Abstract
Non-tubercular mycobacteriae (NTM) are ubiquitous organisms found throughout the environment including soil and water. Incidence of NTM infection or isolation is increasing both in immunocompromised as well as immunocompetent patients and may present with a spectrum of disease similar to tuberculosis. The authors report cases of two patients with a background of cancer who presented with NTM infection mimicking lung cancer. The case report is followed by review of reported cases of NTM infections which presented similarly mimicking cancer. Based on their experience and the review of literature the authors recommend that all efforts should be made to obtain tissue for histology and acid fast bacilli examination in all cases which present with lung masses, nodules, bronchiectasis, air-space shadowing or cavity formation especially over a background of structural lung disease. Such cases should be kept under close clinical and radiological follow-up, as cancer and NTM infections can coexist.
Background
Non-tubercular mycobacteriae (NTM) are ubiquitous organisms found throughout the environment including soil and water. Although previously linked to immunodeficiency states such as AIDS, incidence of NTM infection or isolation is increasing both in immunocompromised as well as immunocompetent patients and is not limited to a particular geographical area.1 Thought to present with a spectrum of clinical disease similar to tuberculosis it may at times present a diagnostic challenge. We report cases of two patients with emphysema and a background of cancer who presented with NTM infection mimicking lung cancer.
Case presentation
The first case is of a 63-year-old lady with a background of emphysema and a 35-pack-year history of smoking who had right upper lobectomy in 1994 for a necrotic carcinoma which was poorly differentiated with mixed squamous and glandular differentiation. Her postoperative period was complicated by numerous lower respiratory tract infections but made eventual good recovery and was discharged from follow-up in June 2000.
In May 2007 she was admitted with an acute exacerbation of chronic obstructive pulmonary disease. She was found to be hypoxic with pO2 of 7.2 kPa and her blood results were as in table 1.
Table 1.
Blood test results on admission
| Haemoglobin | 13 g/l | Sodium | 131 mmol/l | Bilirubin | 28 Gmol/l |
|---|---|---|---|---|---|
| White cell count | 9.3 × 109/l | Potassium | 4.1 mmol/l | Alkaline phosphatase |
251 IU/l |
| C reactive protein | 63 mg/l | Urea | 7.9 mmol/l | Alanine transaminase |
269 IU/l |
| Erythrocyte sedimentation rate | 8 mm/1st h | Creatinine | 72 Gmol/l | Aspartate aminotransferase | 155 IU/l |
On admission, chest radiograph highlighted an incidental mass in the left upper lobe apart from the changes from the lobectomy on the right (figure 1).
Figure 1.
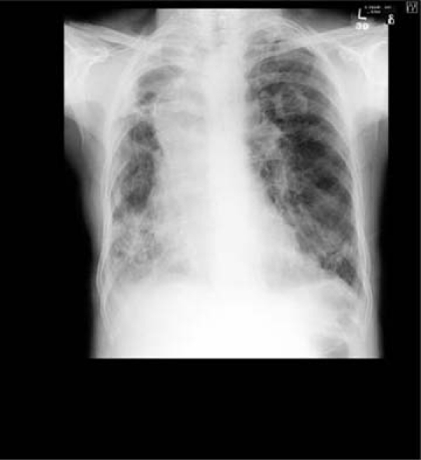
Chest x-ray on admission.
CT followed, which confirmed a cavitating lesion in the apical segment of the left upper lobe with spiculation, extensive mediastinal adenopathy, pericardial effusion, inhomogeneous liver and prominence of both adrenals (figure 2A,B). The overall picture with a background of cancer, investigation results including radiological findings and abnormal liver function tests suggested metastatic malignancy.
Figure 2.
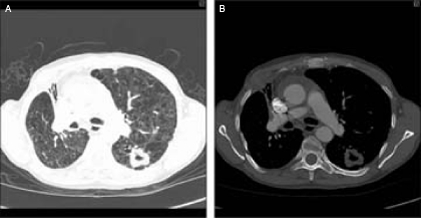
(A,B) Chest CT scan on presentation showing cavitating lesion on left chest.
Further investigations including biopsy of the lesion to confirm the diagnosis were offered to the patient which she declined.
By July 2007, she deteriorated with saturations of less than 85% on air and was started on oxygen therapy. Her deterioration was believed to be secondary to progression of her lung lesion contributed by chronic obstructive airways disease. However, over the next 3 months two separate isolates of Mycobacterium avium intracellulare (MAI) had been obtained from her sputum. With the patient having declined any further investigations for cancer she was started on ethambutol and rifampicin with the left upper lobe cavitating lesion attributed to either cancer recurrence or MAI. She dramatically responded to the treatment with saturations on air in excess of 95% and a repeat chest radiograph following treatment showing resolution of the cavity (figure 3A,B).
