks must be considered in any decision to eliminate radiation. Certainly, improved imaging has enhanced our ability to design radiation fields and the administration of RT to only bulky sites of disease is the current trend. On this trial, our physicians did not always feel comfortable in covering such large fields, especially whole lung and liver, depending on the clinical status of the patient. Maintenance of adequate hematologic parameters was also an issue for patients, and this led to delays in therapy in some cases. Given the concerns about the long-term effects of RT, a pressing goal is to identify the subset of patients in which RT can be eliminated or reduced. The current COG high-risk HL trial is investigating a strategy of delivering RT to sites of bulk disease only in RER patients.
Although the cure rates for high-risk HL are high, there are still several areas for further research. Further reduction of chemotherapy, especially for RER patients, is a strategy to be evaluated. In the management of adults with HL at some centers, early intensification with BEACOPP followed by less intense response-based therapy for RER patients may represent an overall “intensification” of therapy. However, in contrast to the standard of care used by the GHSG, our approach represents a “de-escalation” of therapy. The GHSG has been evaluating reductions in the total number (8 vs 6) or intensity (escalated vs baseline) of BEACOPP cycles.29–31 One consideration is to administer 2 cycles of escalated-dose BEACOPP or the time-intensive BEACOPP-14 instead of 4 cycles of escalated BEACOPP, with subsequent de-escalation for rapid responders.30 Alternatively, substitution of BEACOPP with the dose-dense regimen, ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide), may be an effective strategy as this approach has demonstrated high efficacy with a reduction in the cumulative doses of chemotherapy and radiation below thresholds usually associated with long-term adverse effects.15 An intensified form of ABVE-PC with an increased dose of cyclophosphamide to 1200 mg/m2 per cycle is being investigated in the current COG high-risk trial.
Reductions in the use or fields of RT or numbers of chemotherapy cycles may be gained with greater refinement in response assessment through the use of FDG-PET imaging. Early PET response after 2 courses of ABVD is prognostic,32 although the optimal timing of response assessment by PET in the context of dose-intensive regimens, such as BEACOPP, is not yet known. Retrospective data with the Stanford V regimen suggest that PET evaluation at the end of chemotherapy is as prognostic as interval evaluation.33,34 End-of-chemotherapy PET assessment was prognostic in the GHSG HD-15 trial, in that omission of RT in patients with PET-negative residual tumors was not associated with increased risk of primary progressive disease or early relapses.35 Currently in the HD18 trial, PET is being used to guide chemotherapy duration; patients who are PET-negative after 2 cycles of escalated BEACOPP are being randomly assigned between 8 cycles and only 4 cycles of escalated BEACOPP.29
In conclusion, early intensification followed by less intense response-based therapy for rapidly responding patients is an effective strategy for achieving high EFS in children and adolescents with high risk HL. Further refinements involving reduction in chemotherapy doses and/or duration and in reduction or omission of radiation fields may be achieved through greater use of PET imaging. This will provide additional tailoring of therapy to individual need, with the ultimate goal of reducing relapse rates and long-term toxicities of therapy.
Acknowledgments
This work was supported in part by the National Cancer Institute, National Institutes of Health (Children's Oncology Group Chairman's grants CA-98543 and CA-98413).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.M.K. was the study chair of CCG-59704 and the primary author, and designed and conducted the study; R.S. was the study statistician, performed statistical analyses, designed Kaplan-Meier figures, and reviewed and edited the manuscript; R.H. was the study vice chair, designed the study, and reviewed and edited the manuscript; V.M. was a study committee member, performed central review of RT plans, and reviewed the manuscript; K.M. was a study committee member, performed central reviews of radiology studies, and reviewed and edited the manuscript; S.P. and M.L. were study committee members, performed central pathology reviews, and reviewed the manuscript; D.V. was a study statistician, monitored the study, performed statistical analyses, and reviewed the manuscript; M.W. was CCG Hodgkin lymphoma committee vice chair and mentor to K.M.K., designed the study, and reviewed and edited the paper; R.S. and D.V. performed the statistical analyses; and all authors had access to the primary clinical trial data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kara M. Kelly, MD, Division of Pediatric Oncology, Columbia University Medical Center, 161 Fort Washington Ave, IP-7, New York, NY 10032; e-mail: kk291@columbia.edu.
