Abstract
The myelin sheath wraps large axons in both the CNS and the PNS, and is a key determinant of efficient axonal function and health. Myelin is targeted in a series of diseases, notably multiple sclerosis (MS). In MS, demyelination is associated with progressive axonal damage, which determines the level of patient disability. Few treatments are available for combating myelin damage in MS and related disorders. These treatments, which largely comprise anti-inflammatory drugs, only show limited efficacy in subsets of patients. More-effective treatment of myelin disorders will probably result from early intervention with combinatorial therapies that target inflammation and other processes—for example, signaling pathways that promote remyelination. Indeed, evidence suggests that such pathways might be impaired in pathology and, hence, contribute to the failure of remyelination in such diseases. In this article, we review the molecular basis of signaling pathways that regulate myelination in the CNS and PNS with a focus on differentiation of myelinating glia. We also discuss factors such as extracellular molecules that act as modulators of these pathways. Finally, we consider the few preclinical and clinical trials of agents that augment this signaling.
Introduction
Most large axons in the mammalian nervous system are surrounded by a lipid-rich membrane known as the myelin sheath, which promotes rapid conduction of nerve impulses and protects against axonal damage. Myelin sheaths form during development and consist of compacted spiral wraps of membrane that are supplied by oligodendrocytes in the CNS and Schwann cells in the PNS (Box 1 and Figure 1). These myelinating glia and their target axons form intimate units, with the glia and axons regulating each other's phenotype through the reciprocal exchange of signals (Boxes 2–4).
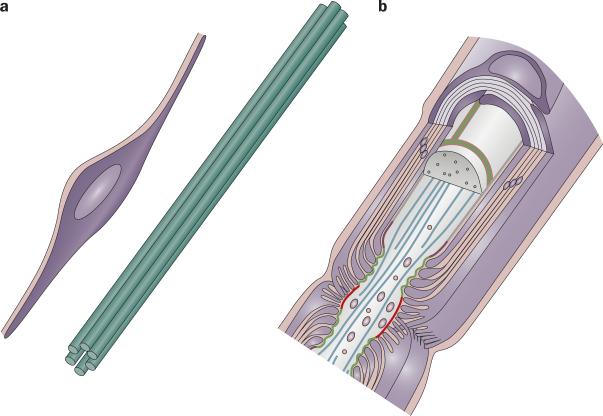
Figure 1.
The myelin–axon unit. The development and maintenance of the myelin–axon unit, here illustrated in the PNS, is dependent on reciprocal signaling between glia and axons. a | Schwann cells sit at the edge of a bundle of axons in prenatal nerves and provide trophic support to neurons (1). In turn, axons and other sources (for example, extracellular matrix) transmit signals that promote the survival and differentiation of glia, as well as myelination (2). b | Eventually, Schwann cells myelinate a segment of one axon. Once the myelin–axon has formed, glia transmit signals that promote axonal health (3), while signals originating from the axon and extracellular matrix promote myelin maintenance (4). Reprinted from Neuron, 40, Salzer, J. L., Polarized domains of myelinated axons, 297–318 © 2003, with permission from Elsevier.
During development, glia provide survival signals to neurons, define the molecular domains of the axolemma and determine the diameter of axons.1,2 In turn, axons provide signals that regulate the proliferation, survival and differentiation of glia, as well as myelin formation.3-5 In adulthood, myelinating glia maintain axolemmal organization, axonal diameter and neuronal health, while axons maintain glial differentiation and myelin integrity.2 At least in the PNS, axonal damage alters these maintenance signals and initiates an active program of Schwann cell dedifferentiation, which probably facilitates myelin reabsorption.6
Multiple diseases target myelin, including multiple sclerosis (MS) and hereditary leukodystrophies in the CNS, and Guillain–Barré Syndrome and hereditary demyelinating neuropathies in the PNS.7 In general, the degree of disability observed in myelin disorders correlates best with the level of associated axonal damage.8, 9 In most disorders, axonal damage is recognized after myelin damage, suggesting that signals from glia or myelin to axons might be altered by disease. New evidence from studies of MS and of globoid (Krabbe) leukodystrophy indicates that axonal damage might occur contemporaneously with myelin damage in some conditions.8, 10 Thus, loss of non-myelin-related glial signals or, even, addition of toxic signals from glia might damage axons. Finally, non-myelin forming Schwann cells are not innocent bystanders. They respond to nearby demyelination by proliferating, thereby impairing their relationship with small caliber axons subserving pain sensation.11 Taken together, these observations call into question which cell type (that is, myelinating or non-myelinating glial cells or neurons) is the most appropriate target for therapy in diseases of myelin.
Treatment of the most common myelin disorders is limited to anti-inflammatory therapies, which can have notable adverse effects.12 Where can we find other therapeutic targets beyond inflammation? Remyelination occurs in both the CNS and PNS after damage and might provide adjunctive targets for therapy that is aimed at limiting destruction of myelin and axons.13 In MS, remyelination occurs in the CNS after initial myelin damage, but fails after multiple episodes of demyelination. This failure of remyelination subsequently leads to augmented axonal degeneration and progressive disability.13 Thus, early intervention with treatments that promote remyelination might slow the progression of MS. Proliferation and migration of oligodendroglial precursor cells (OPCs) near MS plaques has been the focus of much work,13 but failure of differentiation in OPCs has also been recognized. This inability to differentiate might reflect inhibitory influences, or destruction or dysfunction of axons—an important source of differentiation signals for OPCs.13
A starting point for consideration of how differentiation of OPCs might be promoted during repair is the assumption that remyelination depends on signals that are similar to those that occur in developmental myelination. After all, oligodendrocyte precursors or immature Schwann cells very likely recapitulate differentiation as they remyelinate axons.13 Here, we review the various signals that are crucial to the axon–glia interaction (Figure 1). As the molecular basis of glial support of axons is poorly understood,4 the main focus of this Review is on the axon to glia signaling pathways that might promote differentiation and, hence, the formation or maintenance of myelin. We also discuss the extent to which developmental studies can guide remyelination therapies, and provide an overview of the limited number of preclinical and clinical trials that have tested such treatments.
Axolemma-based signaling pathways
Glial myelination can be influenced by axonal contact,14-17 diameter18-20 or electrical activity.21-25 These findings suggest that signals originating from the axonal membrane regulate myelination (Box 2; Figure 2, Figure 3).
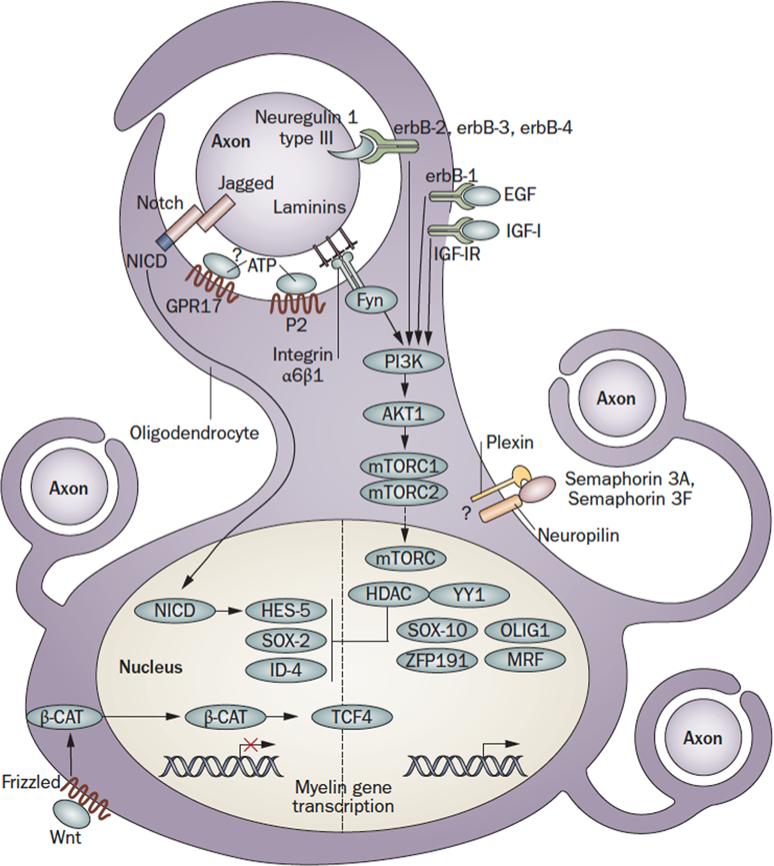
Figure 2.
Axon to glia signaling pathways in myelination. Schematic representation of the main signaling pathways that regulate the onset of myelination during CNS development. The figure depicts an oligodendrocyte just before wrapping. The pathways shown are not comprehensive, but focus on signaling that converges on the glial cell nucleus. Regulatory feedback loops are not displayed. For some molecules, the localization shown is putative. Note that, as oligodendrocytes must integrate signals from multiple axons, with different caliber and electrical activity, a layer of local peri-axonal regulation is likely to be superimposed on nuclear regulation of myelination. Dashed lines indicate signals with uncertain targets. Abbreviations: Nrg: Neuregulin; EGF: Epidermal Growth Factor; IGF1: Insulin-like Growth Factor 1; Sema: Semaphorin; Fyn: Fyn kinase; GPR17: G protein-coupled receptor 17; P2: Purinergic Receptors 2; NICD: Notch-1 intracellular domain: PI3K: phosphatidylinositol-3 kinase; Akt: serine/threonine-specific protein kinaseAkt/PKB; mTOR: mammalian target of rapamycin (mTOR) signaling complexes; HDAC: Histone deacetylase; YY1: YIN-YANG-1; Sox: SRY-box containing transcription factor; Zfp: zinc finger protein; MRF: Myelin gene regulatory factor; Tcf: T-cell factor 4 transcription factor; Hes: hairy and enhancer of split 5 transcription factor; Id: Inhibitor of differentiation transcription factor; β-cat: β-catenin; Wnt: Wingle wingless-related mouse mammary tumor virus integration site protein.