Figure 3.
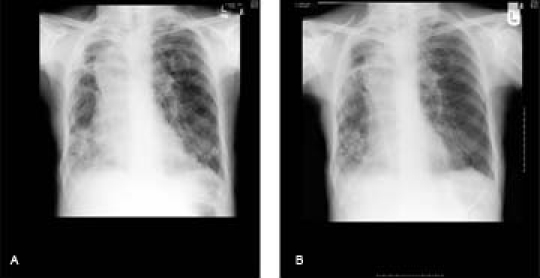
Chest x-ray on presentation (A) and after treatment (B).
The second case is that of a 66-year-old gentleman with a 30-pack-year history of smoking, emphysema and recurrent lower respiratory tract infections. In March 2007, he had a curative oesophagectomy and partial gastrectomy for a grade I oesophageal liposarcoma with the follow-up period complicated by a recurring stricture at the gastro-oesophageal anastomosis site which was treated with repeated dilatations.
In January 2008, he was admitted with recurrent chest infections over preceding few months and weight loss. The admission chest x-ray showed an ill-defined air space opacification within the upper zone of the left lung (figure 4).
Figure 4.
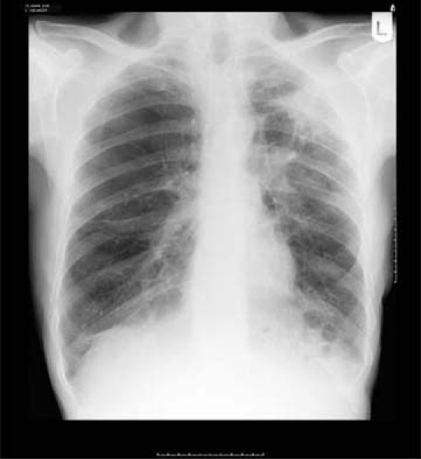
Chest x-ray on presentation.
This was initially thought to be inflammatory in nature but due to its persistence over the next 3 months he underwent a chest CT which showed a large triangular opacity in upper zone of the left lung with a diameter of 7 cm across its base. The lesion appeared to be in close contact with pleural surface and possibly invading the chest wall soft tissue but with no evidence of rib erosion. There was some mediastinal lymphadenopathy and no evidence of metastatic disease elsewhere (figure 5A,B).
Figure 5.
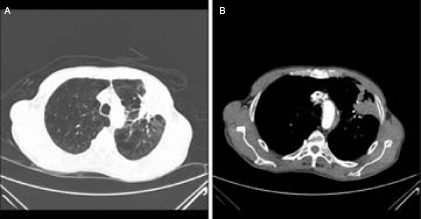
(A,B) CT of the chest pretreatment.
Based on the images alone, it could not be confirmed as to whether the lesion was an inflammatory, primary neoplastic or metastatic process.
Given the clinical presentation and radiological appearance over a background of cancer it was deemed necessary to exclude recurrence of malignancy and he underwent a CT-guided biopsy of the lung lesion. Histology confirmed scar tissue and necrotising granuloma, with no evidence of neoplasia. Bronchoscopic investigations and an interferon (Quantiferon) test were negative for TB. However, sputum culture and genetic probing confirmed two isolates of MAI. Hence he was started on ethambutol and rifampicin. Within 4 weeks of commencing the treatment, the patient improved clinically. A repeat chest radiograph confirmed improvement in the left lung upper lobe lesion indicating MAI infection as the aetiology rather than cancer recurrence (figure 6A,B).
Figure 6.
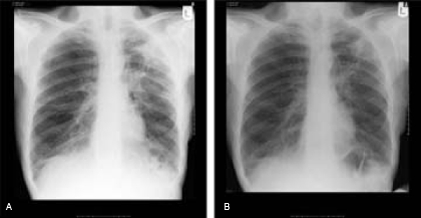
Chest x-ray on presentation (A) and after 4 weeks of treatment (B).
Outcome and follow-up
In summary, we present two patients with a medical history of malignancy and an opacification on chest x-ray suggestive of lung cancer who cultured MAI from their sputum and made a dramatic recovery when treated with ethambutol and rifampicin.