References
- 1.Nachman JB, Sposto R, Herzog P, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin's disease who achieve a complete response to chemotherapy. J Clin Oncol. 2002;20(18):3765–3771. doi: 10.1200/JCO.2002.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med. 2003;348(24):2386–2395. doi: 10.1056/NEJMoa022473. [DOI] [PubMed] [Google Scholar]
- 3.Horning SJ, Hoppe RT, Breslin S, Bartlett NL, Brown BW, Rosenberg SA. Stanford V and radiotherapy for locally extensive and advanced Hodgkin's disease: mature results of a prospective clinical trial. J Clin Oncol. 2002;20(3):630–637. doi: 10.1200/JCO.2002.20.3.630. [DOI] [PubMed] [Google Scholar]
- 4.Hodgson DC, Hudson MM, Constine LS. Pediatric Hodgkin lymphoma: maximizing efficacy and minimizing toxicity. Semin Radiat Oncol. 2007;17(3):230–242. doi: 10.1016/j.semradonc.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Bryne J, Mulvihill JJ, Myers MH, et al. Effects of treatment on fertility in long-term survivors of childhood or adolescent cancer. N Engl J Med. 1987;317(21):1315–1321. doi: 10.1056/NEJM198711193172104. [DOI] [PubMed] [Google Scholar]
- 6.Anselmo AP, Cartoni C, Bellantuono P, Maurisi-Enrici R, Aboulkair N, Ermini M. Risk of infertility in patients with Hodgkin's disease treated with ABVD vs MOPP vs ABVD/MOPP. Haematologica. 1990;75(2):155–158. [PubMed] [Google Scholar]
- 7.Sieniawski M, Reineke T, Josting A, et al. Assessment of male fertility in patients with Hodgkin's lymphoma treated in the German Hodgkin Study Group (GHSG) clinical trials. Ann Oncol. 2008;19(10):1795–1801. doi: 10.1093/annonc/mdn376. [DOI] [PubMed] [Google Scholar]
- 8.Wolden SL, Lamborn KR, Cleary SF, Tate DJ, Donaldson SS. Second cancers following pediatric Hodgkin's disease. J Clin Oncol. 1998;16(2):536–544. doi: 10.1200/JCO.1998.16.2.536. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin's disease. N Engl J Med. 1996;334(12):745–751. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 10.Aisenberg AC, Finkelstein DM, Doppke KP, Koerner FC, Boivin JF, Willet CG. High risk of breast carcinoma after irradiation of young women with Hodgkin's disease. Cancer. 1997;79(6):1203–1210. doi: 10.1002/(sici)1097-0142(19970315)79:6<1203::aid-cncr20>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Horning SJ, Hoppe RT, Kaplan HS, et al. Female reproductive potential after treatment for Hodgkin's disease. N Engl J Med. 1981;304(23):1377–1382. doi: 10.1056/NEJM198106043042301. [DOI] [PubMed] [Google Scholar]
- 12.Bonadonna G, Santoro A, Viviani S, Lombardi C, Ragni G. Gonadal damage in Hodgkin's disease from cancer chemotherapeutic regimens. Arch Toxicol. 1984;7:140–145. doi: 10.1007/978-3-642-69132-4_16. [DOI] [PubMed] [Google Scholar]
- 13.Weiner MA, Leventhal B, Brecher ML, et al. Randomized study of intensive MOPP-ABVD with or without low-dose total-nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin's disease in pediatric patients: a Pediatric Oncology Group study. J Clin Oncol. 1997;15(8):2769–2779. doi: 10.1200/JCO.1997.15.8.2769. [DOI] [PubMed] [Google Scholar]
- 14.Landman-Parker J, Pacquement H, Leblanc T, et al. Localized childhood Hodgkin's disease: response-adapted chemotherapy with etoposide, bleomycin, vinblastine, and prednisone before low-dose radiation therapy-results of the French Society of Pediatric Oncology Study MDH90. J Clin Oncol. 2000;18(7):1500–1507. doi: 10.1200/JCO.2000.18.7.1500. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood. 2009;114(10):2051–2059. doi: 10.1182/blood-2008-10-184143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7(11):1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 17.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 18.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. Hodgkin lymphoma. pp. 321–334. [Google Scholar]
- 19.Yahalom J, Mauch P. The involved field is back: issues in delineating the radiation field in Hodgkin's disease. Ann Oncol. 2002;13(suppl 1):79–88. doi: 10.1093/annonc/13.s1.79. [DOI] [PubMed] [Google Scholar]