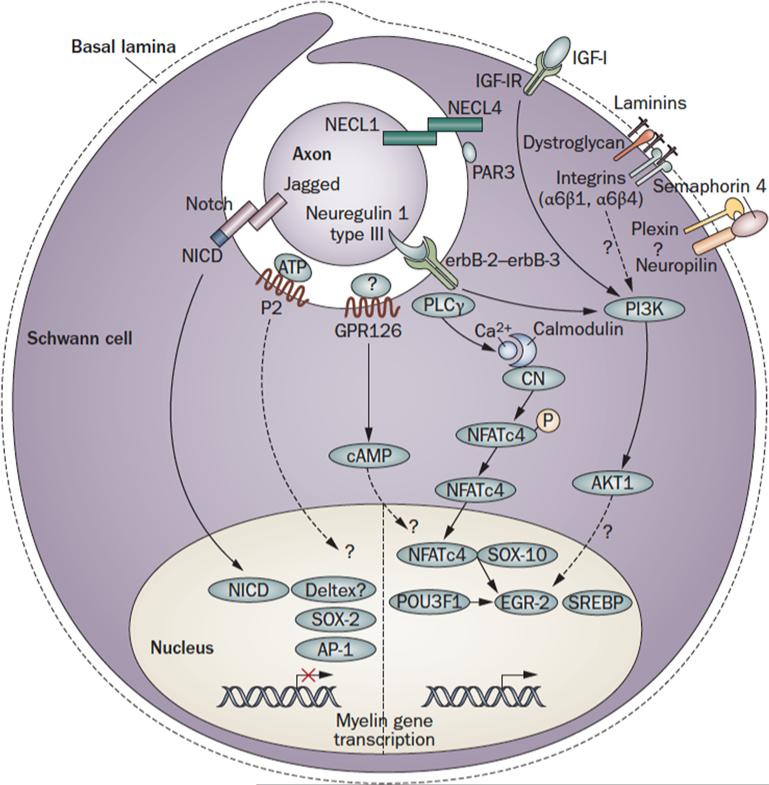
Figure 3.
Axon to glia signaling in myelination. Schematic representation of the main pathways that regulate myelination during PNS development. The figure depicts a promyelinating Schwann cell. The pathways shown are not comprehensive, but focus on signaling that converges on the nucleus. Regulatory feedback loops are not displayed. For some molecules, the localization shown is putative. Dashed lines indicate signals with uncertain targets. NFkB (nuclear factor κB); Pou (Pituitary Octamer Unc-86) 3F1, also known as Tst-1/Oct6/SCIP, Egr (Early growth response) 2, also known as Krox-20; Sox ( SRY-box containing) 10 and SREBP (Sterol Regulatory Element Binding Protein) are transcription factors that activate PNS myelination68, 156. Mutations in these genes are associated with CMT neuropathies9, Waardenburg-Hirschsprung disease and central dysmyelination65, 157. Sox (SRY-box containing) 2 and 4, Id (Inhibitor of differentiation)2, Pax3 and c-Jun-of the activator protein 1 (AP-1 complex, are inhibitory transcription factors active before myelination. Their inappropriate activation might be harmful in neuropathies6. Nrg: Neuregulin; IGF1: Insulin-like Growth Factor 1; Cadm: cell adhesion molecules, also known as IGSF4, SynCAM, Necl, TSLC; PAR: Partition defective 3; GPR: G protein-coupled receptor; P2: Purinergic Receptors 2; NICD: Notch-1 intracellular domain: PI3K: phosphatidylinositol-3 kinase; PLC γ: phospholipase γ; Sema: Semaphorin; NFAT c4: Nuclear factor of activated T-cells, cytoplasmic 4.
Neuregulins and ErbB receptors
In the PNS, the neuregulin (NRG) family of proteins and their receptors, which belong to the ErbB family of tyrosine kinase receptors (heterodimeric ErbB2–ErbB3 in Schwann cells), have emerged as important regulators of most aspects of Schwann cell development (Figure 2).26 The NRGs are encoded by at least four genes, of which Nrg1 is the best characterized.27, 28 Nrg1 encodes multiple isoforms, all containing an epidermal growth factor-like 1 domain. Most isoforms are shed, whereas others remain anchored in the membrane 28. In the PNS, NRG1 type III, which is expressed on the axonal membrane, is a key determinant of whether a Schwann cell will form myelin and, if so, how thick the myelin sheath becomes.29, 30 The potency of NRG1 type III for inducing myelination was demonstrated in cultured superior cervical ganglion neurons, which normally exhibit small-diameter unmyelinated axons. When this NRG was ectopically expressed in such neurons, the associated Schwann cells responded by changing fate and myelinating axons, even if the axons retained their small diameter.30
In the CNS, the role of NRGs in myelination remains controversial. Several studies have reported that rodents deficient in either NRG1 or ErbB receptors had impaired oligodendrocyte differentiation, proliferation, survival and myelination.31-35 These findings suggested that NRGs might be important in CNS myelination. Brinkmann et al., however, reported that mice lacking all NRG1 isoforms or both ErbB3 and ErbB4 receptors showed normal CNS myelination during development. Interestingly, these researchers also showed that NRG1 overexpression in the CNS of transgenic mice induced precocious formation and increased thickness of myelin.36 Taken together, these data suggest that multiple parallel signals probably control myelination in the CNS, and that the regulation of myelination in CNS and PNS differs.35, 36 In support of these assertions, animals with mutations in genes encoding neuronal growth factors,35, 37 extracellular matrix components38 or intracellular mediators39, 40 displayed region-specific myelination in the CNS.
NRG-induced signals are clearly important for myelination during development; however, the involvement of these signals in myelin maintenance, damage or remyelination is unclear. The partial reduction of ErbB2 function in Schwann cells of adult transgenic mice had no effect on myelin, suggesting that neuregulin signals might be dispensible for maintenance of the myelin sheath in the PNS.41 Activation of the ErbB receptors in either myelinating neuron-Schwann cell co-cultures, or upon nerve injury, induced demyelination,42,43 although activation might involve shed NRG1 ligands (not membrane-anchored NRG1 type III). Interestingly, and in agreement with a possible role for ErbB receptors in CNS remyelination, overexpression of ErbB1 in a mouse model of demyelination promoted oligodendrocyte differentiation and remyelination.44
The secretases
In general, the number of growth factors is much lower than the number of biological functions that these molecules regulate. Diversification of function is achieved by altering ligands or downstream signals. For example, a growth factor presented in a soluble or membrane-bound form can activate different signaling pathways. Secretases, like the alpha disintegrin and metalloproteases (ADAM), β-secretase (β-site amyloid precursor protein cleaving enzyme 1; BACE1), or the γ-secretase complex all change the presentation of membrane-associated growth factors.
Many myelin-related growth factors (for example, NRG1) and growth factor receptors (for example, p75 neurotrophin receptor (p75NTR) and Notch-1, undergo proteolytic cleavage at the axonal membrane, suggesting that this process is a common way of regulating these myelin-associated signaling molecules. Identification of the secretases involved in such cleavage and determination of their mechanisms of action could reveal important therapeutic targets for promoting remyelination.
Several ADAMs have been implicated in myelination.45 One study showed that Adam22-null mice exhibited hypomyelinated nerves,46 while data from another study suggested that ADAM22 might bind leucine-rich glioma-inactivated proteins,47 which had been previously implicated in PNS myelination.48 In addition, ADAM19 was upregulated following axonal injury and Adam19-null mice had delayed remyelination.49
Research has revealed that NRG1 type III can be cleaved by BACE1, and that this process is probably regulated by the zinc metallopeptidase nardilysin.50 In agreement with these findings, either BACE1 or nardilysin-null mice showed marked hypomyelination in the PNS50, 51 and in the CNS52, 50. The level of hypomyelination was similar to that observed in mice with a 50% reduction in NRG1 type III expression.29, 30 Taken together, the results from these studies indicate that BACE1 promotes myelination. The data from one study have also revealed that loss of BACE1 impairs remyelination during regeneration after a crush injury to sciatic nerve in mice.53
Inhibition of γ-secretase, enhances the onset and the amount of myelin formed by cultured rat oligodendrocytes.54 Moreover, data from various studies have suggested that γ-secretase might cleave NRG1,55, 56 p75NTR57 or Notch-158, potentially activating multiple intracellular signaling pathways in neurons. Regulated intramembrane proteolysis, a highly conserved method for intracellular communication, appears to be an important control of downstream signals in both axons and glial cells.
The secretases have generated strong interest from pharmaceutical companies as potential therapuetic targets, because these proteases are accessible and multiple secretase inhibitors are already available. Therapeutic strategies that target these proteases must consider the problem of specificity and, hence, collateral effects, as single secretases target multiple molecules. For example, BACE1, a potentiator of myelination, cleaves not only neuregulins, but also amyloid precursor protein, generating the amyloid beta42 peptide, that is probably pathogenetic in Alzheimer disease.