Discussion
Plain radiographic features of NTM infections are thought to be subtle and may take several years to become apparent, leading to a delay in diagnosis.2 CT appearances of NTM lung infection have been described as including bronchiectasis, air-space disease, nodules, scarring, volume loss and less commonly cavities, lymphadenopathy and pleural disease not strongly favouring any lung zone and progressive over a prolonged period of time without treatment. A multifocal presence of bronchiectasis, air-space shadowing and nodules on CT in an otherwise healthy patient has been suggested to be strongly indicative of NTM infection. Similar findings have been reported in a study to characterise CT appearances in Mycobacterium avium complex (MAC) infections in a subgroup of patients predominantly comprising of older women with no underlying malignancy or immunocompromise.3
Sawai et al4 report the case of a patient with a 50-pack-year history of smoking who presented with two nodules on the lung with spiculation and pleural indentation but without any calcification, cavitation or bronchiectasis, findings usually associated with NTM lung infections. Bronchial aspirates from one of the nodules isolated MAC and the patient was treated with antibiotics resulting in significant resolution of one of the nodules but not the other, the subsequent transbronchial biopsy of which resulted in the diagnosis of cancer. Attributing the incidence of NTM infections in cancer patients to the compromised host defences in such patients due to cancer or its treatment the authors highlighted the diagnostic dilemma of NTM infection co-existing with cancer and mimicking it. Cases of a pulmonary infiltrate mimicking lung cancer in a patient with papillary thyroid cancer and MAC infection presenting as a lung mass have similarly been reported.5–7 Kobashi et al8 presented a series of cases with pulmonary MAC infection as a cause of solitary pulmonary nodule that did not show any radiological features of NTM infection as above and were indistinguishable from cancer in showing pleural indentations, notching and spiculation thus necessitating biopsy. A nodule as in the preceding case has been reported to have a high fluorodeoxyglucose uptake on positron emission tomography scan with a standard uptake value of 3.4.9 NTM other than MAI have also been reported in various case reports as mimicking cancers involving other organs.10 11
As in our cases, confirming the diagnosis was important as NTM infections and cancer have different prognostic implications with NTM infections being treatable. Based on our experience and the review of literature we recommend that all efforts should be made to obtain tissue for histology and acid fast bacilli examination in all cases which present with lung masses, nodules, bronchiectasis, air-space shadowing or cavity formation especially over a background of structural lung disease. Such cases should be kept under close clinical and radiological follow-up, as cancer and NTM infections can co-exist.
Learning points.
-
▶
Isolation of NTM is increasing and is not limited to a particular geographical area.
-
▶
Every effort should be made to obtain a tissue sample for confirmation of cancer as NTM infection can present mimicking lung cancer.
-
▶
Confirmed cases of NTM infection on treatment should be kept under close follow-up as NTM can co-exist with cancer.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Management of opportunist mycobacterial infections: Joint Tuberculosis Committee Guidelines 1999 Subcommittee of the Joint Tuberculosis Committee of the British Thoracic Society. Thorax 2000;55:210–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore EH. Atypical mycobacterial infection in the lung: CT appearance. Radiology 1993;187:777–82 [DOI] [PubMed] [Google Scholar]
- 3.Hartman TE, Swensen SJ, Williams DE. Mycobacterium avium-intracellulare complex: evaluation with CT. Radiology 1993;187:23–6 [DOI] [PubMed] [Google Scholar]
- 4.Sawai T, Soda H, Kohno S. Mycobacterium intracellulare pulmonary infection which co-existed and mimicked lung cancer. Inter Med 2008;47:459–62 [DOI] [PubMed] [Google Scholar]
- 5.Gilliard L, Donaldson JC, Stoop DR, et al. Atypical mycobacterium infection mimicking lung cancer. Postgrad Med 1979;65:187, 189 [DOI] [PubMed] [Google Scholar]
- 6.Yano S, Kusumoto M, Asamuara H, et al. A case of Mycobacterium avium complex infection showing solitary pulmonary mass. Radiat Med 2002;20:147–50 [PubMed] [Google Scholar]
- 7.Tezuka Y, Endo S, Yamamoto S, et al. A large nontuberculous mycobacterial granuloma mimicking lung cancer; report of a case. Kyobu Geka 2004;57:1157–60 [PubMed] [Google Scholar]
- 8.Kobashi Y, Fukuda M, Yoshida K, et al. Four cases of pulmonary Mycobacterium avium intracellulare complex presenting as a solitary pulmonary nodule and a review of other cases in Japan. Respirology 2006;11:317–21 [DOI] [PubMed] [Google Scholar]
- 9.Schmeeckle KD, Yankelevitz D, Kim JW, et al. Increased uptake of 18F-fluorodeoxyglucose due to Mycobacterium avium complex in a solitary pulmonary nodule. J La State Med Soc 2008;160:150–2 [PubMed] [Google Scholar]
- 10.Tsai CW, Wang JT, Tsai CC, et al. Disseminated Mycobacterium kansasii infection in an HIV-negative patient presenting with mimicking multiple bone metastases. Diagn Microbiol Infect Dis 2006;54:211–16 [DOI] [PubMed] [Google Scholar]
- 11.Stout JE, Woods CE, Alvarez AA, et al. Mycobacterial bovis peritonitis mimicking ovarian cancer in a young woman. Clin Inf Dis 2001;33:e14–16 [DOI] [PubMed] [Google Scholar]