- 20.Connors JM, Klimo P, Adams G, et al. Treatment of advanced Hodgkin's disease with chemotherapy: comparison of MOPP/ABV hybrid regimen with alternating courses of MOPP and ABVD. A report from the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1997;15(4):1638–1645. doi: 10.1200/JCO.1997.15.4.1638. [DOI] [PubMed] [Google Scholar]
- 21.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley and Sons; 1980. [Google Scholar]
- 22.Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin's lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27(27):4548–4554. doi: 10.1200/JCO.2008.19.8820. [DOI] [PubMed] [Google Scholar]
- 23.Constine LS. Cured of Hodgkin lymphoma, but suffering a broken heart. Leuk Lymphoma. 2008;49(8):1433–1435. doi: 10.1080/10428190802238578. [DOI] [PubMed] [Google Scholar]
- 24.van der Kaaij MA, Heutte N, Le Stang N, et al. Gonadal function in males after chemotherapy for early-stage Hodgkin's lymphoma treated in four subsequent trials by the European Organisation for Research and Treatment of Cancer: EORTC Lymphoma Group and the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2007;25(19):2825–2832. doi: 10.1200/JCO.2006.10.2020. [DOI] [PubMed] [Google Scholar]
- 25.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25(5):571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 26.Hudson MM, Krasin M, Link MP, et al. Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin's disease. J Clin Oncol. 2004;22(22):4541–4550. doi: 10.1200/JCO.2004.02.139. [DOI] [PubMed] [Google Scholar]
- 27.Dörffel W, Lüders H, Rühl U, et al. Preliminary results of the multicenter trial GPOH-HD 95 for the treatment of Hodgkin's disease in children and adolescents: analysis and outlook. Klin Padiatr. 2003;215(3):139–145. doi: 10.1055/s-2003-39372. [DOI] [PubMed] [Google Scholar]
- 28.Mauz-Körholz C, Hasenclever D, Dörffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin's lymphoma: the GPOH-HD-2002 Study. J Clin Oncol. 2010;28(23):3680–3686. doi: 10.1200/JCO.2009.26.9381. [DOI] [PubMed] [Google Scholar]
- 29.Kobe C, Dietlein M, Franklin J, et al. Positron emission tomography has a high negative predictive value for progression or early relapse f or patients with residual disease after first-line chemotherapy in advanced-stage Hodgkin lymphoma. Blood. 2008;112(10):3989–3994. doi: 10.1182/blood-2008-06-155820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engert A, Bredenfeld H, Döhner H, et al. Pegfilgrastim support for full delivery of BEACOPP-14 chemotherapy for patients with high-risk Hodgkin's lymphoma: results of a phase II study. Haematologica. 2006;91(4):546–549. [PubMed] [Google Scholar]
- 31.Engert A, Franklin J, Mueller RP, et al. HD12 randomized trial comparing 8 dose-escalated cycles of BEACOPP with 4 escalated and 4 baseline cycles in patients with advanced-stage Hodgkin lymphoma (HL): an analysis of the German Hodgkin Lymphoma Study Group (GHSG), University of Cologne [abstract]. Blood. 2006;108:33a. Abstract 99. [Google Scholar]
- 32.Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107(1):52–59. doi: 10.1182/blood-2005-06-2252. [DOI] [PubMed] [Google Scholar]
- 33.Advani R, Maeda L, Lavori P, et al. Impact of positive positron emission tomography on prediction of freedom from progression after Stanford V chemotherapy in Hodgkin's disease. J Clin Oncol. 2007;25(25):3902–3907. doi: 10.1200/JCO.2007.11.9867. [DOI] [PubMed] [Google Scholar]
- 34.Iagaru A, Wang Y, Mari C, et al. (18)F-FDG-PET/CT evaluation of response to treatment in lymphoma: when is the optimal time for the first re-evaluation scan? Hell J Nucl Med. 2008;11(3):153–156. [PubMed] [Google Scholar]
- 35.Kobe C, Dietlein M, Franklin J, et al. Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first line chemotherapy in advanced-stage Hodgkin lymphoma. Blood. 2008;112(10):3989–3994. doi: 10.1182/blood-2008-06-155820. [DOI] [PMC free article] [PubMed] [Google Scholar]