The Akt-1 signaling pathway
Akt-1 is emerging as an integrator of various signals that increase myelination in both the CNS and PNS (Box 3). Studies have shown that, Akt-1 is phosphorylated by phosphatidylinositol 3-kinase (PI3K) in response to growth factors that promote myelination, in particular NRG1 type III,30 insulin growth factor 1 (IGF-1)59, 60 and steroids.61 In addition, the expression of constitutively active Akt-1 in mouse oligodendrocytes led to an increase in myelination.62 PI3K–Akt-1 augments myelination through activation of the kinase mammalian target of rapamycin (mTOR), as inhibition of mTOR in vivo was shown to limit myelination in the developing mouse brain.63 mTOR activation induces formation of both mTORC1 and mTORC2 complexes, which in turn regulates the terminal differentiation of oligodendrocytes, and myelin protein and lipid expression.64 The transcription factors targeted by the Akt-1/mTOR signals have yet to be fully elucidated in oligodendrocytes,65 although studies have identified two possible candidates, namely myelin regulatory factor (MRF)66 and zinc finger protein 191 (ZFP191).67
In Schwann cells, most signals that promote myelination act through the transcription factors POU3F1 (POU domain class 3 transcription factor 1; Oct6), EGR2 (early growth response protein 2; Krox20) and SOX-10.68 For example, in cultured mouse Schwann cells, Akt-1 upregulated EGR2-activated myelin protein zero (P0) expression after IGF-1 stimulation.49 Conversely, EGR2 repressed, and was repressed by, dedifferentiation factors, including SOX2 and Notch.69-71 Such reciprocal inhibition permits active, rapid dedifferentiation of Schwann cells after nerve injury, which is necessary for facilitating myelin regeneration.70, 71 Furthermore, these data suggest that the NRG1 type III–Akt-1 intracellular signaling pathway could be a therapeutic target in demyelinating neuropathies where dedifferentiation genes are inappropriately expressed.6, 72.
The calcineurin–NFAT signaling pathway
A study in mice lacking expression of the calcineurin B1 subunit showed that, in Schwann cells, the calcineurin–NFAT (nuclear factor of activated T cells) pathway is involved in activating myelination 73. This signaling pathway is downstream of NRG1 and independent of PI3K. NFAT signal transduction pathways have important roles in multiple tissues, including the developing nervous system, where these pathways promote axonal growth and guidance.74 Nonetheless, the conditional deletion of the gene encoding calcineurin B1 only in Schwann cells in motor roots or only in sensory neurons but not Schwann cells each suggest that NFAT promotes myelination autonomously in Schwann cells 73. These experiments suggest a model in which activation of ErbB2–ErbB3 heterodimers increases intracellular Ca2+ levels via phospholipase Cγ, and, thus, activates calcineurin. In turn, calcineurin activation promotes nuclear translocation of NFATc4, where it complexes with SOX-10 to upregulate EGR2 transcription and myelin gene transcription.73
Nectin-like proteins
Nectin-like proteins (NECLs), now known as cell adhesion molecules (CADMs), comprise five members and have been implicated in axon–glia interactions and myelination. The roles of CADMs 1–4 have been investigated in the rodent nervous system. Studies have reported that heterophilic interactions between NECL1 (CADM3) on the axolemma and NECL4 (CADM4) on Schwann cells might participate in myelination in the PNS.75, 76 Schwann cells must polarize with axonal and basal lamina surfaces to form myelin, and NECLs have been previously implicated to have a role in this process.77 Thus, NECL4 might cooperate with the PAR (partitioning defective) polarity complexes, previously implicated in PNS myelination.78 Surprisingly, transgenic mice lacking expression of NECL1 displayed a mild phenotype, with no effect on PNS myelination and a developmental delay in CNS myelination.79 Functional compensation by other NECLs,76, 80 or unrelated adhesion proteins, might have accounted for this lack of phenotype.
Notch
Notch signaling is fundamental for glial cell development and myelination in both the CNS and PNS. Notch receptors comprise four members, all of which are type I transmembrane proteins. Upon ligand binding, notch receptors are cleaved intracellularly by secretases. The γ-secretase complex generates an intracellular fragment, the notch intracellular domain (NICD), which translocates to the nucleus to activate gene transcription. The ligand engaged on the Notch receptor determines whether the canonical (mediated by the CBF1/Su(H)/Lag-1 proteins, also known as RBPJ) or non-canonical (mediated by Deltex) signaling pathway is activated 81.
In the CNS, canonical Notch1 ligands, which comprise members of the Delta or Serrate/Jagged family, are expressed by neurons at early developmental stages.82 Notch1 is only expressed by oligodendrocytes83. In vitro and in vivo studies have shown that binding of Jagged-1 to Notch1 inhibits OPC differentiation and myelination,83-86 and that such inhibition is mediated via activation of the transcription factor HES-5 (hairy and enhancer of split 5)82. These findings suggest that Notch1 is important for correct spatial and temporal differentiation of OPCs.
Jagged-1 expression is downregulated in retinal ganglion cells after birth; however, in MS, this protein has been reported to be re-expressed in reactive astrocytes surrounding plaques 82. This finding suggested that the failure of OPCs to mature near such lesions could be the result of reactivation of the Notch inhibitory pathway. In support of this assertion, HES-5 expression has been detected in oligodendrocytes near to MS plaques.82 How much of this protein is found in the nuclei of these cells, however, remains controversial 87.
Surprisingly, in adult mice that were exposed to chemicals that induce demyelination, conditional ablation of Notch1 in oligodendrocytes did not produce a marked effect on remyelination.88 Nevertheless, one study has shown that if Notch1 is inactivated in mice during development, oligodendroglial differentiation is accelerated.86 Ultimately, adult myelin was normal in these animals. In this case, remyelination after a chemical lesion was accelerated. Taken together, these results suggest that the timing of Notch 1 inhibition might be critical for achieving remyelination, and that Notch could be a therapeutic target in myelin disorders.
Contactin-1 (also know as neural cell surface protein F3) is a putative non-canonical ligand of Notch that is expressed on axons later than the canonical ligands in development. This Notch ligand has been suggested to promote rather than inhibit OPC differentiation and the expression of myelin genes in the CNS.89 However, the observation that myelination and remyelination are accelerated and are eventually normal in mice lacking Notch in oligodendrocytes argues against this idea 86, 88.
In the rodent PNS, Notch 1 is expressed exclusively by Schwann cells, whereas Jagged-1 is present on both Schwann cells and axons.71 Notch has been shown to promote gliogenesis over neurogenesis in neural crest stem cells in vitro.90 Furthermore, in vivo rodent studies have demonstrated that both canonical and non-canonical Notch1 pathways determine the complex activity of Notch in PNS development and myelination.71 Notch1, via canonical signaling pathways, promotes the transition from precursor to immature Schwann cells71.
By contrast, Notch1 inhibits the onset of PNS myelination. Notch1 expression is tightly downregulated by EGR2—a transcription factor that is necessary for inducing the activation of the myelinating program.91 Moreover, the overexpression of NICD delays myelination and causes hypomyelination. The inhibitory effect of Notch1 on myelination is mediated through non-canonical pathways, as such inhibition seems to be independent to the effects of RBPJ. Interestingly, myelination is also inhibited immediately following nerve injury, to allow proper Schwann cell dedifferentiation and proliferation. Reactivation of Notch1 expression in this context after injury is associated with the RBPJ canonical signal. Thus, the Notch1 pathway is differentially mediated in development and after injury. Thus, regarding the role of Notch1 in the PNS, the molecular events regulating myelination in development differ in part from those in remyelination.71
HES-5 and histone deacetylases
HES-5 can also be activated by Notch-independent signals in OPCs. Activation of this transcription factor can impede the differentiation of these cells during remyelination. For example, epigenetic modifications of chromatin, such as histone deacetylation, blocked the expression of inhibitory transcription factors, including HES-5, SOX-2, ID-2 (inhibitor of DNA binding 2), and ID-4 (inhibitor of DNA binding 4), and promoted myelination in the mouse 92. Inadequate recruitment of histone deacetylases (HDAC) to the transcriptional promoters of such inhibitory factors results in their sustained expression, mirroring what happens in aged rodents, where remyelination is less efficient than in young rodents.92 This finding suggests that activation of HDACs might represent a promising strategy for promoting remyelination. Paradoxically, use of HDAC inhibitors in animal models of inflammatory demyelination have reduced demyelination and limited disability.92, 93 One possible reason for this discrepancy is that HDAC inhibitors may act not only on oligodendrocytes, but also on cells of the immune system or axons. For example, recent in vitro studies have shown that HDAC1 might induce axonal damage in inflammatory demyelinating diseases when the protein is exported from the nucleus to the cytoplasm of neurons.94 This effect could be a potential confounding factor in studies of remyelination using HDAC inhibitors.
Molecules of axoglial junctions
A series of protein and lipid complexes mediate the interaction between axons and myelinating glia. These complexes organize proteins in the axonal and glial membrane (for example, ion channels), and maintain cell vicinity to facilitate the transmission of signals that promote myelination.1 One role for such complexes is in the segregation of voltage-gated sodium (Nav) and potassium channels (Kv) at nodes of Ranvier and adjacent paranodal and juxtaparanodal regions. Such segregation is crucial for proper conduction of axonal impulses (Figure 1).
The combination of functional importance and accessibility renders the axoglial apparatus a prime pathogenetic—but also pharmacological—target. Indeed, early disaggregation of Nav , gliomedin,95 neurofascin96 and contactin-associated protein 97 clusters precedes demyelination in experimental allergic encephalitis (EAE) in rodents or in MS and disaggregation of clusters is also found at the edges of chronic plaques in MS brains.97 Disruption of ion channel clusters at nodes can produce conduction block and early functional disability that accompanies demyelination.95 Furthermore, reclusterization is the first event that marks the onset of remyelination.98 Finally, nodal and paranodal proteins are altered in genetic neuropathies,99 emphasizing the importance of cluster disruption as a general pathogenetic mechanism.
Interestingly, axoglial complexes contain autoantigens that are targeted by the immune system in MS, chronic inflammatory demyelinating neuropathy and Guillan–Barré syndrome. Autoantibodies against neurofascin 186, gliomedin95 and GM1100 (all nodal components), as well as neurofascin 155101 (a paranodal component) have been detected in patients or animal models , and in some cases shown to be pathogenetic. Human autoantibodies against both axonal and glial neurofascins directly inhibited axonal conduction when applied to rodent tissue slices.101 Moreover, when transferred together with pathogenetic T-cells, these autoantibodies exacerbated the severity of EAE in rats in a complement-dependent manner.101 Similarly, anti–GM1 antibodies bound complement proteins and disrupted Nav channel clusters in rabbit nerves.100 Thus, therapeutic strategies aimed at limiting attacks on the axoglial apparatus or promoting its reformation could protect the role of this apparatus in myelination and impulse conduction.
Extracellular modulators
In addition to molecules in the axolemma, secreted extracellular molecules modulate myelination, either independently or in concert with NRGs or other axonal signals (Box 3).6, 13 The accessibility of these extracellular molecules and their potential role in promoting myelination make them appealing therapeutic targets in myelin diseases. Interesting examples of such molecules are laminins, semaphorins and netrins—all secreted molecules involved in axonal guidance during development.
Laminins
Seminal work from the Bunge laboratory showed that high concentrations of laminin could induce myelination in Schwann cell–neuron cultures in the absence of the myelination–promoting factor ascorbate.102, 103 Integrin and dystroglycan receptors mediate the effects of laminin on the survival and differentiation of oligodendrocytes and Schwann cells.104-106. In both cell types, laminin might cooperate with axonal NRG1 to promote survival and differentiation. Adhesion of integrin α6β1 to laminin effectively switches the response of oligodendrocytes to NRG1 from promoting proliferation to promoting differentiation, and allows survival only in those cells in which differentiation is induced 106, 107. In Schwann cells, the absence of laminin impairs the phosphorylation of ErbB2 and the activation of Akt-1 , normally associated with differentiation 104, 105.
Whether the cooperation in survival and differentiation described between laminin receptors and NRG1 extends to myelination is still unclear. In the CNS, laminin and integrin β1 have been shown to activate the pro-myelinating signals Akt-1,38 Fyn108-110 and p38 MAP kinase,111 while a laminin deficiency in dystrophic mice caused regional hypomyelination.38, 109 Whether integrin β1 mediates these effects is controversial, as expression of a dominant-negative integrin β1 in mice delayed the myelination of small-caliber axons,112 but oligodendrocytes lacking integrin β1 have been reported to either cause hypomyelination,113 or myelinate and remyelinate normally.114 In the PNS, myelin thickness is not obviously reduced in laminin-deficient dystrophic mice, nor in the absence of the laminin receptors dystroglycan or integrin α6β4.115-117 The absence of integrin β1 arrests development before myelin formation, precluding its analysis.118
Interestingly, laminin receptors might also protect against demyelination, as acute demyelination has been described in mice lacking both integrin α6β4 and dystroglycan in Schwann cells.117
Semaphorins
Factors other than laminins promote glial recruitment and differentiation, and might be modulated to improve remyelination.13 Among these factors are secreted semaphorins, whose co-receptors are present on myelinating glia.119, 120 Semaphorins 3A and 3F have been found around active, but not chronic, demyelinated plaques in both patients with MS and mouse models of this disease. These observations led Lubetzki and colleagues to suggest that semaphorins might have a role in remyelination.121 Myelinating oligodendrocytes also express semaphorin 4D after injury,122 which limits oligodendrocyte number123 and promotes process collapse.124 Finally, in Neurofibromatosis 1, loss of semaphorin 4F in Schwann cells impairs axon–glia communication, and restoring semaphorin 4F expression normalizes this interaction in vitro; thus, suggesting a means of reducing neurofibroma formation and promoting myelination.125
Netrins
The netrins are a recently described family of factors present on myelinated axons. Netrin-1 and its receptor, DCC (deleted in colorectal cancer), are present on axons and myelinating oligodendroglia, respectively,119, 126 and might be involved in glial recruitment and myelination.119, 127 The absence of Dcc and Netrin in mice did not preclude myelination, but caused disruption of paranodal junctions.128 Since these junctions are disrupted early in demyelinating diseases, activation of netrin-1 could be protective.
Evidence exists for functional or physical interactions between laminins, semaphorins and netrins.129 Thus, these molecules might represent common pharmacological targets for combined therapies that aim to promote both remyelination and axonal regeneration.
Orphan receptors and signals
A few molecules have been shown to modulate myelination and probably mediate axon glia interactions, but their relationship to established ligands, receptors or signaling pathways is unclear.
Serum response factor
In the nervous system, serum response factor (SRF)—an immediate early gene response transcription factor—is important for axonal pathfinding130 and NGF-dependent innervation of sensory neurons.131 Surprisingly, ablation of SRF exclusively in neurons of mice led to hypomyelination (primarily around large caliber axons), impairment of oligodendroglial development and an increase in astrocyte numbers.132 The lack of SRF augments the release of secreted connective tissue growth factor, which associates with the extracellular matrix and, perhaps through sequestration of IGF-1, inhibits oligodendrocyte differentiation and myelination 132.
G protein-coupled receptor 126
Talbot and colleagues have shown, by way of genetic screening, that G protein-coupled receptor (Gpr) 126 is required for peripheral myelination in zebrafish133. In this study, these researchers found evidence that Gpr126 acts in Schwann cells to increase cylic AMP, activate the zebrafish homologue of EGR2 and promote myelination. The ligand for Gpr126 has not yet been identified, and the downstream effects of activating Gpr126 in the CNS remain to be described, although the mammalian homologue of this receptor is under study in mice.
Wnt signaling
In both the CNS and PNS, glial cells must exit from the cell cycle in order to differentiate and initiate the myelination program. This transition is regulated at the transcriptional level,65, 68 and, at least in the CNS, requires histone deacetylation,134 which inhibits the Wnt signaling pathway in part.135 One of the molecules implicated in controlling the exit from the cell cycle is the transcription factor YY1 (yin and yang 1).136 This is a highly conserved nuclear protein that can act either as a repressor or activator.137 Conditional ablation of Yy1 in oligodendrocytes in mice led to impairment of myelination, particularly in the spinal cord. This phenotype was accompanied, at the molecular level, by an arrest of differentiation, with OPCs blocked after exit from the cell cycle. This block was probably the result of a loss of recruitment of YY1 and repressive HDACs to the promoters of transcriptional inhibitors such as TCF4 (transcription factor 4) and ID4.136 The consequence is uncontrolled inhibition of terminal differention of OPCs.
This idea was further supported by the observations that the Wnt–catenin β1 signaling pathway was shown to be active in oligodendroglia surrounding MS lesions, and that dysregulation of these signals in OPCs delayed myelination and remyelination.138, 139 Surprisingly, Tcf4-null mice, which might have been predicted to show derepression from Wnt signaling and premature myelination, actually showed a reduction rather than an increase in expression of oligodendrocyte terminal differentation genes. Myelination could not be directly assessed in this study as Tcf4-null mice die perinatally.140 Ablation of the genes encoding components of the Wnt–catenin β1–TCF4 signaling pathway in oligodendrocytes during myelination will be required to fully understand the role of this pathway in this process and its therapeutic potential.
G-protein coupled receptor 17
Activation of GPR17, a P2Y purinergic GPR, provides further inhibitory regulation of myelination. Mice with loss-of-function mutations in Gpr17 showed premature myelination, whereas overexpression of this gene in mouse brain or cultured OPCs inhibited the maturation of OPCs to myelinating oligodendrocytes, at least in part by increasing ID2 and ID4 expression in the nuclei of OPCs.141 Interestingly, pharmacological activation of GPR17 augmented maturation and MBP expression in cultured OPCs.142 Contrasting results notwithstanding, these data suggest that GPR17 might participate in the purinergic-regulated timing of oligodendroglial differentiation, which was previously postulated by Fields and colleagues 23 to couple electrical activity to the onset of myelination. Furthermore, GPR17 is upregulated in demyelinating lesions in EAE and MS, making this receptor another candidate target for derepression of OPC differentiation in MS.
Does myelination model remyelination?
Many signaling molecules have been characterized in myelin formation during development; however, only a few of these have been evaluated in the context of remyelination. Thus, it is too early to judge whether a molecule's function in development predicts its utility in myelin repair. In some cases, the effect was not found, or paradoxically was opposite of that expected. As Franklin and ffrench-Constant have reviewed,13 these findings might suggest that the intrinsic mechanisms underlying myelin formation in development and after damage differ. Alternatively, the contrasting effects of the signaling molecules might stem from the specific inhibitory effects of the demyelinating environment (for example, inflammatory cytokines), which are not present during development. Moreover, such effects might be explained by making comparisons between diverse processes in myelination and remyelination (for example, comparison of the effects on migration in development with effects on differentiation in demyelination) or, more simply, experimental issues (for example, genetic ablation of one gene might induce compensatory expression of other genes).
One example of a molecule that behaves differently in development and remyelination is Olig1 (oligodendrocyte transcription factor 1), which is a transcription factor that is important for the production and differentiation of OPCs, as well as myelination. Studies in Olig1-null mice suggested that Olig1 is much less important in developing oligodendrocytes than in remyelinating oligodendrocytes.143 Xin and colleagues, however, found that Olig1 was crucial for normal oligodendrocyte development and myelination.144 This discrepancy is probably a consequence of different gene targeting strategies. In the first study 143, the strategy that was used might have activated transcription from the nearby Olig2 gene, which encodes another transcription factor that is essential for oligodendrocyte development and myelination.144 Thus, multiple strategies to assess function, as well as tests in various models of demyelination will be required to validate putative potentiators of myelin repair.145
Preclinical and clinical trials
Two types of therapeutic strategies related to myelination signals have been proposed. The first involves reducing the interference to myelination signals. As noted above, interference of such signals is posited to occur at all levels of signaling pathways, ranging from extracellular ligand–receptor interactions101, 146 to transcription.6 Genetic proof of principle experiments are underway in mouse models of hereditary neuropathies to limit expression of ‘dedifferentiation’ genes such as cJun or Sox2 in Schwann cells; no results have been published thus far.
The second approach is to restore or augment normal myelination signaling during remyelination. The first preclinical and clinical trials along these lines have appeared in the last 6 years. For example, 70% of hereditary demyelinating neuropathies are caused by overexpression of peripheral myelin protein 22 (PMP22) in Charcot-Marie-Tooth disease type 1A [CMT1A]. Therefore, reducing myelination signaling in order to normalize PMP22 expression might be of therapeutic benefit. Preclinical trials of ascorbic acid, which might lower levels of cAMP,147, 148 and the progesterone inhibitor onapristone149, 150 have demonstrated that, in principle, the reduction of PMP22 levels improves myelination in animal models of CMT1A. As a result, several clinical trials with ascorbic acid in patients with CMT1A are underway151-153 and a search is ongoing for less toxic progesterone inhibitors than onapristone.154 Thyroid hormone T4 promotes oligodendrocyte differentiation and myelination in development, and has been shown to promote myelin repair in a preclinical trial in acute EAE.155 IGF-1 also augments myelination in the CNS,59 and a phase II pilot study of the tolerability and efficacy of subcutaneously administered recombinant human IGF-1 (CEP-151) in patients with MS has been completed, although no results have been reported yet.
Conclusions
Continuous reciprocal dialogue between axons and myelinating glia is important during development and maintenance of the myelin sheath. Various types of molecules, acting at diverse regulatory levels, mediate these events. Some of these signaling molecules normally promote myelination, and might be perturbed in myelin diseases, whereas others normally inhibit myelination and can be inappropriately active in such disorders. On the basis of these signals, several therapies are being examined in preclinical trials, with the first clinical trials now underway.
The search for signaling molecules that promote remyelination holds promise for eventually developing combinatorial therapies, including existing anti-inflammatory interventions, for demyelinating diseases. Future research into the development of such treatments will need to address two additional issues. First, for the various diseases of myelin, the initial site of damage (glia or axons) needs to be resolved, as this location might have consequences for where the drug is delivered. Indeed, the cell bodies of neurons and glia that form one myelin–axon unit sometimes reside in different parts of the nervous system (e.g. Schwann cells in peripheral nerve versus motor neurons in the spinal cord), which are characterized by differing pharmacological barriers. Second, as evidence mounts for the important role of axonal injury in disability, signals originating in glia that mediate axonal support need to be identified and characterized. Surprisingly little is known about these signals currently (Box 4).
Box 1 | Myelin formation in mammals.
Rodents
Before birth, oligodendroglial precursors emerge from the ventral and dorsal neural tube and migrate throughout the forming brain and spinal cord.
Perinatally, these cells differentiate and mostly after birth, myelinate segments of multiple axons.158
Before birth, Schwann cell precursors arise from the neural crest and migrate out with extending neurites in forming embryonic peripheral nerves.
Perinatally, immature Schwann cells differentiate, and mostly after birth, Schwann cells begin to form myelin, but unlike oligodendrocytes only myelinate a single segment of one axon.5
Human
Before birth, oligodendroglial and Schwann cell precursors follow a similar developmental pattern as observed in rodents.
Perinatally, myelination has already begun in both the PNS (spinal roots) and CNS (primarily in spinal cord, brainstem and cerebellum, but sparsely above the subcortical nuclei) 159, but myelin formation is significantly more protracted in human than in rodents, extending over the first several years of life.
Box 2 | Axonal signals to glia.
Neuregulins and ErbBs
Notch-1
Neurotrophins
See 161 for an overview of the role of neurotrophins in myelination
BDNF binds to glial p75NTR and promotes myelination78
After myelination, BDNF binds to truncated TrkB molecules on Schwann cells to limit myelination162
In vitro, neurotrophin 3 promotes Schwann cell migration but inhibits myelination. Ntf3-null mice are hypomyelinated163
BDNF binds to full length TrkB molecules on oligodendrocytes and promotes differentiation.
Neurotrophin 3, like platelet-derived growth factor, promotes oligodendrocyte migration, proliferation, survival and differentiation
Neural cell adhesion molecule
Nectin-like proteins
PSA-NCAM
ATP purinergic signaling
Adenosine binds glial purinergic receptors and promotes oligodendroglial differentiation 23
Electrically active neurons (axons) release ATP, which stimulates the production and release of leukemia inhibitory factor from astrocytes, thereby augmenting myelination by oligodendrocytes166.
ATP binds glial P2Y receptors and inhibits Schwann cell proliferation and differentiation 167
Adenosine also binds A2A receptor, which inhibits Schwann cell proliferation but not myelination 168
Neurofascin 186 and contactin-associated protein
Axonal components of the nodes of Ranvier and paranodes could be targets of autoantibodies in MS patients1, 97
Abbreviations: BDNF, brain-derived neurotrophic factor; PSA-NCAM, polysialic acid neural cell adhesion molecule; Trk, tropomyosin receptor kinase
Box 3 | Non-axonal membrane signals to glia.
Laminins, integrins and dystroglycan
Components of the extracellular matrix (laminins) and their receptors (integrins and dystroglycan) are required in Schwann cells for radial sorting and ensheathment of axons, and myelination 169.
Insulin-like growth factor 1
Insulin-like growth factor 1 promotes oligodendrocyte differentiation and survival, as well as myelin integrity and function59
Administration of exogenous recombinant human insulin-like growth factor 1 to rats with experimental autoimmune encephalomyelitis closed the disrupted blood–brain barrier, reduced the number and severity of demyelinating lesions, and improved neurological function170
Progesterone
Thyroid hormone
As for platelet-derived growth factor, thyroid hormone is an instructive signal for oligodendrocyte development and maturation172
Administration of thyroxine (T4) in animal models of demyelination and remyelination, such as experimental autoimmune encephalomyelitis or in cuprizone treated animals, has proven beneficial for remyelination155, 173
Semaphorins
Netrins
Netrins are secreted molecules that are important for axonal pathfinding
In the CNS, the netrin receptor DCC promotes membrane extension127
Box 4 | Glial signals to axons.
Neurotrophins
Glia release neurotrophins to axons, which in response modulate myelination161
In the PNS, brain-derived neutrophic factor is bound to axonal p75NTR and promotes myelination; however, this neutrophic factor, when bound to full length TrkB, inhibits myelination
Nerve growth factor binds TrkA on axons and promotes myelination in the PNS
Glial cell-derived neurotrophic factor probably binds Ret on axons, and promotes myelination of nociceptive neurons in the PNS
In the CNS, nerve growth factor binds TrkA on axons and inhibits myelination
Merlin
Merlin is encoded by the tumor suppressor gene NF2 (implicated in neurofibromatosis type 2 tumors) and is important for the formation of axon–glial junctions174
Lgi4
Lgi4 is an orphan molecule secreted by Schwann cells that regulates PNS myelination, perhaps through effects on axons48
Myelin-associated glycoprotein
Myelin-associated glycoprotein is located on the membrane of glial cells facing axons
The absence of myelin-associated glycoprotein promotes axonal degeneration2
Erytropoietin
Erytropoietin is released by Schwann cells after injury and reduces axonal degeneration175
Sirtuin2
Sirtuin 2 in oligodendrocytes is a putative mediator of axonal degeneration in spastic paraplegia 2 due to mutations in proteolipid protein
lack of proteolipid protein is associated with absence of sirtuin 2 from CNS myelin 176
Fibroblast growth factor receptors
Fibroblast growth factor receptor activation prevents degeneration of unmyelinated sensory axons in the PNS and CNS177
Gliomedin and neurofascin 155
Gliomedin and neurofascin 155 are glial components of the node of Ranvier and paranodal axoglial junctions1
Alteration of gliomedin and neurofascin 155 clusters precedes demyelination in experimental allergic neuritis1, 95
Abbreviation: Trk, tropomyosin receptor kinase.
Acknowledgments
Work in the laboratories of C. Taveggia, M. L. Feltri and L. Wrabetz is supported by grants from FISM (Fondazione Italiana Sclerosi Multipla) Italy; Telethon, Italy; Compagnia di San Paolo, Italy; the NIH, USA; and the European Community. We apologize to colleagues whose relevant work we were unable to cite because of space limitations.
Footnotes
Competing interests
The authors declare no competing interests.
Review criteria
We searched PubMed for articles published up until January 2010 using the terms “axon signaling and glia”, “myelin”, or “remyelination”. We also visited the Inherited Peripheral Neuropathies Mutation Database (http://www.molgen.ua.ac.be/CMTMutations/default.cfm), and consulted our personal collections of literature. In addition, we searched the clinical trial registries http://clinicaltrials.gov/ and http://www.controlled-trials.com/mrct/ with the search terms myelin or remyelination.
References
- 1.Salzer JL, Brophy PJ, Peles E. Molecular domains of myelinated axons in the peripheral nervous system. Glia. 2008;56:1532–40. doi: 10.1002/glia.20750. [DOI] [PubMed] [Google Scholar]
- 2.Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–61. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- 3.Bozzali M, Wrabetz L. Axonal signals and oligodendrocyte differentiation. Neurochem Res. 2004;29:979–88. doi: 10.1023/b:nere.0000021242.12455.75. [DOI] [PubMed] [Google Scholar]
- 4.Simons M, Trajkovic K. Neuron-glia communication in the control of oligodendrocyte function and myelin biogenesis. J Cell Sci. 2006;119:4381–9. doi: 10.1242/jcs.03242. [DOI] [PubMed] [Google Scholar]
- 5.Woodhoo A, Sommer L. Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia. 2008;56:1481–90. doi: 10.1002/glia.20723. [DOI] [PubMed] [Google Scholar]
- 6.Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–65. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- 7.Lazzarini RA, editor. Myelin Biology and Disorders. Elselvier Academic Press; San Diego: 2004. [Google Scholar]
- 8.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–69. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 9.Scherer SS, Wrabetz L. Molecular mechanisms of inherited demyelinating neuropathies. Glia. 2008;56:1578–89. doi: 10.1002/glia.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galbiati F, et al. Combined hematopoietic and lentiviral gene-transfer therapies in newborn Twitcher mice reveal contemporaneous neurodegeneration and demyelination in Krabbe disease. J Neurosci Res. 2009;87:1748–59. doi: 10.1002/jnr.22006. [DOI] [PubMed] [Google Scholar]
- 11.Murinson BB, Archer DR, Li Y, Griffin JW. Degeneration of myelinated efferent fibers prompts mitosis in Remak Schwann cells of uninjured C-fiber afferents. J Neurosci. 2005;25:1179–87. doi: 10.1523/JNEUROSCI.1372-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Diego RS, Weiner HL. Novel therapeutic strategies for multiple sclerosis--a multifaceted adversary. Nat Rev Drug Discov. 2008;7:909–25. doi: 10.1038/nrd2358. [DOI] [PubMed] [Google Scholar]
- 13.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–55. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 14.Langley JN, Anderson HK. On the union of the fifth cervical nerve with the superior cervical ganglion. J Physiol. 1904;30:439–42. doi: 10.1113/jphysiol.1904.sp001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberg HJ, Spencer PS. Studies on the control of myelinogenesis. II. Evidence for neuronal regulation of myelin production. Brain Res. 1976;113:363–78. doi: 10.1016/0006-8993(76)90947-1. [DOI] [PubMed] [Google Scholar]
- 16.Aguayo AJ, Epps J, Charron L, Bray GM. Multipotentiality of Schwann cells in cross-anastomosed and grafted myelinated and unmyelinated nerves: quantitative microscopy and radioautography. Brain Res. 1976;104:1–20. doi: 10.1016/0006-8993(76)90643-0. [DOI] [PubMed] [Google Scholar]
- 17.Lubetzki C, et al. Even in culture, oligodendrocytes myelinate solely axons. Proc Natl Acad Sci U S A. 1993;90:6820–4. doi: 10.1073/pnas.90.14.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan D. A relation between axon diameter and myelination determined by measurement of myelinated spinal root fibers. J Comp Neurol. 1934;60:437–471. [Google Scholar]
- 19.Voyvodic JT. Target size regulates calibre and myelination of sympathetic axons. Nature. 1989;342:430–3. doi: 10.1038/342430a0. [DOI] [PubMed] [Google Scholar]
- 20.Friede RL, Samorajski T. Relation between the number of myelin lamellae and axon circumference in fibers of vagus and sciatic nerves of mice. J Comp Neurol. 1967;130:223–31. doi: 10.1002/cne.901300304. [DOI] [PubMed] [Google Scholar]
- 21.Gyllensten L, Malmfors T. Myelinization of the optic nerve and its dependence on visual function--a quantitative investigation in mice. J Embryol Exp Morphol. 1963;11:255–66. [PubMed] [Google Scholar]
- 22.Tauber H, Waehneldt TV, Neuhoff V. Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci Lett. 1980;16:235–8. doi: 10.1016/0304-3940(80)90003-8. [DOI] [PubMed] [Google Scholar]
- 23.Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–68. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens B, Tanner S, Fields RD. Control of myelination by specific patterns of neural impulses. J Neurosci. 1998;18:9303–11. doi: 10.1523/JNEUROSCI.18-22-09303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demerens C, et al. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93:9887–92. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birchmeier C, Nave KA. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia. 2008;56:1491–7. doi: 10.1002/glia.20753. [DOI] [PubMed] [Google Scholar]
- 27.Falls DL. Neuregulins and the neuromuscular system: 10 years of answers and questions. J Neurocytol. 2003;32:619–47. doi: 10.1023/B:NEUR.0000020614.83883.be. [DOI] [PubMed] [Google Scholar]
- 28.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–52. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michailov GV, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–3. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 30.Taveggia C, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–94. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vartanian T, Fischbach G, Miller R. Failure of spinal cord oligodendrocyte development in mice lacking neuregulin. Proc Natl Acad Sci U S A. 1999;96:731–5. doi: 10.1073/pnas.96.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sussman CR, Dyer KL, Marchionni M, Miller RH. Local control of oligodendrocyte development in isolated dorsal mouse spinal cord. J Neurosci Res. 2000;59:413–20. doi: 10.1002/(SICI)1097-4547(20000201)59:3<413::AID-JNR16>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 33.Sussman CR, Vartanian T, Miller RH. The ErbB4 neuregulin receptor mediates suppression of oligodendrocyte maturation. J Neurosci. 2005;25:5757–62. doi: 10.1523/JNEUROSCI.4748-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy K, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci U S A. 2007;104:8131–6. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taveggia C, et al. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56:284–93. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- 36.Brinkmann BG, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–95. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fruttiger M, et al. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–67. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- 38.Chun SJ, Rasband MN, Sidman RL, Habib AA, Vartanian T. Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J Cell Biol. 2003;163:397–408. doi: 10.1083/jcb.200304154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperber BR, et al. A unique role for Fyn in CNS myelination. J Neurosci. 2001;21:2039–47. doi: 10.1523/JNEUROSCI.21-06-02039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim HJ, et al. WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination. J Neurosci. 2006;26:5849–59. doi: 10.1523/JNEUROSCI.4921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atanasoski S, et al. ErbB2 signaling in Schwann cells is mostly dispensable for maintenance of myelinated peripheral nerves and proliferation of adult Schwann cells after injury. J Neurosci. 2006;26:2124–31. doi: 10.1523/JNEUROSCI.4594-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zanazzi G, et al. Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination. J Cell Biol. 2001;152:1289–99. doi: 10.1083/jcb.152.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guertin AD, Zhang DP, Mak KS, Alberta JA, Kim HA. Microanatomy of axon/glial signaling during Wallerian degeneration. J Neurosci. 2005;25:3478–87. doi: 10.1523/JNEUROSCI.3766-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- 45.Yang P, Baker KA, Hagg T. The ADAMs family: coordinators of nervous system development, plasticity and repair. Prog Neurobiol. 2006;79:73–94. doi: 10.1016/j.pneurobio.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Sagane K, et al. Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 2005;6:33. doi: 10.1186/1471-2202-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sagane K, Ishihama Y, Sugimoto H. LGI1 and LGI4 bind to ADAM22, ADAM23 and ADAM11. Int J Biol Sci. 2008;4:387–96. doi: 10.7150/ijbs.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bermingham JR, Jr., et al. The claw paw mutation reveals a role for Lgi4 in peripheral nerve development. Nat Neurosci. 2006;9:76–84. doi: 10.1038/nn1598. [DOI] [PubMed] [Google Scholar]
- 49.Wakatsuki S, Yumoto N, Komatsu K, Araki T, Sehara-Fujisawa A. Roles of meltrin-beta/ADAM19 in progression of Schwann cell differentiation and myelination during sciatic nerve regeneration. J Biol Chem. 2009;284:2957–66. doi: 10.1074/jbc.M803191200. [DOI] [PubMed] [Google Scholar]
- 50.Ohno M, et al. Nardilysin regulates axonal maturation and myelination in the central and peripheral nervous system. Nat Neurosci. 2009;12:1506–13. doi: 10.1038/nn.2438. [DOI] [PubMed] [Google Scholar]
- 51.Willem M, et al. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–6. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 52.Hu X, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–5. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 53.Hu X, et al. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. Faseb J. 2008;22:2970–80. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watkins TA, Emery B, Mulinyawe S, Barres BA. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron. 2008;60:555–69. doi: 10.1016/j.neuron.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bao J, et al. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat Neurosci. 2004;7:1250–8. doi: 10.1038/nn1342. [DOI] [PubMed] [Google Scholar]
- 56.Bao J, Wolpowitz D, Role LW, Talmage DA. Back signaling by the Nrg-1 intracellular domain. J Cell Biol. 2003;161:1133–41. doi: 10.1083/jcb.200212085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zampieri N, Xu CF, Neubert TA, Chao MV. Cleavage of p75 neurotrophin receptor by alpha-secretase and gamma-secretase requires specific receptor domains. J Biol Chem. 2005;280:14563–71. doi: 10.1074/jbc.M412957200. [DOI] [PubMed] [Google Scholar]
- 58.Fortini ME. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol. 2002;3:673–84. doi: 10.1038/nrm910. [DOI] [PubMed] [Google Scholar]
- 59.Carson MJ, Behringer RR, Brinster RL, McMorris FA. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;10:729–40. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- 60.Ness JK, Mitchell NE, Wood TL. IGF-I and NT-3 signaling pathways in developing oligodendrocytes: differential regulation and activation of receptors and the downstream effector Akt. Dev Neurosci. 2002;24:437–45. doi: 10.1159/000069050. [DOI] [PubMed] [Google Scholar]
- 61.Swamydas M, Bessert D, Skoff R. Sexual dimorphism of oligodendrocytes is mediated by differential regulation of signaling pathways. J Neurosci Res. 2009;87:3306–19. doi: 10.1002/jnr.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flores AI, et al. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28:7174–83. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Narayanan SP, Flores AI, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J Neurosci. 2009;29:6860–70. doi: 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tyler WA, et al. Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. J Neurosci. 2009;29:6367–78. doi: 10.1523/JNEUROSCI.0234-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wegner M. A matter of identity: transcriptional control in oligodendrocytes. J Mol Neurosci. 2008;35:3–12. doi: 10.1007/s12031-007-9008-8. [DOI] [PubMed] [Google Scholar]
- 66.Emery B, et al. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138:172–85. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Howng SY, et al. ZFP191 is required by oligodendrocytes for CNS myelination. Genes Dev. doi: 10.1101/gad.1864510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Svaren J, Meijer D. The molecular machinery of myelin gene transcription in Schwann cells. Glia. 2008;56:1541–51. doi: 10.1002/glia.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mager GM, et al. Active gene repression by the Egr2.NAB complex during peripheral nerve myelination. J Biol Chem. 2008;283:18187–97. doi: 10.1074/jbc.M803330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parkinson DB, et al. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181:625–37. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woodhoo A, et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci. 2009 doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giambonini-Brugnoli G, Buchstaller J, Sommer L, Suter U, Mantei N. Distinct disease mechanisms in peripheral neuropathies due to altered peripheral myelin protein 22 gene dosage or a Pmp22 point mutation. Neurobiol Dis. 2005;18:656–68. doi: 10.1016/j.nbd.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 73.Kao SC, et al. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science. 2009;323:651–4. doi: 10.1126/science.1166562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Graef IA, et al. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell. 2003;113:657–70. doi: 10.1016/s0092-8674(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 75.Maurel P, et al. Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J Cell Biol. 2007;178:861–74. doi: 10.1083/jcb.200705132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spiegel I, et al. A central role for Necl4 (SynCAM4) in Schwann cell-axon interaction and myelination. Nat Neurosci. 2007;10:861–9. doi: 10.1038/nn1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ogita H, Takai Y. Nectins and nectin-like molecules: roles in cell adhesion, polarization, movement, and proliferation. IUBMB Life. 2006;58:334–43. doi: 10.1080/15216540600719622. [DOI] [PubMed] [Google Scholar]
- 78.Chan JR, et al. The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science. 2006;314:832–6. doi: 10.1126/science.1134069. [DOI] [PubMed] [Google Scholar]
- 79.Park J, et al. Disruption of Nectin-like 1 cell adhesion molecule leads to delayed axonal myelination in the CNS. J Neurosci. 2008;28:12815–9. doi: 10.1523/JNEUROSCI.2665-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pellissier F, Gerber A, Bauer C, Ballivet M, Ossipow V. The adhesion molecule Necl-3/SynCAM-2 localizes to myelinated axons, binds to oligodendrocytes and promotes cell adhesion. BMC Neurosci. 2007;8:90. doi: 10.1186/1471-2202-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.D'Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27:5148–67. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.John GR, et al. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8:1115–21. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- 83.Wang S, et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- 84.Genoud S, et al. Notch1 control of oligodendrocyte differentiation in the spinal cord. J Cell Biol. 2002;158:709–18. doi: 10.1083/jcb.200202002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Givogri MI, et al. Central nervous system myelination in mice with deficient expression of Notch1 receptor. J Neurosci Res. 2002;67:309–20. doi: 10.1002/jnr.10128. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, et al. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc Natl Acad Sci U S A. 2009;106:19162–7. doi: 10.1073/pnas.0902834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakahara J, Kanekura K, Nawa M, Aiso S, Suzuki N. Abnormal expression of TIP30 and arrested nucleocytoplasmic transport within oligodendrocyte precursor cells in multiple sclerosis. J Clin Invest. 2009;119:169–81. doi: 10.1172/JCI35440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stidworthy MF, et al. Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination. Brain. 2004;127:1928–41. doi: 10.1093/brain/awh217. [DOI] [PubMed] [Google Scholar]
- 89.Hu QD, et al. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell. 2003;115:163–75. doi: 10.1016/s0092-8674(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 90.Morrison SJ, et al. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101:499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 91.Parkinson DB, et al. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol. 2004;164:385–94. doi: 10.1083/jcb.200307132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen S, et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008;11:1024–34. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Camelo S, et al. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;164:10–21. doi: 10.1016/j.jneuroim.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 94.Kim JY, et al. HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat Neurosci. 2009 doi: 10.1038/nn.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lonigro A, Devaux JJ. Disruption of neurofascin and gliomedin at nodes of Ranvier precedes demyelination in experimental allergic neuritis. Brain. 2009;132:260–73. doi: 10.1093/brain/awn281. [DOI] [PubMed] [Google Scholar]
- 96.Howell OW, et al. Disruption of neurofascin localization reveals early changes preceding demyelination and remyelination in multiple sclerosis. Brain. 2006;129:3173–85. doi: 10.1093/brain/awl290. [DOI] [PubMed] [Google Scholar]
- 97.Wolswijk G, Balesar R. Changes in the expression and localization of the paranodal protein Caspr on axons in chronic multiple sclerosis. Brain. 2003;126:1638–49. doi: 10.1093/brain/awg151. [DOI] [PubMed] [Google Scholar]
- 98.Coman I, et al. Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain. 2006;129:3186–95. doi: 10.1093/brain/awl144. [DOI] [PubMed] [Google Scholar]
- 99.Arroyo EJ, et al. Genetic dysmyelination alters the molecular architecture of the nodal region. J Neurosci. 2002;22:1726–37. doi: 10.1523/JNEUROSCI.22-05-01726.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Susuki K, et al. Anti-GM1 antibodies cause complement-mediated disruption of sodium channel clusters in peripheral motor nerve fibers. J Neurosci. 2007;27:3956–67. doi: 10.1523/JNEUROSCI.4401-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mathey EK, et al. Neurofascin as a novel target for autoantibody-mediated axonal injury. J Exp Med. 2007;204:2363–72. doi: 10.1084/jem.20071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eldridge CF, Bunge MB, Bunge RP. Differentiation of axon-related Schwann cell in vitro: II. Control of myelin formation by basal lamina. J. Neurosci. 1989;9:625–638. doi: 10.1523/JNEUROSCI.09-02-00625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eldridge CF, Bunge MB, Bunge RP, Wood PM. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol. 1987;105:1023–34. doi: 10.1083/jcb.105.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu WM, Feltri ML, Wrabetz L, Strickland S, Chen ZL. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. Journal of Neuroscience. 2005;25:4463–4472. doi: 10.1523/JNEUROSCI.5032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang D, et al. Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J Cell Biol. 2005 doi: 10.1083/jcb.200411158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Colognato H, et al. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002;4:833–41. doi: 10.1038/ncb865. [DOI] [PubMed] [Google Scholar]
- 107.Colognato H, Ramachandrappa S, Olsen IM, ffrench-Constant C. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol. 2004;167:365–75. doi: 10.1083/jcb.200404076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kramer EM, Klein C, Koch T, Boytinck M, Trotter J. Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J Biol Chem. 1999;274:29042–9. doi: 10.1074/jbc.274.41.29042. [DOI] [PubMed] [Google Scholar]
- 109.Relucio J, Tzvetanova ID, Ao W, Lindquist S, Colognato H. Laminin alters fyn regulatory mechanisms and promotes oligodendrocyte development. J Neurosci. 2009;29:11794–806. doi: 10.1523/JNEUROSCI.0888-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Laursen LS, Chan CW, ffrench-Constant C. An integrin-contactin complex regulates CNS myelination by differential Fyn phosphorylation. J Neurosci. 2009;29:9174–85. doi: 10.1523/JNEUROSCI.5942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haines JD, Fragoso G, Hossain S, Mushynski WE, Almazan G. p38 Mitogen-activated protein kinase regulates myelination. J Mol Neurosci. 2008;35:23–33. doi: 10.1007/s12031-007-9011-0. [DOI] [PubMed] [Google Scholar]
- 112.Camara J, et al. Integrin-mediated axoglial interactions initiate myelination in the central nervous system. J Cell Biol. 2009;185:699–712. doi: 10.1083/jcb.200807010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barros CS, et al. Beta1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development. 2009;136:2717–24. doi: 10.1242/dev.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Benninger Y, et al. Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J Neurosci. 2006;26:7665–73. doi: 10.1523/JNEUROSCI.0444-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jaros E, Jenkison M. Quantitative studies of the abnormal axon-Schwann cell relationship in the peripheral motor and sensory nerves of the dystrophic mouse. Brain Res. 1983;258:181–96. doi: 10.1016/0006-8993(83)91141-1. [DOI] [PubMed] [Google Scholar]
- 116.Saito F, et al. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–58. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 117.Nodari A, et al. Alpha6beta4 integrin and dystroglycan cooperate to stabilize the myelin sheath. J Neurosci. 2008;28:6714–9. doi: 10.1523/JNEUROSCI.0326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Feltri ML, et al. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156:199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spassky N, et al. Directional guidance of oligodendroglial migration by class 3 semaphorins and netrin-1. J Neurosci. 2002;22:5992–6004. doi: 10.1523/JNEUROSCI.22-14-05992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bannerman P, et al. Peripheral nerve regeneration is delayed in neuropilin 2-deficient mice. J Neurosci Res. 2008;86:3163–9. doi: 10.1002/jnr.21766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Williams A, et al. Semaphorin 3A and 3F: key players in myelin repair in multiple sclerosis? Brain. 2007;130:2554–65. doi: 10.1093/brain/awm202. [DOI] [PubMed] [Google Scholar]
- 122.Moreau-Fauvarque C, et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003;23:9229–39. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taniguchi Y, et al. Sema4D deficiency results in an increase in the number of oligodendrocytes in healthy and injured mouse brains. J Neurosci Res. 2009;87:2833–41. doi: 10.1002/jnr.22124. [DOI] [PubMed] [Google Scholar]
- 124.Giraudon P, et al. Semaphorin CD100 from activated T lymphocytes induces process extension collapse in oligodendrocytes and death of immature neural cells. J Immunol. 2004;172:1246–55. doi: 10.4049/jimmunol.172.2.1246. [DOI] [PubMed] [Google Scholar]
- 125.Parrinello S, et al. NF1 loss disrupts Schwann cell-axonal interactions: a novel role for semaphorin 4F. Genes Dev. 2008;22:3335–48. doi: 10.1101/gad.490608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Manitt C, et al. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J Neurosci. 2001;21:3911–22. doi: 10.1523/JNEUROSCI.21-11-03911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rajasekharan S, et al. Netrin 1 and Dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA. Development. 2009;136:415–26. doi: 10.1242/dev.018234. [DOI] [PubMed] [Google Scholar]
- 128.Jarjour AA, et al. Maintenance of axo-oligodendroglial paranodal junctions requires DCC and netrin-1. J Neurosci. 2008;28:11003–14. doi: 10.1523/JNEUROSCI.3285-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakamoto T, Kain KH, Ginsberg MH. Neurobiology: New connections between integrins and axon guidance. Curr Biol. 2004;14:R121–3. [PubMed] [Google Scholar]
- 130.Knoll B, et al. Serum response factor controls neuronal circuit assembly in the hippocampus. Nat Neurosci. 2006;9:195–204. doi: 10.1038/nn1627. [DOI] [PubMed] [Google Scholar]
- 131.Wickramasinghe SR, et al. Serum response factor mediates NGF-dependent target innervation by embryonic DRG sensory neurons. Neuron. 2008;58:532–45. doi: 10.1016/j.neuron.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stritt C, et al. Paracrine control of oligodendrocyte differentiation by SRF-directed neuronal gene expression. Nat Neurosci. 2009;12:418–27. doi: 10.1038/nn.2280. [DOI] [PubMed] [Google Scholar]
- 133.Monk KR, et al. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325:1402–5. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shen S, Li J, Casaccia-Bonnefil P. Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain. J Cell Biol. 2005;169:577–89. doi: 10.1083/jcb.200412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ye F, et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009 doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.He Y, et al. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–30. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi Y, Lee JS, Galvin KM. Everything you have ever wanted to know about Yin Yang 1. Biochim Biophys Acta. 1997;1332:F49–66. doi: 10.1016/s0304-419x(96)00044-3. [DOI] [PubMed] [Google Scholar]
- 138.Fancy SP, et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–85. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Feigenson K, Reid M, See J, Crenshaw EB, 3rd, Grinspan JB. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci. 2009;42:255–65. doi: 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 140.Fu H, et al. A genome-wide screen for spatially restricted expression patterns identifies transcription factors that regulate glial development. J Neurosci. 2009;29:11399–408. doi: 10.1523/JNEUROSCI.0160-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen Y, et al. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat Neurosci. 2009;12:1398–406. doi: 10.1038/nn.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lecca D, et al. The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS One. 2008;3:e3579. doi: 10.1371/journal.pone.0003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Arnett HA, et al. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–5. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- 144.Xin M, et al. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J Neurosci. 2005;25:1354–65. doi: 10.1523/JNEUROSCI.3034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Balabanov R, Popko B. Myelin repair: developmental myelination redux? Nat Neurosci. 2005;8:262–4. doi: 10.1038/nn0305-262. [DOI] [PubMed] [Google Scholar]
- 146.Derfuss T, et al. Contactin-2/TAG-1-directed autoimmunity is identified in multiple sclerosis patients and mediates gray matter pathology in animals. Proc Natl Acad Sci U S A. 2009;106:8302–7. doi: 10.1073/pnas.0901496106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Passage E, et al. Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot-Marie-Tooth disease. Nat Med. 2004;10:396–401. doi: 10.1038/nm1023. [DOI] [PubMed] [Google Scholar]
- 148.Kaya F, et al. Ascorbic acid inhibits PMP22 expression by reducing cAMP levels. Neuromuscul Disord. 2007;17:248–53. doi: 10.1016/j.nmd.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 149.Sereda MW, Meyer zu Horste G, Suter U, Uzma N, Nave KA. Therapeutic administration of progesterone antagonist in a model of Charcot-Marie-Tooth disease (CMT-1A). Nat Med. 2003;9:1533–7. doi: 10.1038/nm957. [DOI] [PubMed] [Google Scholar]
- 150.Meyer zu Horste G, et al. Antiprogesterone therapy uncouples axonal loss from demyelination in a transgenic rat model of CMT1A neuropathy. Ann Neurol. 2007;61:61–72. doi: 10.1002/ana.21026. [DOI] [PubMed] [Google Scholar]
- 151.Pareyson D, et al. A multicenter, randomized, double-blind, placebo-controlled trial of long-term ascorbic acid treatment in Charcot-Marie-Tooth disease type 1A (CMT-TRIAAL): the study protocol [EudraCT no.: 2006-000032-27]. Pharmacol Res. 2006;54:436–41. doi: 10.1016/j.phrs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 152.Shy ME. Therapeutic strategies for the inherited neuropathies. Neuromolecular Med. 2006;8:255–78. doi: 10.1385/nmm:8:1-2:255. [DOI] [PubMed] [Google Scholar]
- 153.Burns J, et al. Ascorbic acid for Charcot-Marie-Tooth disease type 1A in children: a randomised, double-blind, placebo-controlled, safety and efficacy trial. Lancet Neurol. 2009;8:537–44. doi: 10.1016/S1474-4422(09)70108-5. [DOI] [PubMed] [Google Scholar]
- 154.Nave KA, Sereda MW, Ehrenreich H. Mechanisms of disease: inherited demyelinating neuropathies--from basic to clinical research. Nat Clin Pract Neurol. 2007;3:453–64. doi: 10.1038/ncpneuro0583. [DOI] [PubMed] [Google Scholar]
- 155.Fernandez M, et al. Thyroid hormone administration enhances remyelination in chronic demyelinating inflammatory disease. Proc Natl Acad Sci U S A. 2004;101:16363–8. doi: 10.1073/pnas.0407262101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Verheijen MH, et al. SCAP is required for timely and proper myelin membrane synthesis. Proc Natl Acad Sci U S A. 2009;106:21383–8. doi: 10.1073/pnas.0905633106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Inoue K, et al. Congenital hypomyelinating neuropathy, central dysmyelination, and Waardenburg-Hirschsprung disease: phenotypes linked by SOX10 mutation. Ann Neurol. 2002;52:836–42. doi: 10.1002/ana.10404. [DOI] [PubMed] [Google Scholar]
- 158.Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7:11–8. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rorke LB, Riggs HE. Myelination of the Brain in the Newborn. J.P. Lippincott Company; Philadelphia: 1969. [Google Scholar]
- 160.Birchmeier C. ErbB receptors and the development of the nervous system. Exp Cell Res. 2009;315:611–8. doi: 10.1016/j.yexcr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 161.Rosenberg SS, Ng BK, Chan JR. The quest for remyelination: a new role for neurotrophins and their receptors. Brain Pathol. 2006;16:288–94. doi: 10.1111/j.1750-3639.2006.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xiao J, et al. BDNF exerts contrasting effects on peripheral myelination of NGF-dependent and BDNF-dependent DRG neurons. J Neurosci. 2009;29:4016–22. doi: 10.1523/JNEUROSCI.3811-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Woolley AG, et al. Developmental loss of NT-3 in vivo results in reduced levels of myelin-specific proteins, a reduced extent of myelination and increased apoptosis of Schwann cells. Glia. 2008;56:306–17. doi: 10.1002/glia.20614. [DOI] [PubMed] [Google Scholar]
- 164.Coman I, Barbin G, Charles P, Zalc B, Lubetzki C. Axonal signals in central nervous system myelination, demyelination and remyelination. J Neurol Sci. 2005;233:67–71. doi: 10.1016/j.jns.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 165.Charles P, et al. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc Natl Acad Sci U S A. 2000;97:7585–90. doi: 10.1073/pnas.100076197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ishibashi T, et al. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–32. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–71. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- 168.Stevens B, Ishibashi T, Chen JF, Fields RD. Adenosine: an activity-dependent axonal signal regulating MAP kinase and proliferation in developing Schwann cells. Neuron Glia Biol. 2004;1:23–34. doi: 10.1017/s1740925x04000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Feltri ML, Wrabetz L. Laminins and their receptors in Schwann cells and hereditary neuropathies. J Peripher Nerv Syst. 2005;10:128–43. doi: 10.1111/j.1085-9489.2005.0010204.x. [DOI] [PubMed] [Google Scholar]
- 170.Genoud S, Maricic I, Kumar V, Gage FH. Targeted expression of IGF-1 in the central nervous system fails to protect mice from experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;168:40–5. doi: 10.1016/j.jneuroim.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 171.Schumacher M, Sitruk-Ware R, De Nicola AF. Progesterone and progestins: neuroprotection and myelin repair. Curr Opin Pharmacol. 2008;8:740–6. doi: 10.1016/j.coph.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 172.Park SK, Solomon D, Vartanian T. Growth factor control of CNS myelination. Dev Neurosci. 2001;23:327–37. doi: 10.1159/000048716. [DOI] [PubMed] [Google Scholar]
- 173.Franco PG, Silvestroff L, Soto EF, Pasquini JM. Thyroid hormones promote differentiation of oligodendrocyte progenitor cells and improve remyelination after cuprizone-induced demyelination. Exp Neurol. 2008;212:458–67. doi: 10.1016/j.expneurol.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 174.Denisenko N, et al. Tumor suppressor schwannomin/merlin is critical for the organization of Schwann cell contacts in peripheral nerves. J Neurosci. 2008;28:10472–81. doi: 10.1523/JNEUROSCI.2537-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hoke A, Keswani SC. Neuroprotection in the PNS: erythropoietin and immunophilin ligands. Ann N Y Acad Sci. 2005;1053:491–501. doi: 10.1111/j.1749-6632.2005.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 176.Werner HB, et al. Proteolipid protein is required for transport of sirtuin 2 into CNS myelin. J Neurosci. 2007;27:7717–30. doi: 10.1523/JNEUROSCI.1254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Furusho M, Dupree JL, Bryant M, Bansal R. Disruption of fibroblast growth factor receptor signaling in nonmyelinating Schwann cells causes sensory axonal neuropathy and impairment of thermal pain sensitivity. J Neurosci. 2009;29:1608–14. doi: 10.1523/JNEUROSCI.5615-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